Abstract
Stigmasterol and sitosterol, important sterols present in plants, are known to influence permeability and fluidity characteristics of the plasma membrane and other organellar membranes. We had previously demonstrated that the Arabidopsis Atcyp710A1 gene, which catalyzes conversion of sitosterol into stigmasterol, plays a role in plasma membrane permeability, thus influencing leakage of cellular nutrients and ions into apoplast. In this study, we investigated the role of this gene in imparting various abiotic stress tolerances in Arabidopsis. By analyzing Atcyp710a1 mutant and AtCYP710A1 overexpressor lines, we found that the AtCYP710A1 gene plays a role in imparting low and high temperature tolerance.
Keywords: membrane stability, thermotolerance, cold tolerance, phytosterol
Introduction
The plasma membrane of plant cells is known to be affected by abiotic stresses; therefore, maintenance of membrane integrity is important to confer abiotic stress tolerance in plants.1-4 Membrane stability positively influences various physiological traits such as water use efficiency5 and osmotic potential6 that condition plants to abiotic stresses. These traits can also influence photosynthesis and other metabolic activities that can affect yield.7 Even though water use efficiency and osmotic potential are extensively studied, plants have not been genetically engineered with altered membrane stability to confer stress tolerance. We have previously shown that Atcyp710a1 Arabidopsis mutant plants showed higher membrane leakage and were susceptible to bacterial pathogens.8 Sitosterol and stigmasterol are two major sterols present in Arabidopsis.9,10 Higher levels of stigmasterol incorporation into the plasma membrane can alter the fluidity and permeability characteristics and restrict leakage of solutes from cytoplasm into the apoplastic region.8,11 Further, sitosterol to stigmasterol ratio in the membrane has been known to influence the response of a cell to various biotic and abiotic stresses.11 In this study, we show that the AtCYP710A1 gene (At2g34500) encoding a C22-sterol desaturase that catalyzes the reaction converting sitosterol into stigmasterol in Arabidopsis9 plays a role in imparting high and low temperature stress tolerance.
Results and Discussion
Perturbation in the cell membrane and subsequent ion leakage are common effects caused by various abiotic stresses, especially temperature fluctuations.12 Therefore, cell membrane stability assays are commonly used to assess the tolerance of plants to various abiotic stresses.13,14 Stability of plasma membrane and other organellar membranes during abiotic stress contributes to the maintenance of cellular metabolic activity.15 In addition, stability of chlorophyll during stress allows cells to maintain a functional chloroplast so that photosynthesis can be revived after the plant’s recovery from stress.16 Hence, estimation of total chlorophyll content can reflect the tolerance of plants to several abiotic stresses. Apart from membrane and chlorophyll stability, which are individual factors representing a portion of tolerance mechanisms, the ability of a plant to survive the stress and accumulate more biomass demonstrates overall plant tolerance. In this study, we used three assays (membrane leakage, chlorophyll reduction and plant survival/biomass) to assess the stress tolerance of Atcyp710a1 mutant and AtCYP710A1 gene overexpressor plants under various abiotic stresses such as high temperature, low temperature, drought, salinity, oxidative and flooding.
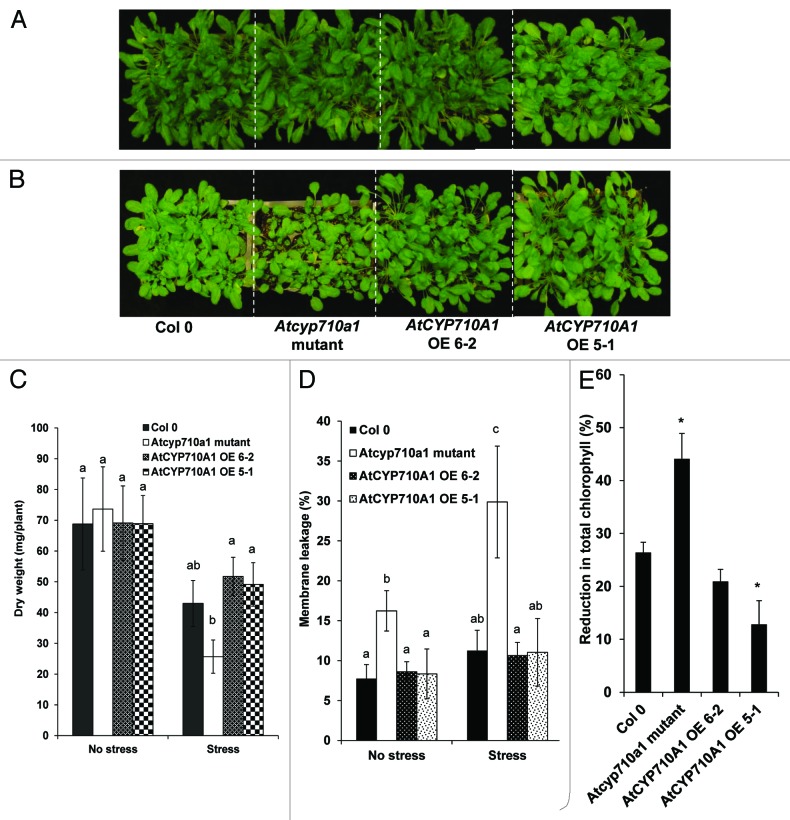
Low temperature stress tolerance
In order to study the relevance of the AtCYP710A1 gene in low temperature stress tolerance, Atcyp710a1 mutant and AtCYP710A1 gene overexpressor Arabidopsis plants were grown at 20° to 22°C under short day conditions as described previously,8 and the stress was imposed by following protocols described earlier.17 The extent of growth reduction under low temperature stress was studied by measuring the dry weight of the plants at the end of the stress period. Under non-stress conditions, both mutant and overexpressor plants had dry weight equal to wild-type plants. However, mutant plants showed a significant reduction in biomass compared with wild-type plants at the end of the stress period. Biomass of overexpressors was similar to wild-type plants (Fig. 1A–C). Further, we assessed the membrane leakage in mutant and overexpressor plants using the previously described protocol.18 Atcyp710a1 mutant plants showed higher membrane leakage under stress (Fig. 1D). Similarly, the extent of chlorophyll reduction was estimated by following the method described earlier19 and the mutant plants showed a significantly higher reduction compared with wild-type plants (Fig. 1E). These results indicate that the AtCYP710A1 gene may have a role in low temperature stress tolerance by imparting membrane and chlorophyll stability. In a separate experiment, the mutant and overexpressor plants were exposed to freezing stress by following the protocol described earlier.17 Briefly, 5-week-old Arabidopsis plants were exposed to low temperature stress under normal light by gradually decreasing the temperature of the growth chamber from 15°C to -1°C over a period of 18h. Later the plants were maintained either at -1°C or -10°C for 12 h under dark, and then the temperature was increased gradually to 21°C under light. Plants were allowed to recover for three days. Both phenotypic observations and plant survival percentages indicated that the susceptibility of both mutants and overexpressors were similar to wild-type plants.
Figure 1. Effect of low temperature stress on Arabidopsis Atcyp710a1 mutant and AtCYP710A1 overexpressors. Arabidopsis wild-type (Col-0), Atcyp710a1 mutant and AtCYP710A1 (OE 6–2 and OE 5–1) overexpressor plants (5 weeks old) were exposed to gradual acclimation stress by reducing the temperature of the growth chamber from 20°C to 7°C over a period of two days and then subjected to low temperature stress (7 ± 1°C) for 30 d. At the end of the stress period, photographs of control plants grown under normal growth conditions (A) and low-temperature-stressed plants (B) were taken. Shoot biomass gained over a period of 33 d of stress was measured (C). Further, membrane leakage (D) and total chlorophyll content at the end of the stress were estimated and expressed as a reduction over no stress controls (E). ANOVA was performed (p = 0.05), and letters above each bar indicate the significance and bars with the same letters are not significantly different. Error bars indicate standard error values for three replicate values. For (E), asterisks over the bars represent statistical significance (student t-test) over corresponding Col-0 values.
High temperature stress tolerance
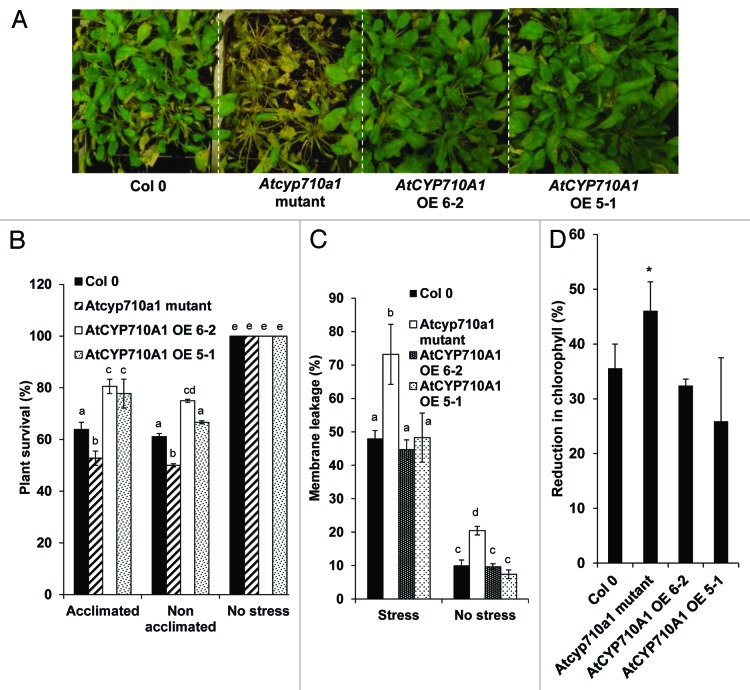
The role of phytosterols under temperature stress has been known for some time.20,21 In order to understand whether the AtCYP710A1 gene plays any role in high temperature stress tolerance, we studied the response of Atcyp710a1 mutant and AtCYP710A1 gene-overexpressing plants under high temperature (45°C for either 3 h or 6 h) stress with or without prior acclimation. Acclimation-based temperature stress imposition was performed according to previous literature.14 The survival percentage of the Atcyp710a1 mutant was lower than the wild-type during both acclimated and non-acclimated high temperature stress. AtCYP710A1 overexpressor plants, either acclimated with mild temperature stress or non-acclimated, showed a significant increase in survival percentage after exposure to severe temperatures of 45°C for 6 h (Fig. 2A and B). Interestingly, the mutant and wild-type plants did not show a difference in survival rates between temperature acclimation and non-acclimation treatments while the overexpressor plants showed a slightly higher survival rate due to temperature acclimation prior to imposition of high temperature stress. Temperature-stress-acclimated plants are known to activate a wide range of stress-responsive pathways, and this can contribute to tolerance upon exposure to severe stress.22 Moreover, this result demonstrates that the high temperature tolerance of mutant and transgenic plants can be best assessed after exposure to acclimation stress. Consistently, earlier studies have shown that acclimation-based high temperature stress imposition was useful to best study the relevance of candidate genes that impart thermotolerance.22,23 Further, the survival frequency was significantly greater in AtCYP710A1 gene overexpressor plants when compared with wild-type and mutant plants. In order to understand the relevance of this gene in maintaining plasma membrane stability under high temperature stress, we assessed the extent of membrane leakage. Atcyp710a1 mutant plants showed up to 70% leakage, whereas wild-type plants showed only 50% under stress (Fig. 2C). However, the overexpressors showed similar leakage to that of wild-type plants under stress. Chlorophyll biosynthesis is affected by high temperature stress,24 and thermotolerant plants are known to retain chlorophyll under stress.25 We assessed the extent of total chlorophyll reduction in Atcyp710a1 mutant and AtCYP710A1 overexpressor plants. The mutant plants showed greater reduction compared with wild-type plants. However, the overexpressor plants did not show any improvement in chlorophyll retention compared with wild-type plants (Fig. 2D). Taken together, these data indicate that the AtCYP710A1 gene has a minor role in imparting thermotolerance by maintaining membrane stability.
Figure 2. Thermotolerance of Arabidopsis Atcyp710a1 mutants and AtCYP710A1 overexpressor plants. Five-week-old Arabidopsis wild-type (Col-0), Atcyp710a1 mutant and AtCYP710A1 (OE 6–2 and OE 5–1) overexpressor plants were exposed to the acclimation temperature and then to severe temperature. Acclimation was done by gradually increasing the temperature from 21°C to 35°C over a period of 8hand then plants were exposed to 40°C for 24 h. After this acclimation treatment, plants were immediately exposed to severe temperatures of 45°C for either 3 h or 6 h. Plants exposed to severe temperature of 45°C for 6 h were allowed to recover under 21°C for five days and photographs of these plants were taken at the end of the recovery period (A) and plant survival was calculated (B). Plants exposed to 45°C for 3h were allowed to recover for 10h under normal growth conditions. Membrane leakage (C) and reduction in total chlorophyll content (D) were measured. ANOVA was performed (p = 0.05), and letters above each bar indicate the significance and bars with the same letters are not significantly different. Error bars indicate standard error values for three replicates. For (D), asterisk over the bar represents statistical significance (student t-test) over the corresponding Col-0 values.
Response of Atcyp710a1 mutant and AtCYP710A1 overexpressor plants to other abiotic stresses
We assessed whether phytosterols play a role in other abiotic stresses mentioned below by exposing the Atcyp710a1 mutant and AtCYP710A1 overexpressor plants to various stresses.
Drought stress
Plants were exposed to gradual drought stress, and the stress responses were observed both at the end of the stress period and also upon recovery of the plants after re-watering. The stress imposition protocols followed here are described in earlier literature.26,27 Briefly, 5-week-old Arabidopsis plants were subjected to mild drought stress (acclimation) by gradually withholding irrigation over a period of two weeks and then exposed to either moderate stress (40% field capacity) or severe stress (25% field capacity) for one week. Results showed that both mutant and overexpressor plants showed phenotypic symptoms similar to that of wild-type plants under drought stress (data not shown). Later, the severely stressed plants were watered (to 100% field capacity) and the recovery phenotype was photographed after five days. The stress recovery phenotype was also similar to wild-type plants (data not shown).
Salinity stress
The response of mutant and overexpressor plants under salinity stress was studied by following salinity stress imposition protocols described previously.27 Briefly, 6-week-old Arabidopsis plants were irrigated with 500 mM NaCl solution for one week. Phenotypic observations were taken seven days after salinity treatment. Results from this experiment indicated that both mutant and overexpressor plants showed similar levels of growth, leaf injury and anthocyanin pigmentation to that of wild-type plants (data not shown).
Oxidative stress
In order to study the oxidative stress response of mutant and overexpressor plants, they were sprayed with 5 µM methyl viologen dichloride solution and exposed to high light intensity for 24 h. Methyl viologen is known to induce oxidative stress in plant cells by blocking the photosynthetic electron transport chain.28 Photo bleaching and cell death phenotype in the mutant and overexpressor leaves were similar to that of wild-type at the end of the stress period (data not shown).
Dessication and flooding stress
Desiccation stress tolerance of these plants was assessed by detached leaf water loss assay,17 and we did not find any difference in water loss among the mutants and overexpressors compared with wild-type. In addition, these plants were also exposed to flooding stress by submerging them in water as described earlier,29 and the extent of recovery after flooding was monitored. Our results showed no difference in recovery growth between mutant/overexpressor plants and the wild-type (data not shown).
In conclusion, our data demonstrated that the AtCYP710A1 gene is important for imparting low and high temperature stress tolerance in Arabidopsis, but not for the other abiotic stresses studied. However, the precise mechanism for the involvement of sitosterol and stigmasterol in regulating membrane permeability characteristics and membrane function are yet to be understood. We speculate that changes in stigmasterol content may either be directly involved in altering membrane permeability characteristics or alter content and composition of other membrane lipids. Another possibility is that stigmasterol and sitosterol may play a role in membrane-associated metabolic processes and signaling pathways influencing the transcript expression of stress-responsive genes. More in-depth studies are needed for a complete understanding of membrane incorporation of these two sterols and its influence on membrane characteristics, including plasma membrane and organellar membranes under various biotic and abiotic stresses.
Acknowledgments
This work was supported by The Samuel Roberts Noble Foundation and Oklahoma Center for the Advancement of Science and Technology (Grant No. PSB09–020). We thank Jackie Kelley for editing the manuscript and Ms. Colleen Elles for plant care.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/23142
References
- 1.Wardlaw IF. Responses of Plants to Environmental Stresses. J. Levitt. Academic Press, New York, 1972. xiv, 698 pp. Physiological Ecology. Science. 1972;177:786. doi: 10.1126/science.177.4051.786. [DOI] [Google Scholar]
- 2.Dias AS, Barreiro MG, Campos PS, Ramalho JC, Lidon FC. Wheat Cellular Membrane Thermotolerance Under Heat Stress. J Agron Crop Sci. 2010;196:100–8. doi: 10.1111/j.1439-037X.2009.00398.x. [DOI] [Google Scholar]
- 3.Matos MC, Campos PS, Passarinho JA, Semedo JN, Marques NM, Ramalho JC, et al. Drought effect on photosynthetic activity, osmolyte accumulation and membrane integrity of two Cicer arietinum genotypes. Photosynthetica. 2010;48:303–12. doi: 10.1007/s11099-010-0038-z. [DOI] [Google Scholar]
- 4.Blum A, Ebercon A. Cell Membrane Stability As A Measure Of Drought And Heat Tolerance In Wheat. Crop Sci. 1981;21:43–7. doi: 10.2135/cropsci1981.0011183X002100010013x. [DOI] [Google Scholar]
- 5.Pimentel C, Zuily-Fodil Y, Laffray D, Pereyra Rossiello RO, Zuily-Fodil Y, Laffray D, Costa França MG. Pham Thi AT. Pereyra Rossiello RO Differences in growth and water relations among Phaseolus vulgaris cultivars in response to induced drought stress. Environ Exp Bot. 2000;43:227–37. doi: 10.1016/S0098-8472(99)00060-X. [DOI] [PubMed] [Google Scholar]
- 6.Singh M, Srivastava JP, Kumar A. Cell Membrane Stability in Relation to Drought Tolerance in Wheat Genotypes. J Agron Crop Sci. 1992;168:186–90. doi: 10.1111/j.1439-037X.1992.tb00997.x. [DOI] [Google Scholar]
- 7.Azizi-e-Chakherchaman S, Mostafaei H, Yari A, Hassanzadeh M, Jamaati-e-Somarin S, Easazadeh R. Study of relationships of leaf Relative Water Content, Cell Membrane Stability and duration of growth period with grain yield of lentil under rain-fed and irrigated conditions. Res J Biol Sci. 2009;4:842–7. [Google Scholar]
- 8.Wang K, Senthil-Kumar M, Ryu C-M, Kang L, Mysore KS. Phytosterols play a key role in plant innate immunity against bacterial pathogens by regulating nutrient efflux into the apoplast. Plant Physiol. 2012;158:1789–802. doi: 10.1104/pp.111.189217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morikawa T, Mizutani M, Aoki N, Watanabe B, Saga H, Saito S, et al. Cytochrome P450 CYP710A encodes the sterol C-22 desaturase in Arabidopsis and tomato. Plant Cell. 2006;18:1008–22. doi: 10.1105/tpc.105.036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnqvist L, Persson M, Jonsson L, Dutta PC, Sitbon F. Overexpression of CYP710A1 and CYP710A4 in transgenic Arabidopsis plants increases the level of stigmasterol at the expense of sitosterol. Planta. 2008;227:309–17. doi: 10.1007/s00425-007-0618-8. [DOI] [PubMed] [Google Scholar]
- 11.Hartmann M-A. Plant sterols and the membrane environment. Trends Plant Sci. 1998;3:170–5. doi: 10.1016/S1360-1385(98)01233-3. [DOI] [Google Scholar]
- 12.Larkindale J, Knight MR. Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol. 2002;128:682–95. doi: 10.1104/pp.010320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farooq S, Azam F. The use of cell membrane stability (CMS) technique to screen for salt tolerant wheat varieties. J Plant Physiol. 2006;163:629–37. doi: 10.1016/j.jplph.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Senthil-Kumar M, Srikanthbabu V, Mohan Raju B, Ganeshkumar, Shivaprakash N, Udayakumar M. Screening of inbred lines to develop a thermotolerant sunflower hybrid using the temperature induction response (TIR) technique: a novel approach by exploiting residual variability. J Exp Bot. 2003;54:2569–78. doi: 10.1093/jxb/erg278. [DOI] [PubMed] [Google Scholar]
- 15.Patil RV, Khanna-Chopra R. Breeding for drought resistance in crops: physiological approaches. J Plant Biol. 2006;33:29–49. [Google Scholar]
- 16.Rosyara U, Subedi S, Duveiller E, Sharma R. The effect of spot blotch and heat stress on variation of canopy temperature depression, chlorophyll fluorescence and chlorophyll content of hexaploid wheat genotypes. Euphytica. 2010;174:377–90. doi: 10.1007/s10681-010-0136-9. [DOI] [Google Scholar]
- 17.Verslues PE, Agarwal M, Katiyar-Agarwal S, Zhu J, Zhu J-K. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. 2006;45:523–39. doi: 10.1111/j.1365-313X.2005.02593.x. [DOI] [PubMed] [Google Scholar]
- 18.Tripathy JN, Zhang J, Robin S, Nguyen TT, Nguyen HT. QTLs for cell-membrane stability mapped in rice (Oryza sativa L.) under drought stress. Theor Appl Genet. 2000;100:1197–202. doi: 10.1007/s001220051424. [DOI] [Google Scholar]
- 19.Hiscox JD, Israelstam GF. A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot. 1979;57:1332–4. doi: 10.1139/b79-163. [DOI] [Google Scholar]
- 20.Dufourc EJ. The role of phytosterols in plant adaptation to temperature. Plant Signal Behav. 2008;3:133–4. doi: 10.4161/psb.3.2.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beck JG, Mathieu D, Loudet C, Buchoux S, Dufourc EJ. Plant sterols in “rafts”: a better way to regulate membrane thermal shocks. FASEB J. 2007;21:1714–23. doi: 10.1096/fj.06-7809com. [DOI] [PubMed] [Google Scholar]
- 22.Senthil-Kumar M, Kumar G, Srikanthbabu V, Udayakumar M. Assessment of variability in acquired thermotolerance: potential option to study genotypic response and the relevance of stress genes. J Plant Physiol. 2007;164:111–25. doi: 10.1016/j.jplph.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Hema R, Senthil-Kumar M, Shivakumar S, Chandrasekhara Reddy P, Udayakumar M. Chlamydomonas reinhardtii, a model system for functional validation of abiotic stress responsive genes. Planta. 2007;226:655–70. doi: 10.1007/s00425-007-0514-2. [DOI] [PubMed] [Google Scholar]
- 24.Tewari AK, Tripathy BC. Temperature-stress-induced impairment of chlorophyll biosynthetic reactions in cucumber and wheat. Plant Physiology (Rockville) 1998;117:851–8. doi: 10.1104/pp.117.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Havaux M, Tardy F. Loss of chlorophyll with limited reduction of photosynthesis as an adaptive response of Syrian barley landraces to high-light and heat stress. Funct Plant Biol. 1999;26:569–78. [Google Scholar]
- 26.Senthil-Kumar M, Govind G, Kang L, Mysore KS, Udayakumar M. Functional characterization of Nicotiana benthamiana homologs of peanut water deficit-induced genes by virus-induced gene silencing. Planta. 2007;225:523–39. doi: 10.1007/s00425-006-0367-0. [DOI] [PubMed] [Google Scholar]
- 27.Senthil-Kumar M, Hema R, Suryachandra TR, Ramegowda HV, Gopalakrishna R, Rama N, et al. Functional characterization of three water deficit stress-induced genes in tobacco and Arabidopsis: an approach based on gene down regulation. Plant Physiol Biochem. 2010;48:35–44. doi: 10.1016/j.plaphy.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Lehoczki E, Laskay G, Gaal I, Szigeti Z. Mode of action of paraquat in leaves of paraquat-resistant Conyza canadensis (L.) Cronq. Plant Cell Environ. 1992;15:531–9. doi: 10.1111/j.1365-3040.1992.tb01486.x. [DOI] [Google Scholar]
- 29.Huynh N, Vantoai T, Streeter J, Banowetz G. Regulation of flooding tolerance of SAG12:ipt Arabidopsis plants by cytokinin. J Exp Bot. 2005;56:1397–407. doi: 10.1093/jxb/eri141. [DOI] [PubMed] [Google Scholar]