Abstract
Adenosine diphosphate (ADP)-receptor antagonists are widely used for thrombus prevention, although reversing their platelet dysfunction is difficult. This study evaluated the ability of desmopressin to reverse clopidogrel-induced platelet dysfunction. Sprague–Dawley rats received either clopidogrel (30 mg/kg) or placebo, followed 4 h later by saline or desmopressin (0.15, 0.3, or 0.6 μg/kg). Bleeding times and platelet aggregation studies were subsequently performed. A bleeding time >25 min was considered “prolonged.” The median bleeding time for clopidogrel-exposed rats was 21 min, vs. 6 min for controls (p < 0.01). Progressively higher doses of 1-deamino-8-d-arginine vasopressin (DDAVP) were associated with a reduced number of rats with prolonged bleeding time (p = 0.001). Higher doses of DDAVP were also associated with a reduction in the median (IQR) bleeding time; 29 (13.5–30) min in rats receiving clopidogrel without DDAVP vs. 19 (12–28) min in rats receiving clopidogrel and 0.6 μg/kg DDAVP. The step-wise dosing of DDAVP resulted in a 54 % reduction in meeting the endpoint of prolonged bleeding time (OR 0.46; p = 0.025; 95 % CI 0.23–0.91). Platelet aggregation was observed in all control rats, but only some of those clopidogrel-treated rats who received 0.6 μg/kg DDAVP. In this model of an ADP-receptor antagonist, DDAVP results in partial reversal of clopidogrel-induced platelet dysfunction.
Keywords: Clopidogrel, DDAVP, Desmopressin, Bleeding, Overdose
Introduction
Acute coronary syndrome is among the most common causes of adult deaths in western society. In the USA, more than 1.5 million Americans are diagnosed annually [1]. This syndrome occurs when vascular endothelium is damaged, clot formation ensues, with the result of a partial or complete occlusion of the blood vessel [2]. Because this condition is so common, numerous medications have been developed in recent years to combat this deadly disease [3]. Clopidogrel is one such medication that is widely utilized for secondary prevention of ischemic events in patients with cardiovascular disease.
Following injury to the vascular endothelium, a complex series of events occur, ultimately resulting in clot formation and stabilization. The damaged endothelium will express both collagen and tissue factor [2]. Platelet activation and aggregation occur as a result of collagen expression, while thrombin production occurs as a result of the expression of tissue factor [4, 5]. Tissue factor forms a complex with factor VIIa, which subsequently is the stimulus for the activation of factor IX, resulting in a complex series of events which culminates in thrombin generation [4, 5]. Thrombin serves several functions, including platelet activation [4]. Following activation, the platelet will release numerous chemicals, including epinephrine, adenosine diphosphate (ADP), thromboxane A2, and serotonin [5–7]. Adenosine diphosphate binds to several G-protein receptors, including P2Y12 and P2X1 [7]. The binding of ADP to the P2X1 receptor results in an influx of calcium, resulting in a conformational change in the platelet shape, thereby further assisting in platelet activation [7]. The ADP-P2Y12 complex ultimately activates the glycoprotein IIb/IIIa receptor, resulting in further thromboxane production and platelet aggregation [8, 9].
In order for platelets to adhere to the vascular endothelium, von Willebrand’s factor (VWF) is needed to combine with glycoprotein 1b-V-IX complex [10]. This binding induces expression of the glycoprotein IIb/IIIa (GP2b/3a) receptor, which is needed for subsequent platelet aggregation [11].
Clopidogrel is a second-generation thienopyridine, which inhibits platelet aggregation via noncompetitive antagonism of the P2Y12 ADP receptor [9, 12]. Clopidogrel is a prodrug which requires metabolism to the active form via the cytochrome P450 isoenzyme 3A4 [9].
While numerous complications and adverse events have been described with clopidogrel, perhaps one of the most concerning events would be the development of bleeding [9]. Unlike anticoagulant medications in which specific therapies exist to reverse the drug should bleeding occur, no such antidote currently exists for antiplatelet agents.
Desmopressin (1-deamino-8-d-arginine vasopressin; DDAVP) is a derivative of antidiuretic hormone [13]. Desmopressin results in an increase in the plasma concentrations of factor VIII and VWF [13] and has been used to increase platelet adhesiveness in not only congenital bleeding disorders (e.g., von Willebrand’s disease) but also in acquired platelet dysfunction (e.g., uremia and possibly aspirin exposure) [13, 14]. One small study involving healthy, non-bleeding volunteers 1 h after receiving a loading dose of clopidogrel [15] as well as case reports of subjects therapeutically taking clopidogrel daily have demonstrated possible improvement in platelet aggregation following the administration of DDAVP [16–18]. However, it is not known if DDAVP will improve platelet aggregation following a clopidogrel overdose. Furthermore, the ideal dose of DDAVP in this setting is not known.
The purpose of this study is to assess if the use of DDAVP can improve platelet aggregation and reduce bleeding in a rodent model of clopidogrel overdose.
Methods
Study Protocol and Bleeding Time
All the animal experiments were approved by the USC Institutional Animal Care and Use Committee. Sixty adult male Sprague–Dawley rats weighing between 200 and 250 g each were purchased from a commercial vendor (Harlan Animal Research Laboratories). On the day prior to the experiment, each animal was housed in routine animal quarters, with ad-lib access to food and water.
Each animal was randomly assigned to one of six treatment groups, receiving a combination of orally administered clopidogrel 30 mg/kg or placebo, followed by an intravenous injection of DDAVP [Sanofi Aventis] or 0.9 % normal saline. The six groups were as follows: oral placebo and IV placebo (group 1); oral placebo + 0.3 μg/kg IV DDAVP (group 2); oral clopidogrel + IV placebo (group 3); oral clopidogrel + 0.15 μg/kg IV DDAVP (group 4); oral clopidogrel + 0.3 μg/kg IV DDAVP (group 5); oral clopidogrel + 0.6 μg/kg IV DDAVP (group 6).
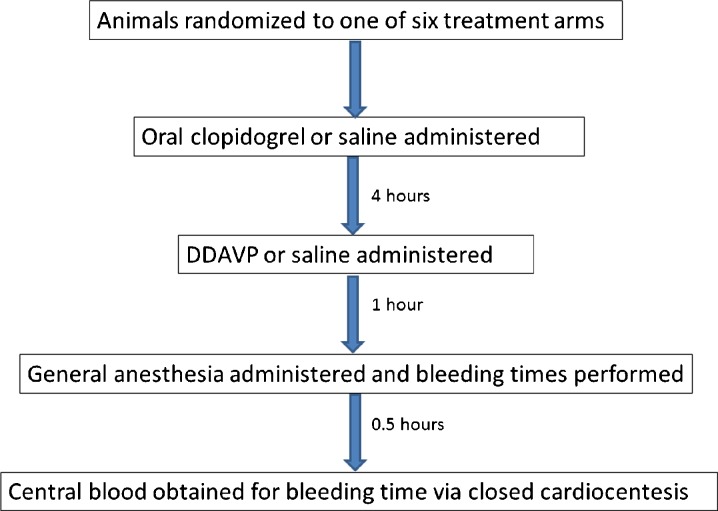
At the start of the experiment (time zero), each rat had an 18-gauge gavage tube inserted in its mouth, and a total of 3 mL of liquid was administered. Those animals assigned to receive clopidogrel received oral clopidogrel (concentration 75 mg/10 mL), followed by enough saline to make a total of 3 mL, or 3 mL saline. The rats were returned to their quarters. Four hours after the gavage, the rats were placed in a commercially available rat immobilizer (Braintree Scientific), and the tail vein was canulated with a 22 gauge needle. An injection of either saline or DDAVP followed by saline was administered in such a manner that ensured each animal received an equivalent volume of 3 mL intravenously. The rats were then returned to their cage for an additional 1 h. Five hours after the start of the experiment, each rat was induced with general anesthesia with 1–4 % isoflurane via nose cone. Once anesthetized, a standard incision (10 mm long, 1.5 mm deep, 8–9 cm from the tail lip) was performed for bleeding time. Bleeding time was measured every minute for up to 30 min, using filter paper to blot until either bleeding stopped or 30 min elapsed (Fig. 1). A prolonged bleeding time was defined a priori as any bleeding time that exceeded five times the upper limit of normal (i.e., 25 min). Thirty minutes after the start of anesthesia (5.5 h after the initial oral drug was administered), the animals were euthanized via exsanguination. Central blood was collected and bleeding studies were performed.
Fig. 1.
Flow diagram of treatment
Platelet Aggregation
In addition to measuring bleeding time, the study also examined platelet aggregation via a Chronalog 490 Platelet aggregometer, using rat platelet-rich plasma (PRP). Whole rat blood was placed into 0.1 M sodium citrate (final concentration 10 mM). The blood was centrifuged at 150 g for 20 min at 22 ° C. The supernatant, PRP, was carefully separated. One milliliter of PRP was subsequently placed in a microcentrifuge and centrifuged for an additional minute. The supernatant was removed and retained as platelet poor plasma (PPP). The PPP was the “blank” for the platelet aggregometer. Inhibition of ADP-induced platelet aggregation was monitored at 37 ° C with the addition of 10 micromolar ADP. The amount of inhibition is determined as a percentage of control aggregation.
Outcomes
The primary outcome measure is a reduction in the bleeding time following the administration of DDAVP. This measurement assessed as a group trend (no DDAVP vs. low-dose DDAVP vs. medium-dose DDAVP vs. high-dose DDAVP). In addition, the number of animals with prolonged bleeding time (defined as bleeding that persisted for greater than 25 min) was compared between groups. The secondary outcome measure is a reduction in time to platelet aggregation during the platelet aggregation studies.
Statistical Analysis
Mean values (with standard deviation) were calculated for the ex vivo platelet aggregation. After determining that the bleeding times were non-normally distributed, medians and interquartile ranges were used to report the bleeding time values. Analysis of variance was used to determine differences between groups (set at alpha level of 0.05). Where medians were significantly different, intergroup post hoc multiple comparisons by pairs were performed by the Tukey HSD test.
The normal bleeding time varies with methodology and species of animal. The estimates of actual bleeding times are far less important, than are estimates of the magnitude of change in those times. Assuming a control bleeding time of 140 ± 70 s, and a post-clopidogrel bleeding time of 280 ± 140 s, the sample size required for 80 % power is 10 animals per group. The power calculation was performed using STATA 12MP (StataCorp, College Station TX), the sample size required for 80 % power is 10 animals per group.
Results
Initial randomization involved 10 rats per subject group. One rat initially assigned to the oral clopidogrel followed by high-dose DDAVP (group 6) was incorrectly placed in the cohort of rats receiving oral and IV placebo. As such, nine animals were in group 6, and 11 animals in group 1. The data were analyzed based on ultimate treatment allocation.
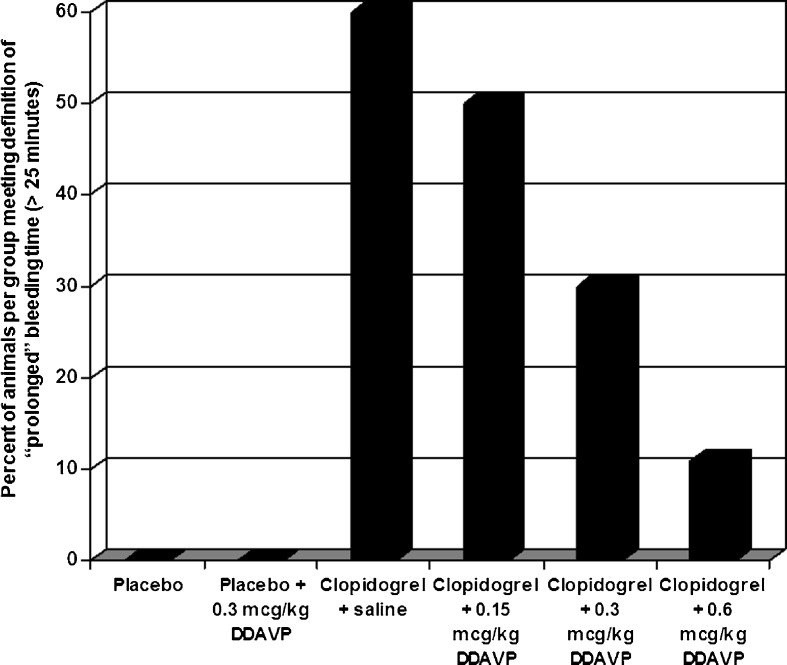
The median (IQR) bleeding time for each animal cohort is demonstrated in Table 1. Median (IQR) bleeding time for clopidogrel-exposed rats, regardless of DDAVP dosing, was 21 (11.5–30) min vs. 6 (5–7) min for the rats receiving oral placebo (p < 0.01). When analyzing clopidogrel-poisoned rats, progressively higher doses of DDAVP were associated with reduced number of rats with a prolonged bleeding time (Fig. 2); p = 0.001, and a reduction in the median (IQR) bleeding time 29 (13.5–30) min in rats receiving clopidogrel without DDAVP vs. 19 (12–28) min in rats receiving clopidogrel and 0.6 μg/kg DDAVP. Comparing a step-wise dosing of DDAVP (none to low; low to medium; and medium to high), there was a 54 % reduction in meeting the endpoint of prolonged bleeding time (OR 0.46; p = 0.025; 95 % CI 0.23–0.91) with each escalation in the dose of DDAVP.
Table 1.
Bleeding times by treatment randomization
| Oral treatment | IV treatment | Median bleeding time (min) | IQR |
|---|---|---|---|
| Saline | Saline | 6 | 3–7 |
| Saline | 0.3 μg/kg DDAVP | 6 | 5.5–7.5 |
| Clopidogrel | Saline | 29 | 11–30 |
| Clopidogrel | 0.15 μg/kg DDAVP | 23.5 | 13–30 |
| Clopidogrel | 0.3 μg/kg DDAVP | 19 | 10–26 |
| Clopidogrel | 0.6 μg/kg DDAVP | 19 | 11–21 |
Fig. 2.
Percentage of animals in each treatment group with prolonged bleeding time
When performing platelet aggregation studies, the rats that received oral placebo followed by either IV saline (group1) or DDAVP (group 2) had a mean (SD) platelet aggregation of 74 (±4) % and 81 (±6) %. Among the rats who received clopidogrel, only those with 0.6 μg/kg DDAVP were able to demonstrate any platelet aggregation (11 (±6) %). Platelet aggregation was not able to be demonstrated in those who got oral clopidogrel followed by saline or lower dose DDAVP.
Discussion
In this rodent model of clopidogrel overdose, DDAVP in any dose partially reversed the antiplatelet effects from both a clinical (bleeding time) standpoint, while only high doses (0.6 μg/kg) reversed platelet aggregation. We found a clear dose–response curve, with increased benefit at higher doses. It is possible further reversal would have occurred with even higher dose of DDAVP. Nonetheless, the median times were not significantly different between the 0.3 and 0.6 μg/kg dosage. This apparent discrepancy is likely due to non-normal distribution of the data. Thus, while the median bleeding time was not different between the medium- and high-dose DDAVP, it was only after administration of the high dose of DDAVP (0.6 μg/kg) that any platelet aggregation was able to be observed.
Leithauser examined the effects of desmopressin on platelet membrane glycoproteins and platelet aggregation in healthy volunteers consuming clopidogrel [15]. In their study, the ristocetin cofactor, platelet reactivity, and platelet aggregation rose significantly following the administration of 300 μg DDAVP nasal spray. The density of the glycoprotein IIb/IIIa receptors per platelet did not change, and the density of the glycoprotein Ib/IX receptor also rose, but not significantly. Their study, like ours, demonstrates laboratory evidence of improvement following desmopressin administration. The current study, however, differs in that it shows not only clinical improvement (bleeding time) but also involves an overdose model, and not simply healthy volunteers.
The observed effect was only partial reversal. The clinical applicability and utility of partial reversal in a patient experiencing a life-threatening hemorrhage is not known. This model was an overdose model; the dose of clopidogrel was nearly 30-fold greater than would be observed in therapeutic dosing. Because clopidogrel binds to the P2Y12 binding site irreversibly, however, it is unlikely that full reversal would occur in any setting. However, it is likely that reversibility would be more pronounced following therapeutic dosing instead of overdose. Nonetheless, to preferentially create longer bleeding times and facilitate detection of a difference with smaller number of animals, an overdose model was chosen.
In this study, DDAVP was administered 4 h after clopidogrel, and platelet aggregation studies were performed 5 h after clopidogrel administration. Clopidogrel is a prodrug and requires transformation into the active metabolite. In humans receiving 600 mg of clopidogrel, the maximal effects of platelet aggregation are observed after 2 h post clopidogrel administration [19]. It is not known definitively after an overdose when maximal platelet inhibition will occur. Therefore, in this study, 5 h was chosen to ensure adequate platelet inhibition had occurred. Based on the differences in bleeding time, it is likely that this time was adequate.
Limitations
No data were collected on potential adverse events associated with the use of DDAVP in this setting. It is known that hyponatremia can occur following DDAVP administration; however, it is unlikely that a single dose of DDAVP would produce clinically relevant hyponatremia. Furthermore, the benefit of reversal of clopidogrel would need to be weighed against the risks of its reversal, such as stent thrombosis with subsequent myocardial infarction. These risks would argue against the use of DDAVP for reversal in the setting of nonlife-threatening bleeds. Thus, the dose needed to do harm in humans is not known, but would likely be quite large. It is now known if the results of this study can be directly extrapolated to humans consuming therapeutic clopidogrel.
Conclusions
Desmopressin produced a dose-dependent reversibility in clopidogrel-induced platelet dysfunction in this overdose model.
Acknowledgments
Conflicts of Interest
There are no financial, litigious, or other conflicts of interest to disclose.
Footnotes
An erratum to this article is available at http://dx.doi.org/10.1007/s13181-013-0308-9.
References
- 1.Kolansky DM. Acute coronary syndromes: morbidity, mortality, and pharmacoeconomic burden. Am J Manag Care. 2009;15(2Suppl):S36–41. [PubMed] [Google Scholar]
- 2.Corti R, Fuster V, Badimon JJ. Pathogenetic concepts of acute coronary syndromes. J Am Coll Cardiol. 2003;41:7–14. doi: 10.1016/S0735-1097(02)02833-4. [DOI] [PubMed] [Google Scholar]
- 3.Valgimigli M, Minarelli M. Triple antiplatelet therapy in acute coronary syndromes. Drugs. 2011;71:1703–19. doi: 10.2165/11594100-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Furie B (2009) Pathogenesis of thrombosis. Hematology Am Soc Hematol Educ Program 255-258 [DOI] [PubMed]
- 5.Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008;359:938–49. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 6.Angiolillo DJ, Capodanno D, Goto S. Platelet thrombin receptor antagonism and atherothrombosis. Eur Heart J. 2010;31:17–28. doi: 10.1093/eurheartj/ehp504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murugappa S, Kunapuli SP. The role of ADP receptors in platelet function. Front Biosci. 2006;11:1977–86. doi: 10.2741/1939. [DOI] [PubMed] [Google Scholar]
- 8.Wallentin L. P2Y(12) inhibitors: differences in properties and mechanisms of action and potential consequences for clinical use. Eur Heart J. 2009;30:1964–77. doi: 10.1093/eurheartj/ehp296. [DOI] [PubMed] [Google Scholar]
- 9.Plosker GL, Lyseng-Williamson KA. Clopidogrel: a review of its use in the prevention of thrombus. Drugs. 2007;67:613–46. doi: 10.2165/00003495-200767040-00013. [DOI] [PubMed] [Google Scholar]
- 10.Berndt MC, Ward CM, DeLuca M, et al. The molecular mechanism of platelet adhesion. Aust NZ J Med. 1995;25:822–30. doi: 10.1111/j.1445-5994.1995.tb02887.x. [DOI] [PubMed] [Google Scholar]
- 11.Arora RR, Rai F. Antiplatelet intervention in acute coronary syndrome. Am J Ther. 2009;16:e29–40. doi: 10.1097/MJT.0b013e31804c7238. [DOI] [PubMed] [Google Scholar]
- 12.Angiolillo DJ. ADP receptor antagonistm: what’s in the pipeline? Am J Cardiovasc Drugs. 2007;7:423–32. doi: 10.2165/00129784-200707060-00005. [DOI] [PubMed] [Google Scholar]
- 13.Coppola A, DiMinno G. Desmopressin in inherited disorders of platelet function. Haemophilia. 2008;14(Suppl 1):31–9. doi: 10.1111/j.1365-2516.2007.01607.x. [DOI] [PubMed] [Google Scholar]
- 14.Franchini M. The use of desmopressin as a hemostatic agent: a concise review. Am J Hematol. 2007;82:731–5. doi: 10.1002/ajh.20940. [DOI] [PubMed] [Google Scholar]
- 15.Leithauser B, Zielske D, Seyfert UT, et al. Effects of desmopressin on platelet membrane glycoproteins and platelet aggregation in volunteers on clopidogrel. Clin Hemorheol Microcirc. 2008;39:293–302. [PubMed] [Google Scholar]
- 16.Naucel FE, de Moraes E, Penido C, et al. Massive nasal bleeding and hemodynamic instability associated with clopidogrel. Pharm World Sci. 2004;26:6–7. doi: 10.1023/B:PHAR.0000013480.17165.6d. [DOI] [PubMed] [Google Scholar]
- 17.Von Heymann C, Schoenfeld H, Sander M, et al. Clopidogrel-related refractory bleeding after coronary artery bypass graft surgery: a rationale for the use of coagulation factor concentrates? Heart Surg Forum. 2005;8:E39–41. doi: 10.1532/HSF98.20041122. [DOI] [PubMed] [Google Scholar]
- 18.Ranucci M, Nano G, Pazzaglia A, et al. Platelet mapping and desmopressin reversal of platelet inhibition during emergency carotid endarterectomy. J Cardiothorac Vasc Anesth. 2007;21:851–4. doi: 10.1053/j.jvca.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Hochholzer W, Trenk D, Frundi D, et al. Time dependence of platelet inhibition after 600-mg loading dose of clopidogrel in a large, unselected cohort of candidates for percutaneous coronary intervention. Circulation. 2005;111:2560–4. doi: 10.1161/01.CIR.0000160869.75810.98. [DOI] [PubMed] [Google Scholar]