Abstract
Introduction
In recent years, cases of severe adverse effects from recreational use of synthetic cannabinoids (SC) have established that these agents represent a novel toxicologic hazard.
Case Report
A 21-year-old male presenting as a vehicular trauma victim was noted with diffuse pulmonary infiltrates related to chronic inhalation of multiple synthetic cannabinoid-containing products. Chest imaging revealed bilateral, subacute lung infiltrates; histopathological analysis of bronchial and alveolar tissues revealed an inflammatory process. An extensive workup failed to identify infectious, malignant, autoimmune, or hematologic causes of the syndrome, and toxicological analysis of the blood and body fluids confirmed the presence of multiple synthetic cannabinoids and metabolites. The patient recovered after an 8-day ICU course, wherein he received antibiotics, steroids, and mechanical ventilation.
Discussion
This case contributes to the currently evolving knowledge about SC agents, adding a rarely described pulmonary complication to the growing list of adverse effects associated with these products.
Keywords: Synthetic cannabinoids, New drugs of abuse, AM-2201, JWH-018, Pulmonary toxicity
Introduction
Synthetic cannabinoids have been distributed as an alternative to Cannabis marijuana over the last several years, and, until very recently, they were considered legal to be sold and distributed [1]. Herbal incense, K2, and Spice are common street names for various products which feature a mixture of psychoactively inert herbs sprayed with synthetic cannabinoids (SC) and sold mainly to youngsters seeking “legal highs” [2, 3]. Identification of the SC agents through routine drug screens is currently not feasible, and specialized laboratory testing must be undertaken to identify one or more among dozens of compounds [4, 5]. Synthetic cannabinoid usage and toxicity is increasing in frequency across the USA and worldwide, with thousands of cases reported to poison control centers and other surveillance networks in the last 4 years [6, 7]. Both animal studies and anecdotal clinical evidence suggest that the SC products may lead to more severe and unusual toxic effects than natural marijuana, possibly due to their potent and unpredictable actions on endogenous cannabinoid receptor systems in the brain [8–13].
A number of adverse effects related to these drugs have been previously reported, including psychosis, seizures, tachycardia, autonomic hyperactivity, and suicidal behavior [5, 14–17]. Presently, we report a case of a 21-year-old male who sustained a prolonged, severe lung injury after chronic inhalational abuse of multiple SC-containing products.
Case Presentation
A 21-year-old man with no prior medical history presented to the emergency department after a motor vehicle collision sustained during a syncopal episode while driving. Per the prehospital reports, as he fainted he drove his vehicle off the road at a slow speed and struck a tree. No passenger space intrusion was noted, and the patient was reportedly wearing his seatbelt.
His initial vitals in the emergency department were as follows: pulse 118, blood pressure 182/108, respiratory rate 45 breaths/min, and oxygen saturation averaging 75 % on room air, which improved to 95 % while breathing 15 L/min oxygen via a non-rebreather face mask. He had no chest wall tenderness but demonstrated worsening dyspnea. He also related a 2-month history of chronic cough, occasional hemoptysis, and at least one prior episode of syncope. On the day prior to presentation, he had begun a course of antibiotics, prescribed by his primary physician, for “strep throat.”
In the emergency department, his respiratory status worsened despite a trial of bilevel positive airway pressure ventilation at 60 % FiO2, necessitating rapid-sequence endotracheal intubation. Almost 200 cc of frank blood was suctioned from the airways after intubation. Aside from minor contusions, no acute traumatic injuries due to the motor vehicle collision were identified.
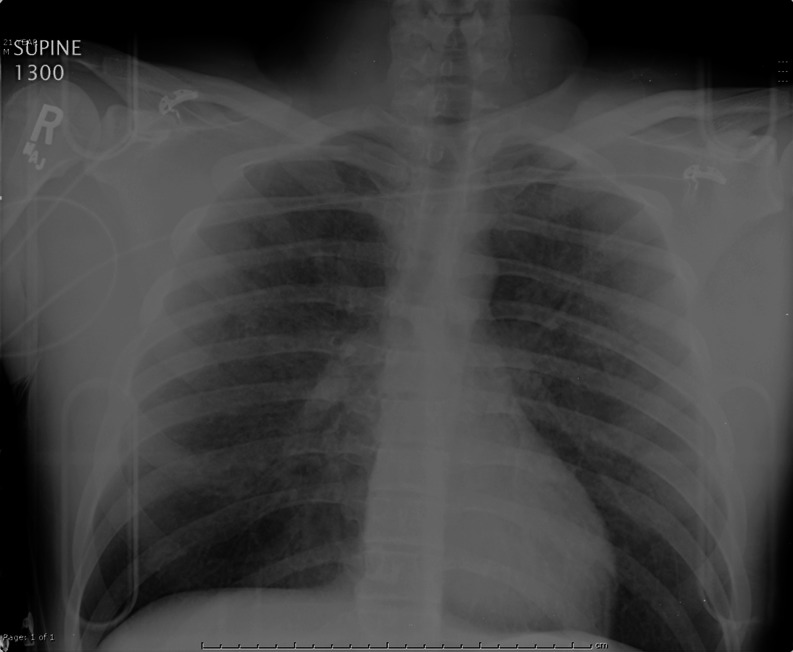
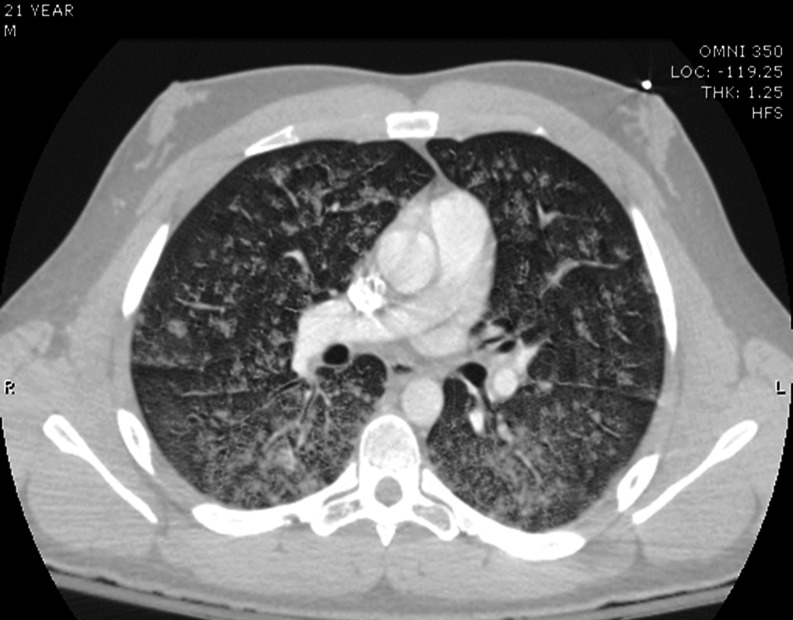
The patient’s initial laboratory results were unremarkable except for leukocytosis (Table 1). Initial chest X-ray (Fig. 1) revealed diffuse, hazy nodular densities throughout both lung fields. A computerized tomography pulmonary angiogram of the chest effectively ruled out acute traumatic pulmonary contusions, but it did reveal extensive and diffuse airspace nodules in a peribronchovascular distribution (Fig. 2). The differential diagnosis for these findings included atypical bacterial infections, infectious viral bronchiolitis, inflammatory bronchiolitis, alveolar hemorrhage, or pulmonary edema.
Table 1.
Initial laboratory data
| Initial result | |
|---|---|
| White blood cell count | 17,600/μL |
| Hemoglobin/hematocrit | 16.3 g/dL/46.9 % |
| Platelets | 334,000/μL |
| Prothrombin time/international normalized ratio | 14.8 s/1.1 |
| Sodium | 138 mEq/L |
| Potassium | 4.2 mg/dL |
| Chloride | 108 mEq/L |
| Bicarbonate | 24 mEq/L |
| Glucose | 168 mg/dL |
| Blood urea nitrogen/creatinine | 14/0.7 mg/dL |
| Creatine kinase | 133 IU/L |
| Arterial pH | 7.27 |
| pCO2 (arterial) | 51 mmHg |
| pO2 (arterial) | 171 mmHg |
| Blood alcohol concentration | 0 mg/dL |
| Lipase, liver enzymes (AST/ALT) | All within normal range |
| Troponin | <0.006 ng/mL |
| Lactic acid | 3.2 mmol/l (normal 0.5–2.2 mmol/L) |
| Urine immunoassay screen for marijuana (delta-9-tetrahydrocannabinol) | Positive |
| Urine immunoassays for amphetamines, barbituates, benzodiazepines, cocaine, opiates, and phencyclidine | Negative |
| Urinalysis (abnormal results) | Protein 70 mg/dL; 11–20 hyaline casts per low power field; no cells |
All values from serum except as noted from urine
Fig. 1.
Chest X-ray on arrival
Fig. 2.
Initial CT pulmonary angiogram
After admission to the medical ICU, the patient remained severely hypoxemic, requiring high peak end-expiratory pressures and 100 % FiO2 on a mechanical ventilator. Broad-spectrum antibiotics, including vancomycin, piperacillin–tazobactam, azithromycin, and fluconazole were initiated, and a mechanical ventilation protocol for ARDS was instituted. High-dose methylprednisolone (1 g daily) was also initiated.
On hospital day 2, the patient was noted to have subcutaneous emphysema on exam, and repeat imaging revealed the presence of pneumomediastinum and a left-sided pneumothorax. A left chest thoracostomy tube was placed, followed by bronchoscopy with transbronchial biopsies of the left lower lobe.
An extensive workup (Table 2) for the cause of his pulmonary lesions was undertaken, which failed to identify infectious, malignant, or autoimmune etiologies for his respiratory failure. Laboratory studies revealed all of the following investigations to be negative: HIV test, Legionella and streptococcal urinary antigen, Mycoplasma IgM antibody, influenza A and B (ELISA), RSV testing by PCR, coccidioidomycosis serology, and final blood cultures. Hence, an infectious etiology appeared to be unlikely. A normal ESR, ANA, ANCA, and complement levels effectively ruled out vasculitis or an autoimmune process. Tumor marker levels (bHCG and alpha-fetoprotein) for metastatic testicular cancer were also unremarkable. A two-dimensional echocardiogram revealed a normal ejection fraction (60–65 %) and no other abnormalities.
Table 2.
Inpatient laboratory data
| Test | Result |
|---|---|
| HIV | Non-reactive |
| Legionella urinary antigen | Negative |
| Streptococcal urinary antigen | Negative |
| Mycoplasma Ig M antibody | Negative |
| Influenza A and B (EIA and PCR) | Negative |
| RSV by PCR | Negative |
| ESR | Negative |
| Blood cultures | Negative |
| ANA (anti-neutrophil antibody) test | Not detected |
| ANCA (anti-neutrophil cytoplasmic antibodies) | <1:20 |
| Complement (C3) | 93.5 (80–170 mg/dl) |
| Complement (C4) | 21.6 (12–36 mg/dl) |
| Beta HCG level | <1 (0–3 IU/L) |
| Alpha-fetoprotein level | 1 (0–9 ng/ml) |
| Coccididomycosis serology (complement fixation and immunodiffusion) | Negative |
| 2-D echocardiogram | Ejection fraction 60–65 % , no other abnormalities |
| Broncho-alveolar lavage (BAL) cell count from left upper lobe | Polymorph nuclear cells, 49 %; lymphocytes, 8 %; macrophages, 43 %; no eosinophils |
| Broncho-alveolar lavage (BAL) gram stain, acid-fast bacilli (AFB) stain and culture, fungal smear and culture, and viral cultures | Negative |
| Cytology | Scattered pulmonary macrophages containing refractile brown pigment of non-ferrous origin on iron-specific staining. No malignant cells; hemosiderin-laden macrophages <1 % |
| Pathology | Carcinoid tumorlett (consistent with non-specific bronchitis) |
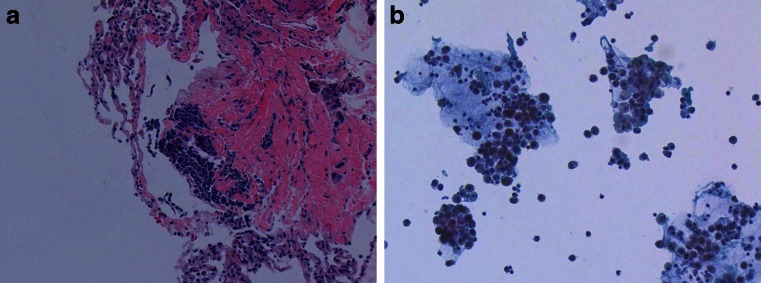
Broncho-alveolar lavage (BAL) with transbronchial biopsies resulted in a non-clearing crimson fluid which did not clear with repeat washings. Histologic analysis revealed lymphocytic infiltrate, and iron-specific cytochemical staining revealed numerous macrophages containing a refractile brown pigment (Fig. 3a, b) that was non-ferruginous in origin. This suggested a process distinct from alveolar hemorrhage with hemosiderin formation. The cell count from the BAL fluid was as follows: polymorphonuclear cells, 49; lymphocytes, 8; macrophages, 43; and no eosinophils. The BAL Gram stain, AFB stains and cultures, fungal smear and culture, and viral cultures were all unremarkable.
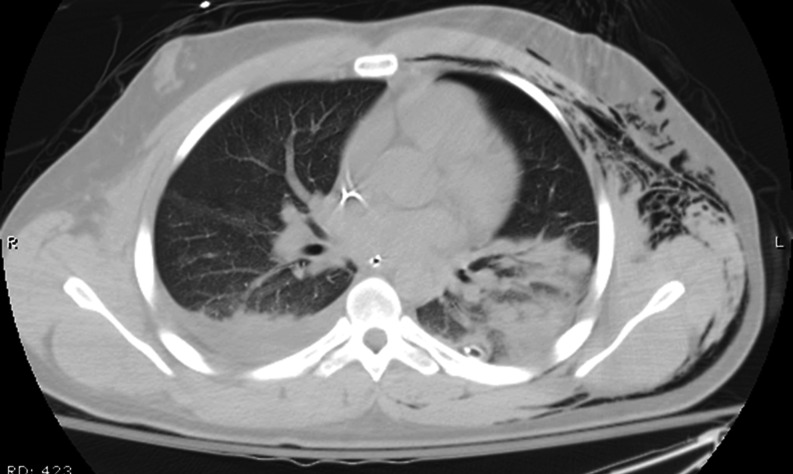
Fig. 3.
Repeat CT scan hospital day 6
Further questioning of his wife, relatives, and workplace supervisors revealed that he had been employed as an aviation mechanic for the military for the prior 2 years, with work-related travel to Afghanistan, Indonesia, and the Middle East, but no known hazardous occupational exposures or close contact with infected persons. The travel history prompted an extensive negative workup for unusual infections, as outlined above. He had smoked one-half pack of regular cigarettes daily, with occasional alcohol and frequent (nearly daily) marijuana use. It also emerged that over the prior 4 months, the patient had smoked synthetic cannabinoids in the form of multiple brands of “Spice” products. His typical habit was to combine and smoke several different Spice products into a pipe or bong (Fig. 4a, b are specimens of unopened packets from his “herbal incense” collection).
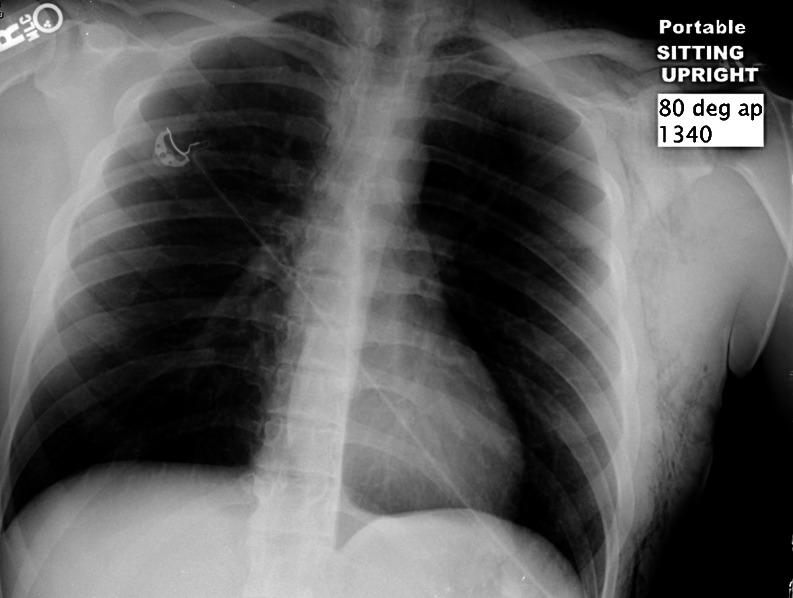
Fig. 4.
Chest X-ray at discharge (hospital day 8)
By the third hospital day, his hypoxemia improved, and, over the course of a week, he improved both clinically and radiographically (Figs. 5 and 6). He was continued on various antibiotics and steroids while the diagnostic workup was completed. Over 8 days, he was successfully weaned from mechanical ventilation, and all medications were stopped or tapered down. He was discharged, neurologically intact, and independently ambulatory, in stable condition.
Fig. 5.
a and b Spice compounds submitted by the patient’s family for analysis, all of which contained AM-2201. Spice, K2, and similar SC agents are sold in colorful, deceptively packaged 1–3-g mixtures containing dried plant products which have been sprayed with one or more synthetic cannabinoids [17]. These products are marketed with deceptive labels such as “herbal incense” or “potpourri” and packets are labeled “not for human consumption”
Fig. 6.
a Hematoxylin and eosin stain at ×40 magnification of transbronchial lung biopsy, showing chronic inflammatory infiltrate in the lung parenchyma. b Broncho-alveolar lavage monolayer showing numerous macrophages with a refractile, non-ferric brown pigment. (×40)
Specimens of his blood, urine, and saliva, all obtained on hospital day 4 and submitted for toxicology analysis using liquid chromatography-tandem mass spectrometry, showed the presence of four SC compounds (AM-2201, JWH-122, JWH-210, and JWH-018) and the absence of over a dozen others (Table 3). Subsequently, time-of-flight mass spectrometry analysis performed on unopened packages from his collection confirmed the presence of AM-2201 in four of five tested specimens (Fig. 5).
Table 3.
Toxicologic analysis for synthetic cannabinoid agents
| Compound (body fluid tested) | Reported results |
|---|---|
| AM-2201 (blood) | 0.75 ng/mL (reporting limit 0.10 ng/ml) |
| JWH-122 (blood) | Positive (reporting limit 0.10 ng/mL) |
| JWH-210 (blood) | Positive (reporting limit 0.10 ng/mL) |
| AM-2201, JWH-122 (oral fluid) | Positive (reporting limit unavailable) |
| JWH-018 N-(5-hydroxypentyl) metabolite (urine) | Positive (reporting limit 0.10 ng/mL) |
| AM-2201 N-(4-hydroxypentyl) metabolite (urine) | Positive (reporting limit 0.10 ng/mL) |
| AM-694, JWH-200, JWH-250, JWH-073, JWH-018, JWH-081, JWH-019, RCS-4, RCS-8 (blood) | None detected (reporting limit 0.10 ng/mL) |
| JWH-073, JWH 018, JWH-019, JWH-210, JWH-081, JWH-200, JWH-250, AM-694, RCS-4, RCS-8 (oral fluid) | None detected |
| JWH-018 N-(4-hydroxypentyl) metabolite (urine) | None detected (reporting limit 0.10 ng/mL |
| JWH-019 N-(5-hydroxypentyl) metabolite (urine) | |
| JWH-073 N-(3-hydroxybutyl) metabolite (urine) | |
| JWH-073 N-(4-hydroxybutyl) metabolite (urine) | |
| JWH-250 N-(4-hydroxypentyl) metabolite (urine) |
Fluids collected for analysis on hospital day 4. All tests were performed using liquid chromatography-tandem mass spectrometry (NMS Laboratories, Willow Grove, PA)
Discussion
Most clinical information regarding toxicity of the SC agents is currently available in case reports, small case series, bulletins from poison control centers and regulatory authorities, and animal models. These agents are potent agonists of the anatomically widespread cannabinoid receptors, which are found within and beyond the CNS [18]. Biological analyses reported in clinical reports confirm that synthetic cannabinoid users are consuming a variety of agents and combinations, which confounds specific cause–effect relationships among this chemically diverse class of agents. The most common adverse effects reported with these agents (Table 4) include a variety of CNS, psychiatric, cardiovascular, and metabolic disturbances such as nausea, vomiting, fevers, mydriasis, hyperglycemia, hypokalemia, psychosis, suicidal behavior, seizures, tachycardia, autonomic hyperactivity, and acute coronary syndromes. This case report describes a young man who developed diffuse pulmonary infiltrates after chronic inhalation of multiple synthetic cannabinoid-containing products. Chest imaging revealed bilateral, subacute lung infiltrates; histopathological analysis of bronchial and alveolar tissues revealed an inflammatory process, and analytical testing confirmed the presence of four SC compounds from biological tissues. The significance of this report can be summarized by two unique and infrequently reported associations with SC product toxicity—the presence of distinct and rapidly resolving pulmonary lesions and the detection of AM-2201, a newer but emerging SC agent in herbal incense products.
Table 4.
| Organ system affected | Symptoms and signs |
|---|---|
| Central nervous system | Agitation, psychosis, irritability, seizures, coma, delirium, paranoia, anxiety |
| Cardiovascular | Tachycardia, hypertension, acute coronary syndrome, chest pain |
| Pulmonary | Tachypnea, diffuse alveolar hemorrhages |
| Other | Nausea, vomiting, fevers, mydriasis, hyperglycemia, hypokalemia |
Pulmonary Findings
Although there is an extensive literature detailing respiratory effects of conventional Cannabis marijuana and non-tobacco additives in cigarettes [19–21], pulmonary toxicity in the context of SC abuse is not as well-reported as toxicity in other organs. This case presented a diagnostic challenge, given the rapid onset of respiratory compromise in an otherwise healthy young man, whose picture did not fit classic patterns of traumatic injury, infection, malignancy, or autoimmune processes. The diagnostic workup and rapid resolution of his radiographic and clinical derangements after administration of corticosteroids suggests an inflammatory process such as allergic alveolitis, although inhalational lung injury due to simple heat and smoke particulates could also account for his findings. Cell cytology of the BAL-recovered fluid demonstrated numerous macrophages containing a refractile brown pigment of non-ferruginous origin. It is possible that this pigment was a component of the SC mixture which the patient has habitually inhaled. Transbronchial biopsies revealed a carcinoid tumorlett, an incidental and non-specific finding associated with bronchitis [22]. Based on his history and radiographic findings, we inferred that the patient developed an inflammatory disorder of his distal airways and alveoli, caused by his habitual inhalation of SC agents.
In 2011, Loschner et al. reported a 19-year-old male who developed coma and bilateral pulmonary hemorrhages after chronic exposure to a SC compound called “greenhouse effect” [23]. The patient improved after mechanical ventilation, methylprednisone, and broad-spectrum antibiotics were initiated, and an extensive workup failed to identify any infectious, hematologic, or autoimmune etiology for the illness. After clinical recovery, the patient admitted to smoking synthetic cannabinoids on a daily basis, typically six to ten times per day. Unfortunately, neither blood/urine concentrations nor product analysis were provided in that brief report.
In our case, as with the Loschner case report, the patient was also a young, otherwise healthy male who developed a chronic, daily, multiple-session habit of inhalational SC consumption in conjunction with conventional Cannabis abuse. Radiographically, a chronic inflammatory infiltrate in both the airways and alveolar spaces was noted, which was also consistent with the direct visualization and cytologic findings. Even though blood was noted in the airways, the present case did not meet the criteria for diffuse alveolar hemorrhage as was seen in Loschner’s case. In contrast to BAL results for classic diffuse alveolar hemorrhage, the BAL fluid analysis lacked hemosiderin-laden macrophages, and the quality of crimson-colored fluid did not clear on subsequent aliquots during the procedure.
Given the inflammatory radiographic and histopathological findings (Fig. 3a, b) and this patient’s rapid clinical improvement, one appealing diagnosis for this case is hypersensitivity pneumonitis (HSP) related to inhaled particulates from the incense materials. Also known as allergic alveolitis, hypersensitivity pneumonitis is primarily a clinical diagnosis and typically occurs as a subacute pulmonary process resulting from the patient’s hypersensitivity to bioaerosols of animal, fungal, or plant origin [24]. Numerous occupational and recreational sources of these offending antigens have been identified, and it is possible that the brown, non-heme pigment found inside the macrophages on this patient’s bronchial washings represent these antigens, perhaps from the herbs or plant products found in one or more SC products. Similar cases and more clinical experience with pulmonary injury following SC abuse may help confirm whether HSP is indeed the etiology of this unique and severe reaction.
Analytical Results
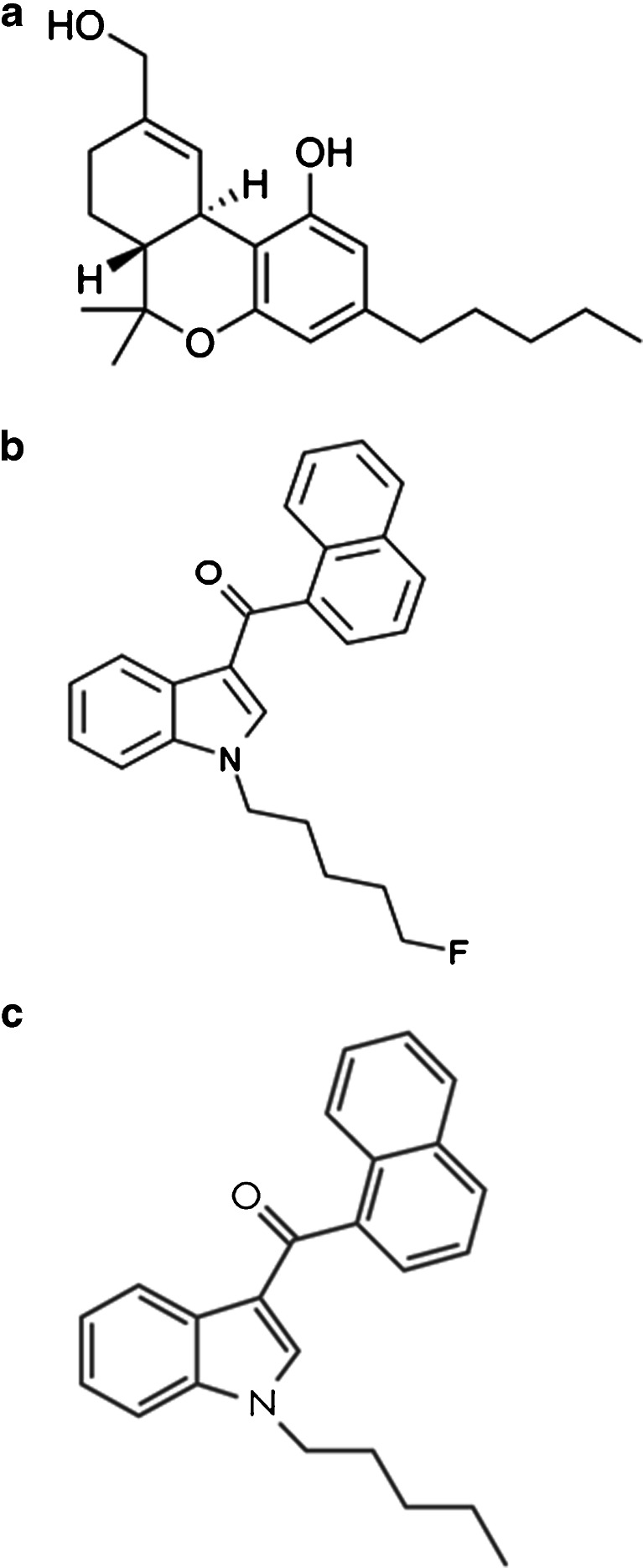
There were four SC compounds identified from his blood, urine, or salivary tissue specimens (Table 3), and about one dozen not detected (NMS Labs, Willow Grove, PA). The identified agents were AM-2201, JWH-122, JWH 210, and a metabolite of JWH-018 (Fig. 7a–c). Notably, this patient also had evidence of tetrahydrocannabinol (THC) on his urine drugs-of-abuse immunoassay screen, which confirmed his historical admission of smoking conventional Cannabis marijuana in addition to multiple blends of synthetic cannabinoids. To date, none of the SC compounds have been demonstrated to trigger a positive THC screen on standard urine toxicology testing.
Fig. 7.
a, b, and c Chemical structures of delta-9-THC, AM-2201, and JWH-018. The immune assay urine drug screen for THC (a) does not detect synthetic cannabinoids (b and c). Note that AM-2201 (middle) is essentially the same structure as JWH-018 (bottom), except that the former has a fluorine attached to its alkane tail
One unique result from analytical biological testing was the presence of AM-2201. Analysis of the unopened SC packages provided by the patient’s relatives also confirmed the presence of AM-2201 in four out of five products tested (product names: Super Strong Incense, Scooby Snax, Mad Hatter, and Kite). This agent is one of multiple “AM-prefix” SC agents (named for their discoverer, A. Makriyannis) which have become more popular following media scrutiny and legal bans on the JWH-prefix SC compounds [25]. It is noteworthy that the structure and metabolism of the fluorinated compound AM-2201 is almost identical to the non-fluorinated agent JWH-018. The presence of a JWH-018 metabolite in the absence of its parent compound in this case may thus represent AM-2201 use and metabolism rather than the exposure to JWH-018 itself, although this pathway has not previously been reported in vivo. Furthermore, an association between AM-2201 and seizures is very recently emerging: a recent case report from the UK, and anecdotal self-reports from users, both describe seizures following the use of a Spice product containing AM-2201 [26, 27]. Considering these newer dispatches, whether our patient presented after a syncopal episode or a brief seizure remains one of many unanswered questions posed by this clinical encounter.
Limitations
This case is limited by an incomplete and perhaps unreliable patient history, combined with a paucity of clinical information about a novel hazardous class of drugs. Conceivably, some occult, unrelated cause or perhaps a combination of unrecognized factors led to his illness and presentation. Moreover, the detection and analysis of specific hazardous substances is fraught with potential errors of omission or misinterpretation, given the sheer number possible exposures from SC compounds in this patient. We hope that this report helps generate additional hypotheses about the clinical pulmonary syndrome we have observed and its relation to the analytical results.
Conclusions
This report adds to the growing body of clinical literature about synthetic cannabinoids with the description of a severe pulmonary syndrome in an otherwise healthy 21-year-old man with a history of habitual SC smoking. The negative workup and rapid resolution of extensive radiographic and clinical derangements after administration of corticosteroids suggested that this developed an inflammatory process such as allergic alveolitis, although inhalational lung injury due to simple heat and smoke particulates could also account for his findings. Laboratory investigations and specific toxicology analysis confirmed the presence of at least four SC compounds, but a firm cause-and-effect association is impossible based on the information currently available. Additional reports and investigations will clarify the association between synthetic cannabinoids and lung injury, but clinicians should be aware that pulmonary hemorrhage, infiltrates, and respiratory failure can occur alongside the diverse array of neuropsychological and hyperdynamic cardiovascular derangements previously described with the use of the SC agents.
References
- 1.Wells DL, Ott CA. The “new” marijuana. Ann Pharmacother. 2011;45(3):414–417. doi: 10.1345/aph.1P580. [DOI] [PubMed] [Google Scholar]
- 2.Vearrier D, Osterhoudt K (2010) A teenager with agitation: higher than she should have climbed. Ped Emerg Care 26(6):462-465 [DOI] [PubMed]
- 3.Seely KA, Lapoint J, Moran JH, Fattore L (2012) Spice drugs are more than harmless herbal blends: a review of the pharmacology and toxicology of synthetic cannabinoids. Prog Neuropsychopharmacol Biol Psychiatry 2012 Apr 26 [DOI] [PMC free article] [PubMed]
- 4.Logan BK, Reinhold LE, Xu A, Diamond FX (2012) Identification of synthetic cannabinoids in herbal incense blends in the United States. J Forensic Sci 2012 Jul 26 [DOI] [PubMed]
- 5.Tofighi B, Lee JD. Internet highs-seizures after consumption of synthetic cannabinoids purchased online. J Addict Med. 2012;6(3):240–241. doi: 10.1097/ADM.0b013e3182619004. [DOI] [PubMed] [Google Scholar]
- 6.Forrester M, Kleinschmidt K, Schwarz E, Young A (2012) Synthetic cannabinoid and marijuana exposures reported to poison centers. Hum Exp Toxicol 2012 Aug 2 [DOI] [PubMed]
- 7.Cohen J, Morrison S, Greenberg J, Saidinejad M. Clinical presentation of intoxication due to synthetic cannabinoids. Pediatrics. 2012;129(4):e1064–e1067. doi: 10.1542/peds.2011-1797. [DOI] [PubMed] [Google Scholar]
- 8.Ashton JC. Synthetic cannabinoids as drugs of abuse. Curr Drug Abuse Rev. 2012;5(2):158–168. doi: 10.2174/1874473711205020158. [DOI] [PubMed] [Google Scholar]
- 9.Castellanos D, Thornton G. Synthetic cannabinoid use: recognition and management. J Psychiatr Pract. 2012;18(2):86–93. doi: 10.1097/01.pra.0000413274.09305.9c. [DOI] [PubMed] [Google Scholar]
- 10.Rosenbaum CD, Carreiro SP, Babu KM. Here today, gone tomorrow…and back again? A review of herbal marijuana alternatives (K2, Spice), synthetic cathinones (bath salts), kratom, Salvia divinorum, methoxetamine, and piperazines. J Med Toxicol. 2012;8(1):15–32. doi: 10.1007/s13181-011-0202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vardakou I, Pistos C, Spiliopoulou C. Spice drugs as a new trend: mode of action, identification and legislation. Toxicol Lett. 2010;197(3):157–162. doi: 10.1016/j.toxlet.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Atwood BK, Huffman JW, Straiker A, et al. JWH018, a common consitiuent of “Spice” herbal blends, is a potent and efficacious cannabinoid CB1 receptor agonist. Br J Pharm. 2010;160:585–593. doi: 10.1111/j.1476-5381.2009.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simmons JR, Skinner CG, Williams J, et al. Intoxication from smoking “Spice. Ann Emerg Med. 2011;57(2):187–188. doi: 10.1016/j.annemergmed.2010.08.039. [DOI] [PubMed] [Google Scholar]
- 14.Schneir AB, Cullen J, Ly BT. “Spice” girls: synthetic cannabinoid intoxication. J Emerg Med. 2011;40(3):296–299. doi: 10.1016/j.jemermed.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Mir A, Obafemi A, Young A, Kane C. Myocardial infarction associated with use of the synthetic cannabinoid K2. Pediatrics. 2011;128(6):e1622–e1627. doi: 10.1542/peds.2010-3823. [DOI] [PubMed] [Google Scholar]
- 16.Hurst D, Loeffler G, McLay R. Psychosis associated with synthetic cannabinoid agonists: a case series. Am J Psychiatry. 2011;168(10):1119. doi: 10.1176/appi.ajp.2011.11010176. [DOI] [PubMed] [Google Scholar]
- 17.Young AC, Schwarz E, Medina G, Obafemi A, Feng SY, Kane C, Kleinschmidt K (2011) Cardiotoxicity associated with the synthetic cannabinoid, K9, with laboratory confirmation. Am J Emerg Med 2011 Jul 28 [DOI] [PubMed]
- 18.Fattore L, Fratta W. Beyond THC: the new generation of cannabinoid designer drugs. Front Behav Neurosci. 2011;5(60):1–12. doi: 10.3389/fnbeh.2011.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tetrault JM, Crothers K, Moore BA, Mehra R, Concato J, Fiellin DA. Effects of marijuana smoking on pulmonary function and respiratory complications: a systematic review. Arch Intern Med. 2007;167:221–228. doi: 10.1001/archinte.167.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lieberman CM, Lieberman BW. Marihuana—a medical review. N Engl J Med. 1971;284:88–91. doi: 10.1056/NEJM197101142840206. [DOI] [PubMed] [Google Scholar]
- 21.Guidotti TL, Laing L, Prakash UB. Clove cigarettes. The basis for concern regarding health effects. West J Med. 1989;151(2):220–228. [PMC free article] [PubMed] [Google Scholar]
- 22.Bennett GL, Chew FS. Pulmonary carcinoid tumorlets. AJR. 1994;162:568. doi: 10.2214/ajr.162.3.8109497. [DOI] [PubMed] [Google Scholar]
- 23.Loschner A, Cihla A, Jalali F, et al. Diffuse alveolar hemorrhage: add “greenhouse effect” to the growing list. Chest. 2011;140:149A. doi: 10.1378/chest.1119854. [DOI] [Google Scholar]
- 24.Selman M, Pardo A, King TE. Hypersensitivity pneumonitis: insights in diagnosis and pathobiology. Am J Respir Crit Care Med. 2012;186(4):314–324. doi: 10.1164/rccm.201203-0513CI. [DOI] [PubMed] [Google Scholar]
- 25.Sobolevsky T, Prasolov I, Rodchenkov G (2012) Detection of urinary metabolites of AM-2201 and UR-144, two novel synthetic cannabinoids. Drug Test Anal. doi:10.1002/dta.1418 [DOI] [PubMed]
- 26.McQuade D, Hudson S, Dargan PI, Wood D (2012) First European case of convulsions related to analytically confirmed use of the synthetic cannabinoid receptor agonist AM-2201. Eur J Clin Pharmacol. doi:10.1007/s00228-012-1379-2 [DOI] [PubMed]
- 27.EkaJ (20 February 2011). “The night i killed my friends”. Erowid.org. Retrieved 2 Dec 2012. http://www.Erowid.org/experiences/exp.php?ID=89294