Abstract
Legumes can form a nitrogen fixing symbiosis with soil bacteria called rhizobia (the RL symbiosis). They can also, like most plants, form symbiotic associations with arbuscular mycorrhizal (AM) fungi, which facilitate plants’ phosphate nutrition. In both interactions, the symbionts are hosted inside the plant root. Nitrogen-fixing rhizobia are housed in intracellular symbiotic structures within nodules, while AM fungi form intracellular symbiotic structures, called arbuscules, within cortical root cells. These two endosymbioses present other similarities, including production by the microsymbionts of lipo-chitooligosaccharidic signals (Nod Factors and Myc-LCOs), and the involvement of common plant signaling elements. In Medicago truncatula, DMI3 encodes a calcium and calmodulin dependent protein kinase that is part of this common signaling pathway, while NFP encodes a LysM domain receptor-like kinase involved in Nod Factor perception. Using tissue specific promoters, we recently uncoupled the roles of NFP and DMI3 in the cortex and the epidermis of the root during the RL symbiosis.1 Here, we provide additional data showing a cell autonomous tissular contribution of DMI3 in the AM symbiosis, and we comment on a non-cell autonomous cortical role of NFP during rhizobial infection.
Keywords: Medicago truncatula, Arbuscular mycorrhizae, CCaMK, LysM-RLK, crosstalk, long distance signaling, rhizobium legume symbiosis, root epidermis/cortex
Introduction
Root endosymbioses are extremely important for the nutrition of plants. The arbuscular mycorrhizal (AM) symbiosis, involving fungi of the Glomeromycota phylum, is formed by 80% of terrestrial plant species and supplies plants with nutrients (mainly phosphate) and water.2 The Rhizobium-legume (RL) symbiosis, which is almost entirely restricted to the legume family, provides combined nitrogen to the plant partner. In the AM symbiosis, plant roots and fungal hyphae are intimately connected. The fungus develops intracellular structures called arbuscules inside the root cortex, and these are thought to be the main site of nutrient exchange between the fungus and the plant.2 In the RL symbiosis, the formation of nitrogen-fixing nodules requires coordination between the initiation of cell divisions in the cortex, responsible for nodule organogenesis and root infection by rhizobia which starts at the epidermis and then progresses through the cortex to the nodule primordium.3,4 Thus, both rhizobial and fungal microsymbionts have to enter root tissues and reach cortical cells to establish a functional symbiotic interaction. Moreover, the entry of both microsymbionts is tightly controlled by the host plant and is prepared in both cases in underlying cell layers by calcium signaling and cellular modifications.5-7 Here, by studying the infection process in Medicago truncatula mutant plants expressing either NFP or DMI3 only in the epidermis or only in the cortex, we investigated whether these key symbiotic genes act in a cell autonomous way or whether they are involved in crosstalk between the epidermis and the cortex required to coordinate microsymbiont penetration of the root.
Results and Discussion
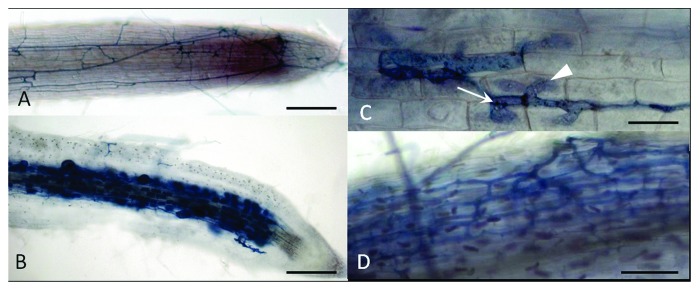
Both rhizobial and mycorrhizal infection are controlled by DMI3 in a cell autonomous way. The M. truncatula gene DMI3 belongs to the common signaling pathway required for the establishment of both the RL and the AM symbiosis. DMI3 encodes a calcium and calmodulin dependent protein kinase (CCaMK) that is required to decipher the calcium oscillations triggered by the perception of the microsymbiont.8,9 During rhizobial infection, DMI3 is expressed both in the epidermis and in the cortex of roots.1dmi3 mutants do not show any rhizobial entry or nodule formation and show only limited responses to Nod Factors (NFs). In the presence of AM fungi, there is also no penetration of the microsymbiont into epidermal cells and, as a result, no arbuscules or vesicules are formed in the root cortex10 (Fig. 1A). Our approach consisted in introducing into dmi3 mutant roots, via Agrobacterium rhizogenes, a DMI3 wild type allele under the control of either pLeEXT1, an epidermis-specific promoter, or pCO2, a cortex-specific promoter,1 followed by analysis of symbiotic phenotypes.
Figure 1. Mycorrhizal phenotypes of Medicago truncatula dmi3 mutant plants complemented by the pLeEXT1:DMI3 construct at 4 weeks post inoculation with Rhizophagus irregularis. (A) dmi3 mutant root carrying an empty vector, showing hyphae developing only on the root surface. (B) dmi3 mutant root complemented with the pDMI3:DMI3 construct showing dense arbuscule and vesicule formation. (C and D) dmi3 mutant root complemented with the pLeEXT1:DMI3 construct. (C) Showing hyphae developing on the root surface (arrow) and intracellular hyphae entering an epidermal cell (arrowhead). (D) intercellular colonisation in the cortex without arbuscule formation. Scale bars: 200 µm in (A and B); 25 µm in (C) and 100 µm in (D).
While the pCO2:DMI3 construct had no effect on the dmi3 mutant phenotype (21 plants tested), roots transformed by the pLeEXT1:DMI3 construct showed penetration of the fungus in the epidermis four weeks after inoculation by Rhizophagus irregularis (formerly known as Glomus intraradices). Most of the time the hyphae formed a coil, but remained blocked in the infected epidermal cell (Fig. 1C). Sometimes they grew between cortical cells (Fig. 1D), as in the positive control. However, hyphae very rarely entered cortical cells and arbuscules were only very occasionally observed (3 out of 36 plants from 4 independent experiments, with 2, 4 and 5 arbuscules, respectively, per plant). Furthermore, no vesicules were observed, while all control (pDMI3:DMI3) dmi3 plants showed dense arbuscule and vesicule formation (Fig. 1B).
Thus, the infection phenotype observed with the epidermal DMI3 construct during the AM symbiosis is comparable to that conferred by this construct during the RL symbiosis.1 Fungal hyphae, like infection threads (ITs), can enter epidermal cells but fail to progress intracellularly into the cortical cell layer where DMI3 is not expressed. This strongly suggests that DMI3 also needs to be present in the cortex for successful intracellular infection both by rhizobia and by AM fungi. We had previously shown that a construct combining DMI3 under the control of the epidermal and the cortical promoters, though functional since it provided a gain of function for nodule organogenesis, did not restore proper rhizobial infection.1 Similarly, this construct did not restore arbuscule formation in the AM symbiosis and the AM infection phenotype was not different from that observed with the epidermal promoter alone. As discussed in the case of the RL symbiosis, we think that this may be due to an insufficient level of activity of the pCO2 promoter in those outer cortical cells underlying infection sites, which consequently does not confer a high enough expression level of DMI3 in the outer cortex for the infection process to occur. . Alternatively, as shown recently for a M. truncatula phosphate transporter deficient mutant, which can be complemented only if the wild type allele is driven by its own promoter,11 a precise spatial and temporal control of DMI3 expression could be required for mycorrhizal infection. The need for cortical expression of DMI3 during infection is consistent with recent data obtained on calcium spiking in cortical cells of M. truncatula, showing that calcium oscillations precede the actual entry of ITs and fungal hyphae into outer cortical cells.7 Thus, it seems that DMI3 controls rhizobial and mycorrhizal infection in a cell autonomous way.
Epidermal rhizobial infection requires NFP-controlled crosstalk between the cortex and the epidermis of the root. NFP (Nod Factor Perception) encodes a receptor-like kinase protein possessing three extracellular LysM domains, presumed to perceive LCOs.12-14 Like dmi3 mutants, M. truncatula nfp mutants are blocked for root hair curling and IT formation but, in contrast to dmi3 mutants, they are not impaired for mycorrhization.15
When NFP was expressed under the control of the epidermal promoter in nfp roots, rhizobia were entrapped in curled root hairs, but no ITs were formed.1 This very early block in the symbiotic process suggests that restoration of IT formation requires expression of NFP not only in the epidermis, but probably also in the cortex, where it is expressed upon rhizobial infection when NFP is driven by its own promoter.1,12
However, as for DMI3, the infection phenotype of nfp roots carrying a construct combining NFP under the control of the epidermal and the cortical promoters was not different from roots in which NFP was only expressed in the epidermis. In this case, the need for a strict control of the timing and level of expression of NFP in the epidermis and in the cortex seems unlikely, since rhizobial infection is fully restored when NFP is expressed under the control of the constitutive p35S promoter. An insufficient expression of NFP due to a low activity of the pCO2 promoter in the outer cortex seems a more likely explanation. Such a need for NFP in the cortex is consistent with the strong activation of the NFP promoter in underlying cortical cells, ahead of IT formation.1,12 Considering that cortical cells prepare for infection and guide IT progression,4 it is possible that NFP is involved in this process through a positive feed-back loop from the cortex to the epidermis. Since cortical expression of DMI3 is not required for IT initiation and progression in root hairs,1 cortical NFP proteins could act by a mechanism independent of DMI3, and thus independent of the activation of the common downstream signaling pathway. This would be consistent with the fact that, in Lotus japonicus, spontaneous nodules obtained with autoactive forms of CCaMK cannot be infected via root hairs in the double NF receptor mutant nfr1/nfr5.16,17 This, together with the fact that NFs are not thought to penetrate into inner root tissues, suggests that the control of infection by signaling from cortical NFP is unlikely to involve NFs.
In the new model illustrating our data (Fig. 2), we propose that IT initiation in epidermal root hair cells is under the control of cortical cells, and that NFP plays an essential role in this mechanism via a non-cell autonomous function of the NFP protein.
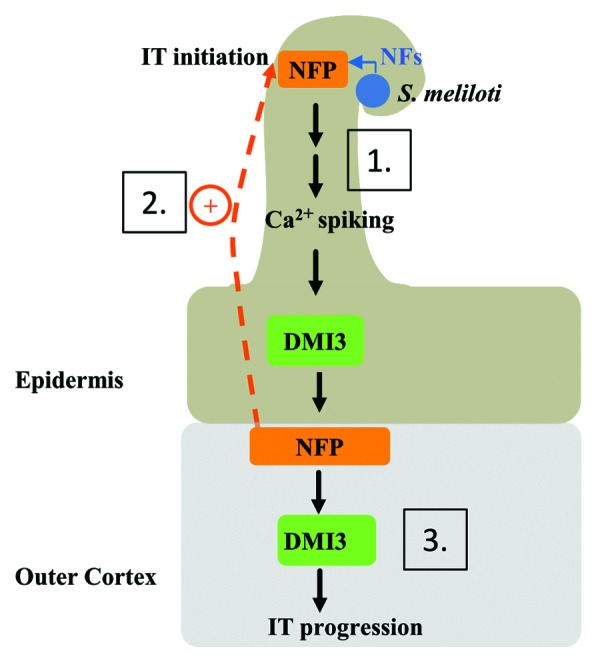
Figure 2. Model for the cellular roles of NFP and DMI3 in the control of rhizobial infection in Medicago truncatula. Nod Factors (NFs) trigger a NFP and DMI3 dependent signaling mechanism in root hairs of the epidermis, which leads to the activation of NFP in underlying cortical cells (1); Cortical NFP, in a NF- and DMI3-independent manner, would signal back to the root hair to control infection thread (IT) initiation (2); Subsequently, cortical DMI3 would be activated to control the progression of the IT into the cortex (3).
Material and Methods
Plant growth conditions, plasmid constructs, rhizobial strains and inoculation and microscopy methods have previously been described.1
AM fungal inoculum was obtained from in vitro-grown hairy root cultures of carrot (Daucus carota) mycorrhized by Rhizophagus irregularis. For this, the entire contents of mycorrhized carrot root cultures grown on M medium as described previously,18 but solidified with 3g/l phytagel, were mixed with 4.25 ml of 0.1 M citric acid and 20.75 ml of 0.1 M sodium citrate in a total volume of 250 ml water. This mixture was blended in a commercial food processor to obtain a semi-liquefied inoculum of spores and fragments of mycorrhized roots that was stored at 4°C. Three weeks after transformation, aliquots of inoculum were put at the same time as transformed plants into sepiolite (Brenntag) in falcon tubes as described,19 and grown in a chamber at 25°C with 18 h light/6 h dark cycles. Roots were colored for mycorrhization by the ink and vinegar protocol20 and observed by light microscopy.
Acknowledgments
We thank Mireille Chabaud for advice in studying mycorrhizal phenotypes and Chrystel Gibelin for preparing the AM inoculant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
P.R. was supported by a fellowship from the French Ministry of Research and Education. This work was funded by the French Agence Nationale de la Recherche (contract ANR-05-BLAN-0243–01 ‘NodBindsLysM’ and contract ANR-09-BLAN-0241 “MycSignaling”) and by the European Community’s Sixth Framework Programme through a Marie Curie Research Training Network (contract MRTN-CT-2006–035546 ‘NODPERCEPTION’). This work was supported by funds from the ‘Laboratoire d’Excellence (LABEX)’ entitled TULIP (ANR-10-LABX-41).
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/22999
References
- 1.Rival P, de Billy F, Bono JJ, Gough C, Rosenberg C, Bensmihen S. Epidermal and cortical roles of NFP and DMI3 in coordinating early steps of nodulation in Medicago truncatula. Development. 2012;139:3383–91. doi: 10.1242/dev.081620. [DOI] [PubMed] [Google Scholar]
- 2.Parniske M. Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nat Rev Microbiol. 2008;6:763–75. doi: 10.1038/nrmicro1987. [DOI] [PubMed] [Google Scholar]
- 3.Oldroyd GE, Murray JD, Poole PS, Downie JA. The rules of engagement in the legume-rhizobial symbiosis. Annu Rev Genet. 2011;45:119–44. doi: 10.1146/annurev-genet-110410-132549. [DOI] [PubMed] [Google Scholar]
- 4.Timmers AC, Auriac MC, Truchet G. Refined analysis of early symbiotic steps of the Rhizobium-Medicago interaction in relationship with microtubular cytoskeleton rearrangements. Development. 1999;126:3617–28. doi: 10.1242/dev.126.16.3617. [DOI] [PubMed] [Google Scholar]
- 5.Genre A, Bonfante P. Check-in procedures for plant cell entry by biotrophic microbes. Mol Plant Microbe Interact. 2007;20:1023–30. doi: 10.1094/MPMI-20-9-1023. [DOI] [PubMed] [Google Scholar]
- 6.Murray JD. Invasion by invitation: rhizobial infection in legumes. Mol Plant Microbe Interact. 2011;24:631–9. doi: 10.1094/MPMI-08-10-0181. [DOI] [PubMed] [Google Scholar]
- 7.Sieberer BJ, Chabaud M, Fournier J, Timmers AC, Barker DG. A switch in Ca2+ spiking signature is concomitant with endosymbiotic microbe entry into cortical root cells of Medicago truncatula. Plant J. 2012;69:822–30. doi: 10.1111/j.1365-313X.2011.04834.x. [DOI] [PubMed] [Google Scholar]
- 8.Lévy J, Bres C, Geurts R, Chalhoub B, Kulikova O, Duc G, et al. A putative Ca2+ and calmodulin-dependent protein kinase required for bacterial and fungal symbioses. Science. 2004;303:1361–4. doi: 10.1126/science.1093038. [DOI] [PubMed] [Google Scholar]
- 9.Wais RJ, Galera C, Oldroyd G, Catoira R, Penmetsa RV, Cook D, et al. Genetic analysis of calcium spiking responses in nodulation mutants of Medicago truncatula. Proc Natl Acad Sci USA. 2000;97:13407–12. doi: 10.1073/pnas.230439797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Catoira R, Galera C, de Billy F, Penmetsa RV, Journet EP, Maillet F, et al. Four genes of Medicago truncatula controlling components of a nod factor transduction pathway. Plant Cell. 2000;12:1647–66. doi: 10.1105/tpc.12.9.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pumplin N, Zhang X, Noar RD, Harrison MJ. Polar localization of a symbiosis-specific phosphate transporter is mediated by a transient reorientation of secretion. Proc Natl Acad Sci USA. 2012;109:E665–72. doi: 10.1073/pnas.1110215109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arrighi JF, Barre A, Ben Amor B, Bersoult A, Soriano LC, Mirabella R, et al. The Medicago truncatula lysin [corrected] motif-receptor-like kinase gene family includes NFP and new nodule-expressed genes. Plant Physiol. 2006;142:265–79. doi: 10.1104/pp.106.084657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bensmihen S, de Billy F, Gough C. Contribution of NFP LysM domains to the recognition of Nod factors during the Medicago truncatula/Sinorhizobium meliloti symbiosis. PLoS ONE. 2011;6:e26114. doi: 10.1371/journal.pone.0026114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lefebvre B, Klaus-Heisen D, Pietraszewska-Bogiel A, Hervé C, Camut S, Auriac M-C, et al. Role of N-glycosylation sites and CXC motifs in trafficking of medicago truncatula Nod factor perception protein to plasma membrane. J Biol Chem. 2012;287:10812–23. doi: 10.1074/jbc.M111.281634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amor BB, Shaw SL, Oldroyd GE, Maillet F, Penmetsa RV, Cook D, et al. The NFP locus of Medicago truncatula controls an early step of Nod factor signal transduction upstream of a rapid calcium flux and root hair deformation. Plant J. 2003;34:495–506. doi: 10.1046/j.1365-313X.2003.01743.x. [DOI] [PubMed] [Google Scholar]
- 16.Madsen LH, Tirichine L, Jurkiewicz A, Sullivan JT, Heckmann AB, Bek AS, et al. The molecular network governing nodule organogenesis and infection in the model legume Lotus japonicus. Nat Commun. 2010;1:10. doi: 10.1038/ncomms1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayashi T, Banba M, Shimoda Y, Kouchi H, Hayashi M, Imaizumi-Anraku H. A dominant function of CCaMK in intracellular accommodation of bacterial and fungal endosymbionts. Plant J. 2010;63:141–54. doi: 10.1111/j.1365-313X.2010.04228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becard G, Fortin JA. EARLY EVENTS OF VESICULAR ARBUSCULAR MYCORRHIZA FORMATION ON RI T-DNA TRANSFORMED ROOTS. New Phytol. 1988;108:211–8. doi: 10.1111/j.1469-8137.1988.tb03698.x. [DOI] [PubMed] [Google Scholar]
- 19.Maillet F, Poinsot V, André O, Puech-Pagès V, Haouy A, Gueunier M, et al. Fungal lipochitooligosaccharide symbiotic signals in arbuscular mycorrhiza. Nature. 2011;469:58–63. doi: 10.1038/nature09622. [DOI] [PubMed] [Google Scholar]
- 20.Vierheilig H, Coughlan AP, Wyss U, Piche Y. Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl Environ Microbiol. 1998;64:5004–7. doi: 10.1128/aem.64.12.5004-5007.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]