Abstract
Soilborne fungal pathogens are highly persistent and provoke important crop losses. During saprophytic and infectious stages in the soil, these organisms face situations of nutrient limitation and lack of essential elements, such as iron. We investigated the role of the bZIP transcription factor HapX as a central regulator of iron homeostasis and virulence in the vascular wilt fungus Fusarium oxysporum. This root-infecting plant pathogen attacks more than hundred different crops and is an emerging human opportunistic invader. Although iron uptake remains unaffected in a strain lacking HapX, de-repression of genes implicated in iron-consuming processes such as respiration, amino acid metabolism, TCA cycle and heme biosynthesis lead to severely impaired growth under iron-limiting conditions. HapX is required for full virulence of F. oxysporum in tomato plants and essential for infection in immunodepressed mice. Virulence attenuation of the ΔhapX strain on tomato plants is more pronounced by co-inoculation of roots with the biocontrol strain Pseudomonas putida KT2440, but not with a mutant deficient in siderophores production. These results demonstrate that HapX is required for iron competition of F. oxysporum in the tomato rhizosphere and establish a conserved role for HapX-mediated iron homeostasis in fungal infection of plants and mammals.
Keywords: iron, virulence, siderophores, rhizosphere, Fusarium, Pseudomonas, HapX
Iron (Fe) is a key element for virtually every organism and functions as an essential cofactor of a wide range of cellular processes. However, the excess of this metal can be highly toxic promoting the production of reactive oxygen species.1 Because of this duality and the limited bioavailability of iron given its conversion into insoluble forms, organisms have developed tightly controlled mechanisms to maintain iron homeostasis, i.e., the balance between uptake, storage and utilization of this element.
Previous studies suggested that human pathogens must cope with the extreme iron-limiting conditions originated by the mammalian immune system to keep invading microorganisms at bay.2-5 Here we investigated the role of iron homeostasis in the soilborne fungal plant pathogen Fusarium oxysporum. Since soluble Fe3+ in natural soils represents only ~10−10 M at equilibrium with soil iron6 and plant roots have efficient iron-sequestering mechanisms,7 we hypothesized that iron homeostasis should play an important role during root infection. F. oxysporum infects and kills both tomato plants and immunodepressed mice, and thus provides an excellent model to study the role of iron homeostasis during fungal infection of plant and mammalian hosts.8
The bZIP transcription factor HapX, which was was originally identified in Schizosaccharomyces pombe9 and Apergillus nidulans,10 has been reported to regulate iron-dependent pathways in several fungal species. HapX transcript levels are upregulated under iron-depleted conditions.2,4,5,11 We found that deletion of hapX in F. oxysporum had no effect on mycelial growth on iron-sufficient media, but dramatically reduced growth under iron-depleted conditions.12 Interestingly, as reported in Candida albicans,4 the F. oxysporum ΔhapX mutant was fully competent in iron uptake. In agreement with this result, transcription of siderophore genes was strongly induced during iron-depleted conditions both in the wild type strain and the ΔhapX mutant.12 Moreover, significant levels of extracellular siderophores were detected in culture supernatants of the fungal strains grown under iron-depleted conditions, but not during iron sufficiency.12 Intracellular siderophore content in the ΔhapX mutant was even higher than that of wild type or the ΔhapX+hapX complemented strain. Collectively, these results suggest that impaired growth of the ΔhapX mutant under iron starvation is unlikely to be caused by a defect in iron acquisition.12
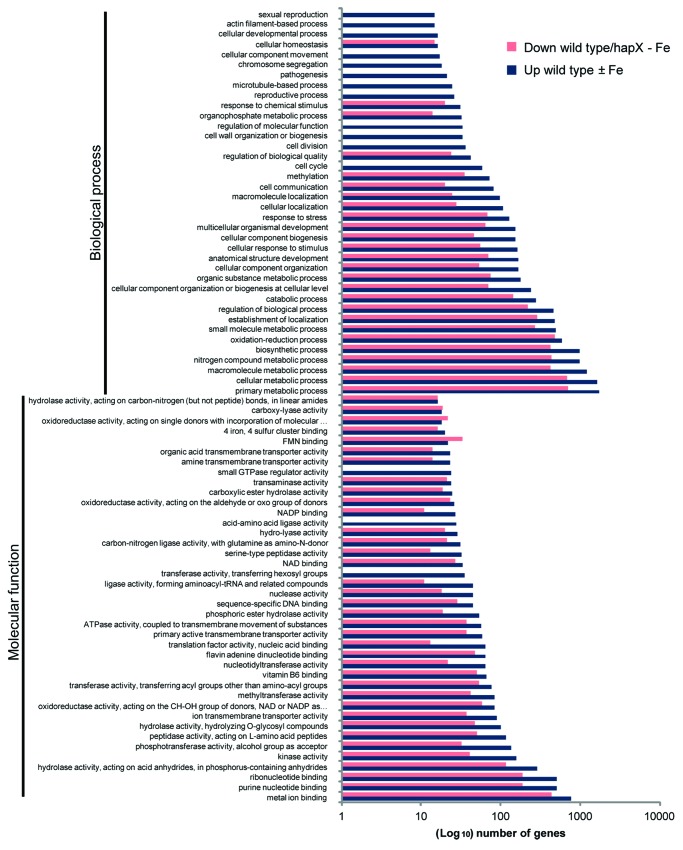
An alternative explanation would be that the ΔhapX mutant wastes iron because it is unable to reduce iron consumption under limiting conditions. We used microarrays to compare genome-wide transcription in the F. oxysporum wild type strain and ΔhapX mutant under steady-state iron starvation vs. iron sufficiency (with or without 50 µM Fe2(SO4)3), with the aim to search for differentially expressed genes (DEGs) downregulated in the wild type but not in the ΔhapX mutant.12 Functional classification of DEGs repressed by HapX under steady-state iron starvation evidenced specific subcategories of molecular function and biological process (wild type ± Fe in Fig. 1), including hexosyl group transferase activity, acid-amino acid ligase activity, small GTPase regulator activity and cell cycle, cell division, cell wall organization and biogenesis, regulation of molecular function, reproductive processes, microtubule-based processes, pathogenesis, chromosome segregation, cellular component movement, cellular developmental process, actin filament-based process, sexual reproduction, respectively.
Figure 1. Functional categories of differentially expressed genes (DEGs) repressed by HapX under steady-state iron starvation. GO functional enrichment analysis of F. oxysporum DEGs that fulfill the following criteria in microarray-based transcriptional profiling: (1) Downregulation in the wild type under steady-state iron starvation vs. iron sufficiency (with or without 50 µM Fe2(SO4)3; wild type ± Fe); (2) Upregulation during steady-state iron-starved growth in ΔhapX compared with the wild type (wild type/hapX - Fe). DEGs were assigned to different functional categories using Blast2GO (version 2.3.5; www.blast2go.org) with the default parameters. The Blast2GO program extracts the GO terms associated with homologies identified with NCBI’s QBLAST and returns a list of GO annotations represented as hierarchical categories of increasing specificity. The level presented in each principal GO category corresponds to 5 and 3 for the molecular function and biological process categories, respectively, with 40 and 38 categories shown, respectively.
Additional molecular function subcategories over-represented in the wild type ± Fe comparison include metal ion binding, purine nucleotide binding, ribonucleotide binding and hydrolase activity acting on acid anhydrides in phosphorus-containing anhydrides with 341, 318, 318 and 173 genes, respectively, more in wild type ± Fe than in the wild type/hapX-Fe comparison. Biological process subcategories such as primary metabolic process, cellular metabolic process, macromolecule metabolic process, nitrogen compound metabolic process and biosynthetic process had 1027, 950, 807, 546, 546 more genes, respectively, in the wild type ± Fe than in the wild type/hapX-Fe comparison (Fig. 1).
In line with the starting hypothesis, this group of DEGs includes a significant number of genes from iron-consuming pathways such as respiration, TCA cycle, Lys biosynthesis or heme biosynthesis.12 Real-time qRT-PCR of representative genes from these categories confirmed that they were strongly repressed during iron starvation in a HapX dependent manner.12 Taken together, these results suggest that growth deficiencies of the ΔhapX mutant under iron starvation are due to de-repression of iron-consuming pathways leading to iron misuse.12
Role of HapX-mediated iron homeostasis in fungal virulence on plants and mammals
When roots of tomato seedlings were inoculated with the different fungal strains, mortality rates of plants infected with the ΔhapX mutant were significantly lower than those of plants infected with the wild type or with the ΔhapX+hapX complemented strain.12 Furthermore, fungal biomass inside the plant roots 7 d after fungal inoculation was decreased in plants inoculated with the ΔhapX mutant relative to those inoculated with the wild type strain. Moreover, mortality rates, as well as the concentration of fungal propagules in kidneys and lungs of immunodepressed mice infected with the ΔhapX mutant were significantly reduced compared those of mice infected with the wild type or with the ΔhapX+hapX complemented strain.12 This is in line with reports from other human pathogens such as A. fumigatus, C. albicans or Cryptococcus neoformans.2-5
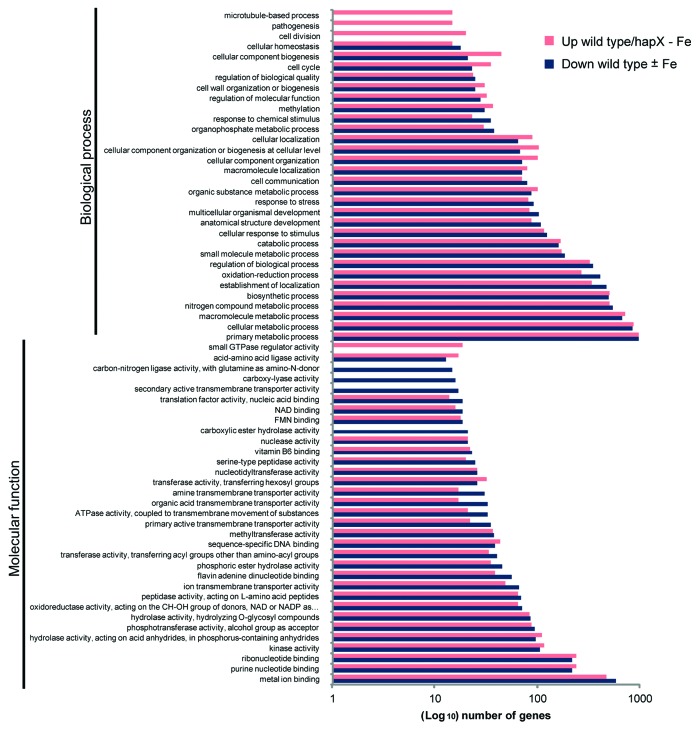
Transcriptional upregulation of genes during iron starvation, including hapX, sidA (siderophore biosynthesis precursor) or srbA (iron acquisition in response to hypoxia), was observed during early stages of plant infection and when F. oxysporum was shifted from minimal medium to human blood.12 Genome-wide transcript profiling revealed a large number of genes whose expression is activated under iron starvation conditions in a HapX-dependent manner. Functional analysis of DEGs identified four molecular functions that were specifically upregulated in the wild type under steady-state iron starvation vs. iron sufficiency (wild type ± Fe): FMN binding, secondary active transmembrane transporter activity, carboxy-lyase activity and carbon-nitrogen ligase activity with glutamine as amido-N-donor. We also detected one molecular function (small GTPase regulator activity) downregulated during steady-state iron-starved growth in the ΔhapX mutant compared with the wild type (wild type/hapX-Fe) (Fig. 2). Among the 30 subcategories under biological processes, transcripts related to cell division, pathogenesis, and microtubule-based process were specifically downregulated genes in the hapX mutant compared with the wild type under iron-limiting conditions (Fig. 2). Other subcategories overrepresented among the genes upregulated in the wild type ± Fe comparison include the molecular function subcategory metal ion binding and the biological process subcategories establishment of localization and oxidation-reduction process. Interestingly, the function of some of these genes is linked to virulence (Fig. 2).
Figure 2. Functional categories of DEGs induced by iron starvation in a HapX-dependent manner. GO functional enrichment analysis of F. oxysporum DEGs that fulfill the following criteria in microarray-based transcriptional profiling: (1) Upregulation in the wild type under steady-state iron starvation vs. iron sufficiency (wild type ± Fe); (2) Downregulation during steady-state iron-starved growth in ΔhapX compared with the wild type (wild type/hapX - Fe). DEGs were assigned to different functional categories using Blast2GO (see Fig. 1) according to molecular function or biological process, with 33 and 30 categories, respectively.
Collectively, these results establish a key role of HapX in reprogramming iron-regulated gene expression during infectious growth of F. oxysporum on plant and mammalian hosts (see model in Fig. 3).
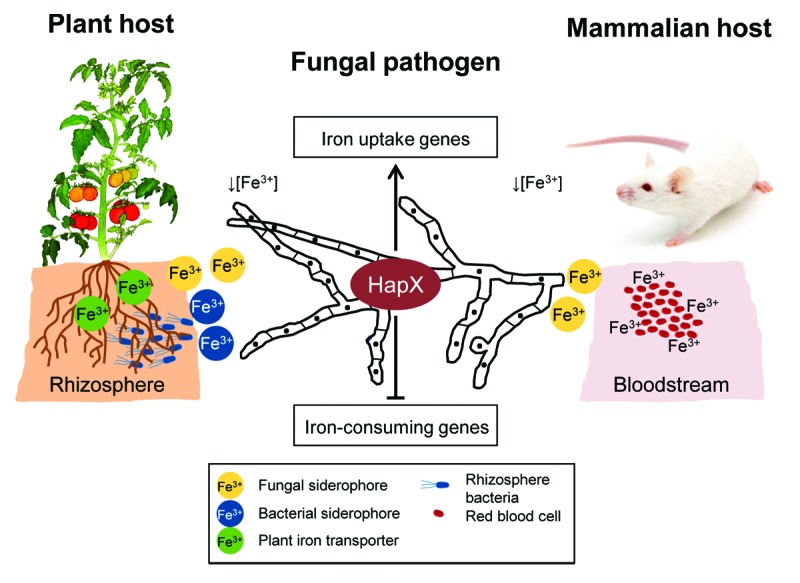
Figure 3. HapX-mediated iron homeostasis is crucial for fungal rhizosphere competence and for virulence on plant and mammalian hosts. During plant infection, F. oxysporum must compete for limited iron resources with rhizosphere colonizing bacteria and with plant roots. During infection of mammalian hosts, free iron levels are very low in serum and tissues due to iron sequestration by iron-sequestering host proteins. Under conditions of iron starvation, HapX mediates upregulation of iron-acquisition genes (e.g., biosynthesis of siderophores) and downregulation of genes functioning in iron-consuming processes (e.g. respiration, TCA cycle, heme biosynthesis).
Iron homeostasis is required for rhizosphere competence of F. oxysporum
The rhizosphere is defined as the area influenced by the root plant system where different types of microorganisms, pathogens or not, may coexist. F. oxysporum must compete for the limited iron with different rhizosphere-inhabiting microorganisms such as siderophore producing bacteria of the genus Pseudomonas.13,14 Two different Pseudomonas strains exhibited an in vitro antagonistic effect against F. oxysporum, which was exacerbated in the ΔhapX mutant, specifically under iron-limiting conditions and dependent on siderophore production.12 Even more striking, coinoculation of tomato roots with F. oxysporum and the pyoverdine (pvd) producing bacterium P. putida KT2440 resulted in a significant delay in plant mortality, confirming the previously reported biocontrol activity of this bacterial isolate. Virulence attenuation by P. putida KT2440 was much more pronounced in plants infected with the ΔhapX mutant than in those infected with the wild type strain. Importantly, the decrease in biomass of the ΔhapX mutant was 2.5× stronger in plants coinoculated with the P. putida wild type strain in comparison with the pvd- mutant.12 Taken together, these results demonstrate that HapX plays a key role during iron competition of F. oxysporum against siderophore producing pseudomonads, and directly affects the ability of the fungus to proliferate in the rhizosphere and cause disease on tomato plants (Fig. 3).
Biological control of plant disease implies any means of controlling disease or reducing the amount or effect of pathogens that relies on biological mechanisms or organisms other than man. Among different reported mechanisms of biocontrol, iron competition mediated by siderophores ranks among the most important ones.15 Our results show that bacterial siderophore production is required for efficient in vitro antagonism of pseudomonads against F. oxysporum under iron-depleted conditions, and that co-inoculation with a siderophore producing P. putida strain causes a clear attenuation of vascular wilt disease caused by F. oxysporum on tomato plants. Interestingly, part of the protecting effect of P. putida was independent of siderophore-mediated iron uptake, since it was detectable both in the bacterial wild type strain and the pvd- mutant. However, plants inoculated with the F. oxysporum ΔhapX mutant displayed an additional decrease in vascular wilt symptoms and fungal biomass, which was specifically observed in combination with the P. putida wild type strain.12 While confirming the presence of additional biocontrol mechanisms other than siderophore-mediated iron competition, these results establish a key role of HapX during iron competition of F. oxysporum with fluorescent pseudomonads and highlight its importance for iron competence in the rhizosphere (Fig. 3).
Clearly, more research is required to improve biocontrol applicability and success rate. The availability of complete genomes from both pathogens and biocontrol organisms should advance our understanding of the different modes of action, leading to an improved production, formulation and application of biocontrol agents.
Acknowledgments
We thank Esther Martínez Aguilera for valuable technical assistance. This research was supported by the following grants: BIO2010 15505 from Ministerio de Ciencia e Innovación (MICINN), European Research Area (ERA)-NET/PathoGenoMics project TRANSPAT (BIO2008–04479-E from MICINN), EUI2009–03942 from MICINN/Plant KBBE, BIO-3847 from Junta de Andalucia to A.D.P., Marie Curie Initial Training Network ARIADNE (FP7-PEOPLE-ITN-237936) to A.D.P. and ERA-NET/Patho- GenoMics project TRANSPAT (FWF I282-B09 from Austrian Science Foundation) to H.H. M.S.L.B. received a PhD fellowship from MICINN.
Glossary
Abbreviations:
- TCA
tricarboxylic acid
- pvd
pyoverdine
- DEGs
differentially expressed genes
- GO
gene ontology
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/23012
References
- 1.Halliwell B, Gutteridge JM. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984;219:1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mercier A, Pelletier B, Labbé S. A transcription factor cascade involving Fep1 and the CCAAT-binding factor Php4 regulates gene expression in response to iron deficiency in the fission yeast Schizosaccharomyces pombe. Eukaryot Cell. 2006;5:1866–81. doi: 10.1128/EC.00199-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hortschansky P, Eisendle M, Al-Abdallah Q, Schmidt AD, Bergmann S, Thön M, et al. Interaction of HapX with the CCAAT-binding complex--a novel mechanism of gene regulation by iron. EMBO J. 2007;26:3157–68. doi: 10.1038/sj.emboj.7601752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schrettl M, Beckmann N, Varga J, Heinekamp T, Jacobsen ID, Jöchl C, et al. HapX-mediated adaption to iron starvation is crucial for virulence of Aspergillus fumigatus. PLoS Pathog. 2010;6:e1001124. doi: 10.1371/journal.ppat.1001124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen C, Pande K, French SD, Tuch BB, Noble SM. An iron homeostasis regulatory circuit with reciprocal roles in Candida albicans commensalism and pathogenesis. Cell Host Microbe. 2011;10:118–35. doi: 10.1016/j.chom.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu PC, Yang CY, Lan CY. Candida albicans Hap43 is a low iron-induced repressor essential for iron-responsive transcriptional regulation and virulence. Eukaryot Cell. 2011;10:207–25. doi: 10.1128/EC.00158-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung WH, Saikia S, Hu G, Wang J, Fung CK, D’Souza C, et al. HapX positively and negatively regulates the transcriptional response to iron deprivation in Cryptococcus neoformans. PLoS Pathog. 2010;6:e1001209. doi: 10.1371/journal.ppat.1001209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haas D, Défago G. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat Rev Microbiol. 2005;3:307–19. doi: 10.1038/nrmicro1129. [DOI] [PubMed] [Google Scholar]
- 9.Jurkevitch E, Hadar Y, Chen Y, Chino M, Mori S. Indirect utilization of the phytosiderophore mugineic acid as an iron source to rhizosphere fluorescent Pseudomonas. Biometals. 1993;6:119–23. doi: 10.1007/BF00140113. [DOI] [PubMed] [Google Scholar]
- 10.Ortoneda M, Guarro J, Madrid MP, Caracuel Z, Roncero MI, Mayayo E, et al. Fusarium oxysporum as a multihost model for the genetic dissection of fungal virulence in plants and mammals. Infect Immun. 2004;72:1760–6. doi: 10.1128/IAI.72.3.1760-1766.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mercier A, Labbé S. Both Php4 function and subcellular localization are regulated by iron via a multistep mechanism involving the glutaredoxin Grx4 and the exportin Crm1. J Biol Chem. 2009;284:20249–62. doi: 10.1074/jbc.M109.009563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.López-Berges MS, Capilla J, Turrà D, Schafferer L, Matthijs S, Jöchl C, et al. HapX-mediated iron homeostasis Is essential for rhizosphere competence and virulence of the soilborne pathogen Fusarium oxysporum. Plant Cell. 2012;24:3805–22. doi: 10.1105/tpc.112.098624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scher FM, Baker R. Effect of Pseudomonas putida and a synthetic iron chelator on induction of soil suppressiveness to Fusarium wilt pathogens. Phytopathology. 1982;72:1567–73. doi: 10.1094/Phyto-72-1567. [DOI] [Google Scholar]
- 14.Simeoni LA, Lindsay WL, Baker R. Critical iron level associated with biological control of Fusarium wilt. Phytopathology. 1987;77:1057–61. doi: 10.1094/Phyto-77-1057. [DOI] [Google Scholar]
- 15.Thomashow LS, Weller DM. Current concepts in the use of introduced bacteria for biological disease control: Mechanisms and antifungal metabolites. In: Stacey G, Keen N, eds. Plant Microbe Interactions, Vol. 1. London: Chapman and Hall Ltd, 1996:187-236. [Google Scholar]