Abstract
The genetic and molecular biological studies mainly in Arabidopsis and in some other plants have begun to uncover the various components of ripening signaling pathway in plants. Although transcriptional regulation of major ripening genes have been studied in detail, information on role of phosphorylation in regulating the activity and stability of core ripening pathway associated proteins in relation to ethylene biosynthesis during fruit ripening is still limited. Recently we have demonstrated the evidence for post-translational regulation of MA-ACS1 (Musa acuminata ACC synthase 1), the rate limiting step enzyme regulating ripening ethylene production in banana, through phosphorylation at the C-terminal Ser 476 and 479 residues by a 41-kDa Ser/Thr protein kinase.1 Here we have further discussed role of protein phosphorylation in regulation of stability and activity of ACS enzymes and the mechanistic and evolutionary perspective of phosphorylation pattern of Type I ACC synthase enzymes.
Keywords: ACC synthase, banana, ethylene, phosphorylation, ripening
Post-Translation Regulation of ACC Synthase Enzymes through Phosphorylation
Ethylene is a well-known gaseous plant hormone involved in many aspects of plant growth and developmental process including its pivotal role in fruit ripening. ACC synthase (ACS), which converts AdoMet (S-adenosyl-l-methionine) to ACC (1-aminocyclopropane-1-carboxylic acid) acts as the rate limiting step enzyme in ethylene biosynthesis pathway. ACS genes have been cloned from various plant species. Genome wide analyses have revealed existence of 12 ACS genes in Arabidopsis, 6 in rice, 10 in grapevine and 11 in poplar respectively. Chromosomal distribution studies suggested that ACS genes are dispersed throughout the respective genomes of the four species, while segmental duplication, tandem duplication and transposition events have been shown as the three major factors contributing to this gene family evolution. Whereas segmental depilation was found to play key role in Arabidopsis and poplar, transposition events were more predominant in rice and grapevine.2 As like tomato, banana ACS is encoded by a small gene family and the members of which are differentially regulated in tissue specific manner during plant development. Although at least nine ACC synthase genes (MACS1–9) exist in banana genome, only ACS1 (MA-ACS1 (Musa acuminata ACC synthase 1) is expressed during banana fruit ripening and has been found to be inducible by exogenous ethylene treatment, suggesting that MA-ACS1 is a key member of the ACC synthase gene family related to banana fruit ripening.3 MA-ACS1 (Musa acuminata ACC synthase 1) is encoded by a single gene in banana genome and has been found to be regulated by various abiotic factors like light, cold, wounding and phytohormones including ethylene and auxin at the transcriptional level.4
Transcriptional regulation of ACS genes has been studied in different plant species in response to various hormonal, developmental and environmental factors.3 However, considerable evidences have also indicated posttranslational regulation of ACS proteins.5 Previous reports have demonstrated posttranslational regulation of ACS proteins by phosphorylation and targeted protein degradation, controlling the enzyme turnover rate instead of regulation of enzyme catalytic activity.6 In tomato cell suspension culture, elicitor induced ACS activity has been found to be blocked by a protein kinase inhibitor, while a phosphatase inhibitor caused rapid increase in ACS activity in the absence of elicitors and also enhanced the elicitor inducible activity of ACS.7 Post-translational regulation of LeACS2 (Lycopersicon esculentum ACS2) by phosphorylation in wounded tomato fruit tissue has been reported previously.8 Biochemical studies indicated that phosphorylation/dephosphorylation of LeACS2 plays key role in regulating the turnover of this protein, upstream of the ubiquitin-26Sproteasome degradation system. Such regulation has been found to be essential in controlling ethylene production in tomato fruit tissue. Furthermore, LeACS2 has been shown to be phosphorylated by CDPK and MAPK at different sites in response to a wounding signal. Phosphorylation at both sites was found to be crucial for LeACS2 stability.9 In cotton, ACC synthase 2 is phosphorylated by a calcium-dependent protein kinase 1 during cotton fiber elongation,10 while Arabidopsis MPK3 and MPK6 have been implicated in phosphorylation of Type 1 ACC synthase (ACS2 and ACS6) and in regulation of expression of AtACS2 and AtACS6 genes through binding with another transcription factor, WRKY33.11 Interestingly, in Arabidopsis, genetic and molecular analyses have revealed role of protein phosphatase 2A (PP2A) in positively regulating the abundance of Type 2 ACS proteins, thus providing a sophisticated regulation of overall ethylene production by differentially regulating the turnover and thus stability of specific classes of ACS enzymes.12 Our recent report has indicated phosphorylation of MA-ACS1, a Type 1 ACS enzyme, at the C-terminal serine 476 and 479 residues by a 41-kDa Ser/Thr family of protein kinase. We found that MA-ACS1 phosphorylation could be associated with the stability of the protein.1 Together all these findings have indicated posttranslational modification of ACS enzymes by phosphorylation which seems to play important role in regulating stability of ACSs.
The C-Terminal Region of ACC Synthase is Crucial for Phosphorylation
One of the crucial components in ethylene biosynthesis cascade is the stability of ACS protein, which appears to be correlated positively with ACS protein activity and ethylene production. Previous studies have revealed that the C-terminal region of ACS proteins plays the key role in the regulation of ACS turnover and activity.8,9 Phytohormones like cytokinins and brassinosteroids have also been found to stabilize multiple ACS proteins and the stabilization has been shown to be C-terminus dependent.13 Phosphorylation of ACS proteins at the C-terminal region has been found to be essential in regulation of ACS protein stability. Studies with eto mutants in Arabidopsis have contributed significantly to our knowledge on mechanism of the posttranslational regulation of ACS. The eto2 mutation contains a single base-pair insertion which changes the C-terminal 12 amino acid residues in AtACS5, while the eto3 mutation carries a single amino acid replacement (Val to Asp) at the C-terminal region of AtACS9. Both mutations have shown to confer increased ACS stability in Arabidopsis. Study of eto1 mutation revealed role of ETO1 (ethylene overproducer 1) in AtACS5 stability in Arabidopsis. ETO1 has been shown to recognize and interact directly with the C-terminal region of AtACS5 and suppresses ACS activity, allowing CUL3 (Cullin 3), a component of ubiquitin E3 ligase, to bind AtACS5, which eventually leads to AtACS5 degradation via the ubiquitin-26S-proteasome complex. The myc-tagged AtACS5 showed increased half-life in eto1 mutant than wild-type plants.9 ETO1 and EOL1 (ETO1-like) were found to be specific for Type 2 ACS proteins in Arabidopsis, and the level of ethylene synthesis has been shown to be increased at the maximum level by elimination of ETO1, then EOL2 and finally EOL1.13 In Rice, 14–3-3 protein was found to interact directly with ACS1 in yeast two-hybrid assays. Rice 14–3-3 proteins have been suggested to play important role in the regulation of ACS activity via binding to the phosphorylated C-terminal tails of ACSs, therefore protecting them from the degradation during ethylene biosynthesis.14 In Arabidopsis, hormonal and mutational analyses have shown that the C-terminal region of the Type 2 ACS proteins play key role in regulating the protein stability and thus results in increased ethylene synthesis.13 These findings have demonstrated that the C-terminal region of ACS is involved in regulating ACS stability.
The C-terminal Ser Residues Constitute the Phosphorylation Sites of ACS Proteins
The deduced amino acid sequences of different isozymes of ACS have indicated that despite high degree of similarity in the rest of protein, the C-terminal regions of ACSs may not be always conserved. The ACS proteins fall into three categories, Type 1, 2 and 3 respectively, specifically based on the amino acid sequence homology of C-terminal region. In addition, the ACS proteins have been shown to possess the (F/L)RLS(F/L) motif at the C-terminal region. Type 1 ACS isozymes contain the Ser residue in the ‘RLSF’ motif, important for CDPK phosphorylation, followed by a long C-terminal tail (27- 33 aa) with the three conserved Ser residues which act as the targets of MPK6 phosphorylation.5 In plum (Prunus sativus), predicted proteins of both Ps-ACS4 and Ps-ACS5 are members of Type1 ACS isozymes. However, these predicted proteins (also closely related to Arabidopsis AtACS2 and AtACS6) lack the Val residue in the conserved C-terminal ‘WVF’ motif. On the other hand, predicted protein Ps-ACS1, which belongs to Type 2 ACS isozymes, has been shown to contain a conserved TOE sequence, the ‘WVF’ sequence motif just before the ‘RLSF’ motif and then followed by a short C-terminal tail.15 Type 2 ACS isozymes, which mainly include the Arabidopsis and tomato ACS proteins, are regulated at the posttranslational level by CDPK phosphorylation.16 Type 3 isozymes, which include the predicted protein Ps-ACS3, contain a long C-terminal or a short C-terminal tail, while lack all the residues important for phosphorylation.
Evolutionary Perspective of ACS Protein Phosphorylation
In Arabidopsis and tomato, ACS proteins are found to be phosphorylated at specific target residues in the conserved C-terminal region.8,9,16 Such studies have suggested a similarity in the pattern of phosphorylation of ACS protein either at the CDPK or MAPK or both sites in the C-terminal region of ACSs in higher plants. This may indicate an evolutionary conservation of the phosphorylation target residues at the C-terminal region of ACS proteins as a part of mechanism of the post-translation regulation. A comparative analysis of the partial C-terminal sequence of Type 1 ACS proteins from different plant species have indicated that the CDPK phosphorylation site RLSF/RLSL motif and MAPK phosphorylation sites (the three Ser residues after the “RLSF/RLSL” motif) were comparatively more conserved in higher plant genomes including both monocot and dicot plant species (Fig. 1), while such phosphorylation sites were found to be less conserved in lower group of plants. In the unicellular green alga, Chlamydomonas reinhardtii, and in bryophytes including the model species Physcomitrella patens and club moss Selaginella moellendorffii, the serine residue within the RLSF/RLSL motif has been found to be replaced by cysteine, while the serine residues for putative MAPK sites were replaced by arginine and lysine residues, respectively, suggesting distinct pattern of evolution of CDPK and MAPK phosphorylation sites from lower group of plants to land plants.
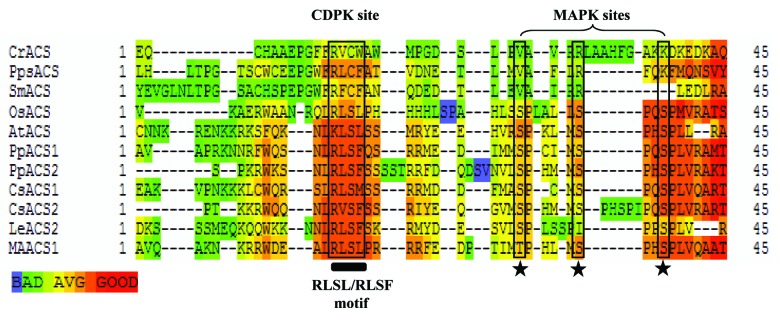
Figure 1. Comparison of C-terminal region of Type I ACC synthase proteins (reported and predicted) from different plant species. In silico analyses was performed using by using T-COFFEE server for comparative analysis of the C-terminal sequence of type I ACS proteins from diverse plant genomes. Cr (Chlamydomonas reinhardtii, v5.3 gene-g7098), Pps (Physcomitrella patens, gene-Pp1s235_83V6), Sm (Selaginella moellendorffii, gene-75495), Os (Oryza sativa, gene-LOC_Os04 g48850), At (Arabidopsis thaliana, gene-AT3G61510), Pp1 and Pp2 (Prunus persica, gene-ppa016458 min.g and ppa004774 min.g), Cs (Citrus sinensis, gene-orange1.1g011570 min.g and 1.1g011801 min.g), Le (Solanum lycopersicum, gene-NM_001247249), MA (Musa acuminata, gene-AY702076). The putative “RLSF/RLSL” motif (CDPK site, (within the box, indicated by bold black line) and the three Ser residues after the “RLSF/RLSL” motif (MAPK sites indicated by asterisk symbol) have been represented.
Summary
The above discussion provides insight into the possible mechanism of post-translational regulation of ACC synthase, one of the key regulatory proteins in the ethylene mediated ripening signaling cascades. Majority of the studies have provided evidence to argue for the role of phosphorylation/dephosphorylation in regulating the ACS protein stability instead of influencing the catalytic activity. This idea was also supported in our study where accumulation of phosphorylated MA-ACS1 was observed in response to ripening during the onset of climacteric in banana fruit, suggesting that MA-ACS1 seems to be stabilized by phosphorylation to ensure enzyme activity during climacteric phase of ripening. However, further detailed research is required to fully understand the mechanism of phosphorylation of MA-ACS1 in regulation of stability and catalytic activity of this protein in relation to ethylene biosynthesis during banana fruit ripening.
Acknowledgment
The work is financially supported by research grant from CSIR project [38(1181)/08/EMR-II], CSIR, Govt. of India to DNSG. The authors are grateful to Jadav Kumar Ghosh for proving necessary technical support.
Glossary
Abbreviations:
- ACC
1-aminocyclopropane-1-carboxylic acid
- CDPK
calcium dependent protein kinase, ETO1, ethylene overproducing 1
- MAPK
Mitogen activated protein kinase
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/23000
References
- 1.Choudhury SR, Roy S, Sengupta DNA. A Ser/Thr protein kinase phosphorylates MA-ACS1 (Musa acuminata 1-aminocyclopropane-1-carboxylic acid synthase 1) during banana fruit ripening. Planta. 2012;236:491–511. doi: 10.1007/s00425-012-1627-9. [DOI] [PubMed] [Google Scholar]
- 2.Xu M, Wang MH, Wang ZM. Genome-wide analysis of 1-amino-cyclopropane-1-carboxylate synthase gene family in Arabidopsis, rice, grapevine and poplar. Afr J Biotechnol. 2012;11:1106–18. [Google Scholar]
- 3.Roy Choudhury S, Roy S, Saha PP, Singh SK, Sengupta DN. Characterization of differential ripening pattern in association with ethylene biosynthesis in the fruits of five naturally occurring banana cultivars and detection of a GCC-box specific DNA binding protein. Plant Cell Rep. 2008;7:1235–49. doi: 10.1007/s00299-008-0547-4. a. [DOI] [PubMed] [Google Scholar]
- 4.Choudhury SR, Roy S, Sengupta DN. Characterization of transcriptional profiles of MA-ACS1 and MA-ACO1 genes in response to ethylene, auxin, wounding, cold and different photoperiods during ripening in banana fruit. J Plant Physiol. 2008;165:1865–78. doi: 10.1016/j.jplph.2008.04.012. b. [DOI] [PubMed] [Google Scholar]
- 5.Chae HS, Kieber JJ. Eto Brute? Role of ACS turnover in regulating ethylene biosynthesis. Trends Plant Sci. 2005;10:291–6. doi: 10.1016/j.tplants.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Spanu P, Grosskopf DG, Felix G, Boller T. The apparent turnover of 1-Aminocyclopropane-1-Carboxylate Synthase in tomato cells is regulated by protein phosphorylation and dephosphorylation. Plant Physiol. 1994;106:529–35. doi: 10.1104/pp.106.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oetiker JH, Olson DC, Shiu OY, Yang SF. Differential induction of seven 1-aminocyclopropane-1-carboxylate synthase genes by elicitor in suspension cultures of tomato (Lycopersicon esculentum) Plant Mol Biol. 1997;34:275–86. doi: 10.1023/A:1005800511372. [DOI] [PubMed] [Google Scholar]
- 8.Tatsuki M, Mori H. Phosphorylation of tomato 1-aminocyclopropane-1-carboxylic acid synthase, LE-ACS2, at the C-terminal region. J Biol Chem. 2001;276:28051–7. doi: 10.1074/jbc.M101543200. [DOI] [PubMed] [Google Scholar]
- 9.Kamiyoshihara Y, Iwata M, Fukaya T, Tatsuki M, Mori H. Turnover of LeACS2, a wound-inducible 1-aminocyclopropane-1-carboxylic acid synthase in tomato, is regulated by phosphorylation/dephosphorylation. Plant J. 2010;64:140–50. doi: 10.1111/j.1365-313X.2010.04316.x. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Mei W, Qin Y, Zhu Y. 1-Aminocyclopropane-1-carboxylic acid synthase 2 is phosphorylated by calcium-dependent protein kinase 1 during cotton fiber elongation. Acta Biochim Biophys Sin (Shanghai) 2011;43:654–61. doi: 10.1093/abbs/gmr056. [DOI] [PubMed] [Google Scholar]
- 11.Li G, Meng X, Wang R, Mao G, Han L, Liu Y, et al. Dual-level regulation of ACC synthase activity by MPK3/MPK6 cascade and its downstream WRKY transcription factor during ethylene induction in Arabidopsis. PLoS Genet. 2012;8:e1002767. doi: 10.1371/journal.pgen.1002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skottke KR, Yoon GM, Kieber JJ, DeLong A. Protein phosphatase 2A controls ethylene biosynthesis by differentially regulating the turnover of ACC synthase isoforms. PLoS Genet. 2011;7:e1001370. doi: 10.1371/journal.pgen.1001370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen MH. Regulation of ethylene biosynthesis via ACC synthase protein stability. 2008
- 14.Yao Y, Du Y, Jiang L, Liu JY. Interaction between ACC synthase 1 and 14-3-3 proteins in rice: a new insight. Biochemistry (Mosc) 2007;72:1003–7. doi: 10.1134/S000629790709012X. [DOI] [PubMed] [Google Scholar]
- 15.Yoshida H, Wang KLC, Chang CM, Mori K, Uchida E, Ecker JR. The ACC synthase TOE sequence is required for interaction with ETO1 family proteins and destabilization of target proteins. Plant Mol Biol. 2006;62:427–37. doi: 10.1007/s11103-006-9029-7. [DOI] [PubMed] [Google Scholar]
- 16.Vogel JP, Woeste KE, Theologis A, Kieber JJ. Recessive and dominant mutations in the ethylene biosynthetic gene ACS5 of Arabidopsis confer cytokinin insensitivity and ethylene overproduction, respectively. Proc Natl Acad Sci USA. 1998;95:4766–71. doi: 10.1073/pnas.95.8.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]


