Abstract
(1,3; 1,4)-β-D-glucan, also known as mixed linkage glucan (MLG), is a polysaccharide that in flowering plants is unique to the cell walls of grasses and other related members of Poales. MLG is highly abundant in endosperm cell walls, where it is considered a storage carbohydrate. In vegetative tissues, MLG transiently accumulates in the primary cell walls of young, elongating organs. In evolutionary distant species such as Equisetum, MLG accumulates predominantly in old tissues in the stems. Similarly, we have recently shown that rice accumulates a large amount of MLG in mature stems, which prompted us to re-evaluate the hypothesis that MLG is solely related to growth in grass vegetative tissues. Here, we summarize data that confirms the presence of MLG in secondary cell walls and mature tissues in rice and other grasses. Along with these results, we discuss additional evidence indicating a broader role for MLG than previously considered.
Keywords: (1,3; 1,4)-β-D-glucan, grass cell walls, hemicelluloses, mixed linkage glucan antibody, secondary cell wall
Plant cell walls are mostly composed of polysaccharides (cellulose, hemicelluloses and pectins) and, in secondary walls, the aromatic polymer lignin. Non-lignified primary walls have important roles during growth and cell expansion, providing structural support and flexibility, while lignified secondary cell walls, present in the vasculature and supporting tissues, are thick, strong, reinforcing structures typically associated with the cessation of growth. Grasses have unique cell walls compared with other flowering plants. They contain high levels of feruloylated arabinoxylan and (1,3; 1,4)-β-D-glucan (also known as mixed linkage glucan or MLG), and low amounts of xyloglucan and pectins.1,2 Among these two unique polysaccharides, MLG has been studied in great detail due to its high abundance in the cell walls of barley, oat and Brachypodium distachyon endosperm,3-5 where it has been speculated to serve as an energy storage polysaccharide. In vegetative organs, MLG has been studied extensively in coleoptiles, where it accumulates to high levels during the elongation phase before being hydrolyzed upon cessation of growth.1,6-9 This transient accumulation in elongating primary cell walls of young organs has led to the widely held belief that MLG in vegetative tissues of grasses is a growth-related polysaccharide that is largely absent from mature, non-elongating tissues and/or secondary cell walls. Interestingly, while characterizing an MLG-deficient rice mutant with loss of function in the CslF6 gene, we found that, although MLG accumulated preferentially in younger tissues, the highest content of MLG in rice occurred in senescing, mature stems.10 We propose here that the current view of grass MLG as either a seed storage or cell expansion-specific polysaccharide needs revision, as its presence in secondary cell walls and mature tissues in rice and other species suggest a broader role for this polymer in plants.
MLG Deposition in Secondary Cell Walls and Mature Tissues of Grasses
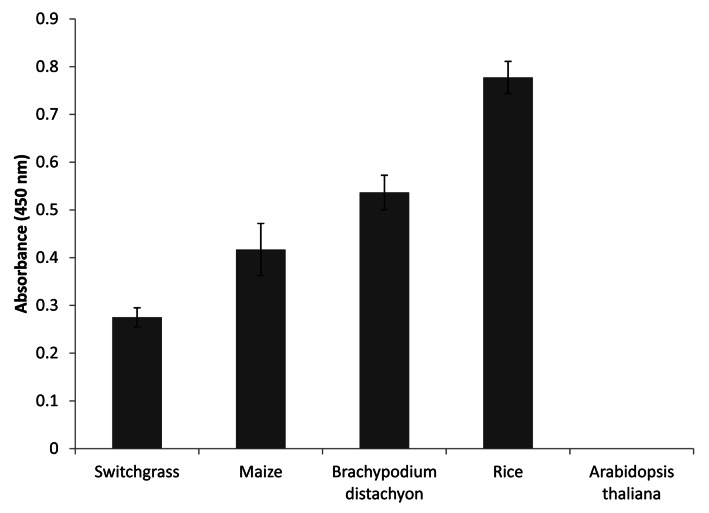
The appearance of MLG in plants is limited to a few evolutionarily distantly related lineages, which suggests that plants acquired this trait independently rather than by common ancestry.11 Among angiosperms, MLG presence is restricted to a few species of the order Poales, which includes the Poaceae (grasses) family.12,13 Recently, MLG has also been found in the cell walls of Equisetum species (horsetails) and the lycophyte Selaginella moellendorffii.14-16 Very few studies have analyzed the distribution of MLG in vegetative tissues other than coleoptiles in grasses.7,17 As shown in Figure 1, MLG accumulates to high levels in sclerenchyma fibers of developing leaf primordia in rice as detected by immunolabeling of tissue sections with a specific monoclonal antibody recognizing (1,3; 1,4)-β-D-glucan. Labeling of the thick secondary walls in the sclerenchyma is just as strong as the labeling in the surrounding primary cell walls of the parenchyma (Fig. 1). Similar observations have been made for mature rice leaves10 and young seedling leaves of barley.11 In contrast, immunogold labeling studies have reported weak detection of MLG in the secondary cell walls of sclerenchyma fibers and xylem vessels in both leaves of the grass Lollium multiflorum and coleoptiles and first leaves of barley.13,17 The apparent discrepancy in MLG detection likely results from differences between grass species (Lollium vs rice and barley) or organ types (coleoptiles vs. leaves). Interestingly, non-grass species in the Poales have been reported to accumulate MLG in mature, non-elongating tissues. For example, in Leptocarpus similis and Restio tetraphyllus, heavy gold labeling was detected in secondary cell walls of immature stems.13 Similarly, in Equisetum plants, MLG accumulates in both young and old tissues, preferentially in aging tissues such as the base of stems,14,15 which is in agreement with our previously reported immunogold labeling results in mature stems of rice.10 In accordance with these studies, we also show here in Figure 2 that MLG occurs in mature, senescing tissues of various grasses [rice, maize, B. distachyon and switchgrass (Panicum virgatum)]. In our previous study, we had suggested that high levels of MLG in mature rice tissues could be specific to rice and not other grasses.10 However, we had performed those preliminary studies using buffer extracted MLG and a less sensitive detection method. The combination of 4M KOH extraction and ELISA shown here allowed us to detect significant amounts of MLG in senescing samples of all grasses tested (Fig. 2). High amounts of MLG in dry ground mature stems and leaves of rice, B. distachyon, switchgrass, Setaria italica (foxtail millet) and Miscanthus have also been detected in a recent glycome profiling study.18 Finally, in barley MLG is present in both elongating and non-elongating root zones, with the highest accumulation in the zone where growth has ceased,19 which indicates that MLG turnover is not the same in non-elongating root tissues as in coleoptiles.
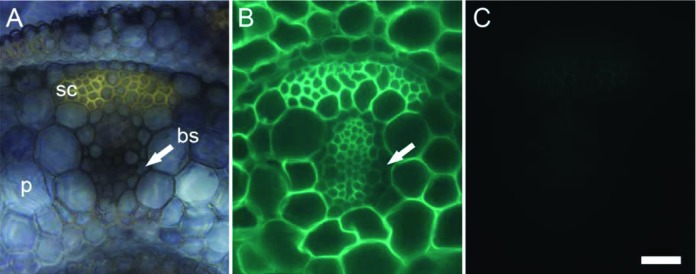
Figure 1. Micrographs of vascular bundles from equivalent transverse sections of developing rice leaf. (A) Bright field micrograph shows the anatomy. The section was stained with Nitro Blue tetrazolium salt which here highlights the sclerenchyma (sc), a tissue type with secondary cell walls. (B) Representative section labeled with a monoclonal antibody25 raised to mixed linkage glucan (MLG). MLG was abundantly detected in the parenchyma (p) as well as in the sclerenchyma fibers. By contrast, the antibody bound only weakly to bundle sheath (bs). (C) After incubation with lichenase, an enzyme that degrades MLG, only the autofluorescence from the sclerenchyma fibers was visible in the sections labeled with the anti-MLG antibody. Together, both (B) and (C) confirm the presence of MLG in cell types with secondary cell walls. The arrows are used to locate the bundle sheath. Scale bar: 20 µm.
Figure 2. ELISA assay using the anti-MLG monoclonal antibody detects abundant MLG in mature stem tissues of several grasses. Senesced stems were ground to a fine powder using a bead beater and treated with 4 M KOH to extract cell wall polysaccharides. The 4 M KOH extracts were used in the ELISA assay, as described before.26 Senesced stems from Arabidopsis plants were used as negative control.
Concluding Remarks
Taken together, the recent scientific literature does not support the commonly held belief that MLG is solely a transient, growth-specific polysaccharide absent in mature tissues in the grasses. The evidence so far suggests that in certain organs, such as the coleoptile and young seedling, MLG turnover from primary cell walls is associated with cessation of growth. In other organs, such as stems and leaves, the presence of MLG at maturity might indicate a role in cell wall strengthening or reinforcement, as recently suggested in stems of rice and Equisetum.10,20 In grass endosperm cell walls, MLG is believed to act as a storage compound. Finally, it is clearly apparent that the heterogeneity in distribution and function of cell wall polysaccharides is not limited to MLG. For example, it is known that xyloglucan has diverse structures and occurrence, and likely different roles in the cell wall, depending on the species or plant tissues considered.21-24
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by the Office of Science, Office of Biological and Environmental Research, of the U. S. Department of Energy (contract no. DE-AC02–05CH11231). We thank Drs. Nicholas Santoro (Great Lakes Bioenergy Research Center, Michigan State University), John Vogel (USDA-ARS, Albany, CA) and Blake Simmons (Sandia National Laboratory and Joint Bioenergy Institute) for maize, B. distachyon and switchgrass samples, respectively.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/23143
References
- 1.Carpita NC. Structure and Biogenesis of the Cell Walls of Grasses. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:445–76. doi: 10.1146/annurev.arplant.47.1.445. [DOI] [PubMed] [Google Scholar]
- 2.Vogel J. Unique aspects of the grass cell wall. Curr Opin Plant Biol. 2008;11:301–7. doi: 10.1016/j.pbi.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Fincher GB. Revolutionary times in our understanding of cell wall biosynthesis and remodeling in the grasses. Plant Physiol. 2009;149:27–37. doi: 10.1104/pp.108.130096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guillon F, Bouchet B, Jamme F, Robert P, Quéméner B, Barron C, et al. Brachypodium distachyon grain: characterization of endosperm cell walls. J Exp Bot. 2011;62:1001–15. doi: 10.1093/jxb/erq332. [DOI] [PubMed] [Google Scholar]
- 5.Burton RA, Fincher GB. Current challenges in cell wall biology in the cereals and grasses. Front Plant Sci. 2012;3:130. doi: 10.3389/fpls.2012.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibeaut DM, Pauly M, Bacic A, Fincher GB. Changes in cell wall polysaccharides in developing barley (Hordeum vulgare) coleoptiles. Planta. 2005;221:729–38. doi: 10.1007/s00425-005-1481-0. [DOI] [PubMed] [Google Scholar]
- 7.Carpita NC, Defernez M, Findlay K, Wells B, Shoue DA, Catchpole G, et al. Cell wall architecture of the elongating maize coleoptile. Plant Physiol. 2001;127:551–65. doi: 10.1104/pp.010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckeridge MS, Rayon C, Urbanowicz BR, Tine MAS, Carpita NC. Mixed linkage (1 -> 3)(1 -> 4)-beta-D-glucans of grasses. Cereal Chemistry. 2004;81:115–27. doi: 10.1094/CCHEM.2004.81.1.115. [DOI] [Google Scholar]
- 9.Christensen U, Alonso-Simon A, Scheller HV, Willats WG, Harholt J. Characterization of the primary cell walls of seedlings of Brachypodium distachyon--a potential model plant for temperate grasses. Phytochemistry. 2010;71:62–9. doi: 10.1016/j.phytochem.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 10.Vega-Sánchez ME, Verhertbruggen Y, Christensen U, Chen X, Sharma V, Varanasi P, et al. Loss of Cellulose synthase-like F6 function affects mixed-linkage glucan deposition, cell wall mechanical properties, and defense responses in vegetative tissues of rice. Plant Physiol. 2012;159:56–69. doi: 10.1104/pp.112.195495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burton RA, Fincher GB. (1,3;1,4)-beta-D-glucans in cell walls of the poaceae, lower plants, and fungi: a tale of two linkages. Mol Plant. 2009;2:873–82. doi: 10.1093/mp/ssp063. [DOI] [PubMed] [Google Scholar]
- 12.Smith BG, Harris PJ. The polysaccharide composition of Poales cell walls: Poaceae cell walls are not unique. Biochem Syst Ecol. 1999;27:33–53. doi: 10.1016/S0305-1978(98)00068-4. [DOI] [Google Scholar]
- 13.Trethewey JA, Campbell LM, Harris PJ. (1->3),(1->4)-beta-d-Glucans in the cell walls of the Poales (sensu lato): an immunogold labeling study using a monoclonal antibody. Am J Bot. 2005;92:1660–74. doi: 10.3732/ajb.92.10.1660. [DOI] [PubMed] [Google Scholar]
- 14.Fry SC, Nesselrode BHWA, Miller JG, Mewburn BR. Mixed-linkage (1-->3,1-->4)-beta-D-glucan is a major hemicellulose of Equisetum (horsetail) cell walls. New Phytol. 2008;179:104–15. doi: 10.1111/j.1469-8137.2008.02435.x. [DOI] [PubMed] [Google Scholar]
- 15.Sørensen I, Pettolino FA, Wilson SM, Doblin MS, Johansen B, Bacic A, et al. Mixed-linkage (1-->3),(1-->4)-beta-D-glucan is not unique to the Poales and is an abundant component of Equisetum arvense cell walls. Plant J. 2008;54:510–21. doi: 10.1111/j.1365-313X.2008.03453.x. [DOI] [PubMed] [Google Scholar]
- 16.Harholt J, Sørensen I, Fangel J, Roberts A, Willats WG, Scheller HV, et al. The glycosyltransferase repertoire of the spikemoss Selaginella moellendorffii and a comparative study of its cell wall. PLoS One. 2012;7:e35846. doi: 10.1371/journal.pone.0035846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trethewey JAK, Harris PJ. Location of (1→3)- and (1→3), (1→4)-beta-D-glucans in vegetative cell walls of barley (Hordeum vulgare) using immunogold labelling. New Phytol. 2002;154:347–58. doi: 10.1046/j.1469-8137.2002.00383.x. [DOI] [PubMed] [Google Scholar]
- 18.Kulkarni AR, Pattahil S, Hahn MG, York WS, O'Neill MA. Comparison of arabinoxylan structure in bioenergy and model grasses. Ind Biotechnol (New Rochelle NY) 2012;8:222–9. doi: 10.1089/ind.2012.0014. [DOI] [Google Scholar]
- 19.Kozlova LV, Snegireva AV, Gorshkova TA. Distribution and structure of mixed linkage glucan at different stages of elongation of maize root cells. Russ J Plant Physiol. 2012;59:339–47. doi: 10.1134/S1021443712030090. [DOI] [Google Scholar]
- 20.Fry SC, Mohler KE, Nesselrode BH, Franková L. Mixed-linkage beta-glucan : xyloglucan endotransglucosylase, a novel wall-remodelling enzyme from Equisetum (horsetails) and charophytic algae. Plant J. 2008;55:240–52. doi: 10.1111/j.1365-313X.2008.03504.x. [DOI] [PubMed] [Google Scholar]
- 21.Fry SC. The structure and functions of xyloglucan. J Exp Bot. 1989;40:1–11. doi: 10.1093/jxb/40.1.1. [DOI] [Google Scholar]
- 22.Hsieh YS, Harris PJ. Xyloglucans of monocotyledons have diverse structures. Mol Plant. 2009;2:943–65. doi: 10.1093/mp/ssp061. [DOI] [PubMed] [Google Scholar]
- 23.Brennan M, Harris PJ. Distribution of fucosylated xyloglucans among the walls of different cell types in monocotyledons determined by immunofluorescence microscopy. Mol Plant. 2011;4:144–56. doi: 10.1093/mp/ssq067. [DOI] [PubMed] [Google Scholar]
- 24.Peña MJ, Kong Y, York WS, O’Neill MA. A Galacturonic Acid-Containing Xyloglucan Is Involved in Arabidopsis Root Hair Tip Growth. Plant Cell. 2012 doi: 10.1105/tpc.112.103390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meikle PJ, Hoogenraad NJ, Bonig I, Clarke AE, Stone BAA. A (1-->3,1-->4)-beta-glucan-specific monoclonal antibody and its use in the quantitation and immunocytochemical location of (1-->3,1-->4)-beta-glucans. Plant J. 1994;5:1–9. doi: 10.1046/j.1365-313X.1994.5010001.x. [DOI] [PubMed] [Google Scholar]
- 26.Verhertbruggen Y, Marcus SE, Haeger A, Ordaz-Ortiz JJ, Knox JP. An extended set of monoclonal antibodies to pectic homogalacturonan. Carbohydr Res. 2009;344:1858–62. doi: 10.1016/j.carres.2008.11.010. [DOI] [PubMed] [Google Scholar]