Abstract
Coumarin effects on gravitropic responses of Arabidopsis thaliana roots were here evaluated. Coumarin alone did not cause any alteration on gravitropic response showing a behavior similar to control plants. In contrast, TIBA and NPA, two auxin transport inhibitors, strongly modified root gravitropic responses. The addition of coumarin to the medium together with TIBA or NPA partially restored the effect of both inhibitors. Simultaneously, a semi-quantitative evaluation of ROS distribution was performed on root tips. TIBA and NPA caused a wide distribution of O2-, ROS oxidant species, around the root tip which disappeared with coumarin addition to both treatments, restoring ROS localized distribution. These results indicated a strong correlation between ROS distribution and coumarin-mediated recovery of root gravitropism.
Keywords: Arabidopsis thaliana, coumarin, gravitropism response, reactive oxygen species
Plants produce a wide range of secondary metabolites that affect several physiological processes. However, their effective role and mechanisms of action are still largely unclear. The involvement of these compounds with plant hormones, in particular with auxin in controlling lateral root formation and gravitropic responses, has been already reported.1 Santelia et al.2 suggested that flavonoids promoted asymmetric PIN auxin efflux carriers shifts during gravity stimulation, redirecting basipetal auxin streams needed for root bending. Successively, Kuhn et al.3 defined the mode of action of flavonols on the auxin transport mechanism. Li et al.4 demonstrated that 4-methylumbelliferone, a coumarin derivative, regulated lateral root formation on Arabidopsis, increasing two auxin efflux facilitator genes (PIN2 and PIN3) expression. They also suggested that auxin redistribution rather than its biosynthesis was responsible of root branching formation after 4-methylumbelliferone exposure.
Coumarin is a secondary metabolite widely distributed in both natural plant communities and crops which play a crucial role in plant-plant allelopathic interactions.5 Released into the environment, coumarin affects plant growth and development of many species, interfering with root system, either to inhibit or to promote its elongation.6 The inhibitory effect of coumarin has already been reported in Avena sp and Phleum pratense roots,7,8 while an increase of lateral root length but not of the primary one has been observed in Arabidopsis at 10−4 M coumarin concentration.9 In the same species, similar effects were usually attributed to auxin that co-regulated two auxin-dependent processes, lateral root development and root gravitropism.10 Indeed, it has been demonstrated that agravitropic root mutants aux1 and axr4 significantly reduced lateral root development.11 Although, Lupini et al.12 suggested an auxin-like pattern on morpho-physiological responses induced by coumarin in maize apical root zones, no information is available for gravitropic root response to this allelochemical. However, current evidence suggested that alongside to auxin, reactive oxygen species (ROS) were signal molecules in the root bending.13 Recently, it was suggested that one of the effect of allelochemicals on target plants may be an enhanced biosynthesis and accumulation of ROS causing the oxidative stress.14,15 For example, gallic acid and (-)-catechin treatments generated high levels of ROS in the treated plant roots.15,16 The aim of this study has been to determine the effects of coumarin on root bending and the involvement of some signal molecules, such as ROS, at downstream level. Root bending quantification and O2∙- root apex distribution in gravistimulated Arabidopsis thaliana seedlings have been evaluated.
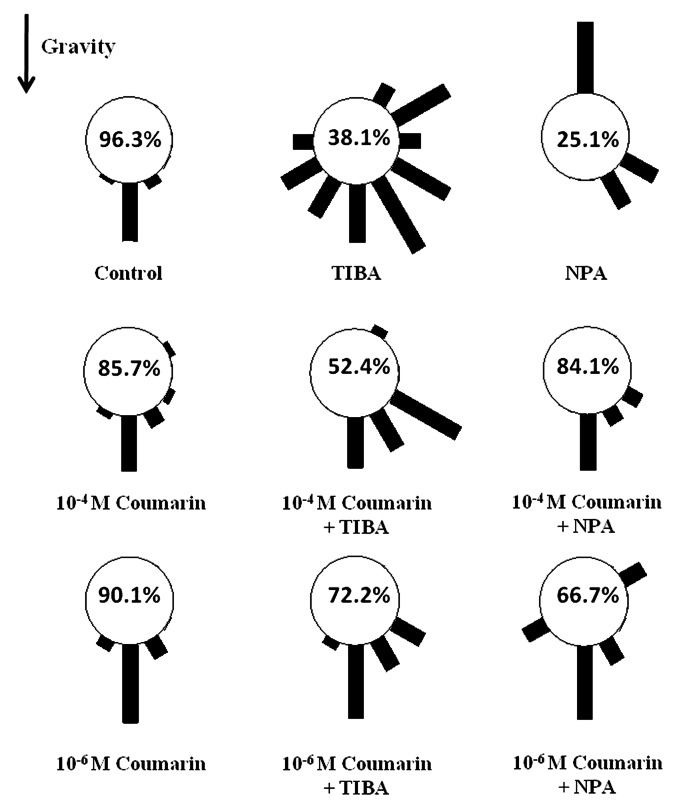
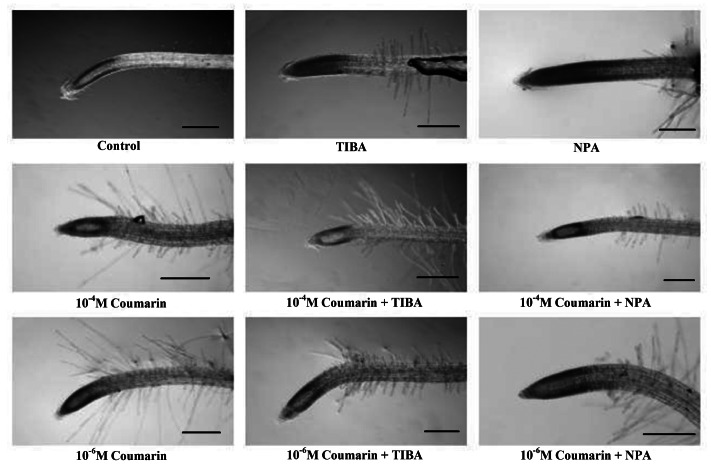
Two coumarin concentrations (10−4 and 10−6 M), able to influence root lateral length of Arabidopsis seedlings,9 were applied to stimulate root gravitropic responses for 24 h (90°) and their responses were showed as distribution frequency in Figure 1. Coumarin, at both concentrations, did not significantly affect gravitropic responses showing 85.7% and 90.1% relative root bending (RRB) values (10−4 and 10−6 M, respectively) similar to that observed in control roots (96.3% RRB) (Fig. 1). Conversely, severe gravitropic responses were induced by TIBA and NPA, two inhibitors of polar auxin efflux, which blocks rootward IAA movement from the root tip.18 Indeed, both inhibitors expanded the distribution frequency along the sectors (38.1 and 25% RRB respectively) (Fig. 1) resulting in an agravitropic phenotype. These results are in agreement to uneven distribution of auxin at different root apex sides. Finally, the same gravitropic assay in the presence of coumarin and TIBA or NPA was performed. At both concentrations, coumarin was partially able to restore root gravitropism induced by both inhibitors. In particular, 10−4 coumarin caused 52.4% and 84.1% RRB values with TIBA and NPA respectively, while 72.2% and 66.7% RRB were observed with 10−6 M coumarin treatment (Fig. 1). These results suggested that, coumarin did not alter over 24 h wild-type gravitropism, but it was able to redirect the asymmetric distribution of auxin, and hence to restore partially root bending. On the other hand, it is well known that ROS generations play a pivotal role as downstream component in auxin-mediated signal transduction in root gravitropim.13 In order to verify the ROS generation involvement in coumarin gravitropic reorientation, after TIBA and NPA exposure, the superoxide anion (O2-) was determined through staining method in root apex of treated plants. The results indicated that coumarin, at both concentrations (10−4 and 10−6 M), caused O2- localized distribution on both sides (up and down) of the epidermis similar to that observed in control plants (Fig. 2). Conversely, in the presence of TIBA and NPA, whole root apex was stained (Fig. 2). The addition of coumarin, at both concentrations, restored localized O2- distribution (Fig. 2). These results indicated that coumarin effect in restoring root curvature did not depend upon auxin redistribution, but was mediated by ROS generation, a downstream component in auxin-mediated signaling.13 How molecular mechanisms involving coumarin and ROS were integrated to induce gravitropic curvature remains to be clarified.
Figure 1. Frequency distribution of gravity responses of Arabidopsis thaliana (Col 0) roots 24 h after reorientation of 90° to horizontal in the presence of coumarin and/or auxin inhibitors (1.510−5 M TIBA, 510−6 M NPA). The percentage represents the sum of 90° and 60° sectors (see Materials and Methods)
Figure 2. Distribution of O2- visualizated by nitroblue tetrazolium (NBT) staining in Arabidopsis root tip reoriented exposed to coumarin and/or auxin inhibitors (1.510−5 M TIBA, 510−6 M NPA).
These results confirmed that coumarin was able to redirected rootward auxin streams necessary for root bending, exhibiting an auxin-like behavior or/and interact with the auxin signaling pathways. The use of agravitropic loss-of-function mutant such as pin2/eir1 could better explain coumarin action.
Materials and Methods
Plant growth conditions
Seeds of Arabidopsis thaliana (Columbia 0) were soaked in distilled water for 30 min and surface sterilized with 95% ethanol and 5% commercial bleach (v/v) for 5 min and rinsed 5 times with distilled sterile water. Thereafter, they were germinated in Petri dish on solidified-agar medium [0.75% (w/v)]19 containing sucrose [0.5% (w/v)], MES (1 g/L), pH 5.75, for 4 d, in a growth chamber (22°C, 65% RH, 14/10 h, 300 μmol photon flux density m–2 s–1).
Gravitropic assay
Gravitropic assay was performed as described by Santelia et al.2 Briefly, five uniform seedlings (4-d old) were transferred to a single plate containing the same nutrient medium supplemented with coumarin (10−4 or 10−6 M) and/or 1.5 × 10−5 M 2,3,5-triiodobenzoic acid (TIBA) or 5 × 10−6 M naphthylphthalamic acid (NPA), concentrations routinely used. Plates were placed vertically at the same environmental condition for 24 h, and after this period, they were turned 90° for other 24 h. Then, root images were captured by digital camera (Olympus C-5050) and the angles of curvature gravitropic were analyzed by Image Tool software v. 3.00 (UTHSC, USA). To simplify the visualization of the gravitropic responses, each gravity stimulated root was assigned to one of 12, 30° sectors. The data were expressed as frequency distribution along the sectors and the sum of 60° and 90° was defined as relative root bending (RRB).2
O2- visualization
In order to visualize O2- in vivo production, plants were harvested after 4 h under gravistimulation treatment. The O2- production was visualized by incubating intact roots in 10 mM K-citrate buffer (pH 6) containing 0.5 mM Nitroblue Tetrazolium chloride (NBT).20,21 The images were captured using the digital camera (Olympus C-5050) and stereoscopic microscopy (Olympus SZX9).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/23156
References
- 1.Davies PJ. Plant hormones: Physiology, Biochemistry and Molecular Biology. Kluwer, London, UK. 1995. [Google Scholar]
- 2.Santelia D, Henrichs S, Vincenzetti V, Sauer M, Bigler L, Klein M, et al. Flavonoids redirect PIN-mediated polar auxin fluxes during root gravitropic responses. J Biol Chem. 2008;283:31218–26. doi: 10.1074/jbc.M710122200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuhn BM, Geisler M, Bigler L, Ringli C. Flavonols accumulate asymmetrically and affect auxin transport in Arabidopsis. Plant Physiol. 2011;156:585–95. doi: 10.1104/pp.111.175976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li X, Gruber MY, Hegedus DD, Lydiate DJ, Gao MJ. Effects of a coumarin derivative, 4-methylumbelliferone, on seed germination and seedling establishment in Arabidopsis. J Chem Ecol. 2011;37:880–90. doi: 10.1007/s10886-011-9987-3. [DOI] [PubMed] [Google Scholar]
- 5.5. Zobel AM, Brown SA. Coumarins in the interaction between the plant and its environment. Allelopathy Journal. 1995;2:9–22. [Google Scholar]
- 6.Brown SA. Coumarins. In: The Biochemistry of Plants. Stumpf PK, Conn EE (eds). Academic Press Inc, New York, USA, 1981; 7:269-99. [Google Scholar]
- 7.Goodwin RH, Avers CJ. The effect of coumarin derivatives on growth of Avena root. Am J Bot. 1950;37:224–7. doi: 10.2307/2437906. [DOI] [Google Scholar]
- 8.Avers CJ, Goodwin RH. Studies on root. IV. Effects of coumarin and scopoletin on the standard root growth pater of Phleum pretense. Am J Bot. 1956;43:612–20. doi: 10.2307/2438877. [DOI] [Google Scholar]
- 9.Abenavoli MR, Nicolò A, Lupini A, Oliva S, Sorgonà A. Effects of different allelochemicals on root morphology of Arabidopsis thaliana. Allelopathy J. 2008;22:245–52. [Google Scholar]
- 10.Lucas M, Godin C, Jay-Allemand C, Laplaze L. Auxin fluxes in the root apex co-regulate gravitropism and lateral root initiation. J Exp Bot. 2008;59:55–66. doi: 10.1093/jxb/erm171. [DOI] [PubMed] [Google Scholar]
- 11.Hobbie L, Estelle M. The axr4 auxin-resistant mutants of Arabidopsis thaliana define a gene important for root gravitropism and lateral root initiation. Plant J. 1995;7:211–20. doi: 10.1046/j.1365-313X.1995.7020211.x. [DOI] [PubMed] [Google Scholar]
- 12.Lupini A, Sorgonà A, Miller AJ, Abenavoli MR. Short-time effects of coumarin along the maize primary root axis. Plant Signal Behav. 2010;5:1395–400. doi: 10.4161/psb.5.11.13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joo JH, Bae YS, Lee JS. Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiol. 2001;126:1055–60. doi: 10.1104/pp.126.3.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weir TL, Park SW, Vivanco JM. Biochemical and physiological mechanisms mediated by allelochemicals. Curr Opin Plant Biol. 2004;7:472–9. doi: 10.1016/j.pbi.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Cruz-Ortega R, Lara-Núňez A, Anaya AL. Allelochemical stress can trigger oxidative damage in receptor plant. Plant Signal Behav. 2007;2:269–70. doi: 10.4161/psb.2.4.3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rudrappa T, Bonsall J, Gallagher JL, Seliskar DM, Bais HP. Root-secreted allelochemical in the noxious weed Phragmites australis deploys a reactive oxygen species response and microtubule assembly disruption to execute rhizotoxicity. J Chem Ecol. 2007;33:1898–918. doi: 10.1007/s10886-007-9353-7. [DOI] [PubMed] [Google Scholar]
- 17.Bais HP, Vepachedu R, Gilroy S, Callaway RM, Vivanco JM. Allelopathy and exotic plant invasion: from molecules and genes to species interactions. Science. 2003;301:1377–80. doi: 10.1126/science.1083245. [DOI] [PubMed] [Google Scholar]
- 18.Casimiro I, Marchant A, Bhalerao RP, Beeckman T, Dhooge S, Swarup R, et al. Auxin transport promotes Arabidopsis lateral root development. Plant Cell. 2001;13:843–52. doi: 10.1105/tpc.13.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt W, Santi S, Pinton R, Varanini Z. Water-extractable humic substances alter root development and epidermal cell pattern in Arabidopsis. Plant Soil. 2007;300:259–67. doi: 10.1007/s11104-007-9411-5. [DOI] [Google Scholar]
- 20.Frahry G, Schopfer P. NADH-stimulated, cyanide-restistant superoxide production in maize coleoptiles analysed with a tetrazoliumbased assay. Planta. 2001;212:175–83. doi: 10.1007/s004250000376. [DOI] [PubMed] [Google Scholar]
- 21.Bielski BHJ, Shine GG, Bajuk S. Reduction of nitroblue tetrazolium by CO2– and O2– radicals. J Phys Chem. 1980;84:830–3. doi: 10.1021/j100445a006. [DOI] [Google Scholar]