Abstract
Background
This study examined the feasibility of a combination prevention intervention for young men who have sex with men (YMSM), an anticipated target population for HIV pre-exposure prophylaxis (PrEP).
Methods
Project PrEPare, a pilot study using a randomized 3-arm design, compared an efficacious behavioral HIV-prevention intervention (3MV) alone, 3MV combined with PrEP (tenofovir/emtricitabine), and 3MV combined with placebo. Eligible participants were 18–22 year old HIV-uninfected men who reported unprotected anal intercourse (UAI) in the past year. Participants were screened for preliminary eligibility at youth venues and community organizations, and were also referred through social networks. Laboratory screening determined final eligibility. Behavioral and biomedical data were collected at baseline and every 4 weeks thereafter for 24 weeks.
Results
Sixty-eight youth (mean age = 19.97 years; 53% African-American, 40% Latino were enrolled; 58 were randomized. Self-reported medication adherence averaged 62% (range 43–83%) while rates of detectable tenofovir in plasma of participants in the FTC/TDF arm ranged from 63.2% (week 4) to 20% (week 24). There were 5 ≥ Grade 2 adverse events possibly/probably related to the study medication. Sexual risk behavior declined from baseline to week 24 in all study arms.
Conclusions
The feasibility of enrolling at risk youth, particularly YMSM of color, into Project PrEPare has been demonstrated. The acceptability of the group intervention along with counseling and testing was high. Self-reported medication adherence and corresponding plasma drug concentrations were low indicating the need for enhanced adherence counseling. Exploration of PrEP use among youth in non-randomized, open label trials is warranted.
INTRODUCTION
Pre-exposure prophylaxis (PrEP) is a promising biomedical intervention for primary HIV prevention. Data released in November 2010 from a multi-national study, the PrEP Initiative (iPrEx, for Iniciativa Profilaxis Pre Exposicion), showed that men who have sex with other men (MSM) at high risk for HIV infection who took a daily tablet containing emtricitabine (FTC) and tenofovir disoproxil fumarate(TDF) [Truvada®] experienced an average of 43.8% fewer HIV infections than those who received a placebo pill (95% CI 15.4 to 62.6%; P=0.005). 1 The protective efficacy of PrEP appeared highly correlated with the level of adherence. A subgroup analysis found a relative reduction in HIV risk of 92% among those with detectable study-drug levels. 1 These groundbreaking results were followed by two additional studies, Partners PrEP and TDF2, demonstrating Truvada® efficacy in serodiscordant couples and heterosexual men and women. 2, 3 In contrast to this growing body of evidence in support of PrEP, two other PrEP studies, both targeting high risk heterosexual women, have been halted or adapted due to futility: 1) Fem-PrEP 4 and 2) oral TDF only and vaginal topical tenofovir gel arms of the Vaginal and Oral Interventions to Control the Epidemic (VOICE) study.5 Based on evidence from these PrEP trials, on July 16th, 2012, the United States Food and Drug Administration approved Truvada® as the first medication for the prevention of sexually-acquired HIV.
Even with demonstrated efficacy and an approved product for adult MSM, questions remain regarding the effective implementation of PrEP. This is especially true among youth, particularly racial and ethnic minority youth, who are increasingly disproportionately impacted by HIV in the United States. 6 As demonstrated in iPrEx as well as the other PrEP trials, adherence to the PrEP medication is highly related to efficacy. Whether for disease management or contraception, adherence to medication is known to be a significant challenge for adolescents and young adults. 7–11 Additionally, questions about the impact PrEP might have on the sexual risk behavior of young people still arise,12, 13 even though there has been no evidence to date of risk compensation in adult PrEP clinical trials. These issues highlight the potential need for behavioral strategies targeting sexual risk and PrEP adherence among youth as well as the need to develop and validate assessment measures for monitoring these behaviors if PrEP is to be effective enough for routine use in such vulnerable populations. The integration of behavioral interventions with PrEP delivery would allow us to comprehensively address and evaluate these issues.
The primary aim of this pilot study was to evaluate the specific components of a PrEP delivery protocol that would be included in future research trials and demonstration projects of PrEP among YMSM. Specifically, the study aimed to: 1) examine the feasibility of enrolling and retaining a cohort of YMSM in a PrEP clinical trial, 2) examine the acceptability of random assignment in a placebo-controlled trial among youth, 3) pilot test measures and approaches for examining risk compensation (defined as an increase in risk behavior attributable to a belief that the use of PrEP will protect against HIV infection) among YMSM enrolled in a PrEP trial, 4) pilot test measures and approaches for examining adherence among YMSM enrolled in a PrEP trial, and 5) examine the overall acceptability, feasibility, and reduction in sexual risk behaviors of a combined biomedical-behavioral HIV risk reduction intervention.
METHODS
Overview
This pilot study combined an evidenced-based behavioral HIV-prevention intervention (Many Men, Many Voices – 3MV) with a biomedical HIV prevention intervention (PrEP). This research study was conducted at two clinical sites in Chicago and funded through the Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN). All study procedures were approved by the Institutional Review Boards of the participating sites.
Participants
Young male participants, between 18 and 22 years of age (inclusive), were approached by study staff at community-based agencies and youth venues for a brief eligibility screening via handheld personalized digital assistant (PDA). Field screening questions assessed age, HIV status, history of unprotected anal intercourse (UAI) in past 12 months, and intent to remain in the area for next 6 months. Participants who were eligible and interested were scheduled for a clinic-based screening visit to confirm eligibility. The target sample size was 100 young men.
Participants were confirmed eligible if they reported at least one episode of UAI with a male within the last 12 months and tested HIV seronegative at screening. Exclusion criteria were sickle cell disease; hypophosphatemia; Creatinine Clearance < 75 ml/min; history of bone fractures not explained by trauma; [≥ 2+] urine dipstick protein or urinary protein-creatinine ratio (Up/cr) ≥ 3.5 g/g; normoglycemic glucosuria ([≥ 1+] urine dipstick); serious psychiatric symptoms; active hepatitis B infection; or use of nephrotoxic drugs, diuretics, non-steroidal anti-inflammatory drugs, other antiretroviral drugs or drugs that may interfere with tenofovir excretion.
Procedures
After participants were confirmed to be eligible and completed their baseline study visit, they were scheduled to attend the group-based behavioral intervention Many Men, Many Voices (3MV).14 3MV was chosen for the behavioral intervention as it is one of the CDC’s Diffusion of Effective Behavioral Interventions (DEBI) and is a group-based HIV-prevention intervention that explores HIV risk among MSM within the context of dual identity as both racial and sexual minorities. For the purposes of this trial, the 3MV intervention was adapted for use with youth groups of mixed racial and ethnic identities. The intervention, facilitated by trained research staff, was conducted as a 2-day seminar at community locations centrally located in Chicago with approximately 8 participants per session.
After the participants completed 3MV, they were scheduled for the Week 0 visit when they were randomized in blocks of six within each site to one of three study arms in a 1:1:1 ratio: 1) daily co-formulated emtricitabine (FTC) and tenofovir disoproxil fumarate (TDF) as PrEP, 2) placebo pill control or 3) “no pill” control. Participants randomized to study pill arms were dispensed a 30-day supply of the study drug at each visit and instructed to take one tablet once daily with or without food at about the same time each day. FTC/TDF tablets and matching placebo tablets were supplied by Gilead Sciences, Inc. in Foster City, CA, USA.
Behavioral and biomedical data were then collected every 4 weeks thereafter for 24 weeks. A medical history, including review of signs and symptoms, and a symptom-directed physical exam were conducted at each visit. Laboratory evaluations included FDA-approved HIV antibody testing, plasma HIV RNA, and urine nucleic-acid amplification testing for gonorrhea and Chlamydia, rapid plasma regain and hepatitis B surface antigen for all participants; for participants in the FTC/TDF or placebo arms, routine chemistries including liver and pancreatic function tests and urine dipstick testing for protein and glucose was also performed. Blood collected at each visit was stored for TDF plasma concentration testing.
All participants received a comprehensive package of HIV prevention services at each visit (risk reduction, condoms, STI screening and treatment, etc.). Behavioral assessments were conducted via audio computer-assisted self-interview (ACASI) at each study visit along with risk reduction counseling, condom distribution, and drug dispensation. No specific adherence counseling was offered to participants because insuring the ability to analyze for product efficacy was not an aim of the study. Participants were provided a $50 incentive for each study visit as well as fare for round-trip public transportation.
Measures
Feasibility was assessed throughout the study via process indicators including number screened, eligible, enrolled, and retained.
Acceptability of the study design was assessed using a 10-item questionnaire at the last study visit. Acceptability of the study pill (i.e., pill size, taste, color) was also assessed at the final study visit.
Adherence to study drug was evaluated per participant using a) Time-Line Follow-Back (TFLB) 15 – this calendar method, which was originally developed to gather information on daily alcohol consumption, was adapted to explore the participant’s self-report of adherence difficulties with PrEP. The TLFB also serves as a temporal ordering cue to help participants piece together their adherence rates over the past month. First, participants are shown a calendar of the previous 30 days. Then they are asked to remember any significant events (i.e., holidays, birthdays, deaths) or daily occurrences (i.e., work/school schedule) that happened during that period. Finally, they are asked about the number of missed doses of medication for each day. A proportion of medication adherence (number of days of missed medication/total number of days) can then be calculated; b) the AIDS Clinical Trial Group Adherence Follow-up Questionnaire (ACTG) 16 is a 13-item scale adapted for PrEP use that asks participants to rate how often they have missed taking their medications over the past month because of a number of reasons on a 4-point Likert scale ranging from Never to Often; c) plasma tenofovir concentrations were collected at each visit, and d) medication gaps were monitored based on pharmacy refill dates. 17
Sexual behaviors were evaluated using a Sexual Activity Questionnaire (ATN Behavioral Leadership Group (BLG) Secondary Prevention Working Group) that includes questions to assess condom/barrier protected and unprotected oral, vaginal, and anal sexual activity with both males and females in the past month.
Risk Compensation was assessed using the Treatment-Related Reduced HIV Risk 18 measure, a 6-item scale adapted for PrEP use that assesses decreased personal concern about engaging in unsafe sex and the potential for infecting others because of the availability of combination therapies.
Data and Safety Monitoring
The Protocol Team monitored the study for clinical adverse events (AE) and aberrant laboratory values using the ATN Table for Grading Severity of Adolescent Adverse Events (October 2006). Expedited Adverse Event (EAE) reporting followed standard reporting requirements as defined in the Manual for Expedited Reporting of Adverse Events to ATN/NICHD, version 1.0, October 2006. An external Data and Safety Monitoring Board (DSMB) also monitored this trial every six months for evidence of early significant benefit or harm to participants.
On November 23rd, 2010, following the release of the iPrEx efficacy results, the DSMB convened a special meeting to discuss the impact of the iPrEx results on Project PrEPare (ATN 082). The DSMB recommended that all participants be immediately notified of the results of the iPrEx study and suggested that all participants currently enrolled should be unblinded, enrollment into the study be discontinued, all participants on the placebo and no pill arms be offered the option of switching to PrEP, and participants on the active PrEP arm continue as scheduled. The recommendations were followed and the results presented in this paper represent data up to the point of unblinding.
Statistical Analysis
Baseline characteristics for all treatment groups were described using frequencies, means, medians, standard deviations, and ranges as appropriate. Similar descriptive statistics will be used to summarize measures of feasibility, acceptability, behavior inhibition and adherence. The Wilcoxon rank sum test (or the Kruskal-Wallis test) was used to test for treatment group differences for quantitative variables. The Pearson Chi-square test (or Fisher’s Exact test) was used for categorical variables. Generalized estimating equations with a logit link and binomial distribution were used to determine if the percentage of actual number of visits conducted, the number of missed medication days (pill arms only) (no missed medication days vs. missed 1 or more medication days), and behavioral disinhibition (no unprotected sex acts vs. 1 or more unprotected sex acts) differed over time between treatment groups. Medication adherence and behavioral disinhibition were also examined as quantitative variables using generalized estimating equations. Statistical analyses were carried out using SAS version 9.2 (SAS Institute Inc, Cary, NC), and p < 0.05 was used to determine statistical significance. No adjustment for multiple comparisons was made due to the exploratory nature of the study.
RESULTS
Recruitment and Participant Retention
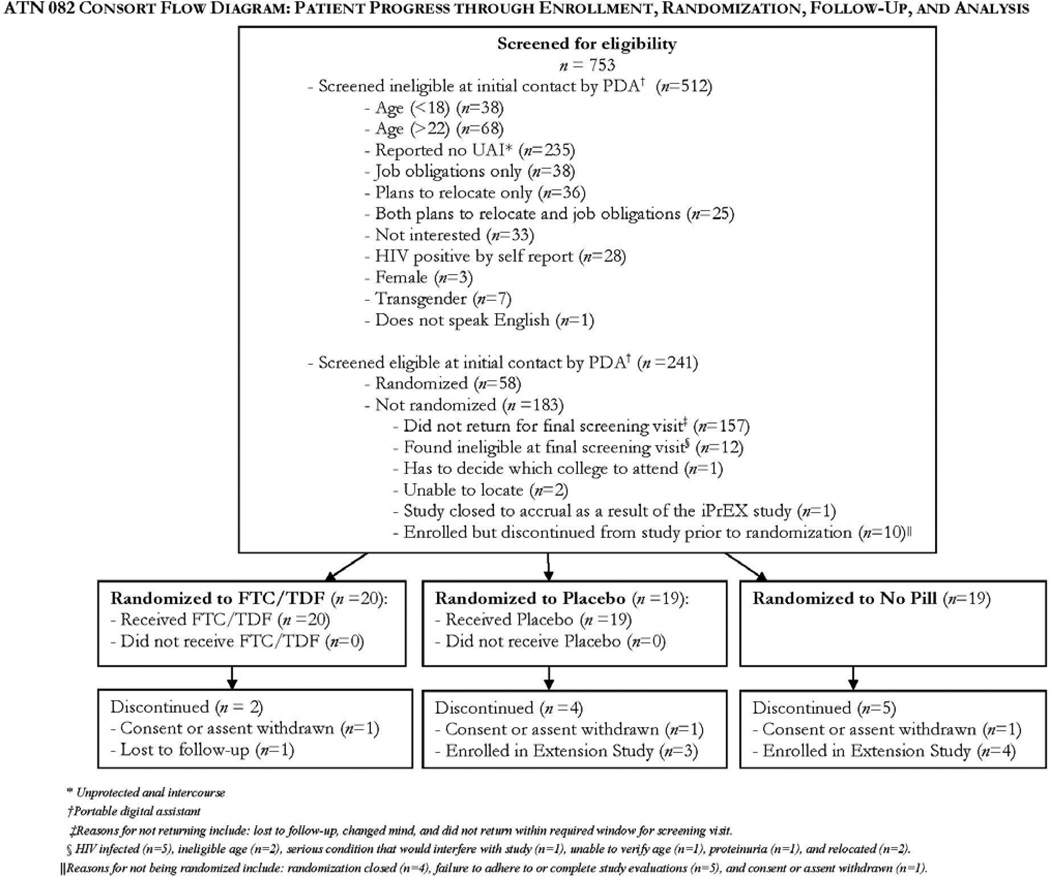
Of the 753 participants screened in the field over a 12 month period, 241 were eligible to proceed with the clinical screening visit, 68 participants were enrolled, and 58 randomized (Fig. 1). Reasons for exclusion included failure to attend screening visit (86%), medically ineligible (7%), relocated or withdrew prior to randomization (5%), and a positive HIV antibody test (3%). Participants were male, mean age 19.97 years and identified as black/African-American (53%), Latino (40%), white (7%), and Native American/Alaskan Native (2%). Thirty-one percent of participants had completed high school and 52% had completed some college coursework. Over half of participants were currently unemployed (55%) and 78% had received some form of public assistance in their lifetime. One-third (32.76%) of participants had spent at least one night in an emergency shelter, 17% had exchanged sex for money or a place to stay, and 16% reported having been kicked out of their house due to their sexual orientation (Table 1.) There were no statistically significant differences between baseline demographics among the three treatment groups. Retention to study visits was 98.5% overall.
Figure 1.
Consort Diagram
Table 1.
Baseline Demographic Data
| Overall | FTC/TDF | Placebo | No Pill | p-value | |
|---|---|---|---|---|---|
| Age at Baseline (years) | |||||
| N | 58 | 20 | 19 | 19 | 0.4763 |
| Mean | 19.97 | 19.80 | 20.26 | 19.84 | |
| Std | 1.30 | 1.44 | 1.45 | 0.96 | |
| Median | 20.00 | 19.50 | 20.00 | 20.00 | |
| Race (%) | |||||
| Black/African American | 31(53.45) | 10(50.00) | 12(63.16) | 9(47.37) | 0.8836 |
| Native American/Alaskan Native | 1(1.72) | 1(5.00) | 0(0.00) | 0(0.00) | |
| White | 4(6.90) | 1(5.00) | 1(5.26) | 2(10.53) | |
| Other/Mixed Race | 22(37.93) | 8(40.00) | 6(31.58) | 8(42.11) | |
| Ethnicity (%) | |||||
| Hispanic or Latino | 23(39.66) | 7(35.00) | 6(31.58) | 10(52.63) | 0.4354 |
| Non-Hispanic or Latino | 35(60.34) | 13(65.00) | 13(68.42) | 9(47.37) | |
| Education | |||||
| Eighth grade or less | 1(1.72) | 0(0.00) | 0(0.00) | 1(5.26) | 0.1448 |
| GED | 2(3.45) | 0(0.00) | 1(5.26) | 1(5.26) | |
| High School Diploma | 18(31.03) | 10(50.00) | 3(15.79) | 5(26.32) | |
| Some College | 30(51.72) | 8(40.00) | 14(73.68) | 8(42.11) | |
| Current Employment | |||||
| Unemployed | 32(55.17) | 12(60.00) | 11(57.89) | 9(47.37) | 0.9669 |
| Full time | 6(10.34) | 2(10.00) | 2(10.53) | 2(10.53) | |
| Part time | 20(34.48) | 6(30.00) | 6(31.58) | 8(42.11) | |
| Housing Experiences (yes) | |||||
| Kicked out of house due to sexual orientation | 9(15.52) | 4(20.00) | 2(10.53) | 3(15.79) | 0.8963 |
| Spent at least one night in shelter | 19(32.76) | 5(25.00) | 6(31.58) | 8(42.11) | 0.5273 |
| Have exchanged sex for money or place to stay | 10(17.24) | 2(10.00) | 3(15.79) | 5(26.32) | 0.3880 |
| Unprotected anal sex with man in past 30 days (%)1 | |||||
| Yes | 24(41.38) | 9(45.00) | 7(36.84) | 8(42.11) | 0.9435 |
| No | 34(58.62) | 11(55.00) | 12(63.16) | 11(57.89) | |
| Unprotected anal or vaginal sex with Woman (%)2 | |||||
| Yes | 3(5.17) | 0(0.00) | 2(10.53) | 1(5.26) | 0.3140 |
| No | 55(94.83) | 20(100.0) | 17(89.47) | 18(94.74) |
The cell size, mean, standard deviation, median and range (min, max) are provided for continuous variables; p-value is from Wilcoxon rank sum test.
Frequency and % are provided for categorical variables; p-value is from Fisher's Exact test.
Acceptability
Acceptability ratings were very high for almost all aspects of the study design, with the exception of randomization. Acceptability of the study drug itself and the prescribed regimen was less favorable (Table 2). Participants were also asked, considering their experience in this PrEP study, how they might use PrEP if it were available in the future (Supplemental Digital Content Table 1). There was a statistically significant difference between treatment groups in the number of subjects that would not take PrEP if it had to be taken more than once a day (p=0.0038). Thirty-eight percent in the No Pill group indicated they would not take PrEP more than once a day, whereas, only 10% in the FTC/TDF group and 0% in the Placebo group indicated they would not take PrEP more than once a day. There was also a statistically significant difference between treatment groups in the number of subjects that would use PrEP only when they knew that their partner was HIV-infected (p=0.0268). Sixty-two percent of the No Pill group indicated they would only use PrEP when they knew that their partner was HIV-infected; however, 30% of the FTC/TDF group and 17% of the Placebo group indicated they would only use PrEP when they knew that their partner was HIV-infected.
Table 2.
Acceptability of study design and study pill
| Overall | FTC/TDF | Placebo | No Pill | p-value | |
|---|---|---|---|---|---|
| Size of the Pill (%) | |||||
| Did not like it at all | 6(27.27) | 3(30.00) | 3(25.00) | - | 0.6921 |
| Did not like | 6(27.27) | 3(30.00) | 3(25.00) | - | |
| Liked | 9(40.91) | 3(30.00) | 6(50.00) | - | |
| Liked a lot | 1(4.55) | 1(10.00) | 0(0.00) | - | |
| Taste of the Pill (%) | |||||
| Did not like it at all | 7(31.82) | 4(40.00) | 3(25.00) | - | 0.4655 |
| Did not like | 5(22.73) | 1(10.00) | 4(33.33) | - | |
| Liked | 9(40.91) | 4(40.00) | 5(41.67) | - | |
| Liked a lot | 1(4.55) | 1(10.00) | 0(0.00) | - | |
| Color of the Pill (%) | |||||
| Did not like it at all | 2(9.09) | 1(10.00) | 1(8.33) | - | 1.0000 |
| Did not like | 5(22.73) | 2(20.00) | 3(25.00) | - | |
| Liked | 9(40.91) | 4(40.00) | 5(41.67) | - | |
| Liked a lot | 6(27.27) | 3(30.00) | 3(25.00) | - | |
| Taking the Pill Everyday (%) | |||||
| Did not like it at all | 4(18.18) | 3(30.00) | 1(8.33) | - | 0.2297 |
| Did not like | 10(45.45) | 3(30.00) | 7(58.33) | - | |
| Liked | 6(27.27) | 2(20.00) | 4(33.33) | - | |
| Liked a lot | 2(9.09) | 2(20.00) | 0(0.00) | - | |
| Taking Part in the Study (%) | |||||
| Did not like it at all | 2(5.71) | 2(20.00) | 0(0.00) | 0(0.00) | 0.1886 |
| Did not like | 1(2.86) | 0(0.00) | 1(8.33) | 0(0.00) | |
| Liked | 3(8.57) | 0(0.00) | 2(16.67) | 1(7.69) | |
| Liked a lot | 29(82.86) | 8(80.00) | 9(75.00) | 12(92.31) | |
| Participating in Group Sessions (%) | |||||
| Did not like it at all | 1(2.86) | 0(0.00) | 1(8.33) | 0(0.00) | 0.1908 |
| Did not like | 3(8.57) | 1(10.00) | 2(16.67) | 0(0.00) | |
| Liked | 6(17.14) | 0(0.00) | 2(16.67) | 4(30.77) | |
| Liked a lot | 25(71.43) | 9(90.00) | 7(58.33) | 9(69.23) | |
| Randomly assigned to a group (%) | |||||
| Did not like it at all | 5(14.71) | 1(11.11) | 4(33.33) | 0(0.00) | 0.2240 |
| Did not like | 7(20.59) | 3(33.33) | 2(16.67) | 2(15.38) | |
| Liked | 13(38.24) | 4(44.44) | 4(33.33) | 5(38.46) | |
| Liked a lot | 9(26.47) | 1(11.11) | 2(16.67) | 6(46.15) | |
| HIV test at Every Visit (%) | |||||
| Did not like | 1(2.86) | 0(0.00) | 1(8.33) | 0(0.00) | 0.1538 |
| Liked | 4(11.43) | 0(0.00) | 3(25.00) | 1(7.69) | |
| Liked a lot | 30(85.71) | 10(100.0) | 8(66.67) | 12(92.31) | |
| Risk Reduction Counseling at Every Visit (%) | |||||
| Did not like it at all | 1(2.86) | 0(0.00) | 1(8.33) | 0(0.00) | 0.2151 |
| Liked | 10(28.57) | 1(10.00) | 5(41.67) | 4(30.77) | |
| Liked a lot | 24(68.57) | 9(90.00) | 6(50.00) | 9(69.23) | |
| Questions about Sexual Behavior at Every Visit (%) | |||||
| Did not like it at all | 1(2.86) | 0(0.00) | 1(8.33) | 0(0.00) | 0.2809 |
| Did not like | 1(2.86) | 0(0.00) | 1(8.33) | 0(0.00) | |
| Liked | 9(25.71) | 1(10.00) | 4(33.33) | 4(30.77) | |
| Liked a lot | 24(68.57) | 9(90.00) | 6(50.00) | 9(69.23) | |
| Contacted by the research team in between visits (%) | |||||
| Did not like | 2(5.88) | 0(0.00) | 2(18.18) | 0(0.00) | 0.1850 |
| Liked | 12(35.29) | 4(40.00) | 5(45.45) | 3(23.08) | |
| Liked a lot | 20(58.82) | 6(60.00) | 4(36.36) | 10(76.92) | |
| Physician exam by a doctor (%) | |||||
| Did not like it at all | 3(8.57) | 0(0.00) | 3(25.00) | 0(0.00) | 0.2366 |
| Did not like | 1(2.86) | 1(10.00) | 0(0.00) | 0(0.00) | |
| Liked | 8(22.86) | 2(20.00) | 3(25.00) | 3(23.08) | |
| Liked a lot | 23(65.71) | 7(70.00) | 6(50.00) | 10(76.92) | |
| Health clinic for study visits (%) | |||||
| Did not like | 2(5.71) | 0(0.00) | 2(16.67) | 0(0.00) | 0.4089 |
| Liked | 11(31.43) | 4(40.00) | 4(33.33) | 3(23.08) | |
| Liked a lot | 22(62.86) | 6(60.00) | 6(50.00) | 10(76.92) |
Frequency and % are provided for categorical variables; p-value is from Fisher's Exact test.
Sexual Risk Behavior and Risk Compensation
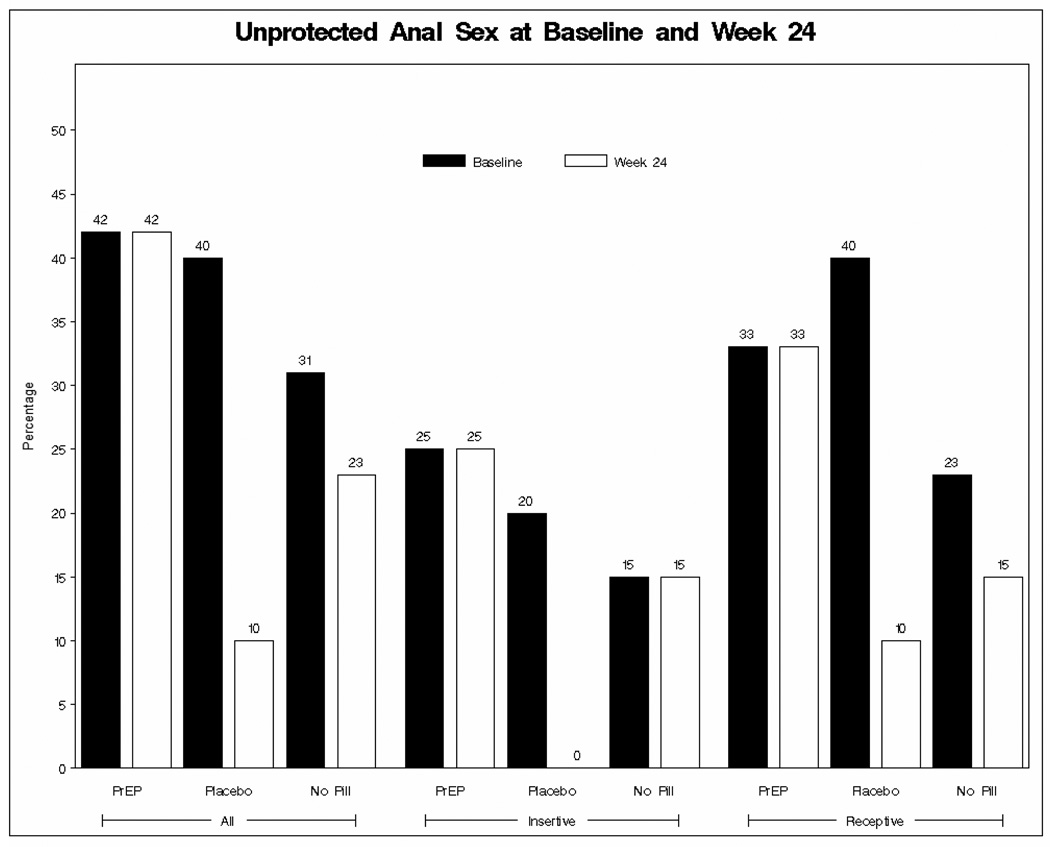
At baseline, 41% of the participants reported unprotected anal intercourse with another man in the past 30 days and 5% reported unprotected vaginal or anal sex with a woman in that same time period. Across all study visits, there were no statistically significant differences in the distribution of male-to-male unprotected anal sex acts among the three treatment groups. While there was a trend from baseline to week 24 of decreasing unprotected anal sex acts across all treatment arms, the differences were not statistically significant (Figure 2).
Figure 2.
Sexual Risk at Baseline vs. Week 24
STI screening at baseline identified 5 participants with syphilis, 2 with Chlamydia, 1 with genital herpes and 1 with molluscum contagiosum. All participants were treated. During the course of the study, additional STI diagnoses included syphilis (4), Chlamydia (7), anogenital human papillomavirus (HPV) (3), genital herpes (1), molluscum contagiosum (1), and gonorrhea (1). There were no HIV seroconversions among enrolled participants during the study.
In order to assess changes in pill-taking behavior based on perceived sexual risk, participants were asked “Thinking back over the past month, did you ever change the way you were taking the study pills based on what was going on in your sex life?.” Ninety-eight percent of participants reported that they did not change their pill taking behaviors based on their sex life.
On the measure of study-related HIV risk behavior (adapted from Vanable, et al.) 18, several statistically significant differences emerged among the treatment groups. For the question “Because I am in this PrEP study, I am less concerned about becoming HIV positive”, differences were found at Weeks 12 and 20. At Week 12, a higher percentage of subjects in the Placebo group (75%) strongly disagreed with the statement compared to the FTC/TDF (40%) and No Pill groups (31.25%). The pattern was similar at Week 20, although the difference between the Placebo group and FTC/TDF was smaller (79% vs. 67%). For the question “I am more willing to take a chance of getting infected now that I am in this PrEP study”, differences emerged at Weeks 8 and 24. At Week 8, a higher percentage of subjects in the Placebo group (100%) strongly disagreed with the statement compared to the FTC/TDF (69%) and No Pill groups (65%). The pattern was similar at Week 24, although the difference between the Placebo group and FTC/TDF was smaller (92% vs. 80%). For the question “I am a lot less worried about 'slipping up' now that PrEP may be taken prior to unprotected sex”, differences were found at Week 16 with a higher percentage of subjects in the Placebo group (93%) strongly disagreed with the statement compared to the FTC/TDF (54%) and No Pill groups (43%). Finally, for the question “I am less concerned about having unprotected anal sex now that I am in this PrEP study”, differences were found at Week 8 with a higher percentage of subjects in the Placebo group (83%) strongly disagreed with the statement compared to the FTC/TDF (47%) and No Pill groups (35%). There were no statistically significant differences between treatment groups at any of the visit weeks for the questions “The availability of PrEP makes me less worried about having unprotected sex” or “I have already risked getting infected with HIV through unsafe sex while I’ve been in this study.”
Medication Adherence
Self-reported adherence to study pill fluctuated across study visits. In the FTC/TDF arm, the median number of doses missed (out of possible 30) was 10, with a range from 5 missed doses (weeks 16 & 20) to 17 missed doses (week 24). Similar fluctuations occurred in the placebo arm with a range from 6 missed doses (week 24) to 19 missed doses (week 16). When calculating missed doses by medication gaps in pharmacy refill dates, median doses missed was 0 across all weeks of the study. There were no statistically significant differences in self-reported adherence between the two study pill arms across all visits. The most common reasons reported for missing doses were: away from home (60%), simply forgot (50%), and too busy (47%).
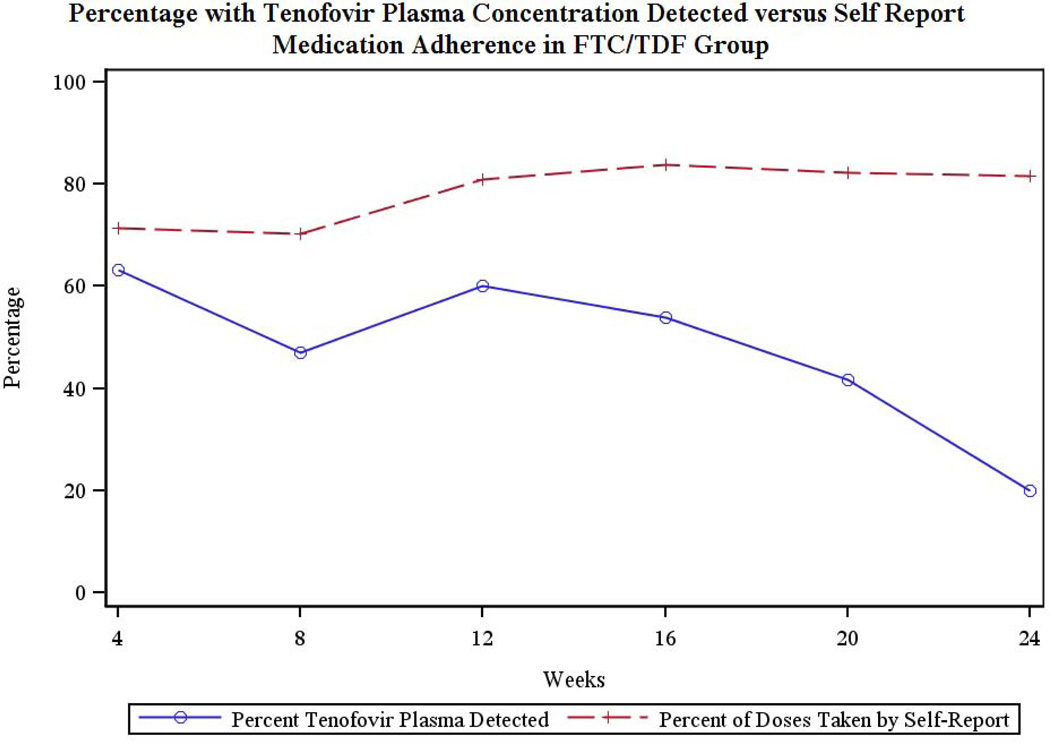
No tenofovir was detected in the two randomly selected plasma samples from each participant in the placebo and no-pill arms. The rates of detectable tenofovir in the plasma of participants in the FTC/TDF arm varied by study visit from 63.2% at week 4 to 20% at week 24. Figure 3 compares the percentage of self-reported doses taken to plasma tenofovir detected.
Figure 3.
Percentages of self-reported doses compared to plasma tenofovir in FTC/TDF arm
Most participants in both pill arms were unable to accurately determine their study-group assignment. There were no statistically significant differences between the FTC/TDF and Placebo group, with the exception of Week 24 where 67% of the placebo group stated that they thought they were on placebo; while only 22% of the FTC/TDF group thought they were on placebo.
Safety
Adverse events Grade 2 and above were reported to the protocol team. There were no serious adverse events and a total of 6 adverse events during the course of the study, 5 of which were possibly or probably related to the study drug. In the FTC/TDF arm, there were one increased blood bilirubin (Grade 3), one proteinuria (Grade 2), one migraine headache (Grade 3) and two instances of decreased creatinine clearance (Grade 3) in one participant. For that participant, the estimated creatinine clearance was 180 mL/min at baseline and showed considerable variability over the course of the study but never declined below 110 mL/min, despite continuation of study drug. In the placebo arm, there was one psychiatric hospitalization resulting from suicidal ideation (Grade 4) that was not related to study participation.
All participants were asked in the ACASI to self-report possible side effects to the study drug, including headache, nausea, vomiting, diarrhea, dizziness. The treatment groups differed at week 8 (p=0.0389) on their reports of nausea, with a higher percentage of subjects in the FTC/TDF group (23.5%) experienced nausea than the Placebo (0%) and No Pill (5.9%) treatment groups. There were no other statistically significant differences among the treatment groups across all other study visits.
DISCUSSION
HIV incidence continues to rise for youth in the United States indicating that our currently available prevention tools still have room for improvement. Powerful biomedical tools, intricately linked to effective HIV risk reduction and PrEP adherence interventions, are needed in order to achieve a decrease in HIV incidence among adolescents and young adults. This study was an initial step toward examining the acceptability, feasibility, and safety of PrEP while also exploring adherence and risk behaviors among young MSM using PrEP.
This study demonstrated the feasibility of enrolling a young cohort of primarily racial and ethnic minority MSM into a PrEP trial and retaining them despite an intensive visit schedule. While the recruitment team became more accurate over time at identifying productive recruitment venues, approximately one person was eligible for every three people screened. This ratio appears, however, to be consistent with recent large HIV prevention trials. 1, 19 Unfortunately, many eligible youth did not attend screening visits due primarily to changes in contact information and the inability of study staff to locate them. Participants reported high acceptability of many of the study components, including monthly HIV testing, physical examinations, group-based intervention sessions, and individual risk reduction counseling. Acceptability of random assignment to study arm and of the study pill itself was not as strong. Interestingly, a substantial number of participants indicated that they would rather not take PrEP if it were to be taken more than once per day or would take it only if they knew their partner was infected. This is consistent with results from other exploratory studies and has significant implications for the design of the next generation of PrEP studies.
Similar to most adult PrEP trials, behavioral disinhibition was not seen among this cohort and an actual decrease in sexual risk behavior was seen over time. This is not surprising given that youth received an intensive, evidence-based HIV prevention intervention prior to randomization as well as individual risk-reduction counseling and condom provision at every study visit. Additionally, youth consistently reported that their pill taking behavior was not altered by events in their sex life. However, given the discrepancies between self-reported adherence and plasma drug detection, the social desirability bias of self-reported behavior must be considered. The strong acceptability of the behavioral interventions and the corresponding decreases in risk behavior from baseline continue to support the use of these types of interventions in conjunction with PrEP.
The identification of previously undiagnosed prevalent cases of HIV during screening as well as incident STIs in this group along with the ability to provide treatment and/or linkage to care for those diagnoses was a success of this study. The ability of a PrEP program to identify an HIV infection rate of 3%, when the range of detection for routine testing in the US is <1%, and to hone in on incident STIs, needs to be further explored, evaluated, and considered as part of the ultimate cost-effectiveness of PrEP rollout. PrEP programs may be an effective HIV and STI (e.g. syphilis) testing and care linkage strategy that could yield successful primary and secondary prevention interventions that are synergistic in decreasing risk for HIV acquisition among youth populations.
Adherence to the study pill was low and poor concordance was seen between self-report and objective measures, with self-report being consistently higher than plasma detection. However, due to the limitations of plasma concentration testing, the low levels only demonstrate that pills were not taken in the 1–2 days prior to the study visit. Many participants did report that simple forgetting and changes in their daily schedules interfered with their ability to adhere to the pill regimen. These low levels of study pill adherence are consistent with the literature on youth adherence to contraception as well as medication for youth living with HIV or other chronic medical conditions. 7−9 Since this study was not intended to measure PrEP efficacy, no structured interventions targeting adherence were offered to participants. PrEP adherence for youth, in the absence of behavioral interventions to improve it in a sustainable fashion, may be insufficient for adequate HIV prevention. Thus, youth-friendly interventions and messaging around adherence will be essential for successful PrEP implementation. Because participants demonstrated a noticeable decline in adherence toward the end of the study, behavioral interventions could incorporate adherence “boosters”, such as text messaging or check-in calls, at times when adherence appears to be waning.
PrEP appeared to be generally safe and well-tolerated by the participants. Nausea was the primary side effect experienced by participants in the FTC/TDF group but seemed to resolve over several weeks. The side effects and adverse events that occurred in this study were similar to other PrEP trials. 1–3
This study successfully demonstrated that a cohort of young, primarily racial and ethnic minority MSM, can be successfully recruited, enrolled and retained in a domestic PrEP study and that the HIV prevention components of such a study are highly acceptable. Future PrEP studies are needed to explore the effectiveness of PrEP among young populations. Such studies should strive to incorporate behavioral interventions aimed at improving both sexual health and adherence, particularly for disadvantaged youth who are unfamiliar with or have limited access to the healthcare system. Furthermore, future demonstration and safety studies should begin to consider the effectiveness of PrEP among adolescents under the age of 18 who are at high risk for acquiring HIV.
Supplementary Material
ACKNOWLEDGMENTS
The Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) is funded by grant Number U01 HD040533-06 from the National Institutes of Health through the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Bill Kapogiannis, MD) with supplemental funding from the National Institutes on Drug Abuse (Nicolette Borek, PhD) and National Institute of Mental Health (Susannah Allison, PhD). We would like to thank Jaime Martinez, MD, PI and Lisa Henry-Reid, MD Co- PI of the Adolescent Trials Unit at John Stroger, Jr. Hospital of Cook County, Chicago, IL and the staff (Kelly Bojan, Christina Brakebill and Rachel Jackson)of the Ruth M. Rothstein CORE Center, Chicago, IL; Robert Garofalo, MD, PI of the Adolescent Trials Unit at Children’s Memorial Hospital and the staff at Howard Brown Health Center (Julia Brennan and Sara Flannigan), Chicago, IL; and the incredibly hard-working Research Assistants at both sites that made recruitment for this project possible (Christopher Balthazar, Ixchell Estes-Ortiz, Carlos Orengo, Pedro Serrano, Alexander Sewell and Michael Slater). ATN 082 has been scientifically reviewed by the ATN's Community Prevention Leadership Group. We would also like to thank Protocol Specialist Nancy Liu and individuals from the ATN Data and Operations Center (Westat, Inc.), Rockville, MD; and individuals from the ATN Coordinating Center at the University of Alabama at Birmingham including Cindy Partlow, Jeanne Merchant and Marcia Berck. Finally, and most importantly, we would like to thank the young men who participated in this study for their willingness to share their lives and their time with us.
Funding: This study was funded under cooperative agreement U01 HD040533-06 from the National Institutes of Health through the Eunice Kennedy Shriver National Institute of Child Health and Human Development with supplemental funding from the National Institute on Drug Abuse and National Institute of Mental Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts: None to declare
REFERENCES
- 1.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, Glidden DV. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J Partners PrEP Study Team. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012 Aug 2;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM TDF2 Study Group. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012 Aug 2;367(5):423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 4.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S FEM-PrEP Study Group. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012 Aug 2;367(5):41–22. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hillier S. 19th Conference on Retroviruses and Opportunistic Infections. Seattle, WA: 2012. HIV prevention research: unanswered questions. [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC) CDC Trials: Pre-Exposure Prophylaxis for HIV Prevention CDC Fact Sheet. 2011 Feb; Available from http://www.cdc.gov/hiv/prep/pdf/PrEP_TrialsFactSheet.pdf.
- 7.Hall KS, White KO, Reame N, Westhoff C. Studying the use of oral contraception: a review of measurement approaches. J Womens Health. 2010 Dec;19(12):2203–2210. doi: 10.1089/jwh.2010.1963. Epub 2010 Oct 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindsey JC, Bosch RJ, Rudy BJ, Flynn PM. Early patterns of adherence in adolescents initiating highly active antiretroviral therapy predict long-term adherence, virologic, and immunologic control. AIDS Patient Care STDS. 2009 Oct;23(10):799–801. doi: 10.1089/apc.2009.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rudy BJ, Murphy DA, Harris DR, Muenz L, Ellen J Adolescent Trials Network for HIV/AIDS Interventions. Patient-related risks for nonadherence to antiretroviral therapy among HIV-infected youth in the United States: a study of prevalence and interactions. AIDS Patient Care and STDS. 2009 Mar;23(3):185–194. doi: 10.1089/apc.2008.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zindani GN, Streetman DD, Streetman DS, Nasr SZ. Adherence to treatment in children and adolescent patients with cystic fibrosis. J Adolesc Health. 2006 Jan;38(1):13–17. doi: 10.1016/j.jadohealth.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 11.de Oliveira BM, Viana MB, Zani CL, Romanha AJ. Clinical and laboratory evaluation of compliance in acute lymphoblastic leukaemia. Arch Dis Child. 2004 Aug;89(8):785–788. doi: 10.1136/adc.2003.030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poynten IM, Zablotska I, Grulich AE. Considerations regarding antiretroviral chemoprophylaxis in MSM. Curr Opin HIV AIDS. 2012 Nov;7(6):549–556. doi: 10.1097/COH.0b013e3283582c71. [DOI] [PubMed] [Google Scholar]
- 13.Golub SA, Kowalczyk W, Weinberger CL, Parsons JT. Preexposure prophylaxis and predicted condom use among high-risk men who have sex with men. JAIDS. 2010;54(5):548–555. doi: 10.1097/QAI.0b013e3181e19a54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilton L, Herbst JH, Coury-Doniger P, et al. Efficacy of an HIV/STI prevention intervention for black men who have sex with men: findings from the Many Men, Many Voices (3MV) project. AIDS Behav. 2009;13:532–544. doi: 10.1007/s10461-009-9529-y. [DOI] [PubMed] [Google Scholar]
- 15.Sobell MB, Maisto SA, Sobell LC, Cooper AM, Cooper T, Sanders B. Developing a prototype for evaluating alcohol treatment effectiveness. In: Sobell LC, Sobell MB, Ward E, editors. Evaluating alcohol and drug absued treatment effectiveness: Recent advances. New York: Pergamon; 1980. pp. 129–150. [Google Scholar]
- 16.Chesney MA, Morin M, Sherr L. Adherence to HIV antiretroviral medicine. Soc Sci Med. 2000;50:1599–1605. doi: 10.1016/s0277-9536(99)00468-2. [DOI] [PubMed] [Google Scholar]
- 17.Berg KM, Arnsten JH. Practical and conceptual challenges in measuring antiretroviral adherence. JAIDS. 2006;1(43 Suppl 1):S79-876. doi: 10.1097/01.qai.0000248337.97814.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vanable PA, Ostrow DG, McKirnan DJ. Viral load and HIV treatment attitudes as correlates of sexual risk behavior among HIV-positive gay men. J Psychosom Res. 2003;54(3):263–269. doi: 10.1016/s0022-3999(02)00483-x. [DOI] [PubMed] [Google Scholar]
- 19.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kmarasamy N HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.