Abstract
The phenethylamines, including 2, 5 dimethoxy-4-iodophenethylamine, commonly referred to as 2C-I, have recently emerged as a new class of designer drugs. Cases of toxicity from these drugs are not well described in the literature. This case report describes a 19 year-old male who insufflated 2C-I. Following the ingestion, the patient developed recurrent seizures, and was taken to the emergency department, where he was noted to be hyperadrenergic and had recurrent seizures. The patient was diagnosed with serotonin syndrome and experienced prolonged respiratory failure, although he ultimately made a full recovery. Comprehensive drug testing revealed the presence of 2C-I. The pharmacologic properties of 2C-I are also discussed.
Keywords: Serotonin syndrome, 2C-I, 2CI, Phenethylamine, Seizure, Rave
Introduction
Phenyethylamines, including 2,5 dimethoxy-4-iodophenethylamine, commonly referred to as 2C-I or “Smiles,” represent an emerging class of recreational drug abuse. This case describes a 19-year-old man who developed recurrent seizures, serotonin syndrome, and prolonged respiratory failure following consumption of 2C-I. Acute overdose was confirmed by high-performance liquid chromatography/time of flight mass-spectrometry (LC/TOF-MS). To our knowledge, this case represents the first case of serotonin syndrome and one of the first cases outlining the toxicity following 2C-I ingestion in the USA.
Case Report
A previously healthy 19-year-old man was in his usual state of health until he attended a “rave” party, where he reportedly insufflated a liquid referred to as “liquid acid.” The patient had no significant past medical history and is not on any medications. He drinks ethanol rarely and no history of other illicit drug use.
Shortly after insufflating the “liquid acid”, he was noted to have a generalized tonic–clonic seizure, prompting activation of the emergency medical services (EMS). Upon arrival of EMS, the patient was noted to be comatose with a heart rate of 143 beats per minute (bpm) and an oxygen saturation of 80 % on room air, with skin described as “cool” and “clammy.” The patient was placed on a non-rebreather at 15 L oxygen/min and was given 2 mg of intravenous naloxone without change in mental status. During transport to the hospital, the patient had two additional generalized tonic–clonic seizures prompting the administration of 2.5 mg intravenous midazolam. His initial vital signs in the emergency department included a blood pressure of 230/104 mmHg, heart rate of 135 bpm, temperature of 39.4 °C, and oxygen saturation of 92 % on a non-rebreather. His Glasgow Coma Score was described as 6 with no further information available.
Shortly after arrival in the emergency department, the patient had an additional generalized tonic–clonic seizure and was administered 2 mg of intravenous lorazepam. The exam was notable for spontaneous clonus and rigidity of the lower extremities. The patient was intubated, sedated with propofol, and chemically paralyzed. The patient’s temperature improved to 38.2 °C.
Initial evaluation in the emergency department included a computed tomography of the head and cervical spine as well as chest radiograph, which were unremarkable. Electrocardiogram was notable only for the presence of sinus tachycardia. Pertinent laboratory findings included a white blood count of 24,600/mm3, glucose 249 mg/dL, creatinine 1.76 mg/dL, and a venous blood gas revealing pH 7.15, paCO2 73, pa02 238, and HCO3 25. His initial aspartate aminotransferase/alanine aminotransferase were normal at 34 and 23 IU/L, respectively. Urine immunoassay drug abuse screen was positive for benzodiazepines. The patient was subsequently transferred to our tertiary care medical center.
On arrival in the intensive care unit, the patient remained agitated with weaning of sedation. The exam revealed diffuse muscular rigidity along with spontaneous clonus in the lower, but not upper, extremities. Due to concern of persistent rigidity and inability to exclude ongoing seizures, the patient received 15 mg/kg of phenobarbital along with cyproheptadine (12 mg via orogastric tube). Despite the administration of phenobarbital and propofol (60 mcg/kg/min), agitation persisted prompting continuous infusions of fentanyl and midazolam. Lumbar puncture was performed and was unremarkable.
On hospital day 2, while sedation was weaned, the patient again became agitated, tachycardic, hypertensive, and febrile with temperature of 40.3 °C. The patient was resedated with resolution of abnormal vital signs. Sputum and blood cultures were obtained and were negative. The patient failed sedation weaning on hospital day 3 due to agitation and required midazolam 6 mg/h, propofol 80 mcg/kg/min, and fentanyl 175 mcg/h to adequately control the agitation. On hospital day 4, the patient was extubated and indorsed visual hallucinations subsequently after extubation. The patient again developed hypertension, tachycardia, and fever (39.9 °C) 2 h post-extubation; these improved after administration of intravenous lorazepam. The agitation and hallucinations resolved on hospital day 5 and the patient was discharged home on hospital day 6.
During the 2 weeks after discharge, the patient reportedly had episodes of forgetfulness, although at follow-up 2 months after discharge, the patient was back to his baseline attending junior college.
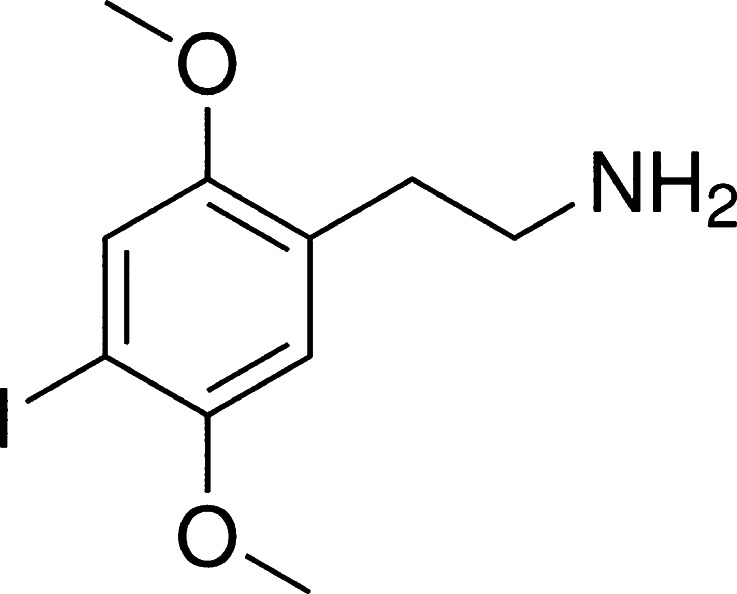
Comprehensive testing of the urine via LC/TOF-MS revealed the presence of 2,5 dimethoxy-4-iodophenethylamine (Fig. 1) commonly referred to as 2C-I or “Smiles.” Additional testing via gas chromatography–mass spectrometry failed to reveal any additional coingestants. Both samples were obtained on admission to the intensive care unit.
Fig. 1.
Chemical structure of 2,5 dimethoxy-4-iodophenethylamine
Discussion
In recent years, the “rave club drug” scene has changed significantly as many new synthetic drugs have become available [1]. One such class of drugs, the phenethylamines, has been a major health problem in Europe [2], and only recently have these drugs began to appear in the USA. 2,5 Dimethoxy-4-iodophenethylamine, or 2C-I, is one such example. Because of rapid metabolism, beta phenethylamine itself is not commonly abused, but the addition of a hydrophobic R group in position 4, along with methoxy groups in positions 2 and 5 make a more stable compound. Collectively, this group of drugs is referred to as the “2C” drugs [3]. As a class, these drugs, including 2C-I, have demonstrated agonism at the 5HT2 receptor, which is believed to be the etiology of the hallucinogenic properties [1, 4]. While 2C-I does have some affinity at the 5HT2A receptor, it binds more potently to the 5HT2C receptor [5]. In addition, in vitro studies have demonstrated the ability of 2C-I to inhibit dopamine, serotonin, and norepinephrine re-uptake [6]. The 2C drugs are also agonists at the alpha-1 adrenergic receptor [1].
Metabolism of the 2C drugs primarily occurs via O-demethylation and demanimation with subsequent oxidation [7, 8]. The demanimation occurs primarily via monoamine oxidase (MAO)-A and MAO-B. The cytochrome P450 isoenzyme 2D6 is a minor contributor in many of the “2C” drugs, but does not appear to be involved in the metabolism of 2C-I [8].
The exact pharmacokinetic and pharmacodynamics effects of 2C-I are not definitively known. Following consumption of a typical “dose” (15–20 mg) of 2C-I, the onset of symptoms can likely be expected within 30 min, although a prolonged (more than 24 h) duration of action is expected [9]. As a class, the phenethylamines display a dose–response curve, with lower doses resulting in stimulating effects, while higher doses are needed for hallucinogenic effects [10]. Following overdose of 2C-I, neurologic and cardiovascular toxicities can be expected. Neuropsychiatric manifestations include hallucinations, anxiety, agitation, and seizures. Hypertension, tachycardia, and respiratory depression have also been described [1].
The drug 4-iodo-2,5-dimethoxy-N-(2-methoxybenzyl)phenethylamine (25I-NBOMe) has also been referred to as “liquid acid” or “liquid LSD,” and 2CI-NBOME. Similar to 2CI, 25I-NBOME is also a potent serotonergic agonist. The LC-MS did not screen for 25-I-NBOME but did confirm the presence of 2C-I. It is, therefore, possible that there was a co-ingestion of 25-I NBOME.
In this case, our patient had a prolonged hospitalization and duration of action, with symptoms persisting for more than 96 h post-ingestion. The presence of altered mental status, increased tone in the lower extremities, clonus, hypertension, tachycardia, and fever met criteria for serotonin syndrome [11, 12]. Based on the mechanism of action of 2C-I, serotonin syndrome mechanistically is feasible, although cases of serotonin syndrome have not been widely described following 2C-I ingestion. It is possible that the prolonged duration of the serotonin syndrome was the result of fentanyl administration in the intensive care unit. Although causality has not been clearly established on rare occasion, fentanyl has been associated with serotonin syndrome [13]. Nonetheless, the patient clearly had toxicity manifested prior to the onset of fentanyl and we feel fentanyl is unlikely to be the primary cause of the relatively long duration of symptoms. It is also possible that the prolonged duration of action was related to the dual mechanism of 2C-I; namely, the direct agonism on the receptor along with its reuptake inhibition.
Conclusion
Overdose of the phenethylamines are relatively new in the USA and serious toxicity from 2C-I has only rarely been described. We believe this represents one of the first laboratory-confirmed cases of 2C-I ingestion in the USA and the only case characterized by a prolonged duration of symptoms and the development of serotonin syndrome.
Acknowledgments
Disclosures
There are no financial, litigational, or other conflicts of interest to disclose.
References
- 1.Haroz R, Greenberg MI. Emerging drugs of abuse. Med Clin N Am. 2005;89:1259–76. doi: 10.1016/j.mcna.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Andreasen MF, Telving R, Birkler RI, et al. A fatal poisoning involving Bromo-Dragonfly. Forensic Sci Int. 2009;183:91–6. doi: 10.1016/j.forsciint.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Theobald DS, Pütz M, Schneider E, et al. New designer drug 4-iodo-2,5-dimethoxy-beta-phenethylamine (2C-I): studies on its metabolism and toxicological detection in rat urine using gas chromatographic-mass spectrometric and capillary electrophoretic/mass spectrometric techniques. J Mass Spectrom. 2006;41:872–86. doi: 10.1002/jms.1045. [DOI] [PubMed] [Google Scholar]
- 4.Johnson MP, Mathis CA, Shuligin AT, et al. [125I]-2-(2, 5-Dimethoxy-4-iodophenyl)aminoethane ([125]-2C-I) as a label for the 5HT2 receptor in rat frontal cortex. Pharmacol Biochem Behav. 1990;35:211–7. doi: 10.1016/0091-3057(90)90228-A. [DOI] [PubMed] [Google Scholar]
- 5.Castillo AC, Villalobos C, Moya PR, et al. Differences in potency and efficacy of a series of phenylisopropylamine/phenylethylamine pairs at 5HT2A and 5HT2C receptors. Br J Pharmacol. 2002;136:510–9. doi: 10.1038/sj.bjp.0704747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagai F, Nonaka R, Satoh Hisashi Kamimura K. The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain. Eur J Pharmacol. 2007;559:132–7. doi: 10.1016/j.ejphar.2006.11.075. [DOI] [PubMed] [Google Scholar]
- 7.Maurer HH. Chemistry, pharmacology, and metabolism of emerging drugs of abuse. Ther Drug Monit. 2010;32:544–9. doi: 10.1097/FTD.0b013e3181eea318. [DOI] [PubMed] [Google Scholar]
- 8.Theobald DS, Maurer HH. Identification of monoamine oxidase and cytochrome P450 isoenzymes involved in the deamination of phenethylamine-derived designer drugs (2c-series) Biochem Pharmacol. 2007;73:287–97. doi: 10.1016/j.bcp.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 9.Drug Enforcement Administration, Office of Forensic Sciences. Microgram Bulletin. US Department of Justice, Drug Enforcement Administration. 2004; 38:48-9
- 10.DeBoer D, Gijzeles MJ, Bosman IJ, et al. More data about the new psychoactive drug 2C-B. J Anal Toxicol. 1999;23:227–8. doi: 10.1093/jat/23.3.227. [DOI] [PubMed] [Google Scholar]
- 11.Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352:1112–20. doi: 10.1056/NEJMra041867. [DOI] [PubMed] [Google Scholar]
- 12.Dunkey EJ, Isbister GK, Sibbritt D, et al. The Hunter serotonin toxicity criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96:635–42. doi: 10.1093/qjmed/hcg109. [DOI] [PubMed] [Google Scholar]
- 13.Kirshner R, Donovan JW. Serotonin syndrome precipitated by fentanyl during procedural sedation. J Emerg Med. 2010;38:477–80. doi: 10.1016/j.jemermed.2008.01.003. [DOI] [PubMed] [Google Scholar]