Fibrin monomers polymerise to generate an insoluble fibrin clot. Activated factor XIII (FXIIIa) catalyses the formation of covalent bonds between adjacent fibrin molecules[1–3]. The interaction between fibrin(ogen) and FXIII has previously been studied[4–9]. The aim of this study was to elucidate FXIII contamination in four different commercially available fibrinogens purified from human plasma, and to characterise two techniques to remove FXIII from fibrinogen preparations.
Commercial fibrinogen was obtained from Calbiochem (human; Merck, Darmstadt, Germany), Enzyme Research Laboratories (human; ERL, Swansea, UK), Haematologic Technologies, Inc. (human; HTI, Essex Junction, VT, USA), Sigma-Aldrich (bovine; St Louis, MO, USA) and FXIII-free human fibrinogen from America Diagnostica Inc (Stamford, CT, USA) and reconstituted according to the manufacturers’ instructions. ERL fibrinogen was further purified to remove any FXIII contamination using one of two methods. The first method is a modification to a previously described ammonium sulphate precipitation method, whereby fibrinogen in solution is treated with ammonium sulphate to 20% (w/v) with 10mM CaCl2 at 4°C, followed by centrifugation at 3,000g for 20 minutes at 4°C, to recover FXIII depleted fibrinogen in the supernatant[7;10]. This method enables large scale purification. The second method employs affinity chromatography with an IF-1 antibody (Kamiya Biomedical, Seattle, WA, USA)[11]. Pooled normal plasma was prepared from 12 healthy volunteers according to the method used by Ariëns et al.[2] FXIII depleted plasma was purchased from ERL. Fibrogammin P, purified using a Sepharose 6B gel filtration column to remove the contaminating albumin and glucose[3], was used as the FXIII positive control for these assays.
FXIII (220ng) or 10μg of fibrinogen samples were subjected to SDS-PAGE gel analysis under reducing conditions. Gels were either stained using coomassie blue or subjected to western blotting and immunoblotted with a HRP-labelled antibody directed against FXIII A-subunit (ERL). Samples were tested for FXIII activity using a modification of a biotin-labelled pentylamine incorporation activity assay previously described by Philippou et al(14). Briefly, microtitre plates were coated with either 10μg/ml casein or 80μg/ml untreated fibrinogen (ERL) and blocked with 1% (w/v) bovine serum albumin (Sigma-Aldrich, Poole, Dorset, UK). Wells were incubated with 10μl of either 1 mg/ml of fibrinogen, 33% (v/v) plasma, or 22 μg/ml FXIII. The cross-linking reaction was initiated by the addition of 90μl of reaction mix containing final concentrations of 100μM DTT (Sigma-Aldrich), 5-(biotinamido)pentylamine (Pierce Chemical Co Rockford, IL, USA), 1mM calcium chloride and 1U/ml or 2U/ml thrombin (Calbiochem) for the fibrinogen or casein coats, respectively. The reaction was stopped by the addition of 200mM EDTA. Biotin-pentylamine incorporation was detected using 10μg/mL streptavidin–alkaline phosphatase (Sigma-Aldrich) followed by 1mg/mL p-nitrophenyl phosphate (Sigma). FXIII activities were determined from the change in absorbance over time and expressed as a percentage of the control FXIII sample.
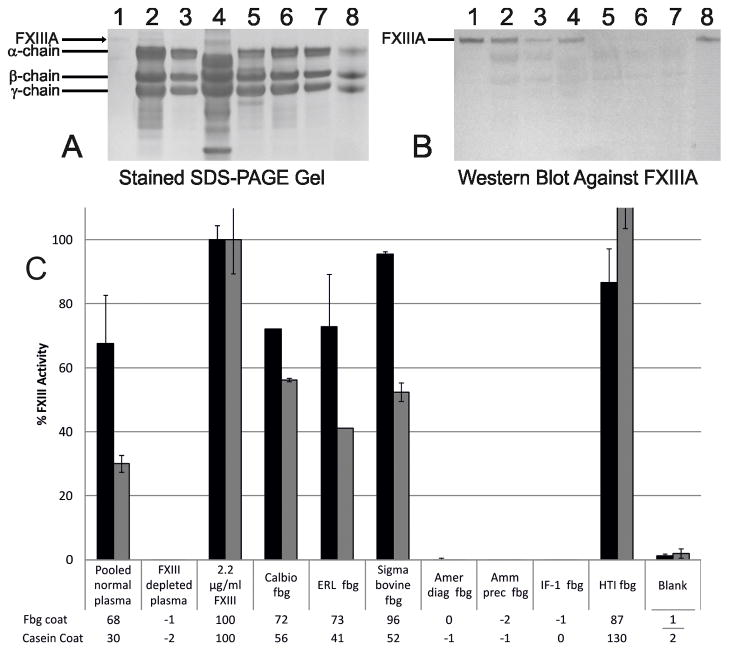
All fibrinogen preparations showed α, β, and γ-chains, as run on an SDS-PAGE gel (Fig. 1A). All four commercial fibrinogens showed FXIII-A subunit was present as identified by Western blot analyses (Fig. 1B). Reassuringly, no FXIII-A subunit could be seen in either the commercial fibrinogen from American Diagnostica that is sold as FXIII-depleted, or in the fibrinogen samples depleted of FXIII in-house (ammonium sulphate precipitation or IF-1 antibody affinity chromatography) (Figure B). This is corroborated by the lack of FXIII activity in these fibrinogen preparations, as shown by the FXIII activity assays (Fig. 1C). All four commercial fibrinogen samples showed varying degrees of FXIII activity whilst the commercial FXIII depleted plasma showed no activity (Fig. 1C). The American Diagnostica product is the only commercially available FXIII-free fibrinogen known to these authors, however this has been discontinued by the company. All other sources have been shown to contain active FXIII, although this can be removed or inactivated through in-house techniques. Iodoacetamide is one of the most commonly used FXIII inhibitors, but unpublished turbidity data from our laboratory has shown this may affect fibrin clot formation at higher concentrations. We present two methods to remove FXIII activity from fibrinogen: ammonium sulphate precipitation and IF-1 antibody affinity chromatography. Neither of these methods are known to affect fibrinogen clottability[7,15]. Separate batch attempts of the ammonium sulphate method sometimes showed faint bands of FXIII-A subunit by western blot analyses; however, these samples did not exhibit any FXIII activity, suggesting that the process of the ammonium sulphate precipitation inactivates any remaining FXIII. In contrast, when fibrinogen is purified using IF-1 chromatography there have been no traces of FXIII antigen or activity in the fibrinogen preparation.
Fig. 1.
The presence and activity of FXIII in fibrinogen samples. Fibrinogen samples were analysed to observe α, β, and γ-chains content using (A) Coomassie stained SDS-PAGE gel, and for the presence of FXIII-A subunit using (B) western blot analyses with a HRP-labelled anti-FXIII-A subunit antibody. Lanes are as follows (1) 220ng FXIII; (2) 10μg Calbiochem fibrinogen; (3) 10μg ERL fibrinogen; (4) 10μg Sigma bovine fibrinogen; (5) 10μg American Diagnostica FXIII-free fibrinogen; (6) 10μg ammonium sulphate precipitated fibrinogen; (7) 10μg IF-1 purified fibrinogen; (8) 10μg HTI fibrinogen. (C) Plasma and fibrinogen samples were tested for FXIII activity relative to purified FXIII using biotin-labelled pentylamine incorporation into fibrin (black) or casein (grey) coated microtitre plates by FXIII. The absorbance of each longest time point is shown with standard error bars of the replicates (n=3).
In summary, fibrinogen from commercial sources is highly contaminated with FXIII antigen and show significant cross-linking activity. In instances where it is key that fibrinogen is free of FXIII contamination we recommend that either ammonium sulphate precipitation is employed where large amounts of fibrinogen are required. However, where small amounts of fibrinogen are required we recommend that the fibrinogen is applied to affinity purification with an IF-1 antibody column.
Acknowledgments
The authors would like to thank Dr Shirley Uitte de Willige for her valuable input into investigating the ammonium sulphate precipitation method. The authors would also like to acknowledge NIH HL31048 (Professor Susan Lord) and MRC G0901546 awards (Drs Ariëns, Philippou).
Footnotes
Disclosure of Conflict of Interest
The authors declare no competing financial interests.
References
- 1.Chen R, Doolittle RF. - cross-linking sites in human and bovine fibrin. Biochemistry. 1971;10:4487–91. doi: 10.1021/bi00800a021. [DOI] [PubMed] [Google Scholar]
- 2.Gorkun OV, Veklich YI, Medved LV, Henschen AH, Weisel JW. Role of the alphaC domains of fibrin in clot formation. Biochemistry. 1994;33:6986–97. doi: 10.1021/bi00188a031. [DOI] [PubMed] [Google Scholar]
- 3.McKee PA, Mattock P, Hill RL. Subunit structure of human fibrinogen, soluble fibrin, and cross-linked insoluble fibrin. Proc Natl Acad Sci USA. 1970;66:738–44. doi: 10.1073/pnas.66.3.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenberg CS, Shuman MA. The zymogen forms of blood coagulation factor XIII bind specifically to fibrinogen. J Biol Chem. 1982;257:6096–101. [PubMed] [Google Scholar]
- 5.Greenberg CS, Dobson JV, Miraglia CC. Regulation of plasma factor XIII binding to fibrin in vitro. Blood. 1985;66:1028–34. [PubMed] [Google Scholar]
- 6.Hornyak TJ, Shafer JA. Interactions of factor XIII with fibrin as substrate and cofactor. Biochemistry. 1992;31:423–9. doi: 10.1021/bi00117a017. [DOI] [PubMed] [Google Scholar]
- 7.Siebenlist KR, Meh DA, Mosesson MW. Plasma factor XIII binds specifically to fibrinogen molecules containing gamma chains. Biochemistry. 1996;35:10448–53. doi: 10.1021/bi9606206. [DOI] [PubMed] [Google Scholar]
- 8.Procyk R, Bishop PD, Kudryk B. Fibrin--recombinant human factor XIII a-subunit association. Thromb Res. 1993;71:127–38. doi: 10.1016/0049-3848(93)90179-r. [DOI] [PubMed] [Google Scholar]
- 9.Smith KA, Adamson PJ, Pease RJ, Brown JM, Balmforth AJ, Cordell PA, Ariëns RA, Philippou H, Grant PJ. Interactions between factor XIII and the alphaC region of fibrinogen. Blood. 2011;117:3460–8. doi: 10.1182/blood-2010-10-313601. [DOI] [PubMed] [Google Scholar]
- 10.Ariens RA, Philippou H, Nagaswami C, Weisel JW, Lane DA, Grant PJ. The factor XIII V34L polymorphism accelerates thrombin activation of factor XIII and affects cross-linked fibrin structure. Blood. 2000;96:988–95. [PubMed] [Google Scholar]
- 11.Takebe M, Soe G, Kohno I, Sugo T, Matsuda M. Calcium ion-dependent monoclonal antibody against human fibrinogen: preparation, characterization, and application to fibrinogen purification. Thromb Haemost. 1995;73:662–7. [PubMed] [Google Scholar]
- 12.Ariens RA, Kohler HP, Mansfield MW, Grant PJ. Subunit antigen and activity levels of blood coagulation factor XIII in healthy individuals. Relation to sex, age, smoking, and hypertension. Arterioscler Thromb Vasc Biol. 1999;19:2012–6. doi: 10.1161/01.atv.19.8.2012. [DOI] [PubMed] [Google Scholar]
- 13.Standeven KF, Carter AM, Grant PJ, Weisel JW, Chernysh I, Masova L, Lord ST, Ariëns RA. Functional analysis of fibrin {gamma}-chain cross-linking by activated factor XIII: determination of a cross-linking pattern that maximizes clot stiffness. Blood. 2007;110:902–7. doi: 10.1182/blood-2007-01-066837. [DOI] [PubMed] [Google Scholar]
- 14.Philippou H, Rance J, Myles T, Hall SW, Ariens RA, Grant PJ, Leung L, Lane DA. Roles of low specificity and cofactor interaction sites on thrombin during factor XIII activation.Competition for cofactor sites on thrombin determines its fate. J Biol Chem. 2003;278:32020–6. doi: 10.1074/jbc.M305364200. [DOI] [PubMed] [Google Scholar]
- 15.Pieters M, Covic N, van der Westhuizen FH, Nagaswami C, Baras Y, Toit LD, Jerling JC, Elgar D, Edmondson KS, van Zyl DG, Rheeder P, Weisel JW. Glycaemic control improves fibrin network characteristics in type-2 diabetes -a purified fibrinogen model. Thromb Haemost. 2008;99:691–700. doi: 10.1160/TH07-11-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]