Abstract
Recent studies suggest that tocopherols could play physiological roles in salt tolerance but the mechanisms are still unknown. In this study, we analyzed changes in growth, mineral and oxidative status in vte1 and vte4 Arabidopsis thaliana mutants exposed to salt stress. vte1 and vte4 mutants lack α-tocopherol, but only the vte1 mutant is additionally deficient in γ-tocopherol. Results showed that a deficiency in vitamin E leads to reduced growth and increased oxidative stress in hydroponically-grown plants. This effect was observed at early stages, not only in rosettes but also in roots. The vte1 mutant was more sensitive to salt-induced oxidative stress than the wild type and the vte4 mutant. Salt sensitivity was associated with (i) high contents of Na+, (ii) reduced efficiency of PSII photochemistry (Fv/Fm ratio) and (iii) more pronounced oxidative stress as indicated by increased hydrogen peroxide and malondialdeyde levels. The vte 4 mutant, which accumulates γ- instead of α-tocopherol showed an intermediate sensitivity to salt stress between the wild type and the vte1 mutant. Contents of abscisic acid, jasmonic acid and the ethylene precursor, 1-aminocyclopropane-1-carboxylic acid were higher in the vte1 mutant than the vte4 mutant and wild type. It is concluded that vitamin E-deficient plants show an increased sensitivity to salt stress both in rosettes and roots, therefore indicating the positive role of tocopherols in stress tolerance, not only by minimizing oxidative stress, but also controlling Na+/K+ homeostasis and hormonal balance.
Keywords: hormones, hydrogen peroxide, oxidative stress, salt stress, vitamin E
Introduction
Salt stress is one of the most important stresses limiting crop yields. When growing in saline soils, plants are subject to three kinds of stresses, including water deficit caused by an osmotic unbalance, ion toxicity due to excess ion accumulation, and nutritional alterations caused by disturbances in the ion transport systems. In addition, the salinity may lead to oxidative stress by increasing reactive oxygen species (ROS) production and/or altered antioxidant defenses.1 Enzymatic and non-enzymatic antioxidants play important roles in alleviating deleterious effects of salt stress.2 Among them, tocopherols are a group of lipid soluble antioxidants that are synthesized only by photosynthetic organisms. Plants accumulate α-tocopherol in leaves, and to a lesser extent its immediate precursor, γ-tocopherol in response to abiotic stress.3 It is well known that tocopherols protect photosynthetic membranes from lipid peroxidation and singlet oxygen.4 Recent studies suggest that tocopherols could play an important role in adaptation to low temperature,5,6 drought7 and heavy metals.8 They participate also in seed dormancy and germination9,10 and cell signaling.7,11
Less information is available on the role of tocopherols in salt stress tolerance. In order to examine this role, the response to salinity has been studied in tocopherol-deficient mutants and transgenic plants over-accumulating tocopherols. Abbasi et al.12 have developed transgenic lines silenced for homogentisate phytyltransferase (HPT) and γ-tocopherol methyltransferase (γ-TMT) and examined the response of these transgenics to salt stress as well as to sorbitol stress and methyl viologen treatments in comparison to wild type plants. Transgenics with low tocopherol content showed an increased sensitivity to all three stresses, whereas in transgenic plants accumulating γ- instead of α-tocopherol, the resistance to osmotic and oxidative stress was increased.12 Rice mutants deficient in tocopherol cyclase (VTE1), and thus in α- and γ-tocopherol, showed less resistance to salt stress, whereas the overexpression of the same gene improved salt tolerance in rice and tobacco.13 The overexpression of γ-TMT, which converts γ-tocopherol to α-tocopherol in Brassica juncea leaves enhanced tolerance to abiotic stresses, including salt stress, showing that α-tocopherol plays an important role in the alleviation of several kinds of stresses.14 In chloroplast of tomato plants subject to moderate and severe salt stress, tocopherols appear to act as antioxidants under moderate stress and in the early phases of severe stress. However upon the last phases of severe stress, it has been suggested to be involved in senescence processes.15 Furthermore, it has been shown that α-tocopherol levels were much higher in the halophyte Cakile maritima than in the glycophyte A. thaliana upon salt stress. The opposite effect was observed with γ-tocopherol.16 These results indicated that α- and γ-tocopherol could have different roles in salt tolerant and sensitive plants. However, mechanisms of salt stress tolerance in tocoherol-deficient plants have not been investigated thus far in detail and the mechanisms remain elusive beyond their role as antioxidants.
In a recent study, we showed that tocopherol-deficient mutants show altered gene expression of ethylene-related genes. Accumulation of γ- instead of α-tocopherol in the vte4 mutant led to elevated transcript levels of ethylene signaling pathway genes (particularly CTR1, EIN2, EIN3 and ERF1) in mature leaves of control plants. However, with salt treatment, transcript levels of most of these genes remained constant or dropped in the vte4 mutant, while they were dramatically induced in the wild type and the vte1 mutant.7 In the present study, we explored the response of tocopherol-deficient mutants to salt stress in hydroponic media. We hypothesized that in this media tocopherol deficiency may be exacerbated and a more dramatic phenotype might be potentially observed. We aimed at unraveling whether or not tocopherols might have a protective function against sodium toxicity in hydroponic media by either limiting oxidative stress and/or through other physiological processes, such as exerting a role on ion homeostasis, the hormonal balance and gene expression.
Results
Differential response to salt stress in vitamin E-deficient mutants
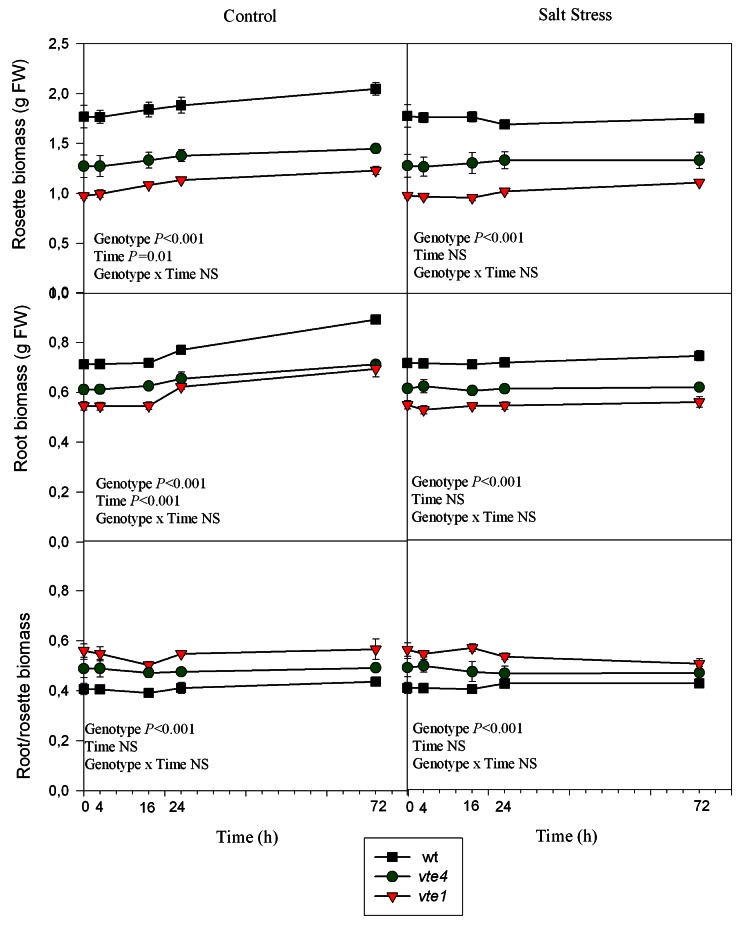
Under control conditions, the growth of roots and rosettes of wild type plants increased gradually throughout the study. In contrast, growth was more moderate in the vte1 and vte4 mutants (Fig. 1). Under salt stress, the same behavior in wild type plants and mutants was observed, salt stress arrested growth in all genotypes (Fig. 1). In both control and salt-stressed plants and among the three genotypes, the highest root/rosette biomass was observed in the vte1 mutant (Fig. 1).
Figure 1. Time course evolution of root and rosette biomass and the root-rosette biomass ratio of wild type and vte1 and vte4 mutants of A. thaliana growing in a hydroponic system exposed to control or salt stress conditions for 72h. Data are the mean ± SE of n = 4 replicates. Results of statistics are given in the panels (ANOVA, P ≤ 0.05). NS, not significant.
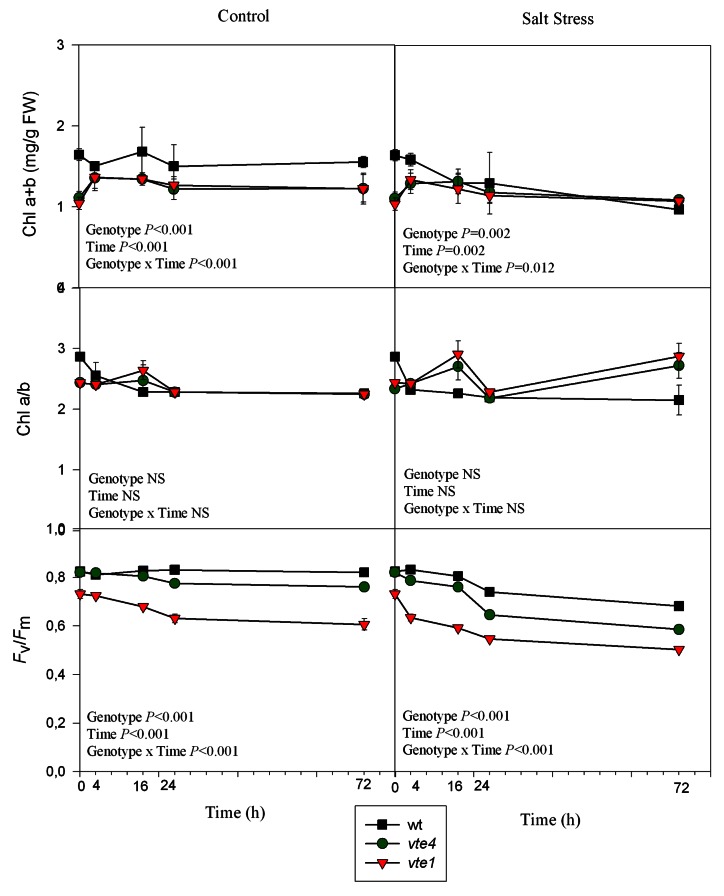
Chlorophyll levels were higher in wild type rosettes compared with those of vte1 and vte4 mutants, which showed similar levels, under control conditions. Under salt stress, chlorophyll levels decreased more dramatically in the wild type than in both mutants, but similar chlorophyll levels were observed after 72 h of treatment in the three genotypes. The Chl a/b ratio remained stable and with similar levels in all genotypes and in both treatments. The maximum efficiency of PSII photochemistry (Fv/Fm ratio) was similar in both wild type plants and the vte4 mutant (0.82 in both cases), but was 11% smaller in the vte1 mutant compared with the other genotypes at the beginning of the experiment. As growth progressed, differences were even more evident between genotypes under control conditions. The salt treatment caused a decrease of 17%, 28% and 31% in wild type plants, vte1 and vte4 mutants, respectively, after 72h (Fig. 2).
Figure 2. Time course evolution of chlorophyll levels (Chl a+b and Chl a/b) and maximum efficiency of PSII photochemistry (Fv/Fm) in leaves of wild type and vte1 and vte4 mutants of A. thaliana growing in a hydroponic system exposed to control or salt stress conditions for 72h. Data are the mean ± SE of n = 4 replicates. Results of statistics are given in the panels (ANOVA, P ≤ 0.05). NS, not significant.
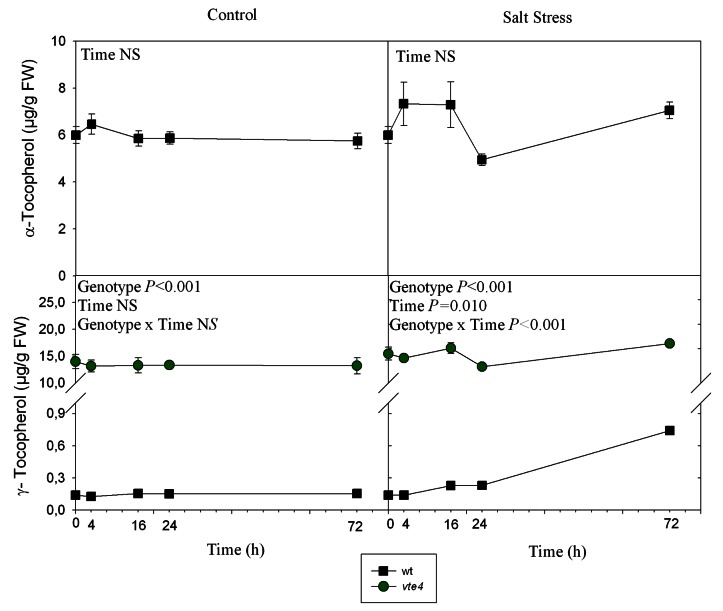
In wild type plants, the levels of α-tocopherol remained unchanged throughout the study under control and salt-stress treatments. The levels of γ-tocopherol were much lower than those of α-tocopherol in wild type plants, but they increased from 0.155 to 0.741 µg g−1 FW after 72h of salt treatment (Fig. 3). As expected, the vte4 mutant showed γ-tocopherol but not α-tocopherol accumulation in rosette leaves with γ-tocopherol levels around 13.2 µg g−1 FW under control conditions, even higher than α-tocopherol in the wild type. As expected, the vte1 mutant did not accumulate either α- or γ-tocopherols in leaves. In roots, tocopherols were not detected in either genotype (detection limit: 0.01 µg/gPF).
Figure 3. Time course evolution of α- and γ-tocopherol levels in leaves of wild type and vte1 and vte4 mutants of A. thaliana growing in a hydroponic system exposed to control or salt stress conditions for 72h. Data are the mean ± SE of n = 4 replicates. Results of statistics are given in the panels (ANOVA, P ≤ 0.05). NS, not significant. α- and γ-tocopherol values were not detected in vte1 mutant
Abscisic acid, hydrogen peroxide and malondialdehyde
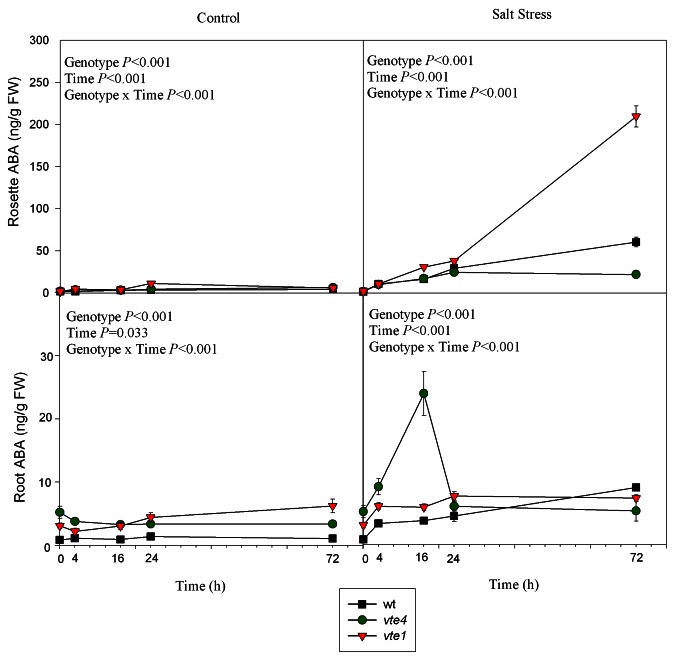
Abscisic acid (ABA) levels increased in the rosettes and roots of salt-treated wild type plants (Fig. 4). In the vte1 mutant, ABA increased also in the rosette of salt-treated plants and attained very high levels when compared with wild type plants and the vte4 mutant, but no significant increases were observed in roots. In contrast, maximal ABA levels were observed in roots of the vte4 mutant, particularly at 16h of salt stress, levels that returned to control values at 72h of treatment (Fig. 4).
Figure 4. Time course evolution of ABA levels in leaves and roots of wild type and vte1 and vte4 mutants of A. thaliana growing in a hydroponic system exposed to control or salt stress conditions for 72h. Data are the mean ± SE of n = 4 replicates. Results of statistics are given in the panels (ANOVA, P ≤ 0.05). NS, not significant.
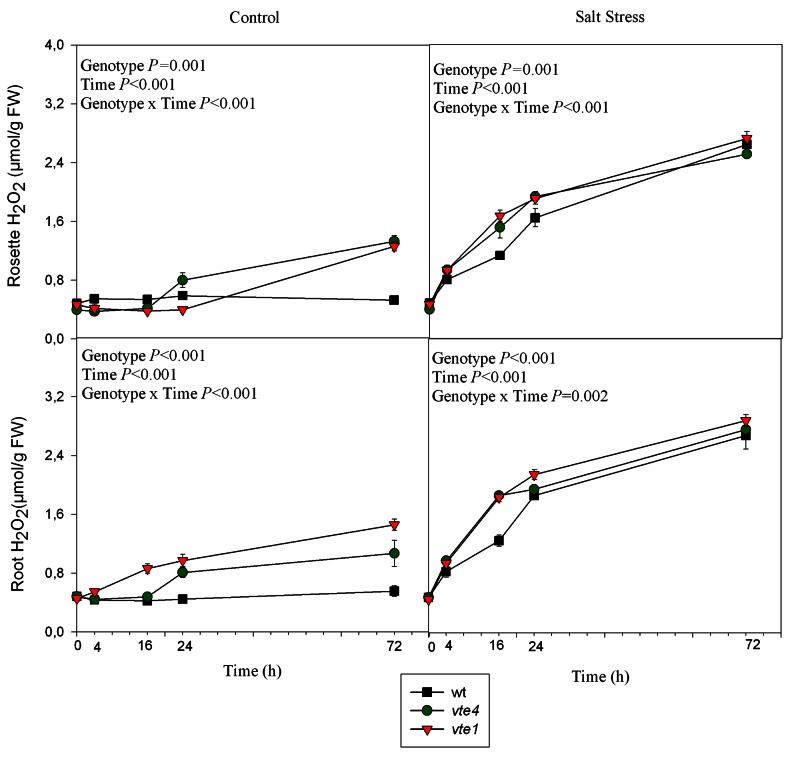
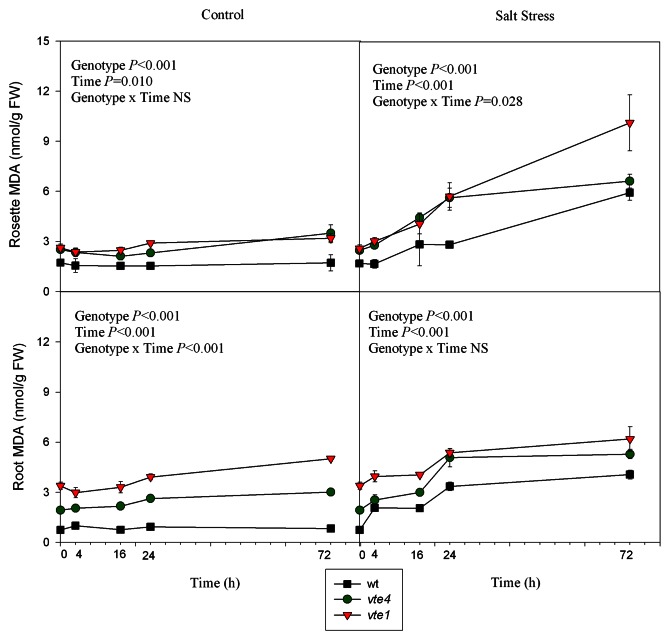
The levels of hydrogen peroxide and malondialdehyde (MDA) were analyzed in rosettes and roots under control and salt stress conditions as oxidative stress markers (Figs. 5 and 6). Under control conditions, hydrogen peroxide and MDA levels were higher in the rosettes and roots of both mutants than in wild type plants. Under salt stress, they increased in wild type and mutant plants; however, their amounts remained always higher in the mutants compared with wild type plants throughout the study. The accumulation of hydrogen peroxide, which was also monitored by DAB staining in roots and leaves, confirmed the quantitative results (Figs. S1 and S2)
Figure 5. Time course evolution of hydrogen peroxide levels in leaves and roots of wild type and vte1 and vte4 mutants of A. thaliana growing in a hydroponic system exposed to control or salt stress conditions for 72h. Data are the mean ± SE of n = 4 replicates. Results of statistics are given in the panels (ANOVA, P ≤ 0.05). NS, not significant.
Figure 6. Time course evolution of malondialdehyde (MDA) levels in leaves and roots of wild type and vte1 and vte4 mutants of A. thaliana growing in a hydroponic system exposed to control or salt stress conditions for 72h. Data are the mean ± SE of n = 4 replicates. Results of statistics are given in the panels (ANOVA, P ≤ 0.05). NS, not significant.
Na+ and K+ homeostasis
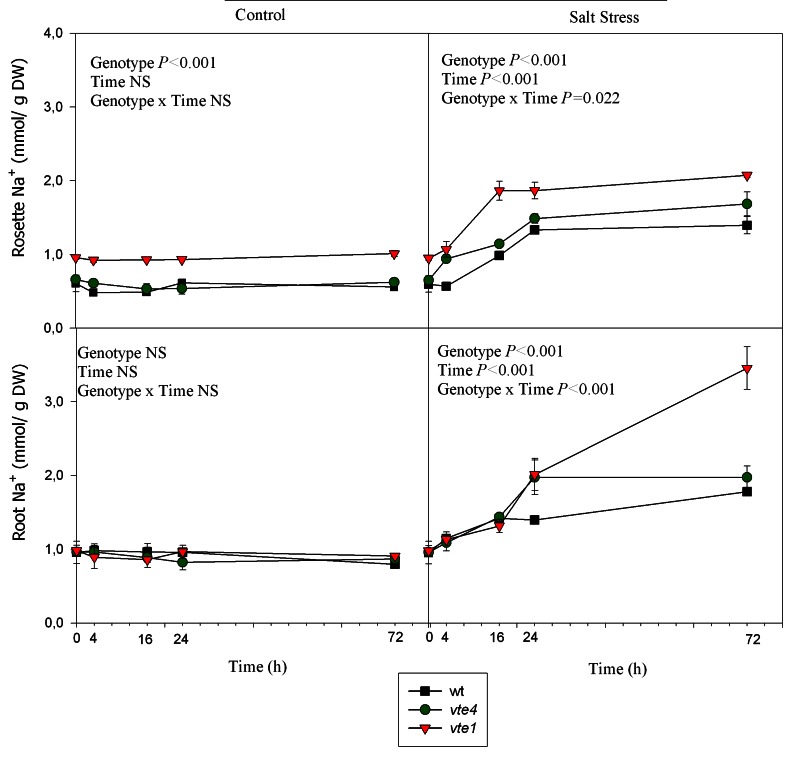
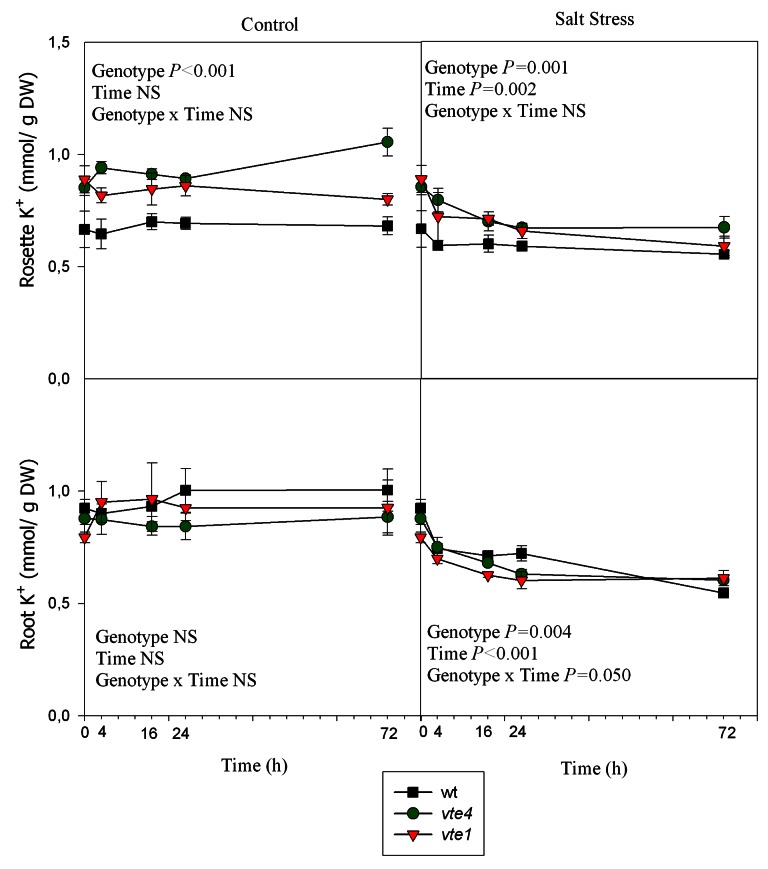
In the three genotypes, rosette Na+ concentrations increased sharply during the first 16h of salt treatment (Fig. 7). Na+ levels of vte4 and vte1 mutants remained higher than those of wild type plants throughout the study. Root Na+ concentrations increased by 2.2-fold compared with controls in wild type plants and the vte4 mutant under salt stress. The increases were even more dramatic in the vte1 mutant, which showed the highest Na+ concentration in roots (3.8-fold higher than of control plants, Fig. 8). K+ contents in rosettes and roots revealed a decrease with salt stress, which occurred at 4h of salt treatment in the three genotypes (Fig. 8).
Figure 7. Time course evolution of Na+ concentrations in roots and rosettes of wild type, vte4 and vte1 mutants of A. thaliana growing in a hydroponic system exposed to control and salt stress conditions for 72h. Data are the mean ± SE of n = 4 replicates. Results of statistics are given in the panels (ANOVA, P ≤ 0.05). NS, not significant.
Figure 8. Time course evolution of K+ concentrations in roots and rosettes of wild type, vte4 and vte1 mutants of A. thaliana growing in a hydroponic system exposed to control and salt stress conditions for 72h. Data are the mean ± SE of n = 4 replicates. Results of statistics are given in the panels (ANOVA, P ≤ 0.05). NS, not significant.
Leaf water contents, JA, ACC and ERF1 expression
To get further insight into the possible causes and cellular signaling mechanisms underlying the increased oxidative stress in leaves, the relative water content (RWC), together with levels of jasmonic acid, 1-aminocyclopropane-1-carboxylic acid and ERF1 expression were examined in rosette leaves of the three genotypes after 72h of salt treatment and controls.
The RWC was significantly lower in the vte4 mutant compared with wild type plants and the vte1 mutant both under control and salt stress conditions, but particularly under salt stress with values below 60% in the vte4 mutant (compared with around 70% in the other genotypes, Fig. 9).
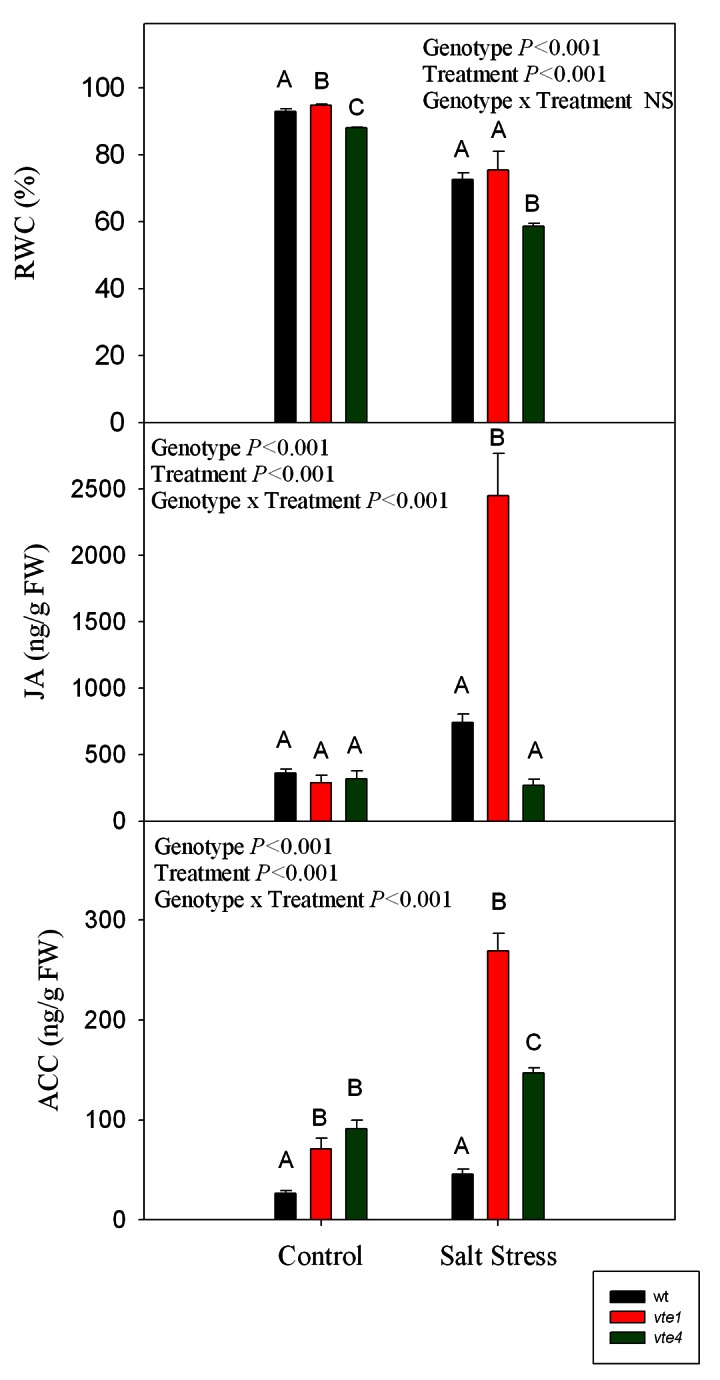
Figure 9. Levels of RWC and the stress-related phytohormones JA and the ethylene precursor ACC, in leaves of wild type and vte1 and vte4 mutants of A. thaliana growing in a hydroponic system exposed to control or salt stress conditions for 72h. Data are the mean ± SE of n = 4 replicates. Results of statistics are given in the panels (ANOVA, P ≤ 0.05). NS, not significant.
JA levels increased dramatically up to 8-fold in the vte1 mutant after 72h of salt treatment compared with controls, but remained unchanged in wild type plants and the vte4 mutant in response to salt stress (Fig. 9). ACC levels were higher in both mutants compared with the wild type under control conditions. ACC levels increased significantly in both mutants under salt stress, in contrast to salt-stressed wild type plants, which maintained the same levels than control plants. The highest JA and ACC levels under salt stress were observed in the vte1 mutant (Fig. 9).
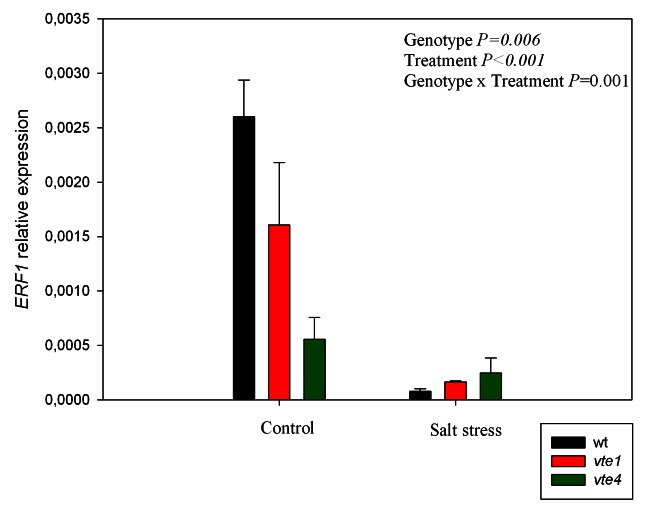
The relative expression levels of the ETHYLENE RESPONSE FACTOR1 (ERF1) gene were also analyzed in rosette leaves (Fig. 10). In the vte1 mutant, and especially in the vte4 mutant, the expression levels were lower than those of wild type plants under control conditions. Under salt stress, gene expression was significantly downregulated, particularly in wild type plants and the vte1 mutant, and attained similar levels in all genotypes (Fig. 10).
Figure 10. Relative expression levels of ERF1, an ethylene and jasmonic acid-responsive gene, in leaves of wild type and vte1 and vte4 mutants of A. thaliana growing in a hydroponic system exposed to control or salt stress conditions for 72h. Data are the mean ± SE of n = 4 replicates. Results of statistics are given in the panels (ANOVA, P ≤ 0.05). NS, not significant.
Discussion
Tocopherol deficiency in A. thaliana negatively affected growth and the physiology of vte1 and vte4 mutants both under control and salt stress conditions. Compared with the vte4 mutant and wild type plants, the vte1 mutant was the most sensitive to salt stress. This sensitivity was associated with (i) more oxidative stress in roots and rosettes, as indicated by hydrogen peroxide and MDA levels, (ii) higher Na+ accumulation in rosettes and roots, and (iii) enhanced ABA levels in rosettes. ABA increases in the vte1 mutant occurred in parallel with increases in JA and ACC levels, but were not caused by reductions in the RWC. In contrast, the reduced capacity to increase ABA levels in rosettes of the vte4 mutant resulted in leaf water loss, as indicated by the higher reductions in the RWC under salt stress in the vte4 mutant compared with the vte1 mutant and wild type plants. A downregulation of ERF1 expression was observed both in wild type plants and the vte1 mutant, but not in the vte4 mutant, which showed low ERF1 expression under control conditions. It appears therefore that while the vte1 mutant upregulates the biosynthesis of ABA, JA and ACC in response to salt stress compared with the wild type, the vte4 mutant is not as efficient in modulating ABA accumulation and suffers an increased water deficit in leaves under salt stress. However, the vte4 mutant was more efficient in counteracting the effects of oxidative stress, as indicated by lower hydrogen peroxide and MDA levels compared with the vte1 mutant both under control and salt stress conditions, thus suggesting that α- and γ-tocopherol play specific roles in (i) keeping the water and ion homeostasis and (ii) controlling hormonal and gene expression levels in plants. Results suggest that γ-tocopherol can partially compensate α-tocopherol deficiency in terms of antioxidant capacity, but at the same time its accumulation leads to dramatic alterations in water and ion homeostasis, leading to a severe water loss under salt stress, at least partially mediated by the inability to increase ABA levels in leaves.
The accumulation of more hydrogen peroxide and MDA in the vte1 and vte4 mutants compared with wild type plants indicated that more oxidative stress takes place in the vitamin E-deficient plants under salt stress. The same results were obtained in the vte1 and vte4 mutants under cadmium and copper stress8 and in outdoor conditions.17 Other studies reported that the complete absence of tocopherols did not impact on growth of A. thaliana under optimal or high light stress conditions,4,18 or even under salt or water stress in soil-grown plants.7 Consequently, it appears that growth conditions are dramatic to provide a given phenotype in these mutants. Such is the case that alterations in sugar transport in these mutants can only be observed under low temperatures.6 In our study, hydroponics caused an increased oxidative stress both in leaves and roots, leading to alterations in chlorophyll levels, PSII efficiency and ion homeostasis in vitamin E-deficient plants. Furthermore, salt stress exacerbated these vitamin E-deficient phenotypes by causing an increased demand for ROS detoxification, since it is known that salt stress increases ROS production in plants.1
The absence of both forms of tocopherol in the vte1 mutant seems to lower the ability of roots to control Na+ and K+ uptake. In the vte4 mutant, Na+ contents were lower than in the vte1 mutant. This suggests that α- and γ-tocopherol play a role in Na+/K+ homeostasis. Farouk19 reported that the application of α-tocopherol alleviated the harmful effect of salinity in wheat due to a reduction of Na+ and increased K+ contents, although the mechanism remains elusive. The antagonistic relations between Na+ and K+ may be considered in the role played by antioxidants in modifying K+/Na+ selectivity under salt stress.20 This positive effect may be due to its role in improving membrane permeability,19 which protects the membrane and membrane-bound enzymes.
The vte4 mutant had an intermediate phenotype, less severe than vte1 mutant, as indicated by growth parameters, the Fv/Fm ratio and MDA levels. This is in accordance with previous reports indicating that γ-tocopherol could take over the function of α-tocopherol under oxidative stress conditions imposed by high light, high temperature or cold.17,21 But in salt-stressed tobacco mutants with silenced γ-TMT, γ-tocopherol was not able to replace α-tocopherol, although tolerance to desiccation was improved.12 Some specific responses appear to be activated in the vte4 mutant. In the vte4 mutant, ABA levels increased rapidly in roots under salt stress and then decreased to values similar to those at the beginning of the treatment. This short-term induction of ABA accumulation may constitute a signal for tissues to increase osmolarity and induce the synthesis of ion transporters.22
It has been suggested that ERF1 acts downstream of the intersection between ethylene and JA signaling and that this transcription factor integrates both signals in plant defense.23 In our study, however, ERF1 expression did not correlate with enhanced JA levels in rosette leaves. While salt stress induced a strong upregulation of JA and ACC biosynthesis, ERF1 expression did not increase, in the salt-stressed vte1 mutant relative to controls. This suggests that additional signaling events (beyond JA and ethylene signaling) may be involved in the regulation of ERF1 expression. Indeed, ERF1 expression was strongly downregulated under salt stress conditions in the three genotypes studied in the present study, while it increased in mature plants of wild type plants and the vte1 mutant in soil-grown A. thaliana plants.7 It is possible that the age of plants and the constitutive stress that represented the hydroponic system for A. thaliana in the present study accounts for such differential response to salt stress. The salt stress imposed on hydroponically-grown wild type plants seemed to lead to a positive response in terms of ABA accumulation, but plants failed to increase ERF1 expression-related defense. Indeed, it has been shown that ABA may suppress both basal and JA-ethylene-activated transcription from defense genes.24 Therefore, it is not surprising that the vte4 mutant was the only phenotype that did not show downregulation of ERF1 expression under salt stress, since this was the only genotype in which ABA did not accumulate in rosette leaves of salt-stressed plants.
Another point to be considered is the dramatic effects observed in roots. Tocopherols did not accumulate in roots; therefore one might not expect observing increased oxidative stress in roots of vitamin E-deficient plants. It should be considered, however, that sampling was made on young plants and during a very active period of growth (as indicated by biomass increases during the 72h of study) and, on the other hand, it has been shown that VTE1 and VTE4 are expressed in meristematic tissues of roots.13,25 Therefore, it is very likely that tocopherols exert a protective function against oxidative stress during periods of growth and that their absence, as it occurs in vte1 and vte4 mutants consequently leads to increased oxidative stress in roots. Therefore, it appears that tocopherol deficiency during periods of active growth may lead to dramatic phenotypes due to its possible role in meristematic tissues, an aspect that warrants further investigations.
Material and Methods
Plant material, conditions of study and sampling
Wild type A. thaliana (ecotype Col-0), the vte4 mutant (deficient in α- but accumulating γ-tocopherol) and the vte1 mutant (deficient in both α- and γ- tocopherol) were used in the present study. Seeds were germinated on agar plates containing 0.8% agar and MES/KOH (pH 5.7). The plates were stacked vertically and maintained under a 16 h light/ 8 h darkness regime in a constant-environment chamber (90–110 μmol quanta m−2 s−1, air temperature between 21 and 23°C). Seedlings were then washed with distilled water and placed in a hydroponic system by means of the Araponics setup (www.araponics.com). Plants were grown in a full Hoagland solution with aeration and the nutrient solution was renewed every three days. Four weeks-old plants were then exposed to NaCl treatment (by adding 100 mM NaCl to the nutrient solution) and sampled at 4, 16, 24 and 72 h. Another group of plants was used as controls (without NaCl addition). Biomass, tocopherols, hydrogen peroxide, MDA and phytohormone levels were measured in both rosettes and roots. Chlorophyll levels, water contents, the maximum efficiency of PSII photochemistry (Fv/Fm) and gene expression were measured in rosette leaves only. For biochemical analyses, samples were collected, immediately frozen in liquid nitrogen and stored at -80°C until analysis. The experimental setup was fully replicated giving the same results.
Plant biomass and leaf water status
The fresh weight (FW) of rosettes and roots were measured at each time of salt treatment to evaluate leaf and root growth. Leaves were weighed and then, leaves were re-hydrated for 24 h at 4°C in darkness and subsequently oven-dried for 72 h at 60°C. The relative leaf water content (RWC) was determined as 100 × (FW-DW)/ (TW-DW), where FW is the fresh weight, TW is the turgid weight after re-hydrating the leaves at 4°C in darkness, and DW is the dry weight after oven-drying the leaves at 60°C to constant weight.
Chlorophyll levels and chlorophyll fluorescence
The chlorophyll levels were determined spectrophotometrically. Leaves were ground in liquid nitrogen and extracted with methanol (100%) using ultrasonication (Vibra-Cell Ultrasonic Processor). The resulting extracts were measured and the specific absorption coefficients were used.26
The maximum efficiency of PSII photochemistry (Fv/Fm) were measured by using a portable fluorimeter mini-PAM (Walz, Effeltrich, Germany) after 1 h of dark adaption, as described previously.27
Determination of Na+ and K+ concentrations
Na+ and K+ concentrations were determined from desiccated leaf and root tissues with 0.5% (v/v) nitric acid and were assayed by flame emission photometry (Ciba, Corning, UK).
DAB staining
Hydrogen peroxide accumulation was measured with 3,3′-diaminobenzidine tetrahydrochloride (DAB) according to the method of Thordal-Christensen.28 Leaves and roots were infiltrated under vacuum with DAB-HCl, pH 3.8 and the pH was adjusted to 7.5 after solubilization. Samples were incubated at room temperature at 8h and maintained in 80% (v/v) ethanol solution for 3 d and embedded in 10% (v/v) glycerol.
Quantitative measurement of hydrogen peroxide
0.5 g of leaves and roots were ground with 3 ml acetone for 30 min at 4°C and the sample was filtered through eight layers of gauze cloth. After the addition of 0.15 g of active carbon, the sample was centrifuged twice at 3.000 g for 20 min at 4°C. 0.2 ml 20% TiCl4 in HCl and 0.2 ml ammonia were next added to 1 ml of the supernatant. The post-reacted compound was centrifuged at 3.000 g for 10 min; the supernatant was discarded and the pellet was dissolved in 3 ml 1 M H2SO4 and the absorbance value was determined at 410 nm.16 The standard curve was made using hydrogen peroxide, and contents calculated from the absorbance at 410 nm compared with the standard curve.
Estimation of lipid peroxidation
The extent of lipid peroxidation was estimated by determining the amount of MDA as described by Ellouzi et al.16
Tocopherol analysis
Tocopherol levels were determined by HPLC as described by Cela et al.7
Phytohormone analyses
Levels of ABA, JA and ACC were determined by UPLC-MS/MS, as described by Müller and Munné-Bosch.29
Gene expression analyses
RNA from the three genotypes was isolated from leaf material using RNeasy Plant Mini Kit (Qiagen), incubated with DNase to eliminate the genomic DNA, following the kit instructions. The cDNA was synthesized using Superscript II Reverse Transcriptase (Invitrogen) and amplified using LightCycler 480 SYBR Green 1 Master (Roche). The primers for the target gene (ERF1, AT3G23240.1) (Forward: TCCTTCAACGAGAACGACTC; Reverse: ACGGATTTGATCGGAAGGTC) and for the reference gene (actin2, AT3G18780) (Forward: TTGAGACCTTTAACTCCCGCTAT; Reverse: CCACTGGCGTACAAGGAGAGA) were purchased from Operon. The conditions were: 45 cycles of: 95°C (10 sec); 63°C (20 sec); 72°C (20 sec). For quantification, the ΔCT method was used with the equation: ratio = 2CT (reference gene)-CT (target gene), where CT is the cycle number at which enough amplified product accumulates to yield a detectable fluorescent signal, the reference gene was actin and the target gene was ERF1.
Statistical analyses
Statistical differences between measurements on different times and genotypes were analyzed following the analysis of variance ANOVA using SPSS (Chicago, IL., USA). Differences were considered significant at a probability level of p ≤ 0.05.
Conclusions
In summary, the results of this study showed an increased sensitivity to salt stress in vitamin E-deficient mutants of A. thaliana, indicating the positive role of tocopherols in stress tolerance. Tocopherols in wild type plants not only served to minimize oxidative stress, but also resulted in an improved Na+/K+ homeostasis and hormonal balance under stress. It appears therefore that tocopherols play a key role in salt stress tolerance not only by reducing the extent of oxidative stress, but also by improving ion homeostasis and the hormonal balance of leaves. Further research is however needed to clarify the signaling cascades connecting tocopherol levels in plastids with ion homeostasis, hormonal responses and gene expression in the nucleus, and particularly the protective role of tocopherols in meristematic tissues, all they aspects that warrant further investigations.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are very grateful to the Serveis Científico-Tècnics and Serveis dels Camps Experimentals (University of Barcelona) for technical assistance. We are indebted to Kathleen Brückner (University of Kiel) for providing the seeds. Support for the research was received through the ICREA Academia prize awarded to S.M.-B., funded by the Generalitat de Catalunya, and the BFU2012-32057 grant funded by the Spanish Government. This work was also supported by Tunisian Ministry of Higher Education and Scientific Research (LR02CB02).
Glossary
Abbreviations:
- ABA
abscisic acid
- ACC, 1-aminocyclopropane-1-carboxylic acid, JA, jasmonic acid, MDA
malondialdehyde
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/23136
References
- 1.Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010;33:453–67. doi: 10.1111/j.1365-3040.2009.02041.x. [DOI] [PubMed] [Google Scholar]
- 2.Szarka A, Tomasskovics B, Bánhegyi G. The ascorbate-glutathione-α-tocopherol triad in abiotic stress response. Int J Mol Sci. 2012;13:4458–83. doi: 10.3390/ijms13044458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munné-Bosch S. The role of α-tocopherol in plant stress tolerance. J Plant Physiol. 2005;162:743–8. doi: 10.1016/j.jplph.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 4.Havaux M, Eymery F, Porfirova S, Rey P, Dörmann P. Vitamin E protects against photoinhibition and photooxidative stress in Arabidopsis thaliana. Plant Cell. 2005;17:3451–69. doi: 10.1105/tpc.105.037036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maeda H, Sage TL, Isaac G, Welti R, DellaPenna D. Tocopherols modulate extraplastidic polyunsaturated fatty acid metabolism in Arabidopsis at low temperature. Plant Cell. 2008;20:452–70. doi: 10.1105/tpc.107.054718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maeda H, Song W, Sage TL, DellaPenna D. Tocopherols play a crucial role in low-temperature adaptation and Phloem loading in Arabidopsis. Plant Cell. 2006;18:2710–32. doi: 10.1105/tpc.105.039404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cela J, Chang C, Munné-Bosch S. Accumulation of γ- rather than α-tocopherol alters ethylene signaling gene expression in the vte4 mutant of Arabidopsis thaliana. Plant Cell Physiol. 2011;52:1389–400. doi: 10.1093/pcp/pcr085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collin VC, Eymery F, Genty B, Rey P, Havaux M. Vitamin E is essential for the tolerance of Arabidopsis thaliana to metal-induced oxidative stress. Plant Cell Environ. 2008;31:244–57. doi: 10.1111/j.1365-3040.2007.01755.x. [DOI] [PubMed] [Google Scholar]
- 9.Sattler SE, Gilliland LU, Magallanes-Lundback M, Pollard M, DellaPenna D. Vitamin E is essential for seed longevity and for preventing lipid peroxidation during germination. Plant Cell. 2004;16:1419–32. doi: 10.1105/tpc.021360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sattler SE, Mène-Saffrané L, Farmer EE, Krischke M, Mueller MJ, DellaPenna D. Nonenzymatic lipid peroxidation reprograms gene expression and activates defense markers in Arabidopsis tocopherol-deficient mutants. Plant Cell. 2006;18:3706–20. doi: 10.1105/tpc.106.044065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munné-Bosch S, Weiler EW, Alegre L, Müller M, Düchting P, Falk J. α-Tocopherol may influence cellular signaling by modulating jasmonic acid levels in plants. Planta. 2007;225:1039–49. doi: 10.1007/s00425-006-0375-0. [DOI] [PubMed] [Google Scholar]
- 12.Abbasi AR, Hajirezaei M, Hofius D, Sonnewald U, Voll LM. Specific roles of α- and γ-tocopherol in abiotic stress responses of transgenic tobacco. Plant Physiol. 2007;143:1720–38. doi: 10.1104/pp.106.094771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ouyang S, He S, Liu P, Zhang W, Zhang J, Chen S. The role of tocopherol cyclase in salt stress tolerance of rice (Oryza sativa) Sci China Life Sci. 2011;54:181–8. doi: 10.1007/s11427-011-4138-1. [DOI] [PubMed] [Google Scholar]
- 14.Yusuf MA, Kumar D, Rajwanshi R, Strasser RJ, Tsimilli-Michael M, Govindjee, Sarin NB et al. Overexpression of γ-tocopherol methyl transferase gene in transgenic Brassica juncea plants alleviates abiotic stress: physiological and chlorophyll a fluorescence measurements. Bioch. Biophys. Acta 2010; 1797:1428–1438. [DOI] [PubMed]
- 15.Sklodowska M, Gapinska M, Gajewska E, Gabara B. Tocopherol content and enzymatic antioxidant activities in chloroplasts from NaCl-stressed tomato plants. Acta Physiol Plant. 2009;31:393–400. doi: 10.1007/s11738-008-0248-1. [DOI] [Google Scholar]
- 16.Ellouzi H, Hamed KB, Cela J, Munné-Bosch S, Abdelly C. Early effects of salt stress on the physiological and oxidative status of Cakile maritima (halophyte) and Arabidopsis thaliana (glycophyte) Physiol Plant. 2011;142:128–43. doi: 10.1111/j.1399-3054.2011.01450.x. [DOI] [PubMed] [Google Scholar]
- 17.Semchuk NM, Lushchak OV, Falk J, Krupinska K, Lushchak VI. Inactivation of genes, encoding tocopherol biosynthetic pathway enzymes, results in oxidative stress in outdoor grown Arabidopsis thaliana. Plant Physiol Biochem. 2009;47:384–90. doi: 10.1016/j.plaphy.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Porfirova S, Bergmüller E, Tropf S, Lemke R, Dörmann P. Isolation of an Arabidopsis mutant lacking vitamin E and identification of a cyclase essential for all tocopherol biosynthesis. Proc Natl Acad Sci U S A. 2002;99:12495–500. doi: 10.1073/pnas.182330899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farouk S. Ascorbic acid and α-tocopherol minimize salt-induced wheat leaf senescence. J Stress Physiol Biochem. 2011;7:58–79. [Google Scholar]
- 20.Azooz MM. Proteins, sugars and ion leakage as a selection criterion for the salt tolerance of three sorghum cultivars at seedling stage grow under NaCl and nicotinamide. Int J Agric Biol. 2004;6:27–35. [Google Scholar]
- 21.Bergmüller E, Porfirova S, Dörmann P. Characterization of an Arabidopsis mutant deficient in gamma-tocopherol methyltransferase. Plant Mol Biol. 2003;52:1181–90. doi: 10.1023/B:PLAN.0000004307.62398.91. [DOI] [PubMed] [Google Scholar]
- 22.Orcutt DM, Nilsen ET. Physiology of plant under stress 2000; John Wiley Sons, New York. [Google Scholar]
- 23.Lorenzo O, Piqueras R, Sánchez-Serrano JJ, Solano R. ETHYLENE RESPONSE FACTOR1 integrates signals from ethylene and jasmonate pathways in plant defense. Plant Cell. 2003;15:165–78. doi: 10.1105/tpc.007468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson JP, Badruzsaufari E, Schenk PM, Manners JM, Desmond OJ, Ehlert C, et al. Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell. 2004;16:3460–79. doi: 10.1105/tpc.104.025833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brady SM, Orlando DA, Lee J-Y, Wang JY, Koch J, Dinneny JR, et al. A high-resolution root spatiotemporal map reveals dominant expression patterns. Science. 2007;318:801–6. doi: 10.1126/science.1146265. [DOI] [PubMed] [Google Scholar]
- 26.Lichtenthaler HK, Wellburn AR. Determinations of total carotenoids and chlorophylls a and b of leafextracts in differentsolvents. Biochem Soc Trans. 1983;11:591–2. [Google Scholar]
- 27.Genty B, Briantais J, Baker NR. The relationship between quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta. 1989;990:87–92. doi: 10.1016/S0304-4165(89)80016-9. [DOI] [Google Scholar]
- 28.Thordal-Christensen H, Zhang ZG, Wei YD, Collinge DB. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997;11:1187–94. doi: 10.1046/j.1365-313X.1997.11061187.x. [DOI] [Google Scholar]
- 29.Müller M, Munné-Bosch S. Rapid and sensitive hormonal profiling of complex plant samples by liquid chromatography coupled to electrospray ionization tandem mass spectrometry. Plant Methods. 2011;7:37. doi: 10.1186/1746-4811-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.