Abstract
We describe the development of an in vitro library selection system (CIS display) that exploits the ability of a DNA replication initiator protein (RepA) to bind exclusively to the template DNA from which it has been expressed, a property called cis-activity. A diverse peptide library is created by ligation of DNA fragments of random sequence to a DNA fragment that encodes RepA. After in vitro transcription and translation, a pool of protein–DNA complexes is formed where each protein is stably associated with the DNA that encodes it. These complexes are amenable to the affinity selection of ligands to targets of interest. Here we show that RepA is a highly faithful cis-acting DNA-binding protein and demonstrate that libraries encoding >1012 random 18-mer peptides can be constructed and used to isolate peptides that bind specifically to disparate targets. The use of DNA to encode the displayed peptides offers advantages over in vitro peptide display systems that use mRNA.
The physical linkage of populations of peptides to their encoding nucleic acids to create large, diverse display libraries has provided a rich source of ligands to a wide range of target molecules. Enrichment of ligands from these libraries is achieved by the coselection of target-binding peptides along with their associated encoding nucleic acids, which allows the subsequent identification of the selected peptide sequences. This approach has been most widely exemplified by using the phage display system (1–4). However, phage display library construction requires the insertion of the library DNA into bacterial cells, and the efficiency of bacterial transformation usually restricts library sizes to the 109 to 1010 range, although in vivo constructed libraries >1010 have been reported (5, 6). Similarly, the display of peptides on bacterial or yeast surfaces or the display of peptides directly on their encoding plasmids all require a transformation step, which imposes a limit to the library sizes that can be constructed (7–9).
In general, it is accepted that a correlation exists between library size and the affinity of ligands that can be isolated from them (10). This correlation has prompted the development of display technologies in which the size-limiting transformation step is unnecessary, allowing ever-larger display libraries to be constructed. These new technologies enable higher affinity ligands to be obtained through the sampling of an increased structural repertoire, which is made possible through the generation of libraries that are up to four orders of magnitude larger than those that can be constructed for phage display. Examples of in vitro display technologies include “in vitro virus” (11) or mRNA display (12–14) and ribosome display (15–19). A common limitation with these technologies is that mRNA is used as the library-encoding nucleic acid, which may be prone to rapid degradation. The selected mRNA also requires a reverse-transcription step before amplification.
Existing DNA-based in vitro selection systems are based on emulsion encapsulation of DNA and are limited to libraries of 109 to 1010 per ml (20, 21). Here we describe an alternate DNA-based approach that does not require any compartmentalization of the library-encoding nucleic acid. The system, termed CIS display, exploits the high-fidelity cis-activity that is exhibited by a group of bacterial plasmid DNA-replication initiation proteins typified by RepA of the R1 plasmid (22, 23). In this context, cis-activity refers to the property of the RepA family of proteins to bind exclusively to the template DNA from which they have been expressed. Other cis-acting proteins exist, such as P2A, which exhibits cis-binding properties similar to RepA (24). In contrast to P2A, RepA does not bind covalently to its target DNA (25, 26). Also, RepA and P2A differ considerably in size (RepA is 33 kDa and P2A is 86kDa), making RepA more attractive for the display of polypeptide libraries.
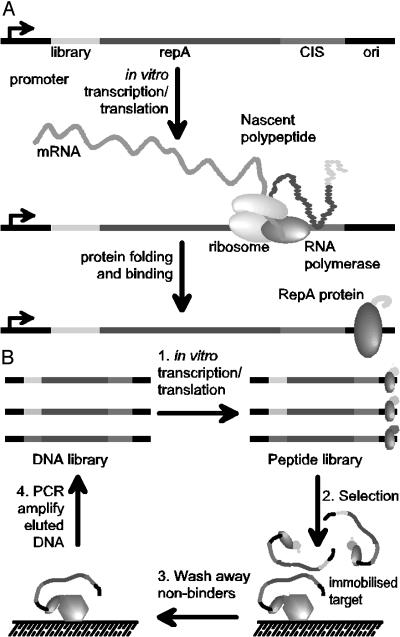
R1 plasmid replication is initiated through the binding of RepA to the plasmid origin of replication (ori). Ori is separated from the RepA-coding sequence by a DNA element termed CIS. This element is thought to be critical in controlling the cis-activity of RepA (Fig. 1; ref. 27). The consensus model for cis-activity is that the CIS element, which contains a rho-dependent transcriptional terminator, causes the host RNA polymerase to stall. This delay allows nascent RepA polypeptide emerging from translating ribosomes to bind transiently to CIS, which in turn directs the protein to bind to the adjacent ori site (28, 29).
Fig. 1.
The principle of the CIS display technology. (A) Template DNA encoding an N-terminal library peptide is ligated to the RepA gene. In vitro transcription is initiated at the promoter and pauses when the RNA polymerase reaches the CIS element. Concurrent translation produces the RepA protein, which transiently interacts with the CIS element, thereby forcing its subsequent binding to the adjacent ori sequence. This process establishes a faithful linkage between a template DNA and the expressed polypeptide that it encodes. (B) CIS display selections begin with the construction of a peptide-encoding DNA library followed by in vitro transcription/translation to form a pool of protein–DNA complexes (step 1). The library pool is incubated with an immobilized target (step 2), nonbinding peptides are washed away (step 3), and the retained DNA that encodes the target-binding peptides is eluted and amplified by PCR (step 4), to form a DNA library ready for the next round of selection. After three to five rounds of selection, recovered DNA is cloned into an appropriate expression vector for the identification of individual target-binding peptide sequences.
By genetically fusing peptide libraries to the N terminus of the RepA protein, we can achieve a direct linkage of peptides to the DNA molecules that encode them; thus, the link between genotype to phenotype that is the common feature of display technologies is established (Fig. 1). In this study we demonstrate that in vitro synthesized RepA has the high-fidelity cis-activity required to allow the display and selection of target-specific ligands. We show >1,000-fold enrichment of peptides in each round of affinity selection against a relevant target and then report the selection of peptide ligands to two well characterized antibodies (anti-P53 DO1 and anti-FLAG M2) and to lysozyme from large, diverse libraries.
Materials and Methods
CIS Display Libraries Construction. All enzymes were purchased from New England Biolabs. All PCR reactions contained 12.5 pmol of each of the primers, 2.5 units of TaqDeepVent DNA polymerase mixture (20:1), 250 μM dNTP (Roche Diagnostics) and 1× ThermoPol Buffer per 50-μl PCR reaction. PCR reactions were carried out on a Techne Techgene PCR machine for one cycle of 1 min and 45 s at 94°C, followed by 20–30 cycles at 94°C, 15 s; at 60°C, 30 s; at 72°C, 1–3 min, followed by a final extension of 5 min at 72°C. The tac-NNB-RepA-CIS-ori PCR construct (sequence is provided in Data Set 1, which is published as supporting information on the PNAS web site) was prepared by appending an 18-mer NNB library (where N is any nucleotide, and B is either C, T, or G to minimize the frequency of TAG stop codons) to the tac promoter by PCR and then ligating it to the RepA-CIS-ori region followed by PCR amplification. The RepA-CIS-ori region was amplified by PCR from the R1 plasmid [obtained from Public Health Laboratory Service, London, U.K. (ECO K12 J53 R1), GenBank accession no. V00351, for the R1 plasmid] by using the primers BSPREPAFOR and ORIREV408 (for all oligonucleotide sequences, see Table 1). The library was constructed by PCR with a tac promoter as template and the primers TACFARUP and NTERM18-mer. Of each of the two reaction products ≈5 μg were digested and ligated with 50 units each of NotI and PspOmI and 2,000 units of T4 DNA ligase in a 300-μl reaction volume, also containing 5 mM ATP (Sigma), 0.1 mg/ml acetylated BSA, and 1× NEB Buffer 4. The amount of full-length ligated product was quantified on an agarose gel, giving a library size of >1012 molecules. The resulting template was further amplified by PCR by using the nesting primers TACFAR1 and ORIREV108 to yield enough template for multiple in vitro transcription/translation reactions.
Table 1. Oligonucleotides (presented 5′–3′).
| BSPREPAFOR, CTGGAGATGGCATCAAGGGCCCCAACTGATCTTCACCAAACGTATTACC |
| ORIREV408, CGTAAGCCGGTACTGATTGA |
| TACFARUP, CAGTTGATCGGCGCGAGATT |
| NTERM18mer, ACATACCGTCATGCGGCCGCTGATCCTCCTCCCCC(VNN)18GGCCATGGTAGATCCTGTTTC |
| TACFAR1, CGCCAATCAGCAACGACTGT |
| ORIREV108, GGGCTTTGTGGTTTCAGTTC |
| TACCKFOR, CAGGAAACAGGATCTACCATGACTGTGGCTGCACCATCTGTCTTC |
| NOTICKREV, CGTTTGGTGAAGATCAGTTGCGGCCGCTGATCCTCCTCCCCCTCCCCTGTTGAAGCTCTTTGTG |
| TACREV, GGTAGATCCTGTTTCCTGTGTG |
| V5REPAFOR, CACAGGAAACAGGATCTACCATGGCCGGAAAACCTATCCCAAACCCTCTCCTAGGACTGGATTCAACGGGGGGAGGAGGATCAGCGGCCGCAACTGATCTTCACCAAACG |
| ECORIGENE8, CGCCGGAATTCTTATCAGCTTGCTTTCGAGG |
| NOTIGENE8, CTGCAGTAATAGGCGGCCGCAGGGGGAGGAGGGTCCGCTGAGGGTGACGATCCCGCA |
| TACFAR2, AACGTGGCTGGCCTGGTTCA |
| TACFAR3, GATAAGAGACACCGGCATAC |
| TACFAR4, GGCGCTATCATGCCATACCG |
| TACFAR5, ACCATTCGGCTAGCGATGAC |
| TAC6, CCCCATCCCCCTGTTGACAATTAATC |
| NOTIRECREV, GGTGAAGATCAGTTGCGGCCGCTGATCCTCCTC |
| All oligonucleotides were purchased from Sigma Genosys (Pampisford, U.K.). The means of oligonucleotide purification were as follows: <35 bases, desalting; 35–50 bases, reverse-phase cartridge purification; >50 bases, PAGE purification. |
V5-RepA and Cκ-RepA Construction. The human Cκ DNA was amplified by PCR from plasmid pDM6 (30) by using primers TACCKFOR and NOTICKREV, followed by assembly to the tac promoter and digestion and ligation to the RepA-CIS-ori region. The tac promoter was amplified by using primers TAC-FARUP and TACREV. The V5 epitope (GKPIPNPLLGLDST; ref. 31) DNA was appended to the RepA-CIS-ori region by PCR with primers V5REPAFOR and ORIREV408 followed by overlapping PCR assembly to the tac promoter (see above).
Construction of M13 gpVIII Phagemid Vector. The M13 gene 8 was PCR-amplified with primers ECORIGENE8 and NOTIGENE8 followed by digestion with EcoRI and NotI. Gel-purified product was ligated into a similarly digested plasmid pDM6 (30) and subsequently electroporated into electrocompetent TG-1 cells.
Affinity Selection. In vitro transcription and translation was performed in an Escherichia coli (strain SL119; ref. 32) S-30 lysate system (33) for up to 30 min at 30°C and then diluted 10-fold with blocking buffer (2–4% Marvel, 0.1 mg/ml herring sperm DNA, 2.5 mg/ml heparin, in PBS or TBS). Typically, 2–4 μg of linear DNA was added per 50 μl of S-30 lysate reaction. When performing library selections 20 μg of library DNA was added to 250 μl of S-30 lysate reaction in the first round; in subsequent rounds, 5 μg of library DNA was added in 100 μl of S-30 lysate reaction.
Solid-Phase Selection. Targets were immobilized on either 4 ml of NUNC Star Immunotubes (Fisher Scientific), Maxisorp plates (VWR Scientific) (both coated overnight at 4°C), or captured by M-280 streptavidin-coated magnetic beads (Dynal, Oslo). Coating concentrations were 50 μg/ml for lysozyme (Sigma), 10 μg/ml for anti-V5 antibody, anti-human κ-chain antibody (AB-CAM, Cambridge, U.K.), DO1 (Oncogene Research, Boston), and anti-corticotropin (anti-ACTH) antibody (Immunologicals Direct, Oxford) in PBS. Immobilized targets were washed twice with PBS and blocked for 1 h at room temperature with Blocking Buffer, and then washed twice with PBS. The diluted transcription/translation reactions were incubated for 1 h at room temperature before washing 6 to 12 times with PBS/0.1%-Tween-20, followed by 6–12 washes with PBS (filled and empty). DNA was eluted with 500 μl of PB solution (Qiagen, Crawley, U.K.) and purified by using QIAquick PCR purification kit (Qiagen). Half of the eluted material was added to a recovery PCR reaction where the N-terminal library region was amplified by using a nested primer for each round (TACFAR2 in round 1, TACFAR3 in round 2, and so forth up to TACFAR5). All recovered PCR product was reattached to the RepA-CIS-ori by restriction ligation (as above) and further amplified, as described above, but with the respective nesting primer to produce input DNA for the next round of selection.
In Solution Selection. Two micrograms of biotinylated anti-FLAG M2 antibody (Sigma) or 25 μg of biotinylated lysozyme (the level of biotin incorporation was determined to be five biotin molecules per lysozyme molecule by using the 2-(4′-hydroxyazobenzene)benzoic acid method; EZ-Link biotinylation kit, Pierce) was added to the diluted transcription/translation reactions (see Solid-Phase Selection) and allowed to bind for 2 h with occasional shaking. Bound library complexes were captured onto- 50 μl of washed and blocked streptavidin-coated magnetic beads (Dynal, Oslo) for 15 min, then washed four times with 1 ml of PBS/0.1% Tween 20 and two times with 1 ml of PBS, before elution with PB solution and processing as above.
ELISA Screening of Selected Peptides. After each round of selection, recovered DNA was PCR-amplified with primers TAC6 and NOTIRECREV and purified and digested with NotI and NcoI. The DNA was then ligated into a similarly digested M13 gpVIII phagemid vector, transformed into E. coli TG-1 cells, and plated on 2% glucose, 2× TY, 100 μg/ml ampicillin plates. Individual colonies were grown for the production of phage particles as described (32). NUNC Maxisorp plates were coated with 100 ng per well of either anti-human Cκ antibody, anti-V5 antibody (ABCAM, Cambridge), anti-FLAG M2 antibody (Sigma), 1 μg/well of DO1 antibody (Oncogene Research), or 5 μg per well of lysozyme (Sigma) in PBS overnight at 4°C. The horseradish peroxidase-conjugated anti-M13 secondary antibody (Amersham Pharmacia Biotech) was diluted 1:5,000 in 2% Marvel in PBS. ELISA assays were performed as described (30). The assay was developed with SureBlue TMB peroxidase substrate (Insight Biotechnology, Middlesex, U.K.) and read at 450 nm.
Results
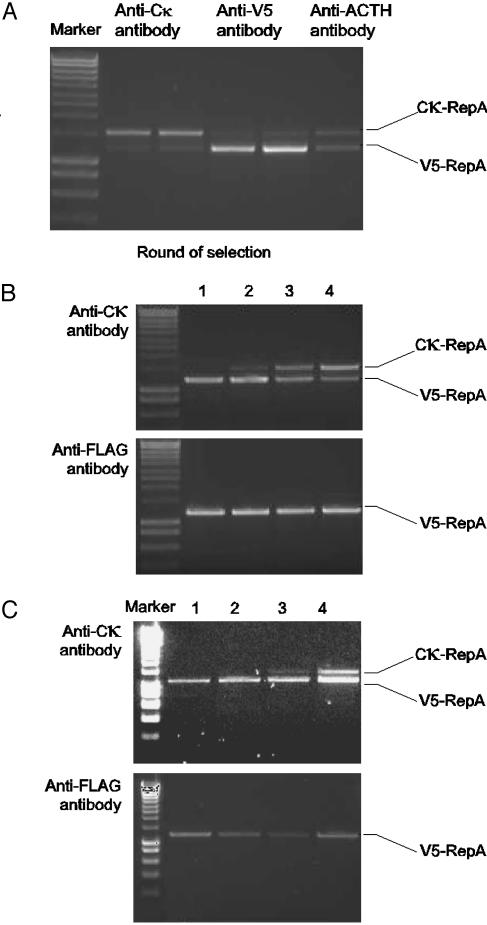
RepA binds to DNA in cis when expressed in vitro from a linear DNA template. To demonstrate cis-activity, two linear RepA DNA constructs containing different-sized inserts, the 14-aa V5 peptide tag (31) and the 105-aa human Cκ Ig constant domain, were mixed in a 1:1 ratio and translated in vitro; the resulting protein–DNA complexes were subjected to affinity selection against three immobilized antibodies. In this selection binding of complexes to anti-Cκ antibodies resulted in the specific recovery of Cκ-RepA DNA but not the V5-RepA DNA and vice versa, as distinguished by their size in agarose gel electrophoresis (Fig. 2A). Binding of complexes to an irrelevant antibody (anti-ACTH) resulted in the recovery of background levels of DNA at the original ratio. These results clearly demonstrate that RepA acts in cis in vitro with high fidelity.
Fig. 2.
Demonstration of target-specific ligand selection by using CIS display. (A) A 1:1 mixture of Cκ-RepA DNA and V5-repA DNA was prepared, transcribed and translated in vitro, and selected against either anti-Ck antibody, anti-V5 antibody, or anti-ACTH antibody. Recovered DNA was amplified with universal primers and separated by agarose gel electrophoresis on the basis of the size difference between the Cκ domain and the V5 peptide tag. The amount of DNA specifically recovered when by using the anti-Cκ and anti-V5 antibodies is shown in duplicate along with a single negative control (anti-ACTH) to reflect background recovery. (B) A 1:108 dilution of Cκ-RepA DNA into V5-repA DNA was prepared and subjected to four rounds of selection against either the anti-Cκ antibody or the anti-FLAG antibody as a negative control. The four rounds of selection are indicated along with the positions of the two recovered PCR products. (C) A 1:1010 dilution of Cκ-RepA DNA into V5-repA DNA was prepared and subjected to four rounds of selection against either the anti-Cκ antibody or the anti-FLAG antibody as a negative control. The four rounds of selection are indicated along with the positions of the two recovered PCR products. Marker refers to the DNA Hyperladder (Bioline, London).
High Level of Enrichment During Sequential Rounds of Affinity Selection. To assess the suitability of CIS display under library selection conditions and to estimate the enrichment levels possible with the system, Cκ-RepA DNA was mixed with V5-RepA DNA at a ratio of either 1:108 or 1:1010 (Cκ/V5) and then subjected to four rounds of selection either against an anti-Cκ antibody or against a negative control antibody target (anti-FLAG M2). DNA recovery after each round of selection with either target was assessed by PCR amplification (Fig. 2 B and C). Only two rounds of selection against anti-Cκ were required to enrich Cκ-RepA complexes from the 1:108 starting ratio so that the Cκ-RepA DNA was visible in the products from the PCR. Because the recovered DNA corresponding to Cκ-RepA comprised ≈1% (calculated by densitometry by using the gel documentation program genetools, SynGene, Cambridge, U.K.) of the total DNA amplified at this stage of the selection process (Fig. 2B, lane 2), this percentage suggests that an enrichment of ≈1,000-fold per round had been achieved under these conditions. For the 1:1010 starting ratio, a third round of selection was required before the Cκ-RepA product was visible (Fig. 2C, lane 3), again suggesting 1,000-fold enrichment per round. As expected, further rounds of selection continued to increase the relative yield of the Cκ-RepA PCR product.
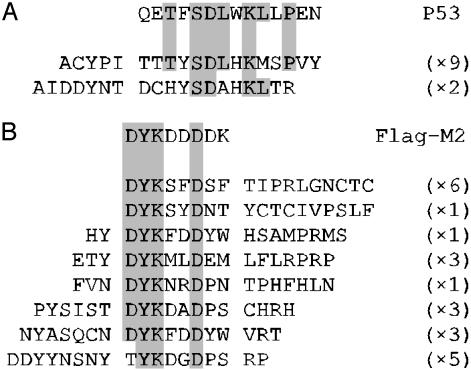
Selection Against Anti-P53, Anti-FLAG Antibody, and Lysozyme. To further validate the technology we selected an 18-mer peptide-RepA library against two antibody targets; the monoclonal anti-P53 antibody DO1 and the monoclonal anti-FLAG antibody M2. DO1 binds to a region within the N terminus of p53 from amino acid residues 11–25 (34). M2 is known to bind with 15 nM affinity to the FLAG epitope sequence (DYKDDDDK) and have a known preference for the sequence DYKXXD, where X is any amino acid (35, 36). A library of 1012 DNA molecules was used as the initial input in the first rounds of selection against DO1 and M2. The recovered DNA was amplified by PCR and used to prepare input DNA for the next round of selection. After each round, recovered DNA was cloned into an M13 gpVIII phagemid vector and tested for specific binding to antibody. Despite the well known sequence and insert-size bias of gpVIII, the pVIII fusion partner was chosen to allow antibody capture of all possible binding peptides. We are investigating both monovalent bacterial expression systems (such as maltose-binding protein) and in vitro expression systems for the characterization of selected peptides.
Eleven ELISA-reactive peptides from the fifth round of selection against DO1 were sequenced, and two different peptide sequences were identified that had homology to the P53 epitope (Fig. 3A). In the selection against M2, 23 specific binding clones were sequenced, and eight different peptide sequences identified. These peptides showed significant homology to the FLAG sequence (Fig. 3B). All sequences contained the required Y2 and K3 and the preferred D6; only one did not contain D1. Other similarities among the selected peptides were identified. A bias existed for Tyr or Asn at position -1, and Pro and Ser were prevalent at positions 7 and 8. In both selections, >90% of clones screened were binding to the target after five rounds, whereas in round 4, the in-solution anti-FLAG selection was still saturating, and the solid-phase anti-p53 selection contained ≈15% of clones binding to the target.
Fig. 3.
Homology between selected binding peptides and respective epitope sequences. The sequences of 11 DO1 (A) and 23 M2-binding peptides (B) are aligned with the P53-DO1 and FLAG-M2 epitope sequences, respectively. Clones from the DO1 selection are taken from round 5, and the M2 peptides originate from rounds 4 and 5. Regions of homology are shaded, and the frequency with which each clone was identified is shown in parentheses.
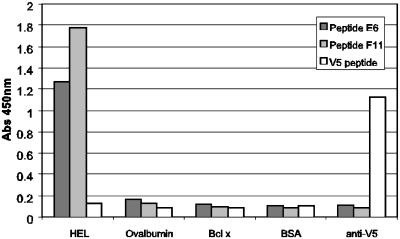
Finally, to demonstrate that the method was capable of deriving peptide ligands to nonantibody targets, a selection was performed against lysozyme. After five rounds of selection, 12% of those clones that were tested were observed to be reactive against lysozyme. The specificities of two representative clones were assessed by ELISA (Fig. 4) by using ovalbumin, BSA, and anti-V5 antibody as negative control targets. The ELISA signals against the lysozyme were between 6- and 12-fold greater than those observed against the control targets.
Fig. 4.
ELISA of peptides selected against lysozyme both on solid-phase and in-solution selection. After five rounds of selection, peptides expressed on phage as M13 gpVIII fusions were checked for specificity of binding to lysozyme (50 μg/ml), ovalbumin (50 μg/ml), Bcl-X (10 μg/ml), BSA (200 μg/ml), and anti-V5 antibody (10 μg/ml). Two examples of the selected clones are shown; E6 and F11, from solid-phase and in-solution selection, respectively. The V5 peptide fused to gpVIII was used as a both a negative control (against lysozyme) and a positive control (against anti-V5 antibody) for the assay.
Discussion
The establishment of a physical linkage between a polypeptide and its encoding nucleic acid is central to the development of display technologies. Of equal importance is that each nucleic acid within a complex pool of nucleic acids can associate exclusively with its own encoded polypeptide. We have shown that a cis-acting DNA-binding protein, RepA, can facilitate this association through the simple in vitro transcription and translation of appropriately constructed DNA templates.
The highly faithful cis-activity of RepA is shown here by the exclusive, target-specific retention of DNA that encoded relevant peptide moieties (Fig. 2A) and by the 1,000-fold target-specific enrichment of the appropriate DNA in each round of selection against a control target (Fig. 2 B and C). A possible explanation for the very low level of trans-activity of RepA is the rapid inactivation of nonbound RepA that has been reported (24).
We have further shown the utility of the CIS display system by achieving successful affinity selection of peptide ligands to the well characterized antibodies DO1 and M2 and to a nonantibody target, lysozyme. The results from these selections compare well with those that have been achieved by using ribosome display, mRNA display, and emulsion-based selections (16, 17, 21, 37). However, CIS display has several advantages over these alternative in vitro selection systems. It is likely that the use of DNA to encode the displayed peptides provides advantages over RNA-based in vitro selection methods. Very large libraries can be rapidly constructed and screened without separating transcription and translation steps and without purification of the protein–DNA complexes before selection. Also, control of divalent cation concentration is not required, and the complexes do not require incubation under sterile or ribonuclease-free conditions. These simplified selection conditions are in contrast to the tight control of these parameters that is normally required when using RNA-based display technologies. Indeed, a method of protecting the mRNA of ribosome display particles using RNA-binding proteins has recently been published in a patent application (38), and protocols for the conversion of the library-encoding mRNA to cDNA in an attempt to stabilize mRNA display complexes before selection have also been reported (39). The high inherent stability of RepA–DNA complexes has been confirmed by incubating in vitro expressed Cκ-RepA with an anti-Cκ antibody for 48 h before elution and recovery of associated DNA. In this experiment no reduction in DNA yield was observed compared with that obtained using standard selection conditions (not shown). Also, preliminary data suggest that we are able to display and demonstrate folding of larger proteins, such as svFv fragments, which further enhances the use of this technology (not shown). Possibly, the most variable factor in the technology is the quality of the library DNA itself. Sequence analysis of cloned unselected library showed that, with different batches of library encoding oligonucleotides, the level of deletions and insertions varied remarkably, e.g., one batch contained 75% deletions and insertions, whereas another contained none. Many further developments of the system can be envisaged. For example, C-terminal display may be possible, but we have avoided testing this at present because of the possibility of disrupting the cis-activity of RepA. In addition, we have found it difficult to fully quantify functional library size, as it seems to depend on the lysate activity, DNA quality, and the method for measuring protein–DNA linkage.
CIS display combines the robustness and ease of use of phage display with the added power and flexibility of in vitro operation. Furthermore, the ease with which the protein–DNA complexes are produced and selections performed should make CIS display well suited to automation, allowing the parallel selection of high-affinity, fine-specificity ligands to multiple targets.
Supplementary Material
Acknowledgments
We thank Dr. David Andrews (McMaster University, Hamilton, Canada) and his research group for provision of S30 lysates and Dr. Andrew Carter at Institute of Food Research, Norwich, for his assistance.
Abbreviation: ACTH, corticotropin.
References
- 1.Smith, G. P. (1985) Science 228, 1315-1317. [DOI] [PubMed] [Google Scholar]
- 2.Scott, J. K. & Smith, G. P. (1990) Science 249, 386-390. [DOI] [PubMed] [Google Scholar]
- 3.Cwirla, S. E., Peters, E. A., Barrett, R. W. & Dower, W. J. (1990) Proc. Natl. Acad. Sci. USA 87, 6378-6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devlin, J. J., Panganiban, L. C. & Devlin, P. E. (1990) Science 249, 404-406. [DOI] [PubMed] [Google Scholar]
- 5.Griffiths, A. D., Williams, S. C, Hartley, O., Tomlinson, I. M., Waterhouse, P., Crosby, W. L., Kontermann, R. E., Jones, P. T., Low, N. M., Allison, T. J., et al. (1994) EMBO J. 13, 3245-3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sidhu, S. S., Lowman, H. B., Cunningham, B. C. & Wells, J. A. (2000) Methods Enzymol. 328, 333-363. [DOI] [PubMed] [Google Scholar]
- 7.Fuchs, P., Breitling, F., Dubel, S., Seehaus, T. & Little, M. (1991) Bio/Technology 9, 1369-1372. [DOI] [PubMed] [Google Scholar]
- 8.Boder, E. T. & Wittrup, K. D. (1997) Nat. Biotechnol. 15, 553-557. [DOI] [PubMed] [Google Scholar]
- 9.Cull, M. G., Miller, J. F. & Schatz, P. J. (1992) Proc. Natl. Acad. Sci. USA 89, 1865-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perelson, A. S. & Oster, G. F. (1979) J. Theor. Biol. 81, 645-670. [DOI] [PubMed] [Google Scholar]
- 11.Nemoto, N., Miyamoto-Sato, E., Husimi, Y. & Yanagawa, H. (1997) FEBS Lett. 414, 405-408. [DOI] [PubMed] [Google Scholar]
- 12.Roberts, R. W. & Szostak, J. W. (1997) Proc. Natl. Acad. Sci. USA 94, 12297-12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keefe, A. D. & Szostak, J. W. (2001) Nature 410, 715-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kreider, B. L. (2000) Med. Res. Rev. 20, 212-215. [DOI] [PubMed] [Google Scholar]
- 15.Palacios, R. & Schimke, R. T. (1973) J. Biol. Chem. 248, 1424-1430. [PubMed] [Google Scholar]
- 16.Mattheakis, L. C., Bhatt, R. R. & Dower W. J. (1994) Proc. Natl. Acad. Sci. USA 91, 9022-9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gersuk, G. M., Corey, M. J., Corey, E., Stray, J. E., Kawasaki, G. H. & Vessella, R. L. (1997) Biochem. Biophys. Res. Commun. 232, 578-582. [DOI] [PubMed] [Google Scholar]
- 18.Amstutz, P., Forrer, P., Zahnd, C. & Pluckthun, A. (2001) Curr. Opin. Biotechnol. 12, 400-405. [DOI] [PubMed] [Google Scholar]
- 19.Hanes, J. & Pluckthun, A. (1997) Proc. Natl. Acad. Sci. USA. 94, 4937-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sepp, A., Tawfik, D. S. & Griffiths, A. D. (2002) FEBS Lett. 532, 455-458. [DOI] [PubMed] [Google Scholar]
- 21.Yonezawa, M., Doi, N., Kawahashi, Y., Higashinakagawa, T. & Yanagawa, H. (2003) Nucleic Acids Res. 31, e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masai, H., Kaziro, Y. & Arai, K. (1983) Proc. Natl. Acad. Sci. USA 80, 6814-6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nikoletti, S., Bird, P., Praszkier, J. & Pittard, J. (1988) J. Bacteriol. 170, 1311-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.FitzGerald, K. (2000) Drug Discovery Today 5, 253-258. [DOI] [PubMed] [Google Scholar]
- 25.Odegrip, R. O. & Haggard-Ljungquist, E. (2001) J. Mol. Biol. 308, 147-163. [DOI] [PubMed] [Google Scholar]
- 26.Giraldo, R. & Diaz, R. (1992) J. Mol. Biol. 228, 787-802. [DOI] [PubMed] [Google Scholar]
- 27.Masai, H. & Arai, K. (1988) Nucleic Acids Res. 16, 6493-6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Praszkier, J. & Pittard, A. J. (1999) J. Bacteriol. 181, 2765-2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Praszkier, J., Murthy, S. & Pittard, A. J. (2000) J. Bacteriol. 182, 3972-3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGregor, D. P. & Robins, S. P. (2001) Anal. Biochem. 294, 108-117. [DOI] [PubMed] [Google Scholar]
- 31.Southern, J. A., Young, D. F., Heaney, F., Baumgartner, W. K. & Randall, R. E. (1991) J. Gen. Virol. 72, 1551-1557. [DOI] [PubMed] [Google Scholar]
- 32.Lesley, S. A., Brow, M. A. D. & Burgess, R. R. (1991) J. Biol. Chem. 266, 2632-2638. [PubMed] [Google Scholar]
- 33.Lesley, S. A. (1995) Methods Mol. Biol. 37, 265-278. [DOI] [PubMed] [Google Scholar]
- 34.Stephen, C. W., Helminen, P. & Lane, D. P. (1995) J. Mol. Biol. 248, 58-78. [DOI] [PubMed] [Google Scholar]
- 35.Slootstra, J. W., Kuperus, D., Plückthun, A. & Meloen, R. H. (1997) Mol. Divers. 2, 156-164. [DOI] [PubMed] [Google Scholar]
- 36.Wegner, G. J., Lee, H. J. & Corn, R. M. (2002) Anal. Chem. 74, 5161-5168. [DOI] [PubMed] [Google Scholar]
- 37.Baggio, R., Burgstaller, P., Hale, S. P., Putney, A. R., Lane, M., Lipovsek, D., Wright, M. C., Roberts, R. W., Liu, R., Szostak, J. W. & Wagner, R. W. (2002) J. Mol. Recognit. 15, 126-134. [DOI] [PubMed] [Google Scholar]
- 38.Holtet, T. & Osbourn, J. (2001) Int. Patent Appl. WO0175097.
- 39.Kurz, M., Gu, K., Al-Gawari, A. & Lohse, P. A. (2001) Chembiochem 2, 666-672. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.