Abstract
New groups of synthetic “designer drugs” have increased in popularity over the past several years. These products mimic the euphoric effects of other well-known illicit drugs but are advertised as “legal” highs and are sold over the internet, at raves and night clubs, and in head shops. The 2C series drugs are ring-substituted phenethylamines that belong to a group of designer agents similar in structure to 3,4-methylenedioxy-N-methylamphetamine (MDMA, Ecstasy). Understanding the pharmacology and toxicology of these agents is essential in order to provide the best medical care for these patients. This review focuses on the pharmacology, pharmacokinetics, clinical effects, and treatment of 2C drug intoxication based on available published literature. Multiple names under which 2C drugs are sold were identified and tabulated. Common features identified in patients intoxicated with 2Cs included hallucinations, agitation, aggression, violence, dysphoria, hypertension, tachycardia, seizures, and hyperthermia. Patients may exhibit sympathomimetic symptoms or symptoms consistent with serotonin toxicity, but an excited delirium presentation seems to be consistent amongst deaths attributed to 2C drugs; at least five deaths have been reported in the literature in patients intoxicated with 2C drugs. 2C drugs are a group of designer intoxicants, many of which are marketed as legal, but may carry risks that consumers are unaware of. These drugs may be characterized by either serotonergic toxicity or a sympathomimetic toxidrome, but a presentation consistent with excited delirium is consistent amongst the reported 2C-related deaths. Treatment of 2C intoxication is primarily supportive, but immediate action is required in the context of excited delirium, hyperthermia, and seizure activity.
Keywords: 2C, Phenethylamine, Shulgin, Hallucinogen, Designer
Introduction
The general public has long been aware of traditional illicit drugs such as cocaine, marijuana, and methamphetamine. Designer drugs were created in the 1960s to avoid the provisions of existing drug laws by preparing analogs or derivatives of currently available drugs. Designer drugs including the 2C class discussed in this article are not new but have been increasing in popularity. Manufacturers of designer drugs purposely change the structure of these drugs to stay ahead of drug laws, making it difficult for clinicians to stay informed. Structure manipulation to produce new compounds, advertisement of these compounds as “legal” highs, and ease of access of designer drugs from the internet, raves, night clubs, and sale in head shops have rejuvenated and promoted the current designer drug resurgence [1, 2].
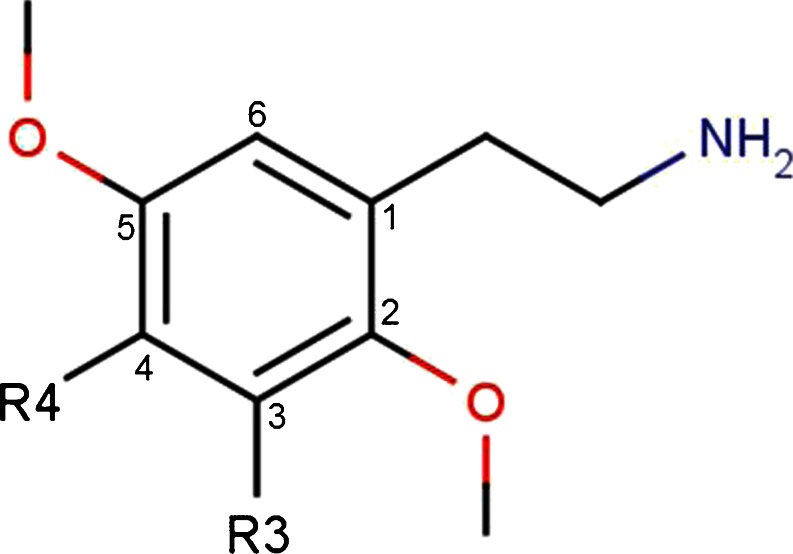
The 2Cs are just one of the designer drug classes currently being promoted and disseminated on the internet and at raves. The phenethylamine-based structure is actually shared among amphetamines, catecholamines, cathinones, and many other drugs. The terminology “2Cs” is in reference to an acronym invented by Alexander Shulgin to describe the two carbons between the amino group and the benzene ring in the chemical structure (Fig. 1) [3, 4]. In this article, we will continue to use “2C” to refer to the group of newly substituted designer hallucinogens with methoxy groups at the 2 and 5 positions on the ring, rather than the larger group of phenethylamine-based compounds that would include epinephrine, dopamine, bupropion, MDMA, methamphetamine, cathinones, and a multitude of other commonly known similarly structured agents.
Fig. 1.
Designer substitution to the 2C structure can result in increased hallucinogenic activity. For example, additions of methoxy groups at the 2 and 5 positions on the base structure ring or the substitution of iodine or bromine at the 4 position results in increased hallucinogenic effects [3, 5]. In 1974, 4-bromo-2,5-dimethoxyphenethylamine (2C-B), the first of the 2Cs, was synthesized by Alexander Shulgin as he was exploring homologs from 2,5-dimethoxy-4-bromoamphetamine [3]. 2C-B was manufactured in the 1980s and early 1990s under the names Nexus, Erox, Performax, Toonies, Bromo, Spectrum, and Venus and marketed as MDMA’s replacement after MDMA became scheduled in the USA [6, 7]. 2C-B was initially intended for psychotherapy use due to its short 1-h duration of action [3]. Due to 2C-B’s significant gastrointestinal effects and lack of empathogenic effects as compared to MDMA, it rapidly fell out of favor for psychotherapy. In 1995, 2C-B was placed on Schedule I of the Controlled Substances Act by the Drug Enforcement Agency (DEA) [6, 7]. However, following the scheduling of 2C-B, other 2C analogues were made available by suppliers as legal alternatives [8].
In 1991, Alexander Shulgin, along with his wife Ann, published PIHKAL, A Chemical Love Story (PIHKAL is an acronym representing “Phenethylamines I Have Known And Loved”). This book detailed synthesis instructions for over 200 psychedelic compounds including bioassays, dosages, and other commentaries [5]. In this book, Shulgin describes the synthesis of 2,5-dimethoxy-4-ethylphenethylamine (2C-E, Europa), 2,5-dimethoxy-4-ethylthiophenethylamine (2C-T-2), 2,5-dimethoxy-4-(n)-propylthiophenethylamine (2C-T-7, Blue Mystic, T7, Beautiful, Tripstay, Tweety-Bird Mescaline), 4-iodo-2,5-dimethoxyphenethylamine (2C-I, i), and many others [3, 4, 8, 9]. It is no surprise that after the publication of Alexander Shulgin’s book, 2C use saw an increase in popularity.
The 2Cs are considered “drugs or chemicals of concern” by the DEA [10]. Substances listed by the DEA as “drugs or chemicals of concern” are Scheduled 1 or II substances under the Controlled Substances Act of 1970 (CSA) and its subsequent amendments or are compounds currently being considered for scheduling under the CSA. Until the summer of 2012, the following were the only 2Cs classified as Schedule I controlled substances: 2,5-dimethoxy-4-bromophenethylamine (2C-B) and 2,5-dimethoxy-4-(n)-propylthiophenethylamine (2C-T-7) [10]. Recently, 2C-E, 2C-D, 2C-C, 2C-I, 2C-T2, 2C-T4, 2C-H, 2C-N, and 2C-P also joined the Schedule 1 restricted list [11]. Under the Analogue Statute of the Controlled Substances Act, it is also illegal to traffic any compounds that are analogous to scheduled phenethylamines, which would include many other previously synthesized and currently available agents [10, 11]. This act, however, does not preclude the possession and use of unscheduled 2Cs [10, 11].
There is scant literature available on the clinical effects of acute 2C intoxication or long-term sequelae of 2C ingestion. Sound medical treatment is predicated on an accurate diagnosis, which in turn requires detailed knowledge of the signs and symptoms associated with specific conditions. To this end, we report a detailed description of the 2C toxidrome with an emphasis on an excited delirium syndrome in 2C fatalities.
Methods
Comprehensive reviews of published literature were conducted using PUBMED and Ovid Medline databases. Reviews were limited to literature published in the English language. Searches were also completed using Google to search for online sources. Any relevant online sources identified were included. Search terms included: phenethylamine, phenylethylamine, designer drug, 2C-B, 2C-I, 2C-E, 2C-T-7, 2C-T-4, 4-bromo-2,5-dimethoxyphenethylamine, 2,5-dimethoxy-4-(n)-propylthiophenethylamine, 4-iodo-2,5-dimethoxyphenethylamine, hallucinogen, hallucinogenic 2C, epidemiology, pattern of use, drug use, drug abuse, clinical presentation, treatment, death, and case report. Bibliographies of the accepted articles were screened for additional literature. Literature was selected based on whether phenethylamines were among the drugs of abuse discussed and whether there was information about the pharmacology, pharmacokinetics, clinical effects, or treatment.
Pharmacology and Pharmacokinetics
There is little information available about the pharmacology of hallucinogenic 2Cs. It has been shown that 2Cs have an affinity for 5-HT2 and alpha-adrenergic receptors, but these compounds can be either agonists or antagonists, which is dependent on the specific receptor subtype [12–14]. The structure of 2Cs responsible for the hallucinogenic effects consists of a primary amine separated by two carbon atoms from the phenyl ring, methoxy groups at positions 2 and 5 on the phenyl ring, and a hydrophobic substituent at position 4 on the phenyl ring (Fig. 1) [3, 15, 16]. It is possible to create new hallucinogenic 2Cs by placing different substituents on the aromatic ring at positions 2, 4, or 5 [5, 16].
Studies examining the pharmacokinetics and pharmacodynamics of 2Cs are limited. The 2Cs are generally available in tablet, capsule, powder, or liquid form, depending on the 2C, and they are ingested orally or insufflated [3, 6–9, 17]. According to reports from the DEA, insufflating the compound produces more rapid and intense effects [8]. For example, oral administration of 2C-T-7 has an onset of 1–2.5 h and duration of action of 5–7 h, whereas insufflation of 2C-T-7 has an onset of 5–15 min and a duration of action of 2–4 h [17].
Shulgin provides anecdotal accounts of onset and duration of euphoric effects after oral ingestion of phenethylamines in PIHKAL, and other anecdotal accounts are available on websites such as www.erowid.org. Table 1 lists some 2Cs and dosages and durations of effect according to Shulgin. However, pharmacokinetics and pharmacodynamics may vary between users, and some users may be more susceptible to toxicity [18]. Also, it is important to emphasize that drugs currently sold on the internet frequently do not contain the substance or dosage for which they are labeled or given when purchased [19].
Table 1.
Reported dosages and duration of action for 2Cs per Shulgin [3]
| 2C | Chemical name | Dosage | Duration (h) |
|---|---|---|---|
| 2C-B | 4-Bromo-2,5-dimethoxyphenethylamine | 12–24 mg | 4–8 |
| 2C-C | 4-Chloro-2,5-dimethoxyphenethylamine | 20–40 mg | 4–8 |
| 2C-D | 4-Methyl-2,5-dimethoxyphenethylamine | 20–60 g | 4–6 |
| 2C-E | 4-Ethyl-2,5-dimethoxyphenethylamine | 10–25 mg | 8–12 |
| 2C-G | 3,4-Dimethyl-2,5-dimethoxyphenethylamine | 20–35 mg | 18–30 |
| 2C-G-3 | 3,4-Trimethylene-2,5-dimethoxyphenethylamine | 16–25 mg | 12–24 |
| 2C-G-5 | 3,4-Norbornyl-2,5-dimethoxyphenethylamine | 10–16 mg | 32–48 |
| 2C-I | 4-Iodo-2,5-dimethoxyphenethylamine | 14–22 mg | 6–10 |
| 2C-N | 4-Nitro-2,5-dimethoxyphenethylamine | 100–150 mg | 4–6 |
| 2C-P | 4-Propyl-2,5-dimethoxyphenethylamine | 6–10 mg | 10–16 |
| 2C-SE | 4-Methylseleno-2,5-dimethoxyphenethylamine | ∼100 mg | 6–8 |
| 2C-T | 4-Methylthio-2,5-dimethoxyphenethylamine | 60–100 mg | 3–5 |
| 2C-T-2 | 4-Ethylthio-2,5-dimethoxyphenethylamine | 12–25 mg | 6–8 |
| 2C-T-4 | 4-Isopropylthio-2,5-dimethoxyphenethylamine | 8–20 mg | 12–18 |
| 2C-T-7 | 4-Propylthio-2,5-dimethoxyphenethylamine | 10–30 mg | 8–15 |
| 2C-T-8 | 4-Cyclopropylmethylthio-2,5-dimethoxyphenethylamine | 30–50 mg | 10–15 |
| 2C-T-9 | 4-(t)-Butylthio-2,5-dimethoxyphenethylamine | 60–100 mg | 12–18 |
| 2C-T-13 | 4-(2-Methoxyethylthio)-2,5-dimethoxyphenethylamine | 25–40 mg | 6–8 |
| 2C-T-15 | 4-Cyclopropylthio-2,5-dimethoxyphenethylamine | >30 mg | Several hours |
| 2C-T-17 | 4-(s)-Butylthio-2,5-dimethoxyphenethylamine | 60–100 mg | 10–15 |
| 2C-T-21 | 4-(2-Fluoroehtylthio)-2,5-dimethoxyphenethylamine | 8–12 mg | 7–10 |
O-demethylation at positions 2 and 5 is the main method of 2C metabolism. The compound is deaminated and then oxidated to either the corresponding acid or base [18, 20, 21]. MAO-A and MAO-B are the major enzymes involved in the deamination of 2Cs [22]. Studied 2Cs have a greater affinity for MAO-A versus MAO-B; MAO-A has a larger binding pocket and can accommodate larger substituents at the 4 position on the phenyl ring [21–23]. Due to the involvement of MAOs in 2C metabolism, there are potential drug–drug interactions with monoamine oxidase inhibitors, which can increase serum 2C concentrations and increase risk for 2C toxicity [22]. CYP2D6 plays a minor role in the metabolism of the following compounds: 2C-D, 2C-E, 2C-T-2, and 2C-T-7 [20, 22]. Limited information suggests that 2C drugs also inhibit reuptake of monoamines including norepinephrine, dopamine, and serotonin [24].
Clinical Effects
Reported effects of 2Cs are a combination of hallucinogenic and stimulating effects [20]. Patients with 2C intoxication will likely present with either a sympathomimetic syndrome, serotonin toxicity, a hallucinogenic picture, or some combination thereof. Signs and symptoms include hallucinations, euphoria, empathy, nausea, vomiting, agitation, tachycardia, hypertension, respiratory depression, and seizures [1, 2, 6]. Users claim increased tactile, visual, auditory, and olfactory sensations [3–9]. Effects from use of 2C-E are reportedly more intense compared to 2C-B and 2C-I [3].
At low doses, 2Cs generally have stimulating effects and increased visual, auditory, and tactile sensations [3]. At moderate doses, hallucinations may be produced. At higher doses, users may experience unpleasant hallucinations and sympathomimetic signs such as tachycardia, hypertension, and hyperthermia [3, 25]. Seizures and delirium have been reported with 2C-T-7 [3, 8].
In PIHKAL, Shulgin notes that during an experience with 25 mg of 2C-E, he became “anxious and sweaty” within a few minutes of taking the substance [3]. He also experienced “toxic psychosis,” and hearing voices talk to him about his worst fears. Symptoms were reported to have lasted for approximately 20 min [3]. Another user notes an “intense” experience after ingesting 16 mg of 2C-E in an oral solution [3, 9]. At the peak of the high, the user experienced “extreme hallucinations” and suicidal thoughts, lasting for approximately 30 min [3, 9].
The combination of clinical effects and the mechanisms of action may give us insight into possible mechanisms of toxicity or death. One clinical effect, which can lead to death and appears to be linked to 2C intoxication, is excited delirium. Although first described in the mid 1800s, excited delirium has only recently been formally recognized as a unique syndrome by the American College of Emergency Physicians [25, 26]. Excited delirium is characterized by the following sequence of events: delirium with agitation, violence, hyperactivity, and in many cases, hyperthermia. This syndrome often results in a sudden and unexpected cardiopulmonary arrest. The pathophysiology of excited delirium is believed to primarily involve dopamine and has been previously described with cocaine use [27]. Chronic cocaine use may cause abnormal dopamine processing in the mesolimbic pathway, resulting in hyperactivity and hyperthermia [28, 29]. Dopamine processing, however, appears to be different in patients exhibiting excited delirium as compared to non-psychotic cocaine users. Recent research has identified several possible explanations for this critical difference. The primary mechanism is believed to result from the inability of the brain to increase the number of drug transporters, which pump dopamine out of the brain. Chronic cocaine users have increased dopamine transporter binding sites. However, excited delirium victims lack these compensatory changes. Thus, excited delirium victims are unable to compensate for rapidly elevating dopamine levels after cocaine use, potentially overstimulating the post-synaptic receptors [30–32]. Similar aberrant dopamine processing has been found in schizophrenic patients suggesting a similar mechanism for excited delirium in these patients [33]
Fatalities have been attributed to 2C overdose. Three deaths have been linked to 2C-T-7, one death to 2C-T-21, two deaths to 2C-I-NBOMe, and one death to 2C-E [34–41]. Table 2 lists the corresponding deaths and their clinical findings. It should be noted that there are recent reports of poor clinical outcomes after exposure to unconfirmed 2Cs, including presumed 2C-I-NBOMe (25i). Other published case reports of 2C intoxication are listed in Table 3 [41–43].
Table 2.
Deaths from 2C reported in the media
| Age/sex | Agent | Route | Dose | Symptoms |
|---|---|---|---|---|
| 20-year-old male [35] | 2C-T-7 | Snorted | 35 mg | Vomiting, hallucinations, agitation, violence/aggression, nasal bleeding, possible seizure activity, pulmonary edema, cardio/pulmonary arrest |
| 17-year-old male [36] | 2C-T-7 | Snorted | Unknown | Agitation, violence, aggression, possible hyperthermia (removal of clothing), rigidity, Cardio-pulmonary arrest |
| Unknown male [37] | 2C-T-7 and MDMA | unknown | Unknown amount of 2CT-7; 200 mg MDMA | Agitation, aggression, violence, seizures, hallucinations, cardio/pulmonary arrest, cerebral hemorrhage |
| 22-year-old male male [38] | 2C-T-21 | ingestion | Unknown (dipped tip of tongue into powder) | Hyperthermia (108 °C), seizures, coma |
| 19-year-old male male [34, 44] | 2C-E | Snorted | Unknown | Aggressive/agitation, hyperthermia, DIC, multi-organ failure |
| 17-year-old male male [39, 40] | 2C-I-NBOMe | Ingestion | Unknown, mixed with chocolate | Hyperventilation, foaming at the mouth |
| 18-year-old male [39, 40] | 2C-I-NBOMe | Unknown | Unknown | Unknown |
Information is taken from that reported in the media and therefore accuracy cannot be verified
Table 3.
Case reports of 2C intoxication
| Age (years) | Agent used | Clinical effects | Outcome | Agent confirmed by laboratory analysis |
|---|---|---|---|---|
| 40-year-old male [42] | Vanilla aroma | Delirium, incoherent | Treated with neuroleptic, symptoms resolved after 17 h | Confirmed as 2-(2,5-dimethoxy-4-isopropylsulfanylphenyl)-ethanamine (2C-T-4) |
| 27-year-old male [43] | 2C-B used 2 days prior to effects | Acute psychosis/hallucinations/paranoid delusions | Onset of symptoms not consistent with pharmacokinetics of 2C-B. Most likely acute psychotic break | No confirmatory testing done |
| 10 individuals ranging in age from 16–23 [34] | Product marketed as 2C-I bought over the internet and distributed at a party | Tachycardia, 2 patients with hypertension with BPs over 220 systolic, eight patients with severe agitation, delirium and hallucinations | Various outcomes, most minor; 1 death, 1 major, 8 minor | Confirmed by analysis as 2C-E |
Treatment
There are currently no antidotes available for 2Cs. Management of acute 2C intoxication consists of focused, symptom-based supportive care. Initial treatment must consist of maintaining airway, breathing, and circulation. Patients with apparent intoxication should have an intravenous line and be placed on a cardiac monitor. Patients presenting with dysphoria should be placed in a calm and quiet environment until signs and symptoms of intoxication are minimized [44]. Rapid sedation, fluid resuscitation, and reduction of hyperthermia are the foundation of excited delirium treatment. If autonomic hyperactivity secondary to abnormal dopamine processing is to blame for the clinical presentation of excited delirium, then rapid sedation of the patient and attenuation of catecholamines is the goal. Benzodiazepines, neuroleptics, or both in combination are commonly used to treat agitated patients. However, to date, there are not published double-blind, randomized, placebo controlled trials comparing the efficacy and safety of benzodiazepines to neuroleptics or the combination of benzodiazepines and neuroleptics in 2C intoxication or excited delirium. Benzodiazepines can be used to treat agitation, hypertension, tachycardia, and hyperthermia [44, 45]. Neuroleptics can be used in the setting of hallucinogen-induced agitation, although some controversy exists regarding their use. Although droperidol, haloperidol, risperidone, and ziprasidone can be used to treat agitated patients, they are purported to potentially exacerbate panic and visual symptomatology [46]. Some excited delirium deaths have been thought to be due to ventricular dysythmias and sudden cardiac death related to QT prolongation. However, most excited delirium deaths that occur while on a monitor have shown asystolic or ventricular escape rhythms and have not been secondary to prolonged QT syndrome degenerating to ventricular tachycardia or ventricular fibrillation [47]. However, given the black box warning regarding neuroleptics causing QT prolongation and small risk for ventricular dysrhythmias, if a patient is known to have QT prolongation, neuroleptics may be avoided. Despite this, neuroleptics have a long clinical history of safe use for chemical restraint in undifferentiated agitation and may offer a mechanism by which to regulate dopamine in excited delirium [46, 48–50]. Studies by Battaglia and Bieniek et al. showed superior efficacy with a combination of haloperidol and lorazapam as compared to either drug alone for psychotic agitation [49].
Despite established efficacy of benzodiazepines and neuroleptics, they have a slow onset of action via the intramuscular (IM) route. Studies examining time to adequate sedation for IM droperidol or IM haloperidol administered for control of acute agitation have demonstrated delays of approximately 15–30 min until peak sedation was achieved [49–51]. Excited delirium patients may not have this amount of time to spare. Burnett et al. suggest ketamine as an alternative chemical restraint agent for patients in excited delirium, and they are not alone in this suggestion [52, 53]. Benefits of ketamine include rapid onset, predictable ability to induce a dissociated state, and lack of cardiovascular or respiratory depression. However, ketamine may result in airway compromise, including laryngospasm and hypoxia, or have stimulatory cardiovascular effects resulting in increased tachycardia and hypertension.
Hyperthermia (temperature ≥40 °C) can be treated with sedation and rapid cooling. Controlling excessive muscle activity is important in treating hyperthermia [54]. For severely hyperthermic patients, sedation, intubation and extended paralysis with rocuronium or veronium may be necessary [54]. Use of these non-depolarizing agents and intubation with mechanical ventilation can be supplemented with external cooling [54]. Antipyretics have no place in the treatment of drug-induced hyperthermia as they work by lowering the hypothalamic set point in febrile patients. There is some concern that hyperthermia occurring in excited delirium results from specific dopamine transport derangements as occurs in neuroleptic malignant syndrome [47]. Therefore, using neuroleptics could be argued as contraindicated. However, as mentioned previously, neuroleptics have a long history of safe use in undifferentiated agitation, and it is felt their benefit of use outweighs this risk.
Conclusion
Ring-substituted phenethylamines, commonly known as 2Cs, are designer drugs that are emerging as new drugs of abuse. Currently, the amount of published literature concerning 2Cs is limited. There are a few 2Cs that have been studied, i.e., 2C-T-7, 2C-B, and 2C-E, so information about the pharmacology, pharmacokinetics, and pharmacodynamics, while low in volume, is becoming available. A syndrome consistent with excited delirium including severe agitation, aggression, violence, seizures, and hyperthermia is consistently depicted in lethal 2C cases. There are currently no antidotes for 2C intoxication. Treatment is limited to supportive care but should include rapid sedation and aggressive treatment of severe hyperthermia in 2C cases presenting with signs and symptoms consistent with a syndrome of excited delirium.
Contributor Information
Be Vang Dean, Email: bevangdean@gmail.com.
Samuel J. Stellpflug, Phone: +1-651-7175845, FAX: +1-651-2545216, Email: samuel.j.stellpflug@healthpartners.com
Aaron M. Burnett, Email: aaron.m.burnett@healthpartners.com
Kristin M. Engebretsen, Email: kristin.m.engebretsen@healthpartners.com
References
- 1.Drug Enforcement Administration [webpage on the Internet]. Drugs of abuse, 2011 edition. Washington, DC: Drug Enforcement Administration [updated 2012 Jun 20]. Available from: http://www.justice.gov/dea/docs/drugs_of_abuse_2011.pdf. Accessed 12 Feb 2013
- 2.Haroz RH, Greenberg MI. New drugs of abuse in North America. Clin Lab Med. 2006;26:147–164. doi: 10.1016/j.cll.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Shulgin AT, Shulgin A. PIHKAL: a chemical love story. Berkeley: Transform Press; 1991. [Google Scholar]
- 4.Erowid[webpage on the Internet]. Erowid 2C-B Vault Basics. Grass Valley, CA; Erowid [updated 2011 Feb 11]. Available from: http://www.erowid.org/chemicals/2cb/2cb_basics.shtml. Accessed 12 Feb 2013
- 5.Wikipedia [webpage on the Internet]. 2C (psychedelics). San Francisco, CA; Wikipedia Foundation, Inc. [updated 2012 Nov 27]. Available from: http://en.wikipedia.org/wiki/2C_%28psychedelics%29. Accessed 12 Feb 2013
- 6.National Drug Intelligence Center [webpage on the Internet]. Information bulletin:2C-B (Nexus) reappears on the club drug scene. Johnstown, PA: National Drug Intelligence Center [updated 2001 May]. Available from: http://www.justice.gov/archive/ndic/pubs0/665/index.htm#Contents. Accessed 12 Feb 2013
- 7.Drug Enforcement Administration [webpage on the Internet]. 4-bromo-2,5-dimethoxyphenethylamine information sheet. Washington, DC: Drug Enforcement Administration [updated 2011 Feb 16]. Available from: http://www.deadiversion.usdoj.gov/drugs_concern/bromo_dmp/bromo_dmp.pdf. Accessed 12 Feb 2013
- 8.European Monitoring Centre for Drugs and Drug Addiction [webpage on the Internet]. 2009 annual report on the state of the drugs problem in Europe. Lisbon: European Monitoring Centre for Drugs and Drug Addiction [updated 2009 Nov 3] Available from: http://www.emcdda.europa.eu/attachements.cfm/att_93236_EN_EMCDDA_AR2009_EN.pdf. Accessed 12 Feb 2013
- 9.Drug Enforcement Administration [webpage on the Internet]. 4-iodo-2,5-dimethoxyphenethylamine information sheet. Washington, DC: Drug Enforcement Administration [updated 2011 Aug 5]. Available from: http://www.deadiversion.usdoj.gov/drugs_concern/2c_i.pdf. Accessed 12 Feb 2013
- 10.Office of Diversion Control [webpage on the Internet]. Drugs and chemicals of concern. Washington, DC: Drug Enforcement Administration [updated 2013 Jan 24] Available from: http://www.deadiversion.usdoj.gov/drugs_concern/index.html. Accessed 12 Feb 2013
- 11.112th Congress [webpage on the Internet]. S. 3187: Food and Drug Administration Safety and Innovation Act. Section 1152. Washington, DC: Library of Congress [updated 2012 Jul 10]. Available from: http://beta.congress.gov/bill/112th-congress/senate-bill/3187. Accessed 12 Feb 2013
- 12.Sanders B, Lankenau SE, Bloom JJ, Hathazi D. Research chemicals: tryptamine and phenethylamine use among high-risk youths. Subst Use Misuse. 2008;43:389–402. doi: 10.1080/00952990701202970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson MP, Mathis CA, Shulgin AT, Hoffman AJ, Nichols DE. [1251]-2-(2,5-dimethoxy-4-iodophenyl)aminoethane ([1251]-2C-I) as a label for the 5-HT2 receptor in rat frontal cortex. Pharmacol Biochem Behav. 1990;35:211–217. doi: 10.1016/0091-3057(90)90228-A. [DOI] [PubMed] [Google Scholar]
- 14.Villalobos CA, Bull P, Saez P, Cassels BK, Huidobro-Toro JP. 4-bromo-2,5-dimethoxyphenethylamine (2C-B) and structurally related phenylethylamines are potent 5-HT2A receptor antagonists in Xenopus laevis oocytes. Br J Pharmacol. 2004;141:1167–1174. doi: 10.1038/sj.bjp.0705722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maurer HH. Chemistry, pharmacology, and metabolism of emerging drugs of abuse. Ther Drug Monit. 2010;32:544–549. doi: 10.1097/FTD.0b013e3181eea318. [DOI] [PubMed] [Google Scholar]
- 16.Monte AP, Marona-Lewicka D, Parker MA, Wainscott DB, Nelson DL, Nichols DE. Dihydrobenzofuran analogues of hallucinogens. Models of 4-substituted (2,5-dimethoxyphenyl) alkylamine derivatives with rigified methoxy groups. J Med Chem. 1996;39:2953–2961. doi: 10.1021/jm960199j. [DOI] [PubMed] [Google Scholar]
- 17.Drug Enforcement Administration [webpage on the Internet]. 2,5-dimethoxy-4-(n)-propylthiophenethylamine information sheet. Washington, DC: Drug Enforcement Administration [updated 2011 Feb 16; cited 2013 Feb 12] Available from: http://www.deadiversion.usdoj.gov/drugs_concern/2ct7.pdf
- 18.Carmo H, Hengstler JG, de Boer D, Ringel M, Remião F, Carvalho F, Fernandes E, dos Reys LA, Oesch F, de Lourdes Bastos M. Metabolic pathways of 4-bromo-2,5-dimethoxyphenethylamine (2C-B): analysis of phase I metabolism with hepatocytes of six species including human. Toxicology. 2005;206:75–89. doi: 10.1016/j.tox.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Davies S, Wood DM, Smith G, Smith G, Button J, Ramsey J, Archer R, Holt DW, Dargan PI. Purchasing “legal highs” on the internet—is there consistency in what you get? Q J Med. 2010;103:489–493. doi: 10.1093/qjmed/hcq056. [DOI] [PubMed] [Google Scholar]
- 20.Meyer MR, Maurer HH. Metabolism of designer drugs of abuse: an updated review. Curr Drug Metabolism. 2010;11:468–482. doi: 10.2174/138920010791526042. [DOI] [PubMed] [Google Scholar]
- 21.Theobald DS, Putz M, Schneider E, et al. New designer drug 4-iodo-2,5-dimethoxy-beta-phenethylamine (2C-I): studies on its metabolism and toxicological detection in rat urine using gas chromatographic/mass spectrometric and capillary electrophoretic/mass spectrometric techniques. J Mass Spectr. 2006;41:872–886. doi: 10.1002/jms.1045. [DOI] [PubMed] [Google Scholar]
- 22.Theobald DS, Maurer HH. Identification of monoamine oxidase and cytochrome P450 isoenzymes involved in the deanimation of phenethylamine-derived designer drugs (2C-series) Biochem Pharmacol. 2007;73:287–297. doi: 10.1016/j.bcp.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 23.Cole MD, Lea C, Oxley N. 4-bromo-2,5-dimethoxyphenethylamine (2C-B): a review of public domain literature. Sci Justice. 2002;42:223–224. doi: 10.1016/S1355-0306(02)71832-7. [DOI] [PubMed] [Google Scholar]
- 24.Nagai F, Nonaka R, Hisashi S, Kamimura K. The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain. Eur J Pharmacol. 2007;559:132–137. doi: 10.1016/j.ejphar.2006.11.075. [DOI] [PubMed] [Google Scholar]
- 25.Vilke GM, DeBard ML, Chan TC, et al. Excited delirium syndrome (ExDS): defining based on a review of the literature. J Emerg Med. 2012;43:897–905. doi: 10.1016/j.jemermed.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 26.Hoffman L. ACEP Recognizes excited Delirium Syndrome. EMNOW’s ACEP Scientific Assembly Edition: October 2009
- 27.Mash DC, Pablo J, Ouyang Q, et al. Dopamine transport function is elevated in cocaine users. J Neurochem. 2002;7:2564–2571. doi: 10.1046/j.1471-4159.2002.00820.x. [DOI] [PubMed] [Google Scholar]
- 28.Stephens BG, Jentzen JM, Karch S, et al. Criteria for the interpretation of cocaine levels human biological samples and their relation to the cause of death. Am J Forensic Med Pathol. 2004;25:1–10. doi: 10.1097/01.paf.0000118960.58334.a9. [DOI] [PubMed] [Google Scholar]
- 29.Staley JK, Talbot JZ, Ciliax BJ, et al. Radioligand binding and immunoautoradiographic evidence for lack of toxicity to dopaminergic nerve terminals in human cocaine overdose victims. Brain Res. 1997;747(2):219–229. doi: 10.1016/S0006-8993(96)01196-1. [DOI] [PubMed] [Google Scholar]
- 30.Little KY, McLaughlin DP, Zhang L, et al. Brain dopamine transporter messenger RNA and binding sites in cocaine users: a postmortem study. Arch Gen Psychiatry. 1998;55:793–799. doi: 10.1001/archpsyc.55.9.793. [DOI] [PubMed] [Google Scholar]
- 31.Wilson JM, Levey AI, Bergeron C, Kalasinsky K, Ang L, Peretti F, Adams VI, Smialek J, Anderson WR, Shannak K, Deck J, Niznik HB, Kish SJ. Striatal dopamine, dopamine transporter, and vesicular monoamine transporter in chronic cocaine users. Ann Neurol. 1996;40:428–439. doi: 10.1002/ana.410400312. [DOI] [PubMed] [Google Scholar]
- 32.Staley JK, Welti CV, Ruttenber AJ. Altered dopaminergic synaptic markers in cocaine psychosis and sudden death. NIDA Res Monogr Series. 1995;153:491. [Google Scholar]
- 33.Schmauss C, Haroutunian V, Davis KL, Davidson M. Selective loss of dopamine D3-type receptor mRNA expression in parietal and motor cortices of patients with chronic schizophrenia. Proc Natl Acad Sci. 1993;90:8942–8946. doi: 10.1073/pnas.90.19.8942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Topeff JM, Ellsworth H, Willhite LA, Bangh SA, Edwards EM, Cole JB. A case series of symptomatic patients, including one fatality, following 2C-E exposure. Clin Toxicol. 2011;49:526. [Google Scholar]
- 35.Erowid [webpage on the Internet]. A reported 2C-T-7 death. Grass Valley, CA; Erowid [updated 2012 Apr 18]. Available from: http://www.erowid.org/chemicals/2ct7/2ct7_death1.shtml. Accessed 16 Feb 2013
- 36.Erowid [webpage on the Internet]. Second reported 2C-T-7 death. Grass Valley, CA; Erowid [updated 2012 Apr 18]. Available from: http://www.erowid.org/chemicals/2ct7/2ct7_death2.shtml. Accessed 16 Feb 2013
- 37.Erowid [webpage on the Internet]. Third confirmed 2C-T-7 death. Grass Valley, CA; Erowid [updated 2012 Apr 18]. Available from: http://www.erowid.org/chemicals/2ct7/2ct7_death3.shtml. Accessed 16 Feb 2013
- 38.Erowid [webpage on the Internet]. LA man’s death leads to drug arrests. Grass Valley, CA; Erowid [updated 2012 Apr 18]. Available from: http://www.erowid.org/chemicals/2ct21/2ct21_article1.shtml. Accessed 16 Feb 2013
- 39.Rupar A [webpage on the Internet]. Adam Budge, 18, charged with murder following friend’s synthetic drug overdose. Minneapolis: City Pages [updated 2012 Jun 21]. Available from: http://blogs.citypages.com/blotter/2012/06/adam_budge_18_charged_with_murder_following_friends_synthetic_drug_overdose.php. Accessed 16 Feb 2013
- 40.Walsh P [webpage on the Internet]. U.S. indicts four in synthetic drug deaths. Minneapolis: Star Tribune [updated 2012 Dec 27]. Available from: http://www.startribune.com/printarticle/?id=184934801. Accessed 16 Feb 2013
- 41.Chanen D [webpage on the Internet]. Blaine man arrested after overdose at house party. Minneapolis: Star Tribune [updated 2011 Mar 18]. Available from: http://www.startribune.com/local/north/118182584.html. Accessed 12 Feb 2013
- 42.Miyajima M, Matsumoto T, Ito S. 2C-T-4 intoxication: acute psychosis caused by a designer drug. Psychiatr Clin Neurosci. 2008;62:243. doi: 10.1111/j.1440-1819.2008.01764.x. [DOI] [PubMed] [Google Scholar]
- 43.Huang HH, Bai YM. Persistent psychosis after ingestion of a single tablet of ‘2C-B’. Prog Neuro-Psychopharmacol Biol Psychiat. 2011;35:293–294. doi: 10.1016/j.pnpbp.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 44.Taylor RL, Maurer J, Tinklenberg JR. Management of “bad trips” in an evolving drug scene. JAMA. 1970;214:422. doi: 10.1001/jama.1970.03170290018003. [DOI] [PubMed] [Google Scholar]
- 45.Spain D, Crilly J, Whyte I, Jenner L, Carr V, Baker A. Safety and effectiveness of high-dose midazolam for severe behavioural disturbance in an emergency department with suspected psychostimulant affected patients. Emerg Med Australas. 2008;20:112. doi: 10.1111/j.1742-6723.2008.01066.x. [DOI] [PubMed] [Google Scholar]
- 46.Nobay F, Simon B, Levitt M, Dresden G. Midazolam versus haloperidol versus lorazepam in the chemical restraint of violent and severely agitated patients. Acad Emerg Med. 2004;11:744–749. doi: 10.1197/j.aem.2003.06.015. [DOI] [PubMed] [Google Scholar]
- 47.Vilke GM, Bozeman WP, Dawes DM, DeMers G, Wilson MP. Excited delirium syndrome (ExDs): treatment options and considerations. J For Leg Med. 2012;19:117–121. doi: 10.1016/j.jflm.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 48.Alexander J, Tharyan P, Adams C, John T, Mol C, Philip J. Rapid tranquillisation of violent or agitated patients in a psychiatric emergency setting. Pragmatic randomised trial of intramuscular lorazepam v. haloperidol plus promethazine. Br J Psychiatry. 2004;185:63–69. doi: 10.1192/bjp.185.1.63. [DOI] [PubMed] [Google Scholar]
- 49.Battaglia J, Moss S, Rush J, Kang J, Mendoza R, Leedom L, Dubin W, McGlynn C, Goodman L. Haloperidol, lorazepam, or both for psychotic agitation? A multicenter, prospective, double-blind, emergency department study. Am J Emerg Med. 1997;15:335–340. doi: 10.1016/S0735-6757(97)90119-4. [DOI] [PubMed] [Google Scholar]
- 50.Thomas H. Droperidol versus haloperidol for chemical restraint of agitated and combative patients. Ann Emerg Med. 1992;21:407–413. doi: 10.1016/S0196-0644(05)82660-5. [DOI] [PubMed] [Google Scholar]
- 51.Martel M, Sterzinger A, Miner J, Clinton J, Biros M. Management of acute undifferentiated agitation in the Emergency Department: a randomized double-blind trial of droperidol, ziprasidone and midazolam. Acad Emerg Med. 2005;12:1167–1172. doi: 10.1111/j.1553-2712.2005.tb01492.x. [DOI] [PubMed] [Google Scholar]
- 52.Burnett AM, Salzman JG, Griffith KR, Kroeger B, Frascone RJ. The Emergency Department experience with prehospital ketamine: a case series of 13 patients. Prehosp Emerg Care. 2012;16:1–7. doi: 10.3109/10903127.2011.627108. [DOI] [PubMed] [Google Scholar]
- 53.Takeuchi A, Ahern TL, Henderson SO. Excited delirium. West J Emerg Med. 2011;12:77–83. [PMC free article] [PubMed] [Google Scholar]
- 54.Rusyniac DE, Sprague JE (2006) Toxin induced hyperthermic syndromes. Med Clin N Am 2005; 89: 1277–1296. Review. Erratum in: Med Clin North Am 90: 261–2 [DOI] [PubMed]