Abstract
Glioblastoma tumors are characterized by their invasiveness and resistance to therapies. The transcription factor STAT3 was recently identified as a master transcriptional regulator in the mesenchymal subtype of GBM, which has generated an increased interest in targeting STAT3. We have evaluated more closely the mechanism of action of one particular STAT3 inhibitor, JSI-124 (cucurbitacin I). In this study, we confirmed that JSI-124 inhibits both constitutive and stimulus-induced JAK2 and STAT3 phosphorylation, and decreases cell proliferation while inducing apoptosis in cultured GBM cells. However, we discovered that prior to the inhibition of STAT3, JSI-124 activates the NF-κB pathway, via NF-κB p65 phosphorylation and nuclear translocation. In addition, JSI-124 treatment induces the expression of IL-6, IL-8 and SOCS3 mRNA, which leads to a corresponding increase in IL-6, IL-8 and SOCS3 protein expression. Moreover, the NF-κB driven SOCS3 expression acts as a negative regulator of STAT3, abrogating any subsequent STAT3 activation and provides a mechanism of STAT3 inhibition following JSI-124 treatment. Chromatin immunoprecipitation analysis confirms that NF-κB p65 in addition to other activating co-factors are found at the promoters of IL-6, IL-8 and SOCS3, following JSI-124 treatment. Using pharmacological inhibition of NF-κB and inducible knockdown of NF-κB p65, we found that JSI-124-induced expression of IL-6, IL-8 and SOCS3 was significantly inhibited, demonstrating an NF-κB dependent mechanism. Our data indicate that although JSI-124 may demonstrate potential anti-tumor effects through inhibition of STAT3, other off-target pro-inflammatory pathways are activated, emphasizing that more careful and thorough pre-clinical investigations must be implemented to prevent potential harmful effects.
Keywords: JSI-124, Glioblastoma, STAT3, NF-κB
Introduction
Glioblastoma (GBM) is the most common malignant tumor of the central nervous system and remains a challenging disease to treat due to the high rate of recurrence and resistance to current treatments (1, 2). Recent efforts have been made elucidating genetic classifications of GBM tumors with the hopes of developing more effective therapies (3, 4). The Cancer Genome Atlas (TCGA) has determined that GBM tumors can be genetically divided into 4 subtypes: classical, mesenchymal, proneural and neural (5). Of these subtypes, mesenchymal tumors have been associated with the worst survival. In addition, it is believed that almost all GBM tumors that recur are of the mesenchymal subtype (3). Two pathways of interest, the Nuclear Factor-κB (NF-κB) and Janus Kinase (JAK)/Signal Transducer and Activator of Transcription (STAT), have been linked to this deadly subtype (5–7).
The NF-κB family consists of five members (p65/RelA, RelB, c-Rel, NF-κB1/p50 and NF-κB/p52) (8). The predominant form of NF-κB is a dimer composed of p65 and p50 proteins and under basal conditions is found in an inactive form in the cytosol through interactions with the inhibitor of NF-κB (IκB) proteins, specifically IκBα. In response to stimuli such as TNF-α, the upstream kinase, IκB Kinase (IKK), phosphorylates IκBα, which leads to ubiquitination and degradation of IκBα (8, 9). The NF-κB p65/p50 dimer then translocates into the nucleus where it becomes phosphorylated, binds to DNA and induces the expression of genes involved in inflammation such as IL-8 and IL-6, as well as anti-apoptotic genes including cIAP2, Bcl-2 and Bcl-xL (10).
The JAK/STAT pathway is commonly activated in response to various immune-mediated and inflammatory stimuli (11). There are four JAK kinases (JAK1, 2, 3, and TYK2) that are responsible for phosphorylating seven STAT proteins (STAT1-5a, 5b and 6). Cytokines of the Interleukin (IL)-6 family (including IL-6, Leukemia Inhibitory Factor (LIF), Oncostatin M (OSM), and IL-11) preferentially phosphorylate and activate JAK2 and STAT3 (12). Cytokine binding to the corresponding extracellular receptor leads to activation of the receptor and in turn causes auto- and trans-phosphorylation of intracellular JAK2 (11). STAT3 then becomes phosphorylated by JAK2, dimerizes, translocates to the nucleus and binds to DNA. STAT3 induces the transcription of several pro-survival genes including IL-6, VEGF and c-Myc (13). Importantly, STAT3 also induces the expression of Suppressor Of Cytokine Signaling (SOCS) proteins, specifically SOCS3, which functions as a negative regulator preventing prolonged STAT3 activation (14).
Numerous studies have demonstrated that the activities of STAT3 and NF-κB are elevated in many cancers, including GBM (10, 13, 15, 16). We and others have observed increased activity and protein levels of both NF-κB and STAT3 in GBM (17–20). Moreover, STAT3 has been determined to be a master regulator of the mesenchymal subtype of GBM (6, 7), and loss of IκBα has also been linked to GBM (21). Due to the increased interest in these two pathways, numerous pharmacological inhibitors have been developed targeting NF-κB and STAT3 with the hopes of benefiting patients with various cancers including GBM (22, 23).
JSI-124 (cucurbitacin I), a chemical compound belonging to the cucurbitacin family, was first discovered as a potent STAT3 inhibitor in multiple cancer cell lines (24). Inhibition of STAT3 activity was attributed to a disruption in STAT3 DNA binding activity and gene expression, and a reduction in subcutaneous lung and breast xenograft tumors was observed with JSI-124 treatment (24), demonstrating anti-tumor potential. Furthermore, JSI-124 has been shown to be a strong activator of apoptosis in multiple tumor cell lines including GBM and has a synergistic effect with other anti-tumor agents (20, 25–27). However, the exact mechanism of STAT3 inhibition by JSI-124 remains unclear. In this study, we set out to examine more closely the effect of JSI-124 treatment on GBM tumor cells in vitro. Consistent with other findings, we found that JSI-124 inhibited both constitutive and stimulus-induced JAK2 and STAT3 phosphorylation, decreased cell proliferation and induced apoptosis in human GBM cells. However, prior to the inhibition of STAT3, time course analysis revealed activation of the NF-κB pathway starting at 15 min post JSI-124 treatment. In addition, we found that JSI-124-induced mRNA expression of IL-8, IL-6 and SOCS3. This increase in mRNA expression translated to protein expression as determined by both IL-8 and IL-6 secretion and cellular SOCS3 protein levels. Pharmacological inhibition and inducible knockdown of NF-κB p65 significantly inhibited JSI-124-induced gene expression. These data reveal that in addition to inhibition of STAT3, additional off-target pathways, including NF-κB, are activated in response to JSI-124 treatment.
Materials and Methods
Reagents and Cells
Antibodies (Ab) to phosphorylated STAT3 (Y705), phosphorylated IKKα/β (S176/180) and phosphorylated p65 (S276) were from Cell Signaling Technologies (Beverly, MA); total IKKα/β, total IκBα, SOCS3, phosphorylated JAK2 (Y1007/1008), total JAK2, and total p65 from Santa Cruz (Santa Cruz, CA); total STAT3, Caspase 3 and PARP from BD Transduction Labs (San Jose, CA); and GAPDH from AbCam (Cambridge, MA). For chromatin immunoprecipitation (ChIP) experiments, Ab to p65 (AbCam), phosphorylated p65 (Cell Signaling), phospho-Pol II (S5; Covance) and STAT3 (Santa Cruz) were used. JSI-124 and BAY-11-7085 were purchased from Calbiochem (La Jolla, CA). Oncostatin M (OSM) was purchased from R&D Systems (Minneapolis, MN). U87-MG and U251-MG cells were maintained as previously described (19). U251-MG cells were authenticated and are the same as the parent line of Dr. Darrell Bigner (Duke University). U87-MG cells were purchased from ATCC and are authentic and consistent with the STR profile in the ATCC database. U251-MG cells that stably express the tetracycline repressor (TetR) protein and inducibly express short hairpin RNA (shRNA) specific for NF-κB p65 (sh-p65) were generated as previously described (19). Briefly, the plasmid carrying shRNA specific for p65 was generated by annealing double-stranded oligonucleotide specific for a 19-bp stretch of the p65 ORF (AA CTG TTC CCC CTC ATC TT) into the pBABE-HI-TetO plasmid, which is under dual control of the tetracycline operator and the HI polymerase (Pol) III promoter. The pBABE-HI-TetO plasmid was a generous gift of Dr. Xinbin Chen (University of California at Davis, CA). Primary astrocyte cultures from C57BL/6 mice were established from neonatal cerebra as described (28).
Immunoblotting
Cells were harvested and lysed in RIPA lysis buffer with protease inhibitors. Protein concentration was determined using the Pierce BCA Assay. Equivalent amounts of protein were run on SDS-PAGE gels and then transferred onto a nitrocellulose membrane. After blocking with 5% milk in Tris Buffered Saline (0.01% Tween) for 1 h, primary Ab were incubated overnight at 4°C, followed by 1 h with biotinylated HRP secondary Ab, and developed with chemiluminescent ECL (Pierce, Rockford, IL), as previously described (29). Immunoblots were initially probed with antibodies directed towards the phosphorylated protein followed by stripping and re-probing with antibodies directed against the total protein levels.
Subcellular Fractionation
Nuclear and cytosolic fractions were isolated as previously described (30). Briefly, cells were washed twice with cold PBS followed by the addition of 0.05% NP-40 lysis solution to each sample. Cells were collected and centrifuged at 2700 X g for 10 min at 4°C. Supernatant (cytosol) was removed, centrifuged at 20,000 X g for 15 min, and the top 100 μl saved as the pure cytosolic fraction. The pellet (nuclei) was resuspended in wash buffer and centrifuged at 2700 X g for 5 min. The supernatant was discarded, pellet resuspended in wash buffer, layered on top of 1 ml of 1 M sucrose and centrifuged at 2700 X g for 10 min. The supernatant was discarded, and the pellet was washed in 0.05% NP-40 lysis solution and centrifuged at 2700 X g for 5 min. The pellet was resuspended in nuclear extraction buffer, incubated on ice for 30 min, and centrifuged at 20,000 X g for 15 min. The supernatant was saved as pure nuclear extract. To verify purity, samples were immunoblotted with caspase 3 and PARP Ab for cytosolic and nuclear fractions, respectively.
Cell Proliferation Assay
Cells were plated in 96 well plates at a density of 1 X 104 cells/well, and the WST-1 Cell Proliferation Assay (Roche) was performed as previously described (31).
ELISA
Cells were treated with JSI-124 for the indicated times and supernatants collected. Human IL-6 and IL-8 ELISA kits were purchased from BioLegend (San Diego, CA) and performed as previously described (32).
Quantitative RT-PCR
Cells were washed with PBS, and total RNA was isolated using TRIzol (Invitrogen) as previously described (28). Approximately 1 μg of RNA per sample was used to generate cDNA by reverse transcription for PCR. Pre-designed Taqman primers (Applied Biosystems) were used to obtain quantitative PCR results using the Applied Biosytems StepOnePlus Real-Time PCR System Thermal Cycling Block and corresponding software analysis for data quantification (StepOneSoftware v2.1, Applied Biosystems). The following Taqman primers and the corresponding Gene Ref # were used: human IL-6 (Hs00174131_m1), human IL-8 (Hs00174103_m1), human NFKBIA (Hs00153283_m1) and human SOCS3 (Hs02330328_s1). Eukaryotic 18s rRNA (Hs99999901_s1) was used as an endogenous control.
Chromatin Immunoprecipitation (ChIP)
ChIP assays were performed as previously described (19, 32). Immunoprecipitation was performed with 5 μg of the appropriate Ab, and immune complexes absorbed with protein A beads or protein G beads blocked with bovine serum albumin and salmon sperm DNA. Immunoprecipitated DNA was subjected to semi-quantitative PCR and analyzed by gel electrophoresis using the following primers: IL-8 Forward 5′-GGG CCA TCA GTT GCA AAT C-3′; IL-8 Reverse 5′-TTC CTT CCG GTG GTT TCT TC-3′; IL-6 Forward 5′-GCG ATG GAG TCA GAG GAA AC-3′; IL-6 Reverse 5′-TGA GGC TAG CGC TAA GAA GC-3′; SOCS3 Forward 5′-GGT CTC CCC TCT GGA ATC TG-3′; SOCS3 Reverse 5′-CCC CCA AAC TTC TCA TTC ACA–3′.
Statistical Analysis
Student’s t-tests were performed for comparison of two values, and ANOVA analysis was performed on appropriate multi-variable analyses. p<0.05 was considered statistically significant.
Results
JSI-124 inhibits constitutive and stimulus-induced JAK2 and STAT3 activation in GBM cells
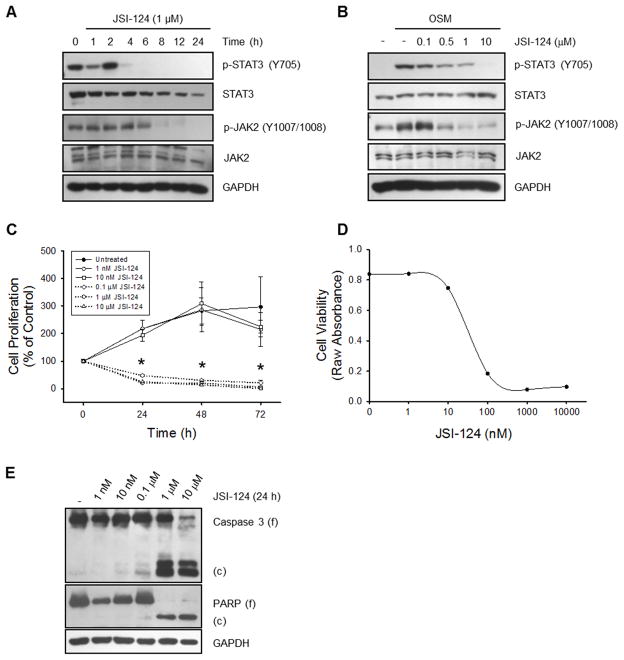
JSI-124 was originally discovered as a STAT3 inhibitor (24). First, we validated the inhibition of STAT3 in cultured human GBM cells. U251-MG cells have basal JAK2 and STAT3 phosphorylation, and JSI-124 (1 μM) inhibited the constitutive phosphorylation of both JAK2 and STAT3 (Fig. 1A). JSI-124 pre-treatment also prevented OSM-induced phosphorylation of JAK2 and STAT3 in a dose-dependent manner (Fig. 1B). Due to the greatly enhanced phosphorylation of STAT3 following OSM stimulation, we have provided an appropriately exposed blot revealing the constitutive STAT3 phosphorylation (Supplemental Fig. 1A). Both constitutive and stimulus-induced JAK2 and STAT3 phosphorylation were also inhibited in U87-MG cells (Supplemental Figs. 1B & C). These results verify the previously established literature regarding JSI-124 inhibition of STAT3 (24).
Figure 1. JSI-124 inhibits constitutive and stimulus-induced JAK2 and STAT3 phosphorylation, decreases cell proliferation and induces apoptosis in glioblastoma cells.
A, U251-MG cells were treated with 1 μM JSI-124 for the indicated times, lysed and immunoblotted with the indicated Ab. B, U251-MG cells were pretreated with various concentrations of JSI-124 for 2 h, followed by treatment with 1 ng/ml OSM for 15 min. Cells were lysed and immunoblotted with the indicated Ab. C, U251-MG cells were plated in 96 well plates and incubated with various concentrations of JSI-124 or vehicle control (DMSO) for the indicated times, and the WST-1 cell proliferation assay was performed. Data represent mean ± SD, replicates of three. Beginning at 24 h, the 0.1 μM, 1 μM and 10 μM concentrations of JSI-124 reached statistical significance (*, p<0.005; ANOVA). D, Cytotoxic drug dose response curve of U251-MG cells with a 24 h treatment with JSI-124. E, U251-MG cells were treated with various concentrations of JSI-124 for 24 h, lysed and immunoblotted with the indicated Ab.
JSI-124 decreases cell proliferation and induces cell death in GBM cells
It has been shown that in addition to inhibition of STAT3, JS1-124 decreased cell proliferation and induced apoptosis (24, 25). It was previously shown that this decrease in cell proliferation was due in part to a JSI-124-induced G2/M arrest and reduction of cyclin D1 (25), but the exact mechanism remains unclear. We also observed a reduction in cell proliferation with JSI-124 treatment of both U251-MG and U87-MG cells (Fig. 1C and Supplemental Fig. 1D). Dose response analysis revealed that 0.1 μM JSI-124 was sufficient to significantly inhibit proliferation by 24 h. Furthermore, a cytotoxic drug dose response curve yields an IC50 value of approximately 28 nM (Fig. 1D). This reduction in cell proliferation was accompanied by an increase in cell death as measured by the presence of cleaved (c) caspase 3 and poly-ADP ribose polymerase (PARP) (Fig. 1E).
JSI-124 treatment activates the NF-κB pathway
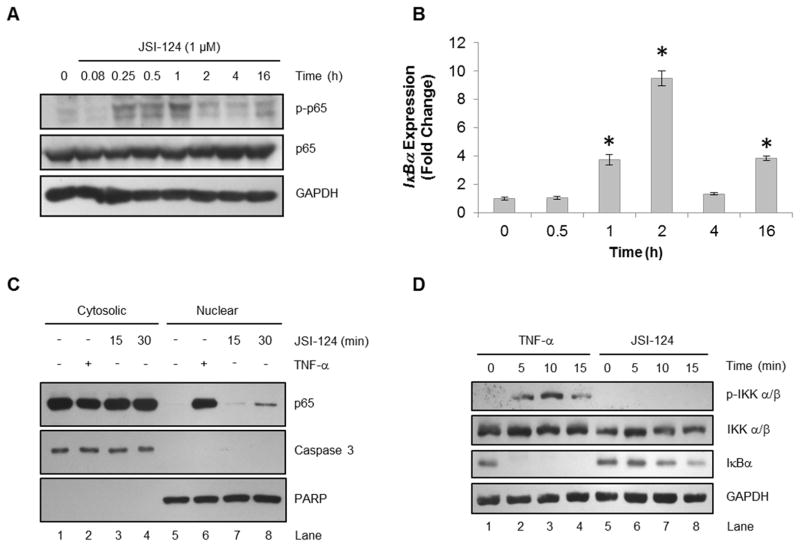
In order to evaluate other potential effects of JSI-124 treatment, U251-MG cells were incubated with JSI-124 (1 μM) for various times. Time course analysis revealed phosphorylation of NF-κB p65, starting as early as 15 min following JSI-124 treatment and decreasing after 1 h (Fig. 2A). Moreover, the JSI-124-induced phosphorylation of NF-κB was accompanied by an increase in the expression of IκBα (Fig. 2B). In addition, both JNK and p38 MAPK, two pathways commonly activated during stress, were also found to be activated (data not shown), which has also recently been shown in leukemia cells treated with JSI-124 (33). Activation of the NF-κB pathway involves nuclear translocation of NF-κB p65, where binding of DNA and transcriptional regulation occurs. Under basal conditions, NF-κB p65 is found sequestered in the cytosol with minimal to no detection in the nucleus (Fig. 2C, Lanes 1 and 5). As a positive control, we observed the presence of nuclear NF-κB p65 following TNF-α treatment in U251-MG cells (Fig. 2C, Lane 6). Moreover, we found that JSI-124 treatment also induced nuclear translocation of NF-κB p65 within 30 min (Fig. 2C, Lane 8). These results indicate that JSI-124 treatment results in the phosphorylation of NF-κB p65 as well as nuclear translocation.
Figure 2. JSI-124 treatment induces NF-κB p65 phosphorylation and nuclear translocation independent of IKK phosphorylation.
A, U251-MG cells were treated with JSI-124 (1 μM) for the indicated times. Cells were lysed and immunoblotted with the indicated Ab. B, U251-MG cells were treated with JSI-124 (1 μM) for the indicated times, followed by isolation of RNA, generation of cDNA and quantitative RT-PCR using Taqman primers. Data represent mean ± SD, replicates of three (*, p<0.001; ANOVA). C, U251-MG cells were treated with TNF-α (10 ng/ml) for 15 min or JSI-124 (1 μM) for 15 or 30 min. Cells were lysed and nuclear and cytosolic fractions were isolated, followed by immunoblotting with the indicated Ab. Caspase 3 and PARP Ab were used to verify purity of cytosolic and nuclear fractions, respectively. D, U251-MG cells were treated with TNF-α (10 ng/ml) or JSI-124 (1 μM) for the indicated times. Cells were lysed and immunoblotted with the indicated Ab.
The NF-κB pathway is activated in response to stimuli such as TNF-α, which leads to phosphorylation of IKK and the degradation of IκBα by the proteasome (8, 9). Using TNF-α as a positive control, we observed IKK phosphorylation and IκBα degradation within 5 min of TNF-α treatment (Fig. 2D). However, we did not observe phosphorylation of IKK in response to JSI-124 treatment. This indicates that activation of the NF-κB pathway in response to JSI-124 is not mediated through IKK phosphorylation, which will be further explained in the discussion. Modest degradation of IκBα by JSI-124 was observed by 15 min (Fig. 2D, Lane 8), which is necessary to allow NF-κB p65 translocation into the nucleus. Overall, these results confirm that JSI-124 treatment activates the NF-κB pathway.
JSI-124 treatment induces IL-6, IL-8 and SOCS3 expression
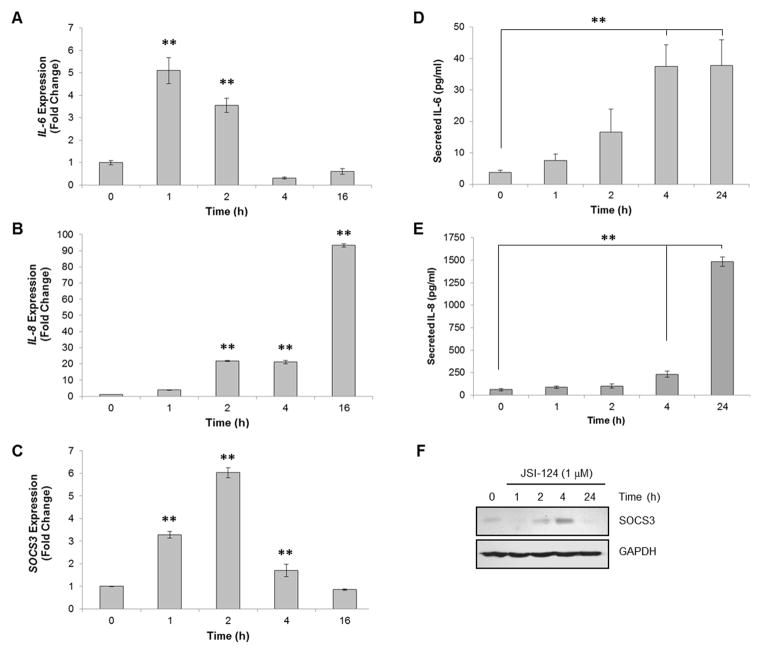
As JSI-124 activates intracellular signaling cascades including NF-κB, we evaluated the induction of several potential downstream genes. We found that JSI-124 treatment induced mRNA expression of IL-6 and IL-8 in both U251-MG (Figs. 3A & B) and U87-MG cells (Supplemental Fig. 2) as measured by quantitative RT-PCR. Both IL-6 and IL-8 are known targets of NF-κB p65 (13). We also observed an increase in the mRNA expression of SOCS3, an endogenous negative regulator of the JAK/STAT3 pathway, which is most often induced by JAK/STAT3 activation (14) (Fig. 3C; Supplemental Fig. 2). The JSI-124-induced gene expression was also validated in human GBM neurospheres (X1066 cells) as well as murine primary astrocytes (Supplemental Fig. 3).
Figure 3. JSI-124 induces the expression of IL-6, IL-8 and SOCS3.
A–C, U251-MG cells were treated with JSI-124 (1 μM) for the indicated times, followed by isolation of RNA, generation of cDNA and quantitative RT-PCR using Taqman primers. Data represent mean ± SD, replicates of three (**, p<0.001; ANOVA). D&E, Supernatants were collected from U251-MG cells treated with JSI-124 (1 μM) for the indicated times, and ELISA performed for detection of IL-6 and IL-8 secreted protein. Data represent mean ± SD, replicates of three (**, p<0.001, ANOVA). F, U251-MG cells were treated with JSI-124 (1 μM) for the indicated times. Cells were lysed and immunoblotted with the indicated Ab.
In order to verify the induction of IL-6 and IL-8 translation to protein, we treated U251-MG cells with JSI-124 for various times, collected supernatants and measured secreted IL-6 and IL-8 by ELISA. We found a significant increase in the amount of secreted IL-6 and IL-8 following JSI-124 treatment (Figs. 3D & E). In addition, beginning at 2 h following JSI-124 treatment, SOCS3 protein expression was increased (Fig. 3F).
Phosphorylated p65 and RNA Polymerase (Pol) II are found at the promoters of IL-6, IL-8 and SOCS3 in cells treated with JSI-124
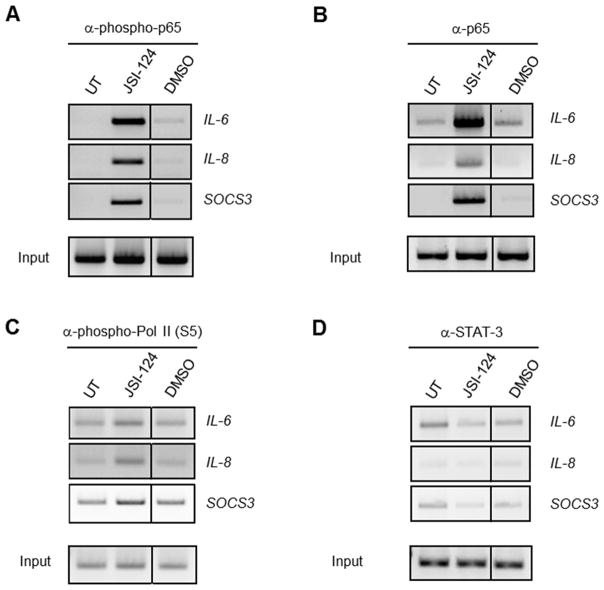
In order to further characterize the role of NF-κB p65 in JSI-124-induced gene expression, the presence of several transcription factors at the promoters of IL-6, IL-8 and SOCS3 was evaluated. Analysis reveals the presence of total and phosphorylated p65 as well as phosphorylated RNA Pol II at the promoters of IL-6, IL-8 and SOCS3 following JSI-124 treatment (Figs. 4A-C). Phosphorylation of RNA Pol II at Serine 5 (S5) is necessary and indicative of transcriptional initiation and activation (34). STAT3 was not at the promoters of the genes analyzed upon JSI-124 treatment, indicating that STAT3 is not activated or responsible for the increase in expression of IL-6, IL-8 or SOCS3 observed following JSI-124 treatment (Fig. 4D). This confirms that in response to JSI-124 treatment, activated NF-κB p65 is recruited to the promoters of IL-6, IL-8 and SOCS3, and is responsible for the increase in gene expression.
Figure 4. ChIP reveals the presence of phosphorylated p65 and RNA Pol II at the promoters of IL-6, IL-8 and SOCS3 of JSI-124 treated cells.
U251-MG cells were left untreated (UT) or treated with JSI-124 (1 μM) or DMSO (vehicle) for 1 h. Immunoprecipitation was performed with 5 μg of Ab to phospho-p65 (A), total p65 (B), phospho-RNA Pol II (C) and STAT3 (D). The immune complexes were absorbed with protein A beads or protein G beads blocked with bovine serum albumin and salmon sperm DNA. Immunoprecipitated DNA was then subjected to semi-quantitative PCR and analyzed by gel electrophoresis for IL-6, IL-8 and SOCS3. The experiment was repeated, and similar results were observed.
Blockade of the NF-κB pathway inhibits JSI-124-induced gene expression
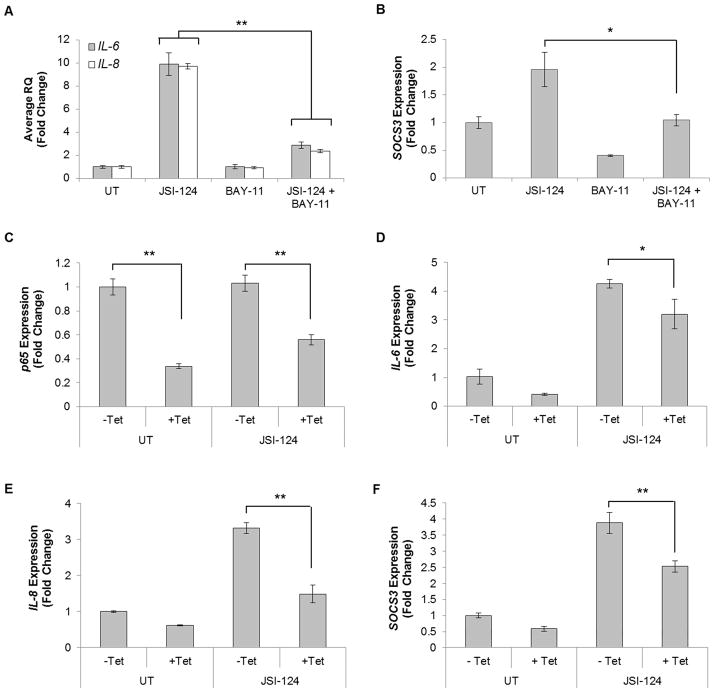
In order to verify the role of the NF-κB pathway in JSI-124-induced gene expression, we used a pharmacological inhibitor of the NF-κB pathway, BAY-11-7085 (BAY-11; Supplemental Fig. 4A) (35). U251-MG cells were pre-treated with BAY-11 for 2 h followed by treatment with JSI-124 for 1 h, and quantitative RT-PCR was performed. JSI-124 treatment induced mRNA expression of IL-6, IL-8 and SOCS3, which was inhibited by pre-treatment with BAY-11 (Figs. 5A & B). This suggests that the NF-κB pathway is necessary for JSI-124 induction of IL-6, IL-8, and SOCS3. To examine the necessity of NF-κB p65 in JSI-124-induced gene expression, we used an inducible p65 knockdown U251-MG derived cell line, U251-TR/sh-p65 (19). U251-TR/sh-p65 cells were grown in the absence or presence of Tetracycline (Tet) for 48 h to effectively decrease p65 levels prior to treatment with JSI-124 (Supplemental Fig. 4B). U251-TR/sh-p65 cells treated with Tet exhibit significantly decreased levels of p65 mRNA (Fig. 5C). Furthermore, loss of p65 significantly inhibited JSI-124-induced IL-8, IL-6 and SOCS3 expression (Figs. 5D–F). Similar to the inhibitor results in Fig. 5A & B, JSI-124 gene expression requires the presence of NF-κB p65.
Figure 5. Blockade of the NF-κB pathway inhibits JSI-124-induced expression of IL-6, IL-8 and SOCS3.
A–B, U251-MG cells were treated with BAY-11-7085 (BAY-11; 10 μM) for 2 h prior to JSI-124 (1 μM) treatment for 1 h. Isolation of RNA, generation of cDNA and quantitative RT-PCR was performed for IL-6 (A), IL-8 (A) and SOCS3 (B) expression. Data represent mean ± SD, replicates of three (*, p<0.01; **, p<0.001; Students t-test). The experiment was repeated, and similar results were observed. C–F, U251-TR/sh-p65 cells were treated with tetracycline (Tet) for 48 h to decrease p65 mRNA expression (C). After 48 h of Tet, cells were then treated with JSI-124 (1 μM) for 1 h. RNA was isolated, followed by generation of cDNA and quantitative RT-PCR was performed for IL-6 (D), IL-8 (E), and SOCS3 (F). Data represent mean ± SD, replicates of three (*, p<0.05; **, p<0.005; Students t-test).
Blockade of the NF-κB pathway does not inhibit JSI-124-induced phenotypic changes or STAT3 inhibition
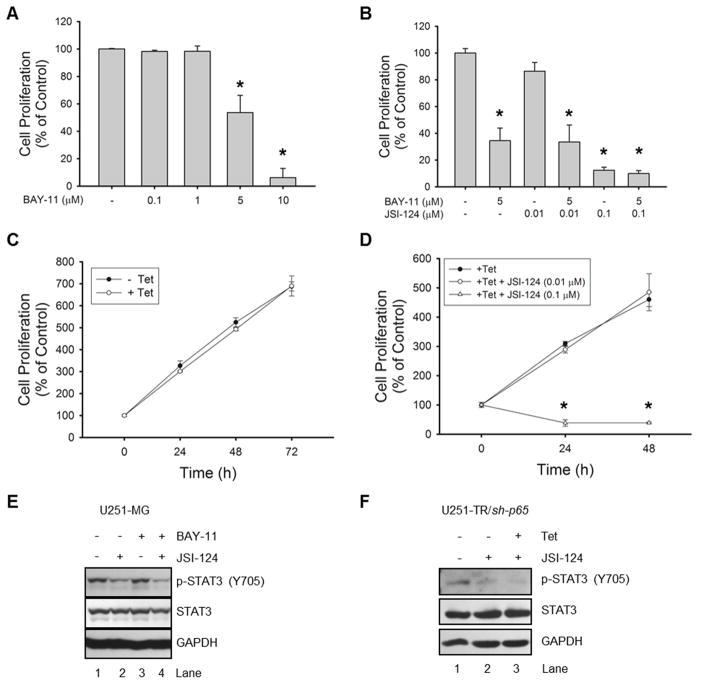
Next, we utilized functional analyses in order to better understand the relationship between NF-κB signaling and the effects of JSI-124 treatment. First, we evaluated a dose response of BAY-11 in GBM cells and observed a dose-dependent decrease in proliferation at 24 h (Fig. 6A). Using 5 μM BAY-11, we tested the combination using two doses of JSI-124 (low and high) to evaluate any possible additive or synergistic effects (Fig. 6B). We observed no enhanced decrease in proliferation with the combination of JSI-124 and BAY-11. In addition, pre-treatment with BAY-11 does not prevent the dramatic decrease in cell proliferation with the higher dose of JSI-124 (0.1 μM; Fig. 6B). In the NF-κB p65 knockdown cells (U251-TR/sh-p65), there is no significant decrease in proliferation with knockdown of NF-κB p65 (Fig. 6C). Using this information, we combined knockdown (+Tet) with two doses of JSI-124 (low and high). Similar to the pharmacological inhibition results, we observed that combination treatment does not enhance or prevent the decrease in proliferation with JSI-124 treatment (Fig. 6D). Furthermore, pharmacological inhibition or shRNA knockdown of NF-κB p65 does not prevent JSI-124-induced cell death (Supplemental Figs. 5A & B). Therefore, JSI-124 efficiently inhibits proliferation and induces cell death in the absence of NF-κB.
Figure 6. Inhibition of NF-κB signaling does not prevent JSI-124 induced phenotypic changes or inhibition of STAT3.
A–B, U251-MG cells were plated in 96 well plates and incubated with various concentrations of JSI-124 and/or BAY-11 for 24 h, and the WST-1 cell proliferation assay was performed. Data represent mean ± SD, replicates of three (*, p<0.001; ANOVA). C–D, U251-TR/sh-p65 cells were plated in 96 well plates and incubated with Tet for the indicated times (C) or pre-treated with Tet for 48 h prior to plating and treatment with various concentrations of JSI-214 for the indicated times (D), and the WST-1 cell proliferation assay was performed. Data represent mean ± SD, replicates of three (*, p<0.001; ANOVA). E–F, U251-MG cells were incubated with BAY-11 (5 μM) for 2 h prior to treatment with JSI-124 (1 μM) for 2 h (E), or U251-TR/sh-p65 cells were pre-treated with or without Tet for 48 h prior to treatment with JSI-124 (1 μM) for 8 h (F). Cells were lysed and immunoblotted with the indicated Ab.
In this report, the induction of SOCS3, a negative regulator of STAT3, via JSI-124-induced NF-κB activation could provide a potential mechanism of the observed STAT3 inhibition. To test this, we evaluated whether blockade of NF-κB would prevent JSI-124-induced STAT3 inhibition. U251-MG as well as U251-TR/sh-p65 cells were treated with JSI-124 and a noticeable inhibition of STAT3 was achieved (Figs. 6E & F, Lane 2). However, we observed that blockade (BAY-11) or downregulation of NF-κB p65 (U251-TR/sh-p65 cells) does not prevent JSI-124-induced inhibition of STAT3 (Figs. 6E & F, Lanes 4 and 3, respectively).
Discussion
In this report, we have determined that the STAT3 inhibitor JSI-124 has the ability to activate signaling pathways in addition to the inhibition of STAT3 in human GBM cells. A signaling schematic illustrating the observed effects of JSI-124 treatment is provided in Figure 7. JSI-124 was originally discovered as a STAT3 inhibitor in multiple cancer cells (24). We first verified the previously established literature regarding the effects of JSI-124, and observed an inhibition of constitutive and stimulus-induced JAK2 and STAT3 phosphorylation in cultured human GBM cells. Inhibition of proliferation and induction of apoptosis was also observed following JSI-124 treatment. These data confirm the literature regarding inhibition of STAT3 signaling, decreased proliferation and induction of apoptosis in cells in vitro (24–26).
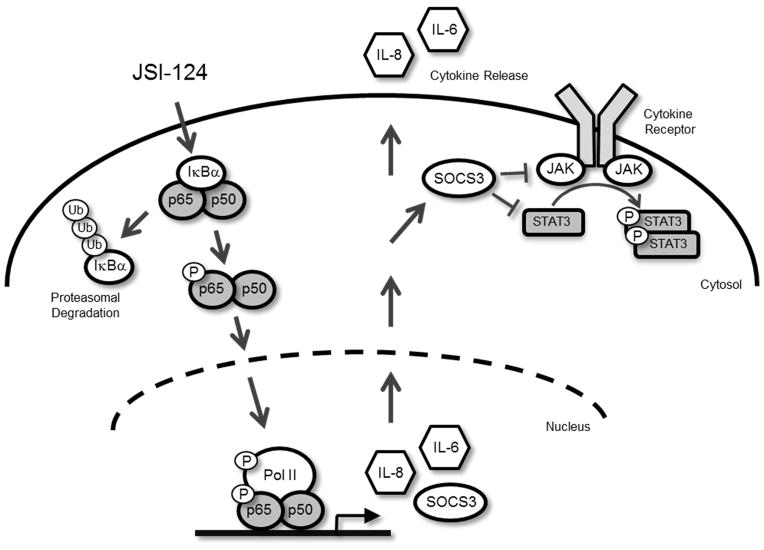
Figure 7. Signaling schematic illustrating the effects of JSI-124 treatment in GBM cells.
JSI-124 treatment activates the NF-κB pathway by phosphorylation (P) of NF-κB p65 protein, subsequent nuclear localization and IκBα degradation (Ub, ubiquitin). Once in the nucleus, phosphorylated NF-κB dimers bind to the promoters of IL-6, IL-8 and SOCS3 and begin gene transcription. IL-6 and IL-8 mRNA are translated into protein and secreted into the medium. SOCS3 mRNA is also translated into protein and acts as a negative regulator of JAK/STAT3, preventing STAT3 activation.
However, we observed that the time needed to inhibit STAT3 phosphorylation was much longer than other known JAK/STAT inhibitors. For example, we and others have shown that AZD1480, a specific JAK1/2 inhibitor, decreases constitutive STAT3 phosphorylation as early as 30 min post treatment (31, 36). Since the exact mechanism of JSI-124 remains unknown, this suggested that the JSI-124 mechanism of STAT3 inhibition was less specific and could be exerting effects on other signaling pathways. Moreover, we observed that prior to the inhibition of STAT3, the NF-κB pathway becomes activated, as observed via phosphorylation of NF-κB p65 as well as nuclear translocation and IκBα degradation (Fig. 7). Similarly, a recent study by Nefedova et al (37) found that JSI-124 treatment of dendritic cells led to the activation of NF-κB. However, in this study, the activation of NF-κB was observed after the inhibition of STAT3 signaling, was IκBα degradation independent, and was attributed to the loss of a dominant negative effect of STAT3 binding to NF-κB family members in these cells.
There are several mechanisms that ultimately lead to the activation of NF-κB (9, 22). Activation of NF-κB through the canonical pathway, via stimuli such as TNF-α or LPS, involves the phosphorylation and activation of the IKK complex. IκBα is then phosphorylated by IKK, which targets IκBα for degradation via the proteasome, and allows NF-κB p65 to translocate to the nucleus. Interestingly, we found that the JSI-124-induced activation of NF-κB p65 was through an IKK-independent mechanism, also known as the atypical pathway (9). In the atypical pathway, reports have suggested that other kinases can also phosphorylate IκBα, leading to dissociation and proteasomal degradation independent of IKK activation (9). For example, in response to UV light or expression of the Her2/Neu oncogene, casein kinase II (CK2) phosphorylates IκBα leading to its degradation and subsequent activation of NF-κB that is dependent on p38 MAPK pathway activation (38, 39). Interestingly, we did observe modest activation of the p38 MAPK pathway in response to JSI-124 treatment (data not shown). Other kinases, including Syk, c-Src and Ribosomal S6 Kinase 1 (RSK1) have also been linked to activation of NF-κB, independent of IKK activation (40–42). Therefore, we suspect other kinases may also become activated following JSI-124 treatment that might lead to the activation of NF-κB p65, independent of classical IKK activation.
Following JSI-124 treatment and NF-κB activation, we measured the expression of inflammatory NF-κB regulated genes including IL-6 and IL-8. This expression was translated into protein as measured by secreted IL-6 and IL-8 in the conditioned medium of cells treated with JSI-124. Both IL-6 and IL-8 expression have been shown to be upregulated in GBM and the corresponding tumor microenvironment both in vitro and in vivo (43–45), and IL-6 gene amplification has been observed in 40–50% of GBM patients and is associated with decreased patient survival (46). Although the role of IL-8 is less characterized in GBM biology, studies have shown that IL-8 acts predominantly as an inflammatory chemoattractant and proangiogenic factor (45). This indicates that JSI-124 induction of IL-6 and IL-8 could have potentially counter-productive effects with regards to inhibiting tumor growth and progression in GBM.
We also observed the induction and expression of SOCS3 following JSI-124 treatment. Upon typical JAK/STAT activation, SOCS3 is expressed in order to provide a mechanism of negative feedback. Once expressed, SOCS3 interacts with various cytokine receptors and/or JAKs to prevent subsequent STAT3 phosphorylation. In this study, we found the SOCS3 induction was driven by NF-κB, not STAT3. Although NF-κB induced SOCS3 is uncommon, we and others have shown that under certain conditions, SOCS3 expression can be induced by NF-κB activation (47–49). We found that the expression of SOCS3 was induced by NF-κB as observed by the presence of phosphorylated NF-κB p65 at the promoter of SOCS3 (Fig. 4A). The requirement for NF-κB in SOCS3 expression was validated using pharmacological inhibition and shRNA knockdown of NF-κB. The induction of SOCS3, a negative regulator of STAT3, via JSI-124-induced NF-κB activation could provide a potential mechanism of the observed STAT3 inhibition. Therefore, we evaluated whether inhibition of NF-κB would prevent JSI-124-induced STAT3 inhibition, and observed that the blockade (BAY-11) or downregulation of NF-κB p65 (U251-TR/sh-p65 cells) does not prevent JSI-124-induced inhibition of STAT3 (Figs. 6E & F). This could be due, in part, to the fact that NF-κB inhibition prior to JSI-124 treatment significantly reduces, but does not completely abrogate SOCS3 expression (Figs. 5B & F). Therefore, following JSI-124 treatment, the NF-κB driven SOCS3 expression acts as a negative regulator of STAT3, abrogating any subsequent STAT3 activation and provides a mechanism of STAT3 inhibition (Fig. 7).
In conclusion, there have been several papers evaluating combination therapies with other anti-cancer agents and JSI-124. Premkumar et al. (27) illustrated that Dasatinib, a small molecule tyrosine kinase inhibitor, synergizes with JSI-124 to increase apoptosis and inhibit growth and migration of GBM cells. However, several chemotherapeutic agents including doxorubicin and cisplatin have also been shown to activate NF-κB, similar to JSI-124 (50). Overall, this report reveals additional off-target effects of JSI-124 treatment, in addition to the inhibition of STAT3. Given the link between NF-κB, inflammation and cancer progression, continued research of these pre-clinical therapeutic agents must be performed to fully understand and anticipate potential harmful effects.
Supplementary Material
Acknowledgments
Grant Support
This work was supported by T32NS048039 (B.C.M.), R01CA158534 (E.N.B) and R01CA138517 (S.E.N.) from the National Institutes of Health, American Brain Tumor Association Basic Research Fellowship in Honor of Paul Fabbri (B.C.M.), and funding from the Southeastern Brain Tumor Foundation (E.N.B.).
Footnotes
Conflict-of-interest disclosure: The authors declare no conflicts of interest.
References
- 1.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 2.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157–73. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 4.Network CGAR. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–8. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carro MS, Lim WK, Alvarez MJ, Bollo RJ, Zhao X, Snyder EY, et al. The transcriptional network for mesenchymal transformation of brain tumours. Nature. 2010;463:318–25. doi: 10.1038/nature08712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhat KP, Salazar KL, Balasubramaniyan V, Wani K, Heathcock L, Hollingsworth F, et al. The transcriptional coactivator TAZ regulates mesenchymal differentiation in malignant glioma. Genes Dev. 2011;25:2594–609. doi: 10.1101/gad.176800.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayden MS, Ghosh S. NF-kappaB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 2012;26:203–34. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 10.Karin M. NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol. 2009;1:a000141. doi: 10.1101/cshperspect.a000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Shea JJ, Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity. 2012;36:542–50. doi: 10.1016/j.immuni.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aggarwal BB, Kunnumakkara AB, Harikumar KB, Gupta SR, Tharakan ST, Koca C, et al. Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship? Ann N Y Acad Sci. 2009;1171:59–76. doi: 10.1111/j.1749-6632.2009.04911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7:454–65. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- 15.Atkinson GP, Nozell SE, Benveniste ET. NF-kappaB and STAT3 signaling in glioma: targets for future therapies. Expert Rev Neurother. 2010;10:575–86. doi: 10.1586/ern.10.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bromberg J, Wang TC. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell. 2009;15:79–80. doi: 10.1016/j.ccr.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brantley EC, Nabors LB, Gillespie GY, Choi YH, Palmer CA, Harrison K, et al. Loss of protein inhibitors of activated STAT-3 expression in glioblastoma multiforme tumors: implications for STAT-3 activation and gene expression. Clin Cancer Res. 2008;14:4694–704. doi: 10.1158/1078-0432.CCR-08-0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mizoguchi M, Betensky RA, Batchelor TT, Bernay DC, Louis DN, Nutt CL. Activation of STAT3, MAPK, and AKT in malignant astrocytic gliomas: correlation with EGFR status, tumor grade, and survival. J Neuropathol Exp Neurol. 2006;65:1181–8. doi: 10.1097/01.jnen.0000248549.14962.b2. [DOI] [PubMed] [Google Scholar]
- 19.Nozell S, Laver T, Moseley D, Nowoslawski L, De Vos M, Atkinson GP, et al. The ING4 tumor suppressor attenuates NF-kappaB activity at the promoters of target genes. Mol Cell Biol. 2008;28:6632–45. doi: 10.1128/MCB.00697-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lo HW, Cao X, Zhu H, Ali-Osman F. Constitutively activated STAT3 frequently coexpresses with epidermal growth factor receptor in high-grade gliomas and targeting STAT3 sensitizes them to Iressa and alkylators. Clin Cancer Res. 2008;14:6042–54. doi: 10.1158/1078-0432.CCR-07-4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bredel M, Scholtens DM, Yadav AK, Alvarez AA, Renfrow JJ, Chandler JP, et al. NFKBIA deletion in glioblastomas. N Engl J Med. 2011;364:627–37. doi: 10.1056/NEJMoa1006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perkins ND. The diverse and complex roles of NF-kappaB subunits in cancer. Nat Rev Cancer. 2012;12:121–32. doi: 10.1038/nrc3204. [DOI] [PubMed] [Google Scholar]
- 23.Sansone P, Bromberg J. Targeting the interleukin-6/Jak/stat pathway in human malignancies. J Clin Oncol. 2012;30:1005–14. doi: 10.1200/JCO.2010.31.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blaskovich MA, Sun J, Cantor A, Turkson J, Jove R, Sebti SM. Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res. 2003;63:1270–9. [PubMed] [Google Scholar]
- 25.Su Y, Li G, Zhang X, Gu J, Zhang C, Tian Z, et al. JSI-124 inhibits glioblastoma multiforme cell proliferation through G(2)/M cell cycle arrest and apoptosis augment. Cancer Biol Ther. 2008;7:1243–9. doi: 10.4161/cbt.7.8.6263. [DOI] [PubMed] [Google Scholar]
- 26.Ishdorj G, Johnston JB, Gibson SB. Inhibition of constitutive activation of STAT3 by curcurbitacin-I (JSI-124) sensitized human B-leukemia cells to apoptosis. Mol Cancer Ther. 2010;9:3302–14. doi: 10.1158/1535-7163.MCT-10-0550. [DOI] [PubMed] [Google Scholar]
- 27.Premkumar DR, Jane EP, Agostino NR, Scialabba JL, Pollack IF. Dasatinib synergizes with JSI-124 to inhibit growth and migration and induce apoptosis of malignant human glioma cells. J Carcinog. 2010;9:1–14. doi: 10.4103/1477-3163.65448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma X, Reynolds SL, Baker BJ, Li X, Benveniste EN, Qin H. IL-17 enhancement of the IL-6 signaling cascade in astrocytes. J Immunol. 2010;184:4898–906. doi: 10.4049/jimmunol.1000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao X, Laver T, Hong SW, Twitty GB, Jr, Devos A, Devos M, et al. An NF-kappaB p65-cIAP2 link is necessary for mediating resistance to TNF-alpha induced cell death in gliomas. J Neurooncol. 2011;102:367–81. doi: 10.1007/s11060-010-0346-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meares GP, Jope RS. Resolution of the nuclear localization mechanism of glycogen synthase kinase-3: functional effects in apoptosis. J Biol Chem. 2007;282:16989–7001. doi: 10.1074/jbc.M700610200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McFarland BC, Ma JY, Langford CP, Gillespie GY, Yu H, Zheng Y, et al. Therapeutic potential of AZD1480 for the treatment of human glioblastoma. Mol Cancer Ther. 2011:2384–93. doi: 10.1158/1535-7163.MCT-11-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nozell S, Laver T, Patel K, Benveniste EN. Mechanism of IFN-beta-mediated inhibition of IL-8 gene expression in astroglioma cells. J Immunol. 2006;177:822–30. doi: 10.4049/jimmunol.177.2.822. [DOI] [PubMed] [Google Scholar]
- 33.Ishdorj GJJ, Gibson SB. Cucurbitacin-I (JSI-124) activates the JNK/c-Jun signaling pathway independent of apoptosis and cell cycle arrest in B leukemic cells. BMC Cancer. 2011;11:1–12. doi: 10.1186/1471-2407-11-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phatnani HP, Greenleaf AL. Phosphorylation and functions of the RNA polymerase II CTD. Genes Dev. 2006;20:2922–36. doi: 10.1101/gad.1477006. [DOI] [PubMed] [Google Scholar]
- 35.Pierce JW, Schoenleber R, Jesmok G, Best J, Moore SA, Collins T, et al. Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J Biol Chem. 1997;272:21096–103. doi: 10.1074/jbc.272.34.21096. [DOI] [PubMed] [Google Scholar]
- 36.Hedvat M, Huszar D, Herrmann A, Gozgit JM, Schroeder A, Sheehy A, et al. The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell. 2009;16:487–97. doi: 10.1016/j.ccr.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nefedova Y, Cheng P, Gilkes D, Blaskovich M, Beg AA, Sebti SM, et al. Activation of dendritic cells via inhibition of Jak2/STAT3 signaling. J Immunol. 2005;175:4338–46. doi: 10.4049/jimmunol.175.7.4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kato T, Jr, Delhase M, Hoffmann A, Karin M. CK2 is a C-terminal IkappaB kinase responsible for NF-kappaB activation during the UV response. Mol Cell. 2003;12:829–39. doi: 10.1016/s1097-2765(03)00358-7. [DOI] [PubMed] [Google Scholar]
- 39.Romieu-Mourez R, Landesman-Bollag E, Seldin DC, Sonenshein GE. Protein kinase CK2 promotes aberrant activation of nuclear factor-kappaB, transformed phenotype, and survival of breast cancer cells. Cancer Res. 2002;62:6770–8. [PubMed] [Google Scholar]
- 40.Takada Y, Mukhopadhyay A, Kundu GC, Mahabeleshwar GH, Singh S, Aggarwal BB. Hydrogen peroxide activates NF-kappa B through tyrosine phosphorylation of I kappa B alpha and serine phosphorylation of p65: evidence for the involvement of I kappa B alpha kinase and Syk protein-tyrosine kinase. J Biol Chem. 2003;278:24233–41. doi: 10.1074/jbc.M212389200. [DOI] [PubMed] [Google Scholar]
- 41.Abu-Amer Y, Ross FP, McHugh KP, Livolsi A, Peyron JF, Teitelbaum SL. Tumor necrosis factor-alpha activation of nuclear transcription factor-kappaB in marrow macrophages is mediated by c-Src tyrosine phosphorylation of Ikappa Balpha. J Biol Chem. 1998;273:29417–23. doi: 10.1074/jbc.273.45.29417. [DOI] [PubMed] [Google Scholar]
- 42.Bohuslav J, Chen LF, Kwon H, Mu Y, Greene WC. p53 induces NF-kappaB activation by an IkappaB kinase-independent mechanism involving phosphorylation of p65 by ribosomal S6 kinase 1. J Biol Chem. 2004;279:26115–25. doi: 10.1074/jbc.M313509200. [DOI] [PubMed] [Google Scholar]
- 43.Van Meir E, Sawamura Y, Diserens AC, Hamou MF, de Tribolet N. Human glioblastoma cells release interleukin 6 in vivo and in vitro. Cancer Res. 1990;50:6683–8. [PubMed] [Google Scholar]
- 44.Inda MM, Bonavia R, Mukasa A, Narita Y, Sah DW, Vandenberg S, et al. Tumor heterogeneity is an active process maintained by a mutant EGFR-induced cytokine circuit in glioblastoma. Genes Dev. 2010;24:1731–45. doi: 10.1101/gad.1890510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brat DJ, Bellail AC, Van Meir EG. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro Oncol. 2005;7:122–33. doi: 10.1215/S1152851704001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tchirkov A, Khalil T, Chautard E, Mokhtari K, Veronese L, Irthum B, et al. Interleukin-6 gene amplification and shortened survival in glioblastoma patients. Br J Cancer. 2007;96:474–6. doi: 10.1038/sj.bjc.6603586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akhtar LN, Qin H, Muldowney MT, Yanagisawa LL, Kutsch O, Clements JE, et al. Suppressor of cytokine signaling 3 inhibits antiviral IFN-beta signaling to enhance HIV-1 replication in macrophages. J Immunol. 2010;185:2393–404. doi: 10.4049/jimmunol.0903563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pauli EK, Schmolke M, Wolff T, Viemann D, Roth J, Bode JG, et al. Influenza A virus inhibits type I IFN signaling via NF-kappaB-dependent induction of SOCS-3 expression. PLoS Pathog. 2008;4:e1000196. doi: 10.1371/journal.ppat.1000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li X, Massa PE, Hanidu A, Peet GW, Aro P, Savitt A, et al. IKKalpha, IKKbeta, and NEMO/IKKgamma are each required for the NF-kappa B-mediated inflammatory response program. J Biol Chem. 2002;277:45129–40. doi: 10.1074/jbc.M205165200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakanishi C, Toi M. Nuclear factor-kappaB inhibitors as sensitizers to anticancer drugs. Nat Rev Cancer. 2005;5:297–309. doi: 10.1038/nrc1588. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.