Abstract
Rationale
Exercise appears to be a promising non-pharmacological treatment for nicotine addiction that may be useful for the vulnerable adolescent population.
Objectives
To determine if wheel running, an animal model of aerobic exercise, during an abstinence period would decrease subsequent nicotine-seeking in rats that had extended access to nicotine self-administration during adolescence.
Methods
Male adolescent rats (n = 55) were trained to self-administer saline or nicotine infusions (5 or 10 μg/kg) under a fixed ratio 1 schedule with a maximum of 20 infusions/day beginning on postnatal day 30. After 5 days, access was extended to 23-hr/day with unlimited infusions for a total of 10 days. After the last self-administration session, rats were moved to polycarbonate cages for a 10-day abstinence period where they either had access to a locked or unlocked running wheel for 2-hr/day. Nicotine-seeking was examined following the 10th day of abstinence under a within-session extinction/cue-induced reinstatement paradigm.
Results
Intake was higher at the 10 μg/kg dose as compared to the 5 μg/kg dose; however, intake did not differ within doses prior to wheel assignment. Compared to saline controls, rats that self-administered nicotine at either dose showed a significant increase in drug-seeking during extinction, and consistent with our hypothesis, exercise during abstinence attenuated this effect. Nicotine led to modest, but significant levels of cue-induced reinstatement; however, in this adolescent-onset model, levels were variable and not affected by exercise.
Conclusions
Exercise may effectively reduce relapse vulnerability for adolescent-onset nicotine addiction.
Keywords: adolescent, nicotine, self-administration, wheel-running, exercise, nicotine-seeking, extinction, rat, reinstatement
Introduction
Cigarette smoking is the leading cause of preventable death in the United States and is a major health concern worldwide (Danaei et al. 2009; World Health Organization 2003). Most smokers initiate use during adolescence and those that do find it more difficult to quit later in life (Breslau and Peterson 1996). Adolescents are also known to progress to addiction more rapidly despite smoking less than adults (Tanski et al. 2004). Although rates of smoking have tapered off over recent decades, an alarming number of individuals begin smoking each year, and these rates are on the rise among adolescents (i.e., 1.5 million in 2009 compared to 1.3 million in 2002; SAMHSA 2010). Although there are FDA approved cessation treatments for nicotine addiction, none have been approved for adolescent populations, and the use of such pharmacotherapies is controversial in adolescents due to ongoing neurodevelopment (Kaplan and Ivanov 2011).
Exercise appears to be a promising non-pharmacological treatment for nicotine addiction that may be useful for the vulnerable adolescent population. Clinical studies in adults have shown that acute bouts of exercise decreases cigarette craving, withdrawal symptoms, and cue-elicited craving (Bock et al. 1999; Taylor and Katomeri 2007; Janse Van Rensburg et al. 2009, Haasova et al. 2012). However, the long-term effect of exercise in maintaining abstinence in adults is less clear and requires more rigorous effort and attention in the field (for review, see Ussher et al. 2012). In animals, wheel running, a model of aerobic exercise, appears to have both and short and long-term beneficial effects. Specifically, wheel running has been found to effectively reduce the self-administration of other psychostimulant drugs such as cocaine and methamphetamine and to decrease drug-seeking when contemporaneously available (Miller et al. 2012; Zlebnik et al. 2012; Smith and Pitts 2011; Smith et al. 2011; Zlebnik et al. 2010; Smith et al. 2008; Cosgrove et al. 2002). Wheel running during abstinence has also been shown to decrease subsequent cocaine-seeking even when not contemporaneously available (Lynch et al. 2010). Much less information is available on the effects of wheel running in adolescents and on nicotine self-administration.
The purpose of this study was to determine if wheel running during an abstinence period would prevent subsequent nicotine-seeking in rats that began self-administering nicotine during adolescence. An extended access paradigm was used to approximate human access conditions (Paterson and Markou 2004; O’Dell et al. 2007). Specifically, rats given extended access to nicotine (23 hr/day) have been reported to achieve daily nicotine levels that are comparable to those observed in humans (i.e. 0.18–1.38 versus 0.14–1.14 mg/kg nicotine, in rats and humans, respectively; Valentine et al. 1997). Importantly, such conditions also lead to the development of physical dependence (i.e. withdrawal symptoms; O’Dell and Koob, 2007) and after prolonged abstinence, produce increased subsequent nicotine-seeking (e.g., increased drug-seeking behavior and increased motivation to obtain the drug; Abdolahi et al. 2010). The effects of 2 hr/day access to a running wheel on nicotine-seeking were assessed at a time when levels of nicotine-seeking are known to be high (Abdolhai et al. 2010) using a within-session extinction/cue-induced reinstatement paradigm. Two doses of nicotine were used in this study to model light versus moderate-to-high levels of consumption. Additional groups of rats were given access to saline infusions in order to determine whether nicotine was functioning as a reinforcer under these extended access conditions. Additionally, given previous findings showing that light/auditory cues have reinforcement value, these saline controls established baseline levels of responding in the presence and absence of cues and allowed us to then determine whether prior nicotine self-administration affects subsequent extinction and reinstatement responding. These saline groups also served as a control for nonspecific effects of wheel running on subsequent lever responding during extinction and reinstatement. We expected to find a differential effect of wheel running in attenuating nicotine-seeking, with the greatest benefit seen in rats that formerly self-administered the lower nicotine dose since these rats were expected to self-administer less nicotine and have lower levels of subsequent nicotine-seeking as compared to rats that formerly self-administered the higher dose.
Materials and Methods
Animals
Male Sprague-Dawley (Charles River Laboratories, Portage, ME, USA) rats (N=55) arrived at the laboratory on postnatal day (PND) 22 with nicotine self-administration taking place from PND 30 to PND 45. Upon arrival, animals were individually housed in self-administration chambers within sound attenuating boxes (Med Associates, St Albans, VT, USA). Rats were maintained on a 12 hour light/dark schedule (lights on at 0700 and off at 1900) and had free access to food and water throughout the experiment. To ensure rapid acquisition of nicotine self-administration during the narrow window of adolescence, rats were pre-trained to lever-press for sucrose pellets (45 mg) as described previously (Lynch 2008). Briefly, on PND 25, rats were permitted daily 23-hour access to lever press for sucrose pellets under a fixed ratio 1 (FR1) schedule, in which a response on the active lever resulted in the delivery of a sucrose pellet from an automated food hopper. No light or auditory cues were used in these pre-training sessions. Pre-training was terminated after 2 days of responding for 50 or more sucrose pellets. Animals were weighed upon arrival, prior to surgery, for the 2 days following surgery and 3 times a week thereafter. All procedures were approved by the Animal Care and Use Committee at the University of Virginia.
Drug
Nicotine bitartrate (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in 0.9% sterile saline (pH 7.4) and passed through a microfilter; the doses are expressed as the free base weight. Two doses of nicotine were used in this study; a low (5 μg/kg/infusion) and a moderate (10 μg/kg/infusion) dose were selected because our pilot studies indicated these doses lead to nicotine intake that were comparable to light and moderate-to-heavy smoking, respectively. Infusions of saline or nicotine (5 μg/kg/infusion and 10μg/kg/infusion) were delivered at a rate of 0.1 ml/sec. Infusion duration was based on each individual’s weight which was adjusted 3 times/week. Nicotine solution was stored in the dark at 4°C but was available at room temperature during self-administration.
Surgery
On PND 28, rats were implanted with a chronic indwelling silastic catheter (0.51 and 0.94 mm o.d.; Dow Corning Corporation, Midland, MI, USA) to allow intravenous self-administration of nicotine or saline as described previously (Lynch 2009). Briefly, rats were anesthetized with a combination of dexmedetomidine (0.2 mg/kg) and ketamine (40 mg/kg). Between 1.6 – 2.0 cm of the catheter was inserted into the right jugular vein and anchored in place with suture. The other end was led subcutaneously to a small incision between the scapulae and connected to a metal cannula within a silicone harness that the animal wore for the remainder of the experiment. To ensure the patency of the catheter, it was flushed with heparinized saline every other day prior to the daily sessions. The animals were given 2 days to recover from surgery.
Nicotine Self-administration
Self-administration sessions began on PND 30 in the self-administration chambers in which the rats were housed. Chambers contained a house light (4.76 W) that was illuminated from 0700 to 1900 daily, 2 levers; a retractable active lever (drug-associated lever) and a stationary inactive lever, and holders for a food receptacle and water bottle. Above the active lever, there was a light (4.76W) that was illuminated during infusions. The pump containing the drug syringe was mounted within the sound attenuating chamber; therefore the rat also received auditory cues from the pump during infusions. To signal the beginning of a session the active lever was extended into the chamber. All self-administration occurred under a FR1 schedule, in which a single response on the active lever led to an infusion of nicotine. A response on the inactive lever was recorded but had no programmed consequence. A schematic of behavioral experiments is depicted in Fig. 1.
Fig 1.
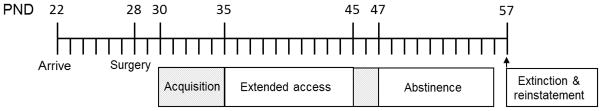
Schematic of behavioral protocol by postnatal day (PND). Self-administration began with a 5-day acquisition period in which rats were limited to 20 infusions/day under a fixed ratio 1 (FR1) schedule. Subsequently, rats were permitted an unlimited number of infusions for 23-hours/day during the 10-day extended access period. Next, infusions were again limited to 20 infusions/day for 2 days prior to forced abstinence. During the 10-day abstinence period rats had 2-hour access to either a locked or unlocked running wheel each day. After the last exercise session rats were returned to self-administration boxes and the following day began a within-session extinction/cue-induced reinstatement paradigm
Rats were initially trained to self-administer nicotine (5 or 10 μg/kg/infusion, N=21 or 18, respectively) or saline (N=16) under limited access conditions. Beginning on PND 30, rats were permitted a maximum of 20 infusions/day for 5 days (first shaded box in Fig. 1). Once 20 infusions had been delivered within a session, the active lever was retracted from the self-administration chamber. Animals were said to have acquired nicotine self-administration after 2 consecutive days of receiving all 20 infusions with a 2:1 preference for the active lever over the inactive lever. Beginning on PND 35, self-administration access was extended to 23-hours/day with an unlimited number of infusions available per session. Rats were then switched back to limited access conditions with a maximum of 20 infusions per session for 2 days prior to abstinence (second shaded box in Fig. 1) in order to normalize nicotine intake within each dose condition prior to abstinence.
Wheel running
On PND 47, rats were moved to polycarbonate cages with a wheel attachment (diameter: 35.6 cm; Med Associates, St Albans, Vermont, USA) for the 10 day abstinence period. Rats were randomly assigned to either 2-hr/day access to a locked (saline n = 8; 5 μg/kg dose n = 11; 10 μg/kg dose n = 9) or unlocked (saline n = 8; 5 μg/kg dose n = 10; 10 μg/kg dose n = 9) running wheel condition. A revolution counter attached to the wheel measured every quarter turn of the wheel and revolutions were recorded after each daily 2-hr session. In the unlocked wheel condition rats were free to run in the wheel during the daily access sessions. Rats in the locked wheel condition were able to enter the wheel, however the wheel was stationary and they were not able to run. Wheel access sessions occurred between 1000 and 1200, during the light phase of the light/dark cycle.
Extinction and cue-induced reinstatement
Following the last wheel session, animals were moved back to their operant conditioning chambers for the remainder of the day. Nicotine-seeking was then tested the following day (PND 57) under a within-session extinction and cue-induced reinstatement paradigm. Extinction responding was examined in at least 5 sessions that were each 1 hour in duration until responding extinguished (defined as fewer than 15 responses in the last session). Each extinction session began with the extension of the active lever into the self-administration chamber. Responses on the active lever were recorded but had no programmed consequence. At the end of each session there was a 5-minute timeout period in which the active lever was retracted to separate each extinction sessions. The 1-hour reinstatement session began after the final extinction session and a 5-minute timeout with the introduction of the active lever into the chamber and presentation of the discrete cues formerly associated with nicotine (i.e. the stimulus light and the sound of the pump) for 5 seconds. Each response on the active lever during this session resulted in the presentation of these cues but did not produce an infusion of nicotine or saline.
Data analysis
All data are presented as mean ± standard error of mean (SEM). Repeated measures analysis of variance (ANOVA) was used to analyze group differences in extended access self-administration, wheel-running during the abstinence period, and lever responses during extinction and reinstatement sessions. The main dependent measures included daily number of infusions during extended access, average daily intake during extended access, daily distance run during abstinence, and total responding during the extinction and reinstatement sessions. In addition to these analyses we also further examined rates of extinction by comparing the number of sessions required to meet the extinction criterion using univariate analysis of variance. Specifically, although a minimum of 5 extinction sessions were run some animals met the extinction criterion in fewer than 5 sessions and some required 1(two rats within the 5 μg/kg dose locked wheel condition, two within the 5 μg/kg dose unlocked wheel condition, and one rat within the 10 μg/kg unlocked wheel condition) or more additional sessions (one rat within the 10 μg/kg dose locked wheel condition). All post-hoc analyses were done using the Bonferroni corrected t-test. One tailed t-tests were used to test all a priori predicted comparisons. Specifically, we predicted that nicotine as compared to saline would lead to higher levels of responding under both extended access conditions and extinction/reinstatement testing conditions, that intake during extended access conditions and levels of responding during extinction/reinstatement would be highest in the 10 μg/kg dose group, and that wheel running would decrease levels of extinction/reinstatement responding, particularly in the 5 μg/kg dose group. The Pearson correlation coefficient was used to determine the associations between wheel running and subsequent extinction and reinstatement responding. Statistical analyses were performed using SPSS (version 20) and alpha was set to 0.05 for all tests.
Results
Extended access
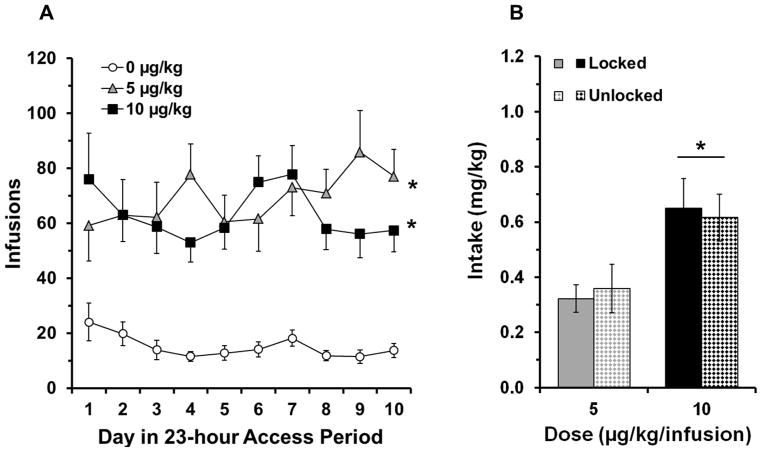
During the extended access period, rats that self-administered nicotine at the 5 and 10 μg/kg doses, obtained significantly more infusions than rats that self-administered saline (Fig. 2A; overall effect of group, F2, 52 = 18.2, p < 0.001; 5 μg/kg versus saline, t35 = 5.6, p < 0.001; 10 μg/kg versus saline, t32 = 6.6, p < 0.001). Although the number of infusions obtained did not differ between the 5 and 10 μg/kg groups, average nicotine intake was significantly greater for the 10 μg/kg group as compared to the 5 μg/kg group (effect of dose; F1, 35 = 14.0, p < 0.01; Fig. 2B). Importantly, within each dose, intake did not differ for rats that would later be in the unlocked and locked groups. Thus, any potential differences found in subsequent nicotine-seeking behavior cannot be attributed to differing levels of nicotine intake.
Fig 2.
Nicotine is reinforcing in adolescent rats. (a) Number of infusions (mean ± SEM) is plotted for each of the 10 days of extended access period. The open circles represent saline data points (n = 16), gray triangles represent the 5 μg/kg data points (n = 21), and black squares represent the 10 μg/kg data points (n = 18). An * indicates a significant difference from saline (p < 0.05). (b) Average daily intake during extended access (mean ± SEM) is plotted for all nicotine groups. Solid gray bar represents the locked wheel condition in the 5 μg/kg dose (n = 11), gray checkered bar represents the unlocked wheel condition in the 5 μg/kg dose (n = 10), black solid bar represents the locked wheel condition in the 10 μg/kg dose (n = 9) and black checkered bar represents the unlocked wheel condition in the 10 μg/kg dose (n = 9). The bar and * indicates a significant effect of dose (p < 0.05)
Inactive lever responding was comparable to the low levels of active lever responding observed among saline controls and did not differ significantly between groups. The average daily inactive lever responses during the extended access period were 22.5 ± 6.6, 31.6 ± 6.9, and 27.6 ± 5.4 for saline, 5 μg/kg dose, and 10 μg/kg dose groups, respectively. Animal weights were the same across all conditions at the onset of self-administration (saline, 102 ± 3 g; 5 μg/kg dose, 97 ± 2 g; 10 μg/kg dose, 98 ± 3 g). At the end of the self-administration period, however, the nicotine groups weighed slightly less than the saline group, although this effect did not reach statistical significance (saline, 225 ± 6 g; 5 μg/kg dose, 204 ± 7 g; 10 μg/kg dose, 213 ± 5 g).
Wheel running during abstinence
Although time-dependent changes in daily levels of running were observed where levels of running increased over time (Fig. 3A), overall levels of running did not differ between doses. A repeated measures ANOVA revealed a significant effect of day (F9,216 = 4.3, p < 0.001), but a non-significant effect of dose and a non-significant interaction of group by day. The similarity between the doses for levels of running is further illustrated in Fig. 3B where data are plotted as average daily distance run for the 0, 5, and 10 μg/kg groups.
Fig 3.
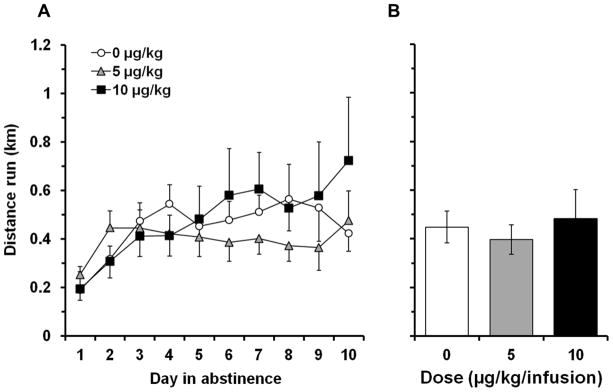
Nicotine self-administration does not affect wheel-running. (a) Number of revolutions (mean ± SEM) is plotted for each of the 10 days during the abstinence period. The open circles represent saline data points (n = 8), gray triangles represent the 5 μg/kg data points (n = 10), and black squares represent the 10 μg/kg data points (n = 9). (b) Average daily distance run in kilometers (mean ± SEM) is plotted between groups. The white bar represents the saline condition, the gray bar represents the 5 μg/kg dose, and the black bar represents the 10 μg/kg dose
Extinction and cue-induced reinstatement
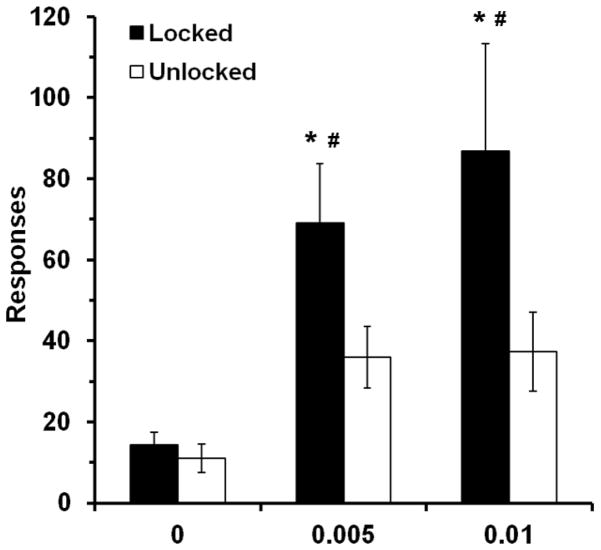
Levels of extinction responding were highest in rats that self-administered nicotine and were given access to a locked wheel during abstinence (Fig. 4). A repeated measures ANOVA on responding across the 5 extinction sessions revealed a significant effect of dose (F2, 49 = 6.5, p < 0.01) and wheel-condition (F1, 49 = 6.2, p < 0.05) (Fig. 4). Subsequent analysis of total extinction responding within each dose, revealed a significant effect of wheel condition within both the 5 and 10 μg/kg nicotine groups (t19 = 2.0, p < 0.05; t16 = 1.9, p < 0.05, respectively), but not within the saline group. Within the locked wheel condition, levels of extinction responding were significantly higher within both nicotine groups as compared to saline controls (effect of dose, F2, 25 = 4.0, p < 0.05; 5 μg/kg versus saline, t17 = 3.1, p < 0.01; 10 μg/kg versus saline, t15 = 3.0, p < 0.01; Fig. 5). Similarly, under unlocked wheel conditions, both nicotine groups had higher levels of responding during extinction as compared to saline (effect of dose, F2,24 = 3.5, p < 0.05; 5 μg/kg versus saline t16 = 2.7, p < 0.05; 10 μg/kg versus saline t15 = 2.4, p < 0.05) however, no difference was observed between the two nicotine dose conditions. Extinction responding was higher in the initial sessions particularly the first session as compared to later sessions with results from the overall ANOVA revealing a significant effect of time (F4, 196 = 5.4, p < 0.001). Although no significant interaction of time by dose was observed when levels of responding were examined across the 5 sessions of extinction, analysis of the number of sessions required to meet the extinction criteria revealed a significant effect of dose (F2, 49 = 4.2, p < 0.05), where both nicotine groups took significantly longer to extinguish responding as compared to saline (5 μg/kg versus saline, t35 = 2.4, p < 0.05; 10 μg/kg versus saline, t32 = 3.0, p < 0.01). However, no overall effect of wheel condition was observed on rates of extinction. There was also no association between the levels of wheel running and subsequent extinction responding. Analysis of the inactive lever responses during extinction revealed no effect of dose or wheel condition. Inactive lever responding for locked and unlocked wheel conditions were 8.3 ± 3.2 and 11.0 ± 3.4, 15.9 ± 2.8 and 14.8 ± 4.0, 21.0 ± 6.8 and 6.2 ± 2.5 for saline, 5 μg/kg dose, and 10 μg/kg dose groups, respectively. Thus, nicotine-seeking as assessed under extinction conditions was enhanced following abstinence in both nicotine groups, and wheel running during abstinence attenuated this effect.
Fig 4.
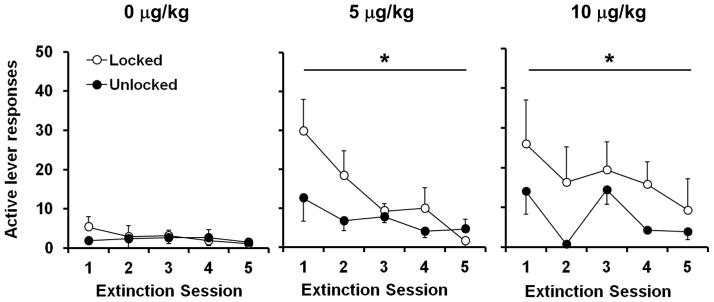
Wheel running during abstinence period significantly attenuates nicotine-seeking during extinction. Number of responses (mean ± SEM) is plotted for each of the first 5 extinction sessions. Open circles represent the locked wheel condition (saline, n = 8; 5 μg/kg dose, n = 11; 10 μg/kg dose n = 9) condition and black circles represent the unlocked wheel condition (saline, n = 8; 5 μg/kg dose, n = 10; 10 μg/kg dose, n = 9). A bar and * indicates a significant effect of exercise condition (p < 0.05)
Fig 5.
Wheel running effectively reduces during extinction responding in both nicotine doses. Total number of responses during the first 5 extinction sessions (mean ± SEM) is plotted by dose and wheel condition. White bars represent the locked wheel condition and black bars represent the unlocked wheel condition. The * indicates a significant difference from saline counterpart (p < 0.05) and the # indicates a significant difference from unlocked wheel group within dose (p < 0.05)
In contrast to the effects seen in extinction responding, levels of reinstatement responding did not differ by wheel condition. In fact, levels of reinstatement responding were relatively low and although a significant effect of time was observed when comparing levels of responding during the last extinction session to those observed during the reinstatement session (F1, 49 = 13.8, p < 0.01; Table 1), the interactions of time by wheel condition and time by wheel condition by dose were not significantly different. We did, however, observe a trend for a significant time by dose interaction (p = 0.053) with subsequent comparisons within each dose revealing significant effect of time in both the 5 and the 10 μg/kg doses (F1, 19 = 19.7, p < 0.001; F1, 16 = 4.7, p < 0.05, respectively) but not the saline, suggesting a tendency for nicotine to induce reinstatement responding. In order to further explore this possibility we examined responding during just the reinstatement session and found a significant effect of dose (F2, 49 = 3.4, p < 0.05) with reinstatement responding higher in both nicotine doses as compared to saline (5 μg/kg dose versus saline t35 = 3.7, p < 0.01; 10 μg/kg dose versus saline t32= 2.2, p < 0.05). Importantly, total number of responses during the last extinction session did not differ significantly by dose or wheel conditions. There was no association between the levels of wheel running and subsequent reinstatement responding. Analysis of the inactive lever responses during reinstatement revealed no effect of dose or wheel condition. Inactive lever responding for locked and unlocked wheel conditions were 0 ± 0 and 2.3± 1.1, 1.5 ± 0.7 and 2.5 ± 1.5, 0.7 ± 0.3 and 1.0 ± 0.8 for saline, 5 μg/kg dose, and 10 μg/kg dose groups, respectively. Although nicotine induced subsequent reinstatement responding, levels were modest and variable and did not differ by wheel condition.
Table 1.
Wheel running does not attenuate cue-induced reinstatement
| Group | Last Extinction Responses | Reinstatement Responses |
|---|---|---|
| 0 μg/kg | ||
| Locked | 1.1 (1.1) | 0.5 (0.4) |
| Unlocked | 1.5 (0.9) | 1.0 (0.6) |
|
| ||
| 5 μg/kg | # | |
| Locked | 0.7 (0.5) | 7.1 (2.5) |
| Unlocked | 1.5 (0.7) | 9.6 (2.8)* |
|
| ||
| 10 μg/kg | # | |
| Locked | 1.4 (0.8) | 13.6 (7.7) |
| Unlocked | 3.9 (1.8) | 7.8 (4.2)* |
The mean (± SEM) number of active lever responses
indicates a significant increase in responding from last extinction (p < 0.05)
indicates a significant increase from saline (p < 0.05)
Discussion
The goal of this study was to determine if wheel-running during an abstinence period would attenuate subsequent nicotine-seeking in an adolescent-onset model of nicotine addiction. Consistent with our hypothesis, wheel running during abstinence significantly attenuated nicotine-seeking. However, in contrast to our hypothesis, levels of nicotine-seeking were comparable between the two dose conditions, and wheel running produced a similar decrease under both dose conditions. The effects of wheel running were also apparent under extinction conditions, but not under cue-induced reinstatement conditions where levels of responding were relatively low and variable. The present study is the first to show that wheel running effectively reduces drug-seeking in an adolescent-onset model, as well as the first to demonstrate its efficacy for nicotine.
Wheel running during abstinence attenuated subsequent extinction responding in animals that had previously self-administered nicotine. This finding is consistent with previous work in which wheel running during an abstinence period significantly attenuated subsequent cocaine-seeking in adult rats (Lynch et al. 2010). Furthermore, as was found in Lynch et al. (2010), in this study there was no association between levels of wheel running and drug-seeking. It is also important to note that the rats were permitted to run during the light phase of their light/dark cycle where the levels of wheel running are expected to be lower as compared to the dark phase. Despite the time of day and modest levels of running in the wheel, we observed a significant attenuation of extinction responding 10 days after the last self-administration session and 1 day after the last wheel running session. Taken together, these data suggest that even modest levels of exercise may produce a beneficial effect on nicotine-seeking that persists beyond the acute bout of exercise.
Surprisingly, levels of cue-induced reinstatement were not affected by prior wheel running. These findings are in contrast to studies with cocaine, where wheel and treadmill running have been found to decrease subsequent cocaine-seeking under both extinction and reinstatement conditions (Lynch et al. 2010; Smith et al. 2012; Thanos et al. 2013). One possible explanation for these discrepant results is that exercise differentially affects drug-seeking during extinction versus reinstatement in rodents that previously self-administered cocaine versus nicotine. However, a more likely explanation is that the effects of wheel running during reinstatement were obscured in the present study by a floor effect. Specifically, levels of reinstatement responding under control locked wheel conditions were relatively low (mean, 9 ± 4) and variable (0–74 responses). Indeed, within the locked wheel condition, levels of reinstatement responding were roughly one third of the average observed in these same animals during the first hour of extinction, and comparable to the wheel running-attenuated levels observed during hour one of extinction within the unlocked wheel condition. The levels of reinstatement responding observed here are also much lower than those observed previously for cue-induced reinstatement following cocaine self-administration. In addition to differences in the levels of reinstatement responding induced following cocaine versus nicotine self-administration, age may account for this floor effect. Specifically, recent findings with cocaine, show that while a cocaine prime or stressor led to robust reinstatement in adolescents, the presentation of cues did not reinstate cocaine-seeking in this age group. However, in the adult comparison group, cues, stress, and cocaine prime all reinstated cocaine-seeking (Anker and Carroll 2010). These findings suggest that cue-induced reinstatement is not sufficiently robust in adolescents to reveal an effect of exercise. Cue-induced reinstatement is known to rely on the prefrontal cortex (Koya et al. 2008), and given that this brain region continues to develop throughout adolescence (Counotte et al. 2011; Geidd 2004), it is possible that adolescents are less vulnerable than adults to drug-seeking in response to cues. Further research is needed to address this possibility.
During the self-administration period, nicotine maintained significantly higher levels of responding than did saline, indicating that nicotine was functioning as a reinforcer in these adolescent rats. As has been previously reported with extended access conditions (O’Dell et al. 2007; Valentine et al. 1997), the higher (10 μg/kg) dose of nicotine maintained significantly higher levels of intake than did the lower (5 μg/kg) dose of nicotine. However, despite the differences in levels of intake and in contrast to our hypothesis, we found that levels of nicotine-seeking were similar between rats that had self-administered either the higher or lower dose of nicotine, and that wheel running during abstinence was equally effective at reducing nicotine-seeking during extinction between both nicotine groups. These results suggest that exercise may be equally effective in both light and moderate to heavy smokers. It should be noted, however, that because rats metabolize nicotine faster than humans, both doses of nicotine may have resulted in lower levels of nicotine in the brain as compared to those achieved in human smokers (Matta et al. 2007). Although exercise can also affect drug metabolism (Døssing. 1985), this would not have been a contributing factor in this study because wheel running did not overlap with nicotine self-administration.
There are currently no FDA-approved drug therapies for smoking cessation in adolescent smokers. The findings from this study showing that wheel running during abstinence attenuates subsequent nicotine-seeking in an adolescent-onset model suggests that exercise may effectively prevent smoking relapse in teens. Our current findings in rats are also consistent with recent findings in adolescent humans showing that high school students enrolled in an after school program with an exercise component were more likely than other high school students to abstain from smoking (Horn et al. 2011). One factor to consider is that while wheel running is highly reinforcing in rats, humans have different affinities for various types of exercise (Ekkekakis et al. 2008) and may benefit most from exercise that they “like” to do. In the present study, rats that ran in the wheel showed a significant benefit as compared to rats that could only sit or climb on the wheel and thus appear to benefit from aerobic-like exercise. Future studies should explore the benefits of aerobic and non-aerobic exercise on adolescent smoking behavior.
Acknowledgments
This work was supported by grants from Virginia Foundation for Healthy Youth 8520667 and 8520893 (D.H.B.), NIDA RO1DA024716-F1 (W.J.L.), and NIH training grant T32 HD007323 (V.S.).
Footnotes
The authors have no conflicts of interest to disclose.
References
- Abdolahi A, Acosta G, Breslin FJ, Hemby SE, Lynch WJ. Incubation of nicotine-seeking is associated with enhanced protein kinase A-regulated signaling of dopamine- and cAMP-regulated phosphoprotein of 32 kDa in the insular cortex. Eur J Neurosci. 2010:31. doi: 10.1111/j.1460-9568.2010.07114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Reinstatement of cocaine seeking induced by drugs, cues, and stress in adolescent and adult rats. Psychopharmacology. 2010;208:211–222. doi: 10.1007/s00213-009-1721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock BC, Marcus BH, King TK, Borrelli B, Roberts MR. Exercise effects on withdrawal and mood among women attempting smoking cessation. Addict Behav. 1999;24:399–410. doi: 10.1016/s0306-4603(98)00088-4. [DOI] [PubMed] [Google Scholar]
- Breslau N, Peterson EL. Smoking cessation in young adults: Age at initiation of cigarette smoking and other suspected influences. Am J Public Health. 1996;86:214–220. doi: 10.2105/ajph.86.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav. 2001;70:515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Counotte DS, Smit AB, Pattij T, Spijker S. Development of the motivational system during adolescence, and its sensitivity to disruption by nicotine. Dev Cogn Neurosci. 2011;1:430–443. doi: 10.1016/j.dcn.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP, Hunter RG, Carroll ME. Wheel-running attenuates intravenous cocaine self-administration in rats: sex differences. Pharmacol Biochem Behav. 2002;73:663–71. doi: 10.1016/s0091-3057(02)00853-5. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Bossert JM, Koya E, Shaham Y. Context-induced relapse to drug seeking: a review. Phil Trans Soc B. 2008;363:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, et al. The Preventable Causes of Death in the United States: Comparative Risk Assessment of Dietary, Lifestyle, and Metabolic Risk Factors. PLoS Med. 2009;6(4):e1000058. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diergaarde L, de Vries W, Raaso H, Schoffelmeer ANM, De Vries TJ. Contextual renewal of nicotine-seeking in rats and its suppression by the cannabinoid-1 receptor antagonist Rimonabant (SR141716A) Neuropharmacology. 2008;55:712–716. doi: 10.1016/j.neuropharm.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Døssing M. Effect of acute and chronic exercise on hepatic drug metabolism. Clin Pharmacokinet. 1985;10:426–431. doi: 10.2165/00003088-198510050-00004. [DOI] [PubMed] [Google Scholar]
- Ekkekakis P, Hall EE, Petruzzello SJ. The relationship between exercise intensity and affective responses demystified: To crack the 40-year-old nut, repace the 40-year-old nutcracker. Ann Behav Med. 2008;35:136–149. doi: 10.1007/s12160-008-9025-z. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann NY Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Kaplan G, Ivanov I. Pharmacotherapy for substance abuse disorders in adolescence. Pediatr Clin North Am. 2011;58:243–258. doi: 10.1016/j.pcl.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Haasova M, Warren FC, Ussher M, Janse Van Rensburg K, Faulkner G, Cropley M, Byron-Daniel J, Everson-Hock ES, Oh H, Taylor AH. The acute effects of physical activity on cigarette cravings: systematic review and meta-analysis with individual participant data. Addiction. 2012 doi: 10.1111/j.1360-0443.2012.04034. [DOI] [PubMed] [Google Scholar]
- Horn K, Dino G, Branstetter SA, Zhang J, Noerachmanto N, Jarrett T, Taylor M. Effects of physical activity on teen smoking cessation. Pediatrics. 2011;128:801–811. doi: 10.1542/peds.2010-2599. [DOI] [PubMed] [Google Scholar]
- Janse Van Rensburg K, Taylor A, Hodgson T. The effects of acute exercise on attentional bias towards smoking related stimuli during temporary abstinence from smoking. Addiction. 2009;104:1910–1917. doi: 10.1111/j.1360-0443.2009.02692.x. [DOI] [PubMed] [Google Scholar]
- Koya E, Uejima JL, Wihbey KA, Bossert JM, Hope BT, Shaham Y. Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology. 2009;56:177–85. doi: 10.1016/j.neuropharm.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ. Acquisition and maintenance of cocaine self-administration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacology. 2008;197:237–246. doi: 10.1007/s00213-007-1028-0. [DOI] [PubMed] [Google Scholar]
- Lynch WJ. Sex and ovarian hormones influence vulnerability and motivation for nicotine during adolescence in rats. Pharmacol Biochem Behav. 2009;94:43–50. doi: 10.1016/j.pbb.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Piehl KB, Acosta G, Peterson AB, Hemby SE. Aerobic exercise attenuates reinstatement of cocaine-seeking behavior and associated neuroadaptations in the prefrontal cortex. Biol Psychiatry. 2010;68:774–777. doi: 10.1016/j.biopsych.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, et al. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology. 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- Miller ML, Vaillancourt BD, Wright MJ, Jr, Aarde SM, Vandewater SA, Creehan KM, Taffe MA. Reciprocal inhibitory effects of intravenous d-methamphetamine self-administration and wheel activity in rats. Drug Alcohol Depend. 2011;121:90–6. doi: 10.1016/j.drugalcdep.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Koob GF. ‘Nicotine deprivation effect’ in rats with intermittent 23-hour access to intravenous nicotine self-administration. Pharmacol Biochem Behav. 2007;86:346–353. doi: 10.1016/j.pbb.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen CM, Winder DG. Stimulus dynamics increase the self-administration of compound visual and auditory stimuli. Neurosci Lett. 2012;511:8–11. doi: 10.1016/j.neulet.2011.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Prolonged nicotine dependence associated with extended access to nicotine self-administration in rats. Psychopharmacology. 2004;173:64–72. doi: 10.1007/s00213-003-1692-7. [DOI] [PubMed] [Google Scholar]
- Smith MA, Pennock MM, Walker KL, Lang KC. Access to a running wheel decreases cocaine-primed and cue-induced reinstatement in male and female rats. Drug Alcohol Depend. 2012;121:54–61. doi: 10.1016/j.drugalcdep.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Pitts EG. Access to a running wheel inhibits the acquisition of cocaine self-administration. Pharmacol Biochem Behav. 2011;100:237–43. doi: 10.1016/j.pbb.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Walker KL, Cole KT, Lang KC. The effects of aerobic exercise on cocaine self-administration in male and female rats. Pyschopharmacology. 2011;218:357–69. doi: 10.1007/s00213-011-2321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Schmidt KT, Iordanou JC, Mustroph ML. Aerobic exercise decreases the positive-reinforcing effects of cocaine. Drug Alcohol Depend. 2008;98:129–135. doi: 10.1016/j.drugalcdep.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. NSDUH Series H-38A, HHS Publication No SMA 10-4856 Findings. Office of Applied Studies; Rockville, MD: 2010. Results from the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings. [Google Scholar]
- Tanski SE, Prokhorov AV, Kein JD. Youth and tobacco. Minerva Pediatr. 2004;56:553–565. [PubMed] [Google Scholar]
- Taylor A, Katomeri M. Walking reduces cue-elicited cigarette cravings and withdrawal symptoms, and delays ad libitum smoking. Nicotine Tob Res. 2007;9:1183–1190. doi: 10.1080/14622200701648896. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Stamos J, Robinson LS, Heyman G, Tucci A, Wang GJ, Robinson JK, Anderson BJ, Volkow ND. Daily treadmill exercise attenuates cocaine cue-induced reinstatement and cocaine induced locomoter responses but increases cocaine-primed reinstatement. Behav Brain Res. 2013;239:8–14. doi: 10.1016/j.bbr.2012.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine JD, Hokanson JS, Matta SG, Sharp BM. Self-administration in rats allowed unlimited access to nicotine. Psychopharmacology. 1997;133:300–304. doi: 10.1007/s002130050405. [DOI] [PubMed] [Google Scholar]
- World Health Organization. World health report: Shaping the future. 2003 http://www.who.int/whr/2003/en/, retrieved 8 Aug 2012.
- Zlebnik NE, Anker JJ, Gliddon LA, Carroll ME. Reduction of extinction and reinstatement of cocaine seeking by wheel running in females. Pyschopharmacology. 2010;209:113–125. doi: 10.1007/s00213-010-1776-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlebnik NE, Anker JJ, Carroll ME. Exercise to reduce the escalation of cocaine self-administration in adolescent and adult rats. Pyschopharmacology. 2012 doi: 10.1007/s00213-012-2760-7. Published online: 03 July 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]