Summary
Background
Tissue factor pathway inhibitor (TFPI) is an alternatively spliced protein with two isoforms, TFPIα and TFPIβ, which differ in their carboxy-terminal structure and cellular localization. Detailed characterization of their inhibitory activity is needed to define potentially unique inhibitory roles in tissue factor (TF) mediated thrombotic and inflammatory disease and to understand how pharmaceuticals targeted to different structural regions of the TFPI isoforms alter hemostasis in hemophilia patients.
Methods
The TF inhibitory activity of TFPIβ localized to the surface of CHO cells was compared to soluble TFPIα using in vitro and in vivo assays.
Results
In TF-FVIIa-mediated FXa generation assays, TFPIβ was a slightly better inhibitor than TFPIα, which was ~3-fold better than TFPI- 160, a soluble, altered form of TFPI similar to TFPIβ. In direct FXa inhibitory assays, TFPIβ had an IC50 2.5-fold lower than TFPIα and 56-fold lower than TFPI-160. TFPIβ inhibited TF-mediated CHO cell migration though Matrigel, while TFPIα or TFPI-160 were poor inhibitors, demonstrating that TFPIβ effectively blocks TF-initiated signaling events during cellular migration through matrices not permeable to soluble forms of TFPI. Further, TFPIβ inhibited TF-dependent CHO cell infiltration into lung tissue following tail vein injection into SCID mice and blocked development of consumptive coagulopathy.
Conclusions
When compared to TFPIα, TFPIβ is a slightly better inhibitor of TF procoagulant activity. As a surface associated protein, TFPIβ is a much better inhibitor of TF-mediated cellular migration than soluble TFPIα and may distinctly act in the inhibition of TF-mediated signaling events on inflamed endothelium and/or monocytes.
Keywords: alternative splicing, hemophilia, migration, tissue factor, TFPI
Introduction
Tissue factor (TF) is located within extravascular tissues and initiates blood coagulation following vascular injury by binding to factor (F) VIIa present in the blood [1]. The TF-FVIIa complex activates FIX and FX [2], which initiate hemostatic cascades that restore vascular integrity through formation of a meshwork of fibrin and platelets. In addition to preventing hemorrhage, TF-FVIIa can promote various cellular activities, including cellular migration through the extracellular matrix [3,4].
The primary physiological inhibitor of TF-FVIIa is tissue factor pathway inhibitor (TFPI) [5,6]. TFPI is a multivalent Kunitz-type serine protease inhibitor that simultaneously inactivates FXa and FVIIa, immediately following activation of FX by TF-FVIIa [7]. Alternative splicing results in the production of two isoforms in humans, TFPIα and TFPIβ [8]. Both isoforms have the same N-terminal sequence, containing two Kunitz-type inhibitor domains that bind and inhibit FVIIa and FXa, but differ at their C-termini. The C-terminus of TFPIα has a third Kunitz domain (K3) followed by a stretch of amino acids rich in arginine and lysine residues, while the C-terminus of TFPIβ encodes a glycosylphosphatidyl inositol (GPI)-anchor attachment site [9].
TFPIα protein has been identified in human plasma [10], placenta [11], and platelets [12]. In human plasma TFPIα is 50–80% truncated at its carboxy-terminus [13], but increases 2- to 4-fold upon heparin infusion [10,14] suggesting the presence of an in vivo pool of full-length TFPIα that is non-specifically bound to endothelial glycosaminoglycans. However, heparin-releasable TFPIα is not present on the surface of cultured endothelial cells [15,16] but is localized within an intracellular compartment and released following treatment with heparin or thrombin [15–17]. TFPI present on the surface of cultured endothelial cells is removed with phosphatidlyinositol phospholipase C (PIPLC), indicating that it has a GPI-anchor [11,18]. Consistent with this finding, TFPIβ protein has been identified as the isoform present in all major vascular beds of adult mice [19] and in cultured human endothelial cells and human placental microsomes [20].
Previous studies comparing the inhibitory activities of TFPIα and soluble forms of TFPI that mimic TFPIβ, such as TFPI-160 (which contains the K1 and K2 domains), have demonstrated that TFPIα is the more effective inhibitor of FXa in amidolytic assays [21–23]. However, unlike TFPI-160, TFPIβ is linked to the cell surface through a GPI-anchor, which may significantly alter its activity compared to soluble forms of TFPI [24]. Studies examining the inhibitory activity of TFPIβ using small-interfering RNA (siRNA) techniques to limit TFPIα expression have suggested that it effectively inhibits TF-FVIIa-mediated generation of FXa on the surface of ECV304 cells [25], and the TF-mediated migration of MDA-MB-231 cells [26]. However, these inhibitory studies are limited by residual TFPIα produced by the cells and potential off-target effects of the siRNA, both of which complicate the identification of specific TFPIβ inhibitory functions.
The inhibitory activity of cell-associated TFPIβ, and how it compares to soluble TFPIα, is not well understood. A CHO cell model system in which human TF and human TFPIβ are expressed on the cell surface was developed to further define the biological activities of cell-associated TFPIβ and compare these activities to soluble TFPIα and TFPI-160 in a series of in vitro and in vivo assays. This model system has a distinct advantage in that the cells do not produce TFPIα, allowing for accurate determination of the amount of TFPIβ on the cell surface and quantitative comparisons of TFPIα and TFPIβ inhibitory activities. TFPIβ is shown to be the more potent inhibitor of several TF-mediated physiological processes, particularly TF-mediated cellular migration.
Materials and methods
Production and characterization of CHO cells expressing TF and TFPIβ
CHO (K1) cells were transfected with a hygromycin-resistant plasmid containing human full-length TF (gift of Dr. Wolfram Ruf, Scripps Research Institute, La Jolla, CA) to produce CHO-TF cells. CHO-TF cells were then transfected with a neomycin-resistant plasmid containing human TFPIβ to produce CHO-TF/TFPIβ cells. Cells were prepared for flow cytometry as previously described [27]. To verify the presence of a GPI-anchor, transfected CHO-TF/TFPIβ cells were treated with 1 U/ml PIPLC for 1 hour at 37°C [27] and analyzed by flow cytometry.
Standardization of cell preparations
Cells were washed, harvested, pelleted by centrifugation (180 x g, 15 minutes), and resuspended in 1 mL of PBS. A 100 μl aliquot was pelleted and lysed in 100 μl CHAPS buffer for protein determination of each individual sample. Measurement of total protein was used to standardize cell concentration for all assays. This is a more reliable and reproducible method than cell counts.
TF-initiated amidolytic assay
Initially, the CHO-TF and CHO-TF/TFPIβ cells were examined to confirm equal surface TF activity. Intact cells (30 μg/well), untreated or treated with PIPLC or anti-TFPI polyclonal antibody, were added to FVIIa (10 pM) and 0.5 mM factor Xa substrate (MeO-CO-D-CHD-Cly-Arg-pNA.AcOH (American Diagnostica, Stamford, CT)). Reactions were initiated with FX (20 nM). Maximum FXa generation was determined by comparison of the peak slope obtained to a standard curve of FXa generation. In assays to determine IC50 values CHO-TF/TFPIβ cells or CHO-TF cells, alone or with indicated concentrations TFPIα (either glycosylated from HEK expression or non-glycoyslated from E.coli expression) or TFPI-160 [21], were incubated with FX (20nM) and reactions initiated with 10 pM FVIIa. The total cellular protein concentration (CHO-TF and/or CHO-TF/TFPIβ) was 90 μg/ml in all reactions to ensure equal amounts of TF. Aliquots were removed at timed intervals over 6 minutes and quenched in 33 mM EDTA. FXa present at time points was determined by comparison to the standard curve. IC50 values were determined using a variable slope dose-response curve (GraphPad Prism V5, La Jolla, CA).
Factor Xa Inhibition Assay
Reaction mixtures were prepared containing CHO-TF cells (90 μg/mL), varying concentrations of glycosylated or non-glycosylated TFPIα, TFPI-160, or CHO-TF/TFPIβ cells, and FXa chromogenic substrate (0.5 mM). The reaction was initiated by addition of FXa (0.1 nM), and monitored for 45 min at 405 nm. The steady-state concentration of free FXa was determined by comparison to a FXa standard curve. IC50 values were determined as described for the TF-FVIIa inhibition assays.
TFPI ELISA
A total TFPI ELISA used a mouse monoclonal anti-K2 antibody [28] for capture and rabbit polyclonal TFPI antibody [11] for detection. Secondary anti-rabbit HRP antibody was detected using QuantaRed Enhanced Chemifluorescent HRP Substrate. TFPI released from the cells was determined by comparison to a TFPIα standard curve.
TF-dependent Matrigel cellular migration assays
Transwell inserts (8.0 μm) were coated with Matrigel (1 mg/mL), a protein mixture from a murine tumor containing over 1851 proteins including FII, FVII and FX [29]. After coating, inserts were incubated at 37°C, washed with warm serum-free media and seeded with CHO, CHO-TF, or CHO-TF/TFPIβ cells (2.5 X 104/well). After 36 hours non-migrating cells were removed, the insert membranes fixed with 1.5% formaldehyde and stained with crystal violet. In some experiments, cells were incubated with 10 μM argatroban, SCH79797, recombinant tick anticoagulant peptide (rTAP), or recombinant nematode anticoagulant protein C2 (rNAPC2) (rTAP and rNAPC2 gifts from Dr. Sriram Krishnaswamy, University of Pennsylvania), or 100 nM soluble TFPIα or TFPI-160, prior to seeding. Migrating cells were counted using a Nikon Eclipse E600 light microscope at 20X field magnification.
In vivo TF-dependent cellular migration assays
All animal experiments were approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee. CHO-TF or CHO-TF/TFPIβ cells were harvested and re-suspended in sterile PBS (35 μg/100 μL) and injected into the tail vein of 6–12 week old CB.17 SCID mice (C.B-Igh-1b/IhcrTac-Prkdcscid). On day 10, mice were anesthetized, whole blood was collected for complete blood count as described [30], and lungs were harvested for Trichrome staining and examination of cellular burden in a blinded fashion using the Nikon light microscope.
Statistical Analyses
To test for statistical significance, the students’ t-test or one-way ANOVA were performed using GraphPad Prism. Bonferroni’s Multiple Comparison test was performed to test for differences between groups when the one-way ANOVA was significant. A p-value less than 0.05 was considered statistically significant.
Results
Production and characterization of CHO-TF and CHO-TF/TFPIβ cells
Human TFPIβ was co-expressed with human TF in CHO cells, providing a model system that allowed testing and quantitative comparisons of TFPIα and TFPIβ inhibitory activities. Flow cytometry experiments demonstrated presence of similar TF expression on CHO-TF and CHO-TF/TFPIβ cell lines (Fig. 1A). TF-FVIIa-mediated FXa generation experiments demonstrate equal TF activity on the cell lines following neutralization of TFPIβ activity with an anti-TFPI antibody TFPIβ or its removal with PIPLC (Fig. 1B). TFPI ELISA assay of PIPLC treated cellular supernatant quantified 92.8 pg TFPIβ/μg total cellular protein on the CHO-TF/TFPIβ cell surface.
Fig. 1.
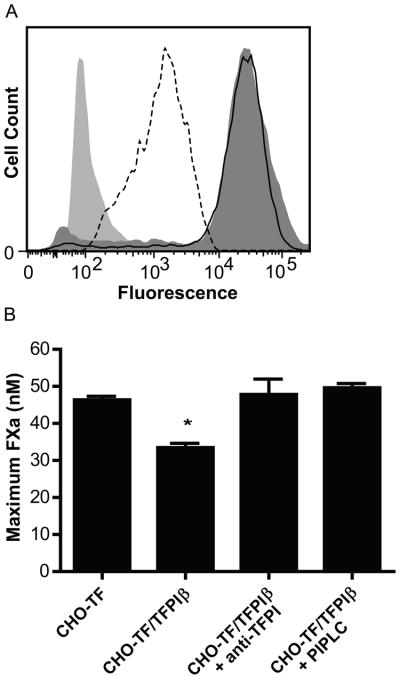
CHO-TF and CHO-TF/TFPIβ cells have similar TF expression and activity. (A) Flow cytometry for TF on CHO-TF (filled dark grey) and CHO-TF/TFPIβ cells (solid line) and for TFPI on CHO-TF/TFPIβ cells (dotted line). The isotype control is shaded light grey. The data are representative of 3 experiments. (B) TF-FVIIa-mediated FXa generation on CHO-TF cells and CHO-TF/TFPIβ cells. TFPIβ decreases the amount of FXa generated (p<0.001) and can be totally reversed by anti-TFPI polyclonal antibody or by treatment of the cells with PIPLC (mean ± SEM, n=3).
TFPIβ is a more potent inhibitor of TF-FVIIa and FXa than either TFPIα or TFPI-160
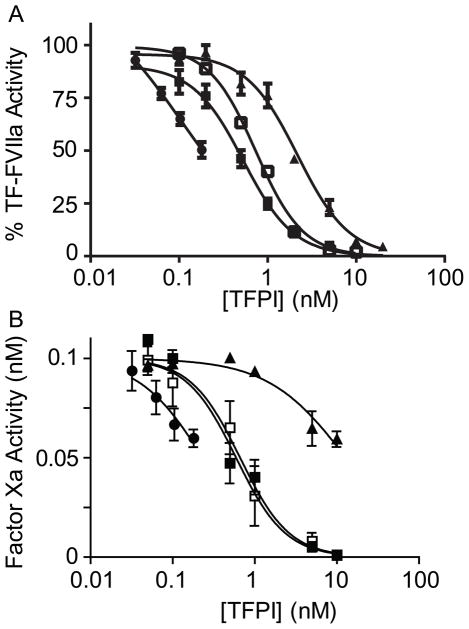
TFPIβ activity was quantified and compared to that of soluble glycosylated and non-glycosylated TFPIα and TFPI-160 in TF-FVIIa and FXa-initiated assays (Fig. 2). Activity of soluble forms of TFPI toward TF-FVIIa was determined using CHO-TF cells as the source of TF (Fig. 2A). Experiments with TFPIβ contained mixtures of CHO-TF/TFPIβ and CHO-TF cells to yield the indicated TFPIβ concentrations while maintaining a constant total amount of TF. TFPIβ inhibited TF-FVIIa to a greater extent than glycosylated or non-glycosylated TFPIα or TFPI-160 at all concentrations. Extrapolation of data points for TFPIβ yielded an estimated IC50 of 0.11 nM, slightly lower than IC50 values for glycosylated TFPIα (IC50=0.74 nM), non-glycosylated TFPIα (IC50=0.53 nM), or TFPI-160 (IC50=2.18 nM). This IC50 value assumes that TFPIβ equivalently inhibits co-expressed TF and TF expressed on a different cell, a scenario which may not be true. Rather, it is probable that TFPIβ is a potent inhibitor of co-expressed TF and a relatively poorer inhibitor of TF expressed on an adjacent cell. Thus, the IC50 of TFPIβ towards co-expressed TF may be significantly lower than 0.11 nM.
Fig. 2.
TFPIβ is an effective inhibitor TF-FVIIa and FXa. (A) TF-FVIIa-mediated FXa generation (mean ± SEM, n=3) and (B) FXa activity (mean ± SEM, n=3) were measured in the presence of glycosylated TFPIα (□), non-glycosylated TFPIα (■), TFPI-160 (▲) or CHO-TF/TFPIβ cells (●). Cellular TF was constant in all experiments. Data are shown as percent activity compared to the “no TFPI” control in (A) or as uninhibited FXa activity in (B).
Reactions examining direct FXa inhibition by TFPIα or TFPI-160 contained 90 μg/ml CHO-TF cells, mimicking the cellular concentration in reactions with CHO-TF/TFPIβ (Fig. 2B). Glycosylated (IC50= 0.66 nM) and non-glycosylated (IC50=0.59 nM) TFPIα were better inhibitors of FXa than TFPI- 160 (IC50=12.90 nM). TFPIβ (extrapolated IC50=0.23 nM) was a slightly better inhibitor of FXa than TFPIα (2- to 3-fold) and substantially better (56-fold) than TFPI-160.
TFPIβ inhibits TF-dependent cellular migration through Matrigel
A transwell assay system utilizing Matrigel, a protein mixture derived from a murine tumor that contains FII, FVII and FX [29], was utilized to compare the abilities of TFPIβ, glycosylated and non-glycosylated TFPIα, and TFPI-160 to inhibit TF-mediated cellular migration. CHO cells migrated poorly, while CHO-TF cells had a 15-fold increase in cellular migration, demonstrating TF-dependent migration in this assay. When compared to CHO-TF cells, glycosylated TFPIα, non-glycosylated TFPIα, and TFPI-160 (100 nM) inhibited migration by approximately 30% (p<0.01), while CHO-TF/TFPIβ cells (expressing only approximately 21 pM TFPIβ per well) inhibited migration by 83% (p< 0.001) (Fig. 3A).
Fig. 3.
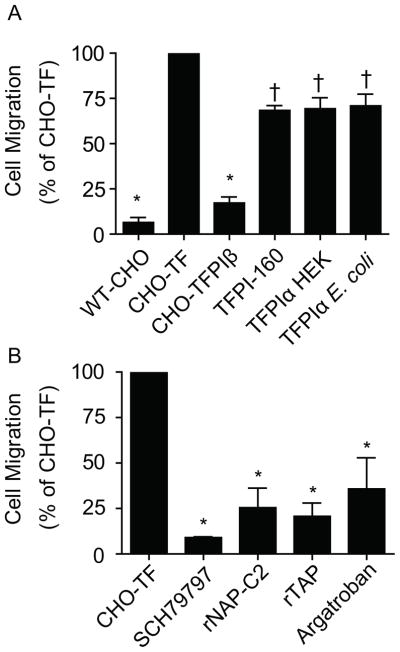
TFPIβ blocks CHO-TF cell migration through Matrigel by preventing thrombin generation and activation of PAR-1. Cellular migration was standardized to that of CHO-TF cells. (A) Migration of CHO-TF/TFPIβ cells compared to CHO cells, CHO-TF cells and CHO-TF cells in the presence of 100nM/well recombinant TFPI-160, glycosylated TFPIα or non-glycosylated TFPIα (mean ± SEM, n=3). (B) Migration of CHO-TF cells in the presence of SCH79797 (PAR-1 antagonist), rNAP-C2 (TF-FVIIa inhibitor), rTAP (FXa inhibitor), or argatroban (thrombin inhibitor), all at 10 μM (*p<0.001, †p<0.01) (mean ± SEM, n=3).
The PAR-1 antagonist SCH79797 greatly reduced cellular migration demonstrating that it is dependent on PAR-1 activation (p<0.001) (Fig. 3B). Next, specific inhibitors were used to determine the protease responsible for PAR-1 cleavage. Inhibitors of TF-FVIIa (rNAP-C2), FXa (rTAP), and thrombin (argatroban) all greatly reduced cellular migration, demonstrating that thrombin, generated via TF-mediated activation of coagulation, is the PAR-1 cleaving protease (p<0.001) (Fig. 3B).
TFPIβ inhibits TF-dependent cellular infiltration and consumptive coagulopathy
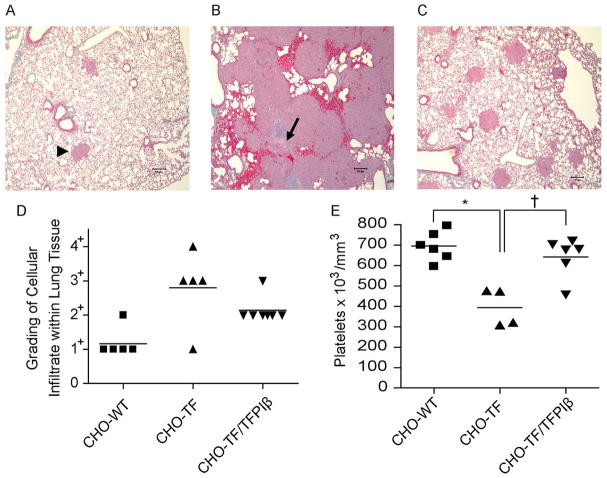
As TFPIβ markedly inhibited the TF-mediated migration of cells in vitro, we directly tested its ability to inhibit tissue infiltration in a well-characterized murine model system [3]. In this assay CHO, CHO-TF, or CHO-TF/TFPIβ cells were injected into the tail vein of SCID mice and cellular infiltration into lung tissue was assessed ten days later. Histological analyses demonstrated normal lung parenchyma with only rare cellular infiltrate detected in mice injected with CHO cells (Fig. 4A, arrowhead). In contrast, mice injected with CHO-TF cells often had large sheets of cells with very little remaining normal lung parenchyma (Fig. 4B). The lung parenchyma had alveoli with thickened walls and fibrin clots within the vasculature (Fig. 4B, arrow). Mice injected with CHO-TF cells had greatly decreased platelet counts compared to those injected with CHO cells (400 × 103/mm3 vs. 700 × 103/mm3, p<0.0001), suggesting the presence of a consumptive coagulopathy (Fig. 4E). Mice injected with CHO-TF/TFPIβ cells had reduced cellular infiltrate with a moderate amount of normal lung tissue present (Fig. 4C and 4D) and, importantly, had platelet counts similar to mice injected with CHO cells, suggesting that TFPIβ completely prevented development of a consumptive coagulopathy.
Fig. 4.
TFPIβ reduces TF-mediated tissue infiltration and consumptive coagulopathy. Histology of the lungs from SCID mice ten days following injection with (A) CHO; (B) CHO-TF; or (C) CHO-TF/TFPIβ cells. (A) Arrowhead indicates a small cluster of CHO cells within the lung. (B) Arrow indicates a large intravascular thrombus. (D) Histopathological grading (1+–4+) of lungs ten days following injection cells. (E) Platelet counts ten days following injection of cells (*p<0.001, † p<0.05).
Discussion
The expression of TFPIα in platelets and TFPIβ on the surface of vascular endothelium suggests they may have distinct biological activities. As a first step to define these, our laboratory developed a CHO-cell model system to compare the inhibitory activities of different TFPI isoforms. This model system, in which CHO cells co-express human TF and human TFPIβ, simulates disease states, such as infection or cancer, where TF and TFPI may be present on endothelium, monocytes or tumor cells [31–33]. The results demonstrate that while cell-surface associated TFPIβ is only a slightly more effective inhibitor of TF-FVIIa and FXa than TFPIα in assays using purified proteins, it is a much more effective inhibitor in cellular migration assays. Further, using a SCID mouse model it was demonstrated that TFPIβ is a highly effective inhibitor of TF-mediated cellular migration and induction of disseminated coagulopathy. Thus, TFPIβ may distinctly inhibit intravascular TF-mediated cell signaling events on inflamed endothelium or monocytes.
Consistent with the findings of Riewald and co-workers [34], the CHO cell system demonstrated that TFPIβ is a potent inhibitor of TF-FVIIa-mediated FXa generation. TFPIβ could inhibit either TF co- expressed on the same cell, which seems most likely, or on an adjacent cell. As these two scenarios cannot be differentiated, an accurate IC50 value for inhibition of TF-FVIIa by TFPIβ could not be calculated. However, at every concentration tested, TFPIβ was a more potent inhibitor of TF-FVIIa- mediated FXa generation than TFPIα or TFPI-160. TFPIβ also was a potent direct inhibitor of FXa. Based on IC50 concentrations, it inhibits FXa two-fold better than TFPIα and 56-fold better than TFPI-160. The rapid FXa inhibition by TFPIβ is somewhat unexpected. Since the third Kunitz domain and the C- terminal region are necessary for rapid FXa inhibition in solution phase assays [21–23], one may predict that TFPIβ is a poor FXa inhibitor. However, the GPI-anchoring of TFPIβ to the membrane compensates for the lack of these domains allowing rapid FXa inhibition. Finally, the inhibitory activity of the different forms of TFPI used in these experiments does not appear to depend on their glycosylation, since glycosylated and non-glycosylated TFPIα had similar inhibitory activity.
CHO-TF cells readily migrated through Matrigel, which contains FVII and FX [29]. This migration was mitigated by 83% with CHO-TF/TFPIβ cells. Although the CHO-TF/TFPIβ cells expressed more TF than TFPIβ, it appears that in these assays the amount of FVIIa in Matrigel is limiting and that TFPIβ is a very effective inhibitor of TF-mediated cell signaling required for migration. In contrast, high concentrations (100 nM) of soluble TFPIα (either glycosylated or non-glycosylated) or TFPI-160 inhibited CHO-TF cell migration only 30%. These results were somewhat surprising, since all forms of TFPI at 100 nM totally inhibit TF-FVIIa mediated FXa generation in amidolytic assays (Fig. 2A). Thus, TF-FVIIa may be inhibited by membrane associated forms of TFPI but protected from inhibition by soluble forms of TFPI during Matrigel migration. Alternatively, soluble forms of TFPI may be poorer inhibitors of TF-mediated cell signaling events than would be predicted from the TF-FVIIa inhibition assays. Consistent with this notion, Ahamed and colleagues have reported that 50- to 100-fold more TFPIα is required to inhibit TF-FVIIa mediated cellular signaling than is needed to inhibit TF-FVIIa mediated generation of FXa [35]. Thus, it appears that TFPIβ, when localized to the same cellular surface as TF, as may occur on the surface of inflamed endothelium, monocytes or tumor cells [31–33], is a highly effective inhibitor of TF-mediated cellular migration, while circulating plasma TFPIα would be a relatively poor inhibitor. These findings have important implications for the use of TFPIα as a therapeutic agent, as is being considered for severe sepsis associated with community acquired pneumonia [36,37], and are consistent with the high concentration of human TFPIα (3049 ng mL-1 or 9 μM) necessary to observe therapeutic attenuation of coagulation, inflammation and bacterial growth in a murine model of pneumococcal pneumonia [38].
The presence of TF on the CHO cell surface greatly enhanced their infiltration into SCID mouse lung tissue following tail vein injection [3] even though there is ample (44 nM) soluble TFPI in mouse plasma [30]. TF has been reported to support cell adhesion, migration, homing and extravasation [39,40], any or all of which may produce the cellular infiltration observed in this model. In various model systems TF has been shown to induce cellular infiltration through PAR-1 [41] or PAR-2 [4]. The Matrigel migration assay data suggests the underlying mechanism for CHO-TF cells is thrombin generation with activation of PAR-1. Importantly, the expression of relatively small amounts of TFPIβ on the CHO-TF cell surface dampened infiltration of CHO-TF cells into lung tissue and totally protected the mice from the decreased platelet count associated with a TF-induced consumptive coagulopathy, demonstrating that TFPIβ effectively down-regulates TF-mediated procoagulant and cellular signaling events in vivo.
TFPIα, a soluble protein released from endothelial cells and platelets, is an effective anticoagulant that limits thrombus growth in murine vascular injury models [30] and is a physiological regulator of bleeding in murine hemophilia [42]. Further, TFPIα may have unique functions, not performed by TFPIβ, that are mediated by its third Kunitz domain, which binds to protein S and enhances inhibition of FXa [43], and its basic C-terminal region. The data presented here suggest that expression of TFPIβ on endothelial cells is optimized to dampen intravascular TF activity, where it is available at a high localized concentration to inhibit potentially detrimental TF-mediated coagulation and/or cell signaling events that occur during inflammation. The localization of TFPIβ within lipid rafts/caveolae in endothelial cells has been shown to further enhance its TF inhibitory activity [27,44].
Continued characterization of the distinct inhibitory activities of TFPIα and TFPIβ is needed to define their mechanism of action in TF mediated thrombotic and inflammatory disease and has increasing importance with the development of pharmaceutical agents for treatment of hemophilia that block TFPI anticoagulant activity[28,45,46]. These agents act by targeting different structural domains of TFPI; and the target selected may have important therapeutic implication. For example, depletion of hematopoietic cell TFPI, which is predominantly TFPIα within platelets, is sufficient for restoring hemostasis in FVIII deficient mice suggesting that compounds specifically targeting TFPIα may be effective hemostatic agents with decreased risk for inducing a generalized procoagulant state, since they would not alter the anticoagulant activity of TFPIβ expressed on endothelium [42]. The data presented here suggest that TFPIα and TFPIβ distinctly inhibit different TF functions and provide clues for targeting different regions of the protein that could limit off-target effects that may occur for TFPI blocking agents designed to treat hemophilia.
Supplementary Material
Footnotes
Addendum
S. A. Maroney planned and performed experiments, analyzed data and contributed to writing the manuscript. P. E. Ellery analyzed data and contributed to writing the manuscript. J. P. Wood performed experiments, analyzed data and contributed to writing the manuscript. J. P. Ferrel and N. D. Martinez performed experiments. A. E. Mast planned experiments, analyzed data and contributed to writing the paper.
Disclosure of conflict of interests
This work was supported by NHLBI grants HL068835 to AEM and HL091469 to SAM. JPW gratefully acknowledges training grant support from NHLBI grant HL007209. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Further support was provided through a research grant from Novo Nordisk to AEM.
References
- 1.Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. Am J Pathol. 1989;134:1087–97. [PMC free article] [PubMed] [Google Scholar]
- 2.Ratnoff OD, Davie EW. The Activation of Christmas Factor (Factor IX) by Activated Plasma Thromboplastin Antecedent (Activated Factor XI)*. Biochemistry. 1962;1:677–85. [Google Scholar]
- 3.Mueller BM, Ruf W. Requirement for Binding of Catalytically Active Factor VIIa in Tissue Factor-dependent Experimental Metastasis. J Clin Invest. 1998;101:1372–8. doi: 10.1172/JCI930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hjortoe GM, Petersen LC, Albrektsen T, Sorensen BB, Norby PL, Mandal SK, Pendurthi UR, Rao LVM. Tissue factor-factor VIIa-specific up-regulation of IL-8 expression in MDA-MB-231 cells is mediated by PAR-2 and results in increased cell migration. Blood. 2004;103:3029–37. doi: 10.1182/blood-2003-10-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Girard TJ, Warren LA, Novotny WF, Likert KM, Brown SG, Miletich JP, Broze GJ., Jr Functional significance of the Kunitz-type inhibitory domains of lipoprotein-associated coagulation inhibitor. Nature. 1989;338:518–20. doi: 10.1038/338518a0. [DOI] [PubMed] [Google Scholar]
- 6.Pedersen B, Holscher T, Sato Y, Pawlinski R, Mackman N. A balance between tissue factor and tissue factor pathway inhibitor is required for embryonic development and hemostasis in adult mice. Blood. 2005;105:2777–82. doi: 10.1182/blood-2004-09-3724. [DOI] [PubMed] [Google Scholar]
- 7.Baugh RJ, Broze GJ, Jr, Krishnaswamy S. Regulation of extrinsic pathway factor Xa formation by tissue factor pathway inhibitor. J Biol Chem. 1998;273:4378–86. doi: 10.1074/jbc.273.8.4378. [DOI] [PubMed] [Google Scholar]
- 8.Chang JY, Monroe DM, Oliver JA, Roberts HR. TFPIbeta, a second product from the mouse tissue factor pathway inhibitor (TFPI) gene. Thromb Haemost. 1999;81:45–9. [PubMed] [Google Scholar]
- 9.Zhang J, Piro O, Lu L, Broze GJ., Jr Glycosyl phosphatidylinositol anchorage of tissue factor pathway inhibitor. Circulation. 2003;108:623–7. doi: 10.1161/01.CIR.0000078642.45127.7B. [DOI] [PubMed] [Google Scholar]
- 10.Novotny WF, Palmier M, Wun TC, Broze GJ, Jr, Miletich JP. Purification and properties of heparin-releasable lipoprotein- associated coagulation inhibitor. Blood. 1991;78:394–400. [PubMed] [Google Scholar]
- 11.Mast AE, Acharya N, Malecha MJ, Hall CL, Dietzen DJ. Characterization of the association of tissue factor pathway inhibitor with human placenta. Arterioscler Thromb Vasc Biol. 2002;22:2099–104. doi: 10.1161/01.atv.0000042456.84190.f0. [DOI] [PubMed] [Google Scholar]
- 12.Maroney SA, Haberichter SL, Friese P, Collins ML, Ferrel JP, Dale GL, Mast AE. Active tissue factor pathway inhibitor is expressed on the surface of coated platelets. Blood. 2007;109:1931–7. doi: 10.1182/blood-2006-07-037283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Broze GJ, Jr, Lange GW, Duffin KL, MacPhail L. Heterogeneity of plasma tissue factor pathway inhibitor. Blood Coagul Fibrinolysis. 1994;5:551–9. [PubMed] [Google Scholar]
- 14.Sandset PM, Abildgaard U, Larsen ML. Heparin induces release of extrinsic coagulation pathway inhibitor (EPI) Thromb Res. 1988;50:803–13. doi: 10.1016/0049-3848(88)90340-4. [DOI] [PubMed] [Google Scholar]
- 15.Lupu C, Poulsen E, Roquefeuil S, Westmuckett AD, Kakkar VV, Lupu F. Cellular effects of heparin on the production and release of tissue factor pathway inhibitor in human endothelial cells in culture. Arterioscler Thromb Vasc Biol. 1999;19:2251–62. doi: 10.1161/01.atv.19.9.2251. [DOI] [PubMed] [Google Scholar]
- 16.Hansen JB, Svensson B, Olsen R, Ezban M, Osterud B, Paulssen RH. Heparin induces synthesis and secretion of tissue factor pathway inhibitor from endothelial cells in vitro. Thromb Haemost. 2000;83:937–43. [PubMed] [Google Scholar]
- 17.Lupu C, Lupu F, Dennehy U, Kakkar VV, Scully MF. Thrombin induces the redistribution and acute release of tissue factor pathway inhibitor from specific granules within human endothelial cells in culture. Arterioscler Thromb Vasc Biol. 1995;15:2055–62. doi: 10.1161/01.atv.15.11.2055. [DOI] [PubMed] [Google Scholar]
- 18.Sevinsky JR, Rao LV, Ruf W. Ligand-induced protease receptor translocation into caveolae: a mechanism for regulating cell surface proteolysis of the tissue factor- dependent coagulation pathway. J Cell Biol. 1996;133:293–304. doi: 10.1083/jcb.133.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maroney SA, Ferrel JP, Pan S, White TA, Simari RD, McVey JH, Mast AE. Temporal expression of alternatively spliced forms of tissue factor pathway inhibitor in mice. J Thromb Haemost. 2009;7:1106–13. doi: 10.1111/j.1538-7836.2009.03454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Girard TJ, Tuley E, Broze GJ., Jr TFPIbeta is the GPI-anchored TFPI isoform on human endothelial cells and placental microsomes. Blood. 2012;119:1256–62. doi: 10.1182/blood-2011-10-388512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lockett JM, Mast AE. Contribution of regions distal to glycine-160 to the anticoagulant activity of tissue factor pathway inhibitor. Biochemistry. 2002;41:4989–97. doi: 10.1021/bi016058n. [DOI] [PubMed] [Google Scholar]
- 22.Wesselschmidt R, Likert K, Girard T, Wun TC, Broze GJ., Jr Tissue factor pathway inhibitor: the carboxy-terminus is required for optimal inhibition of factor Xa. Blood. 1992;79:2004–10. [PubMed] [Google Scholar]
- 23.Lindhout T, Willems G, Blezer R, Hemker HC. Kinetics of the inhibition of human factor Xa by full-length and truncated recombinant tissue factor pathway inhibitor. Biochem J. 1994;297 (Pt 1):131–6. doi: 10.1042/bj2970131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen HH, Vicente CP, He L, Tollefsen DM, Wun TC. Fusion proteins comprising annexin V and Kunitz protease inhibitors are highly potent thrombogenic site-directed anticoagulants. Blood. 2005:2004–11. doi: 10.1182/blood-2004-11-4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piro O, Broze GJ. Comparison of cell-surface TFPIalpha and beta. Journal of Thrombosis and Haemostasis. 2005;3:2677–83. doi: 10.1111/j.1538-7836.2005.01636.x. [DOI] [PubMed] [Google Scholar]
- 26.Stavik B, Skretting G, Aasheim HC, Tinholt M, Zernichow L, Sletten M, Sandset PM, Iversen N. Downregulation of TFPI in breast cancer cells induces tyrosine phosphorylation signaling and increases metastatic growth by stimulating cell motility. BMC Cancer. 2011;11:357. doi: 10.1186/1471-2407-11-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maroney SA, Ellery PE, Wood JP, Ferrel JP, Bonesho CE, Mast AE. Caveolae optimize tissue factor-Factor VIIa inhibitory activity of cell-surface-associated tissue factor pathway inhibitor. Biochem J. 2012;443:259–66. doi: 10.1042/BJ20111994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hilden I, Lauritzen B, SORENSEN BB, Clausen JT, Jespersgaard C, Krogh BO, Bowler AN, Breinholt J, Gruhler A, Svensson LA, Petersen HH, Petersen LC, Balling KW, Hansen L, Hermit MB, Egebjerg T, Friederichsen B, Ezban M, Bjorn SE. Hemostatic effect of a monoclonal antibody mAb 2021 blocking the interaction between FXa and TFPI in a rabbit hemophilia model. Blood. 2012;119:5871–8. doi: 10.1182/blood-2012-01-401620. [DOI] [PubMed] [Google Scholar]
- 29.Hughes CS, Postovit LM, Lajoie GA. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics. 2010;10:1886–90. doi: 10.1002/pmic.200900758. [DOI] [PubMed] [Google Scholar]
- 30.Maroney SA, Cooley BC, Ferrel JP, Bonesho CE, Mast AE. Murine hematopoietic cell tissue factor pathway inhibitor limits thrombus growth. Arterioscler Thromb Vasc Biol. 2011;31:821–6. doi: 10.1161/ATVBAHA.110.220293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Contrino J, Hair G, Kreutzer DL, Rickles FR. In situ detection of tissue factor in vascular endothelial cells: correlation with the malignant phenotype of human breast disease. Nat Med. 1996;2:209–15. doi: 10.1038/nm0296-209. [DOI] [PubMed] [Google Scholar]
- 32.Hu Z, Garen A. Targeting tissue factor on tumor vascular endothelial cells and tumor cells for immunotherapy in mouse models of prostatic cancer. Proc Natl Acad Sci U S A. 2001;98:12180–5. doi: 10.1073/pnas.201420298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang H, Ivanciu L, Popescu N, Peer G, Hack E, Lupu C, Taylor FB, Jr, Lupu F. Sepsis-Induced Coagulation in the Baboon Lung Is Associated with Decreased Tissue Factor Pathway Inhibitor. Am J Pathol. 2007;171:1066–77. doi: 10.2353/ajpath.2007.070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schuepbach RA, Velez K, Riewald M. Activated protein C up-regulates procoagulant tissue factor activity on endothelial cells by shedding the TFPI Kunitz 1 domain. Blood. 2011;117:6338–46. doi: 10.1182/blood-2010-10-316257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahamed J, Belting M, Ruf W. Regulation of tissue factor-induced signaling by endogenous and recombinant tissue factor pathway inhibitor 1. Blood. 2005;105:2384–91. doi: 10.1182/blood-2004-09-3422. [DOI] [PubMed] [Google Scholar]
- 36.Abraham E, Reinhart K, Opal S, Demeyer I, Doig C, Rodriguez AL, Beale R, Svoboda P, Laterre PF, Simon S, Light B, Spapen H, Stone J, Seibert A, Peckelsen C, De DC, Postier R, Pettila V, Artigas A, Percell SR, et al. Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis: a randomized controlled trial. JAMA. 2003;290:238–47. doi: 10.1001/jama.290.2.238. [DOI] [PubMed] [Google Scholar]
- 37.Laterre PF. Beyond antibiotics in severe community-acquired pneumonia: the role and rationale for tissue factor pathway inhibition. Crit Care. 2008;12 (Suppl 6):S4. doi: 10.1186/cc7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Den Boogaard FE, Brands X, Schultz MJ, Levi M, Roelofs JJ, van’ V, van der PT. Recombinant human tissue factor pathway inhibitor exerts anticoagulant, anti-inflammatory and antimicrobial effects in murine pneumococcal pneumonia. J Thromb Haemost. 2011;9:122–32. doi: 10.1111/j.1538-7836.2010.04089.x. [DOI] [PubMed] [Google Scholar]
- 39.Fischer EG, Riewald M, Huang HY, Miyagi Y, Kubota Y, Mueller BM, Ruf W. Tumor cell adhesion and migration supported by interaction of a receptor-protease complex with its inhibitor. J Clin Invest. 1999;104:1213–21. doi: 10.1172/JCI7750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pendurthi UR, Alok D, Rao LV. Binding of factor VIIa to tissue factor induces alterations in gene expression in human fibroblast cells: up-regulation of poly(A) polymerase. Proc Natl Acad Sci U S A. 1997;94:12598–603. doi: 10.1073/pnas.94.23.12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bromberg ME, Bailly MA, Konigsberg WH. Role of protease-activated receptor 1 in tumor metastasis promoted by tissue factor. Thromb Haemost. 2001;86:1210–4. [PubMed] [Google Scholar]
- 42.Maroney SA, Cooley BC, Ferrel JP, Bonesho CE, Nielsen LV, Johansen PB, Hermit MB, Petersen LC, Mast AE. Absence of hematopoietic tissue factor pathway inhibitor mitigates bleeding in mice with hemophilia. Proc Natl Acad Sci U S A. 2012;109:3927–31. doi: 10.1073/pnas.1119858109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hackeng TM, Sere KM, Tans G, Rosing J. Protein S stimulates inhibition of the tissue factor pathway by tissue factor pathway inhibitor. Proc Natl Acad Sci U S A. 2006;103:3106–11. doi: 10.1073/pnas.0504240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lupu C, Hu X, Lupu F. Caveolin-1 Enhances Tissue Factor Pathway Inhibitor Exposure and Function on the Cell Surface. J Biol Chem. 2005;280:22308–17. doi: 10.1074/jbc.M503333200. [DOI] [PubMed] [Google Scholar]
- 45.Prasad S, Lillicrap D, Labelle A, Knappe S, Keller T, Burnett E, Powell S, Johnson KW. Efficacy and safety of a new-class hemostatic drug candidate, AV513, in dogs with hemophilia A. Blood. 2008;111:672–9. doi: 10.1182/blood-2007-07-098913. [DOI] [PubMed] [Google Scholar]
- 46.Waters EK, Genga RM, Schwartz MC, Nelson JA, Schaub RG, Olson KA, Kurz JC, McGinness KE. Aptamer ARC19499 mediates a procoagulant hemostatic effect by inhibiting tissue factor pathway inhibitor. Blood. 2011;117:5514–22. doi: 10.1182/blood-2010-10-311936. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.