Abstract
Activation of Cu/Zn superoxide dismutases (CuZnSODs) is aided by Cu incorporation and disulfide isomerization by Cu chaperone of SOD (CCS). As well, an Fe-S cluster scaffold protein, ISU, might alter the incorporation of Fe or Mn into yeast MnSOD (ySOD2), thus leading to active or inactive ySOD2. However, metallochaperones involved in the activation of FeSODs are unknown. Recently, we found that a chloroplastic chaperonin cofactor, CPN20, could mediate FeSOD activity. To investigate whether Fe incorporation in FeSOD is affected by CPN20, we used inductively coupled plasma mass spectrometry to analyze the ability of CPN20 to bind Fe. CPN20 could bind Fe, and the Fe binding to FeSOD was increased with CPN20 incubation. Thus, CPN20 might be an Fe chaperone for FeSOD activation, a role independent of its well-known co-chaperonin activity.
Keywords: Arabidopsis, chaperonin 20, FeSOD, iron chaperone, iron homeostasis, SOD activation
Superoxide dismutases (SODs) are antioxidant enzymes in the first-line defense against reactive oxygen species (ROS) activity by converting O2− to O2 and H2O2, which requires a specific metal cofactor for activity.1,2 In Arabidopsis thaliana, CuZnSOD activation involves two pathways: the CCS-dependent pathway, which requires Cu chaperone of SOD (CCS), and an alternative pathway that acts in the absence of CCS.3-6
FeSODs are found in prokaryotes and plants but not in non-photosynthetic bacteria and animals7,8 and have an important role in plants. For instance, products of three FeSOD genes (FSD1, 2, 3) are found in Arabidopsis chloroplasts; FSD2 and FSD3 are essential for chloroplast development.9 FeSOD requires cofactor Fe for its activity; however, how it obtains the prerequisite Fe and becomes active is not known. Recent research discovered that co-chaperonin 20 (CPN20) can activate FeSOD activity in Arabidopsis.10 CPN20 is a chloroplastic co-chaperonin protein,11-15 but its function in FeSOD activation is independent of its chaperonin activity.10
CPN20 Binds Fe and Enhances Fe Incorporation into FeSOD
We analyzed the ability of Fe to bind CPN20 and the Fe loading of Holo-FSD1 incubated with CPN20 by inductively coupled plasma mass spectrometry (ICP-MS). The affinity-purified GST-CPN20 was tested as described.10 The molecular weight of both GST and CPN20 is ~26 kD, so the equal-amount input of GST (26 kD) and GST-CPN20 (53 kD) used here represents. The amount of Fe bound to GST-CPN20 was 2-fold greater than that to the equal loading of GST control (Fig. 1A); however, in molar terms, the amount of Fe bound to GST-CPN20 could be 4-fold higher than that to GST control. So CPN20 can itself bind Fe in vitro. Moreover, the Fe incorporation of Holo-FSD1 was significantly increased (~1.7-fold) when incubated with GST-CPN20 (Fig. 1B). Thus, CPN20 can bind Fe and facilitate its incorporation into Holo-FSD1 in vitro.
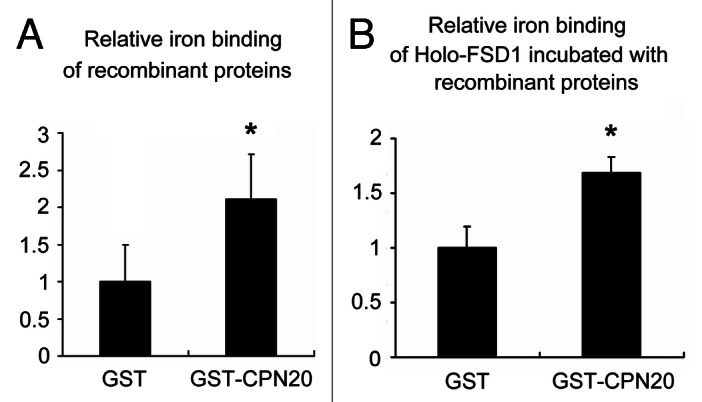
Figure 1. ICP-MS analysis of amount of Fe. (A) Fe binding of GST or GST-CPN20. Five μg GST or GST-CPN20 incubated with 2 μM ferrous ammonium sulfate was tested. (B) Fe binding of Holo-FSD1 on incubation with GST or GST-CPN20. Five μg Holo-FSD1 incubated with 5 μg GST or GST-CPN20 in 2 μM ferrous ammonium sulfate solution was tested. Data are relative to the GST control (mean ± SD, n = 5). *, p < 0.05 (two-tailed Student’s t-test).
CPN20 Might act as a Fe Chaperone for FeSOD Activation
Apo-FSD1, with the lost cofactor Fe, became inactive with alkaline denaturation of Holo-FSD1.16 Apo-FSD1 could not recover its activity with exogenous Fe unless CPN20 or cellular extract was present.10 In contrast, when Apo-FSD1 was incubated with CPN20, its activity was recovered even without exogenous Fe. CPN20 alone can also enhance Holo-FSD1 activity without exogenous Fe.10 Our finding that CPN20 binds Fe and the Fe incorporation of FSD1 was enhanced by CPN20 (Fig. 1A and B) implies that CPN20 might activate FSD1 by transferring its bound Fe into FSD1. For CuZnSOD activation, Cu-loaded CCS has been found to activate Apo-CuZnSOD without exogenous Cu.17 The current model underlying this phenomenon proposes that CCS transfers its bound Cu to Apo-CuZnSOD.18 Our results led to a similar conclusion: CPN20 binds Fe and transfers the bound Fe to FeSOD.
So far, the known cases of Fe chaperones are rare; only two Fe chaperones are known in mammals and yeast. One is frataxin, the mitochondrial Fe chaperone for Fe-S clusters and heme biosynthesis.19 Frataxin interacts with aconitase in a citrate-dependent manner to protect the Fe-S cluster of aconitase against disassembly and to promote its reactivation. The other is human poly(rC)-binding protein 1 (PCBP1), which delivers cytosolic Fe to ferritin.20 Here, we propose that CPN20 could be an Fe chaperone for FeSOD activation in chloroplasts. Among the three chaperones, CPN20 and frataxin both help in activating other proteins. PCBP1 has a dual function as an RNA-binding protein and an Fe chaperone for ferritin. Thus, both CPN20 and PCBP1 have a function other than their classic roles. Interestingly, these proteins all localize to different compartments: PCBP1 to the cytosol, frataxin to mitochondria and CPN20 to chloroplasts.
Interaction Between CPN20 and FSD1 Might be Transient
The binding and release of co-chaperonins to chaperonins is necessary for the operation of the chaperonin system.15 Hence, for CPN20, a transient interaction with chaperonin 60 (CPN60) or FeSOD seems logical. For CCS and CuZnSOD, their interaction is transient.21 Yeast yCCS showed no interaction with wild-type ySOD1 but rather with ySOD1H48F, a mutant that cannot accept the Cu cofactor from yCCS normally. According to the current model, CuZnSOD is released from CCS and becomes active as a dimer after the Cu is transferred and the disulfide isomerization reaction takes place. Interactions between CPN20 and FeSOD have been confirmed by yeast two-hybridization and fluorescence resonance energy transfer (FRET) analyses.10 However, results of both native-PAGE and gel filtration analyses indicated no major signal shift of FSD1 after incubation with CPN20, even though the same treatments enhanced FSD1 activity.10 Similarly, interactions between CCS and CuZnSOD were found with yeast two-hybridization3,22 but not immunoprecipitation or native gel analysis.21,23 Thus, the interaction between CPN20 and FSD1 could be transient, similar to with CCS and CuZnSODs, chaperonins and co-chaperonins.
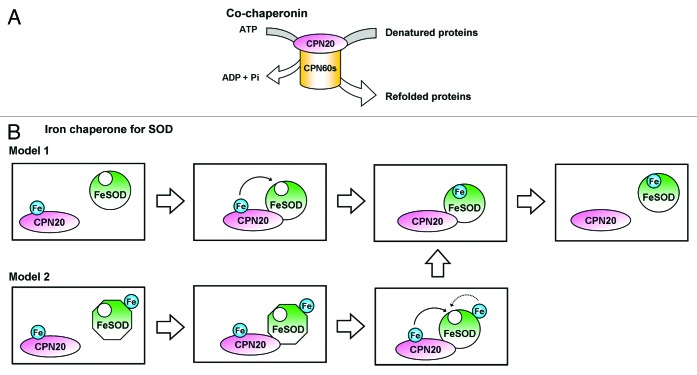
Possible Models for the Role of CPN20 in FeSOD Activation
According to our observations, the novel role of CPN20 in FeSOD activation could be as an Fe chaperone for SOD (Fig. 2). We suggest two models for the role of CPN20 in FeSOD activation: Model 1, CPN20 binds Fe, interacts with FeSOD, transfers its bound Fe to FeSOD, and then releases the active FeSOD; or Model 2, CPN20 might facilitate Fe incorporation in FeSOD by certain conformation changes or modifications, which makes FeSOD more accessible for Fe to be incorporated properly in its reaction site.
Figure 2. Summary of CPN20 functions. (A) CPN20 functions as a co-chaperonin, which assists the chaperonin CPN60 in folding the denatured proteins in an ATP-dependent way. (B) We demonstrate the additional function of CPN20 in FeSOD activation. We propose two possible models for the mechanism: Model 1, CPN20 transfers its bound Fe to FeSOD, thus activating FeSOD; Model 2, CPN20 might help in conformational changes or modifications of the FeSOD for the co-factor Fe to be incorporated into the reaction site properly.
Materials and Methods
Fe binding tests
To determine the amount of Fe bound by GST and GST-CPN20, 2 μM ferrous ammonium sulfate solution was incubated with 5 μg GST (6.4 μM) or GST-CPN20 (3.1 μM) at 30°C for 3 min. To determine the amount of Fe bound by Holo-FSD1, 5 μg Holo-FSD1 (7 μM) was added into the mixture as described above for 25 min incubation at 30°C. Each reaction mixture was supplied with 5 μM EDTA for incubation at room temperature for 1 min, followed by the addition of 1 mL Milli-Q water, and then subjected to Amicon Ultra-4 10K centrifugal filter device (Millipore). The concentrates were then washed with 2 mL Milli-Q water, and 3 mL collected filtrates was boiled at 100°C for 10 min with 2% HNO3, then subjected to ICP-MS Fe analysis.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank the Instrument Center, Taipei Medical University, Taiwan, for ICP-MS analysis. This work was supported by grants from the National Science Council, Taiwan (98−2311−B−002−007−MY3 and 101−2311−B−002−001), and partially supported by National Taiwan University (101R892003) to TLJ.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/23074
References
- 1.Beyer W, Imlay J, Fridovich I. Superoxide dismutases. Prog Nucleic Acid Res Mol Biol. 1991;40:221–53. doi: 10.1016/S0079-6603(08)60843-0. [DOI] [PubMed] [Google Scholar]
- 2.Bowler C, Van Montagu M, Inzé D. Superoxide dismutase and stress tolerance. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:83–116. doi: 10.1146/annurev.pp.43.060192.000503. [DOI] [Google Scholar]
- 3.Chu CC, Lee WC, Guo WY, Pan SM, Chen LJ, Li HM, et al. A copper chaperone for superoxide dismutase that confers three types of copper/zinc superoxide dismutase activity in Arabidopsis. Plant Physiol. 2005;139:425–36. doi: 10.1104/pp.105.065284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdel-Ghany SE, Burkhead JL, Gogolin KA, Andrés-Colás N, Bodecker JR, Puig S, et al. AtCCS is a functional homolog of the yeast copper chaperone Ccs1/Lys7. FEBS Lett. 2005;579:2307–12. doi: 10.1016/j.febslet.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 5.Huang CH, Kuo WY, Weiss C, Jinn TL. Copper chaperone-dependent and -independent activation of three copper-zinc superoxide dismutase homologs localized in different cellular compartments in Arabidopsis. Plant Physiol. 2012;158:737–46. doi: 10.1104/pp.111.190223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang CH, Kuo WY, Jinn TL. Models for the mechanism for activating copper-zinc superoxide dismutase in the absence of the CCS Cu chaperone in Arabidopsis. Plant Signal Behav. 2012;7:428–30. doi: 10.4161/psb.19192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alscher RG, Erturk N, Heath LS. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot. 2002;53:1331–41. doi: 10.1093/jexbot/53.372.1331. [DOI] [PubMed] [Google Scholar]
- 8.Fink RC, Scandalios JG. Molecular evolution and structure--function relationships of the superoxide dismutase gene families in angiosperms and their relationship to other eukaryotic and prokaryotic superoxide dismutases. Arch Biochem Biophys. 2002;399:19–36. doi: 10.1006/abbi.2001.2739. [DOI] [PubMed] [Google Scholar]
- 9.Myouga F, Hosoda C, Umezawa T, Iizumi H, Kuromori T, Motohashi R, et al. A heterocomplex of iron superoxide dismutases defends chloroplast nucleoids against oxidative stress and is essential for chloroplast development in Arabidopsis. Plant Cell. 2008;20:3148–62. doi: 10.1105/tpc.108.061341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuo WY, Huang CH, Liu AC, Cheng CP, Li SH, Chang WC, et al. CHAPERONIN 20 mediates iron superoxide dismutase (FeSOD) activity independent of its co-chaperonin role in Arabidopsis chloroplasts. New Phytol. 2012 doi: 10.1111/j.1469-8137.2012.04369.x. In press. [DOI] [PubMed] [Google Scholar]
- 11.Bertsch U, Soll J, Seetharam R, Viitanen PV. Identification, characterization, and DNA sequence of a functional “double” groES-like chaperonin from chloroplasts of higher plants. Proc Natl Acad Sci U S A. 1992;89:8696–700. doi: 10.1073/pnas.89.18.8696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baneyx F, Bertsch U, Kalbach CE, van der Vies SM, Soll J, Gatenby AA. Spinach chloroplast cpn21 co-chaperonin possesses two functional domains fused together in a toroidal structure and exhibits nucleotide-dependent binding to plastid chaperonin 60. J Biol Chem. 1995;270:10695–702. doi: 10.1074/jbc.270.18.10695. [DOI] [PubMed] [Google Scholar]
- 13.Hirohashi T, Nishio K, Nakai M. cDNA sequence and overexpression of chloroplast chaperonin 21 from Arabidopsis thaliana. Biochim Biophys Acta. 1999;1429:512–5. doi: 10.1016/S0167-4838(98)00268-4. [DOI] [PubMed] [Google Scholar]
- 14.Koumoto Y, Shimada T, Kondo M, Takao T, Shimonishi Y, Hara-Nishimura I, et al. Chloroplast Cpn20 forms a tetrameric structure in Arabidopsis thaliana. Plant J. 1999;17:467–77. doi: 10.1046/j.1365-313X.1999.00388.x. [DOI] [PubMed] [Google Scholar]
- 15.Weiss C, Bonshtien A, Farchi-Pisanty O, Vitlin A, Azem A. Cpn20: siamese twins of the chaperonin world. Plant Mol Biol. 2009;69:227–38. doi: 10.1007/s11103-008-9432-3. [DOI] [PubMed] [Google Scholar]
- 16.Yamakura F. A study on the reconstitution of iron-superoxide dismutase from Pseudomonas ovalis. J Biochem. 1978;83:849–57. doi: 10.1093/oxfordjournals.jbchem.a131981. [DOI] [PubMed] [Google Scholar]
- 17.Rae TD, Schmidt PJ, Pufahl RA, Culotta VC, O’Halloran TV. Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science. 1999;284:805–8. doi: 10.1126/science.284.5415.805. [DOI] [PubMed] [Google Scholar]
- 18.Culotta VC, Yang M, O'Halloran TV. Activation of superoxide dismutases: putting the metal to the pedal. Biochim Biophys Acta 2006; 1763:747-58. [DOI] [PMC free article] [PubMed]
- 19.Bulteau AL, O’Neill HA, Kennedy MC, Ikeda-Saito M, Isaya G, Szweda LI. Frataxin acts as an iron chaperone protein to modulate mitochondrial aconitase activity. Science. 2004;305:242–5. doi: 10.1126/science.1098991. [DOI] [PubMed] [Google Scholar]
- 20.Shi H, Bencze KZ, Stemmler TL, Philpott CC. A cytosolic iron chaperone that delivers iron to ferritin. Science. 2008;320:1207–10. doi: 10.1126/science.1157643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torres AS, Petri V, Rae TD, O’Halloran TV. Copper stabilizes a heterodimer of the yCCS metallochaperone and its target superoxide dismutase. J Biol Chem. 2001;276:38410–6. doi: 10.1074/jbc.M104790200. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt PJ, Kunst C, Culotta VC. Copper activation of superoxide dismutase 1 (SOD1) in vivo. Role for protein-protein interactions with the copper chaperone for SOD1. J Biol Chem. 2000;275:33771–6. doi: 10.1074/jbc.M006254200. [DOI] [PubMed] [Google Scholar]
- 23.Furukawa Y, Torres AS, O’Halloran TV. Oxygen-induced maturation of SOD1: a key role for disulfide formation by the copper chaperone CCS. EMBO J. 2004;23:2872–81. doi: 10.1038/sj.emboj.7600276. [DOI] [PMC free article] [PubMed] [Google Scholar]