Abstract
Background
Intervertebral disc degeneration, a main cause of back pain, is an endemic problem and a big economic burden for the health care system. Current treatments are symptom relieving but do not address underlying problems—biological and structural deterioration of the disc. Tissue engineering is an emerging approach for the treatment of intervertebral disc degeneration since it restores the functionality of native tissues. Although numerous studies have focused on the nucleus pulposus tissue engineering and achieved successes in laboratory settings, disc tissue engineering without annulus fibrosus for the end stage of disc degeneration is deemed to fail. The purpose of this article is to review the advancement of annulus fibrosus tissue engineering.
Material and Methods
Relevant articles regarding annulus fibrosus tissue engineering were identified in PubMed and Medline databases.
Results
The ideal strategy for disc regeneration is to restore the function and integrity of the disc by using biomaterials, native matrices, growth factors, and cells that producing matrices. In the past decades there are tremendous advancement in annulus fibrosus tissue engineering including cell biology, biomaterials, and whole disc replacement. The recent promising results on whole disc tissue engineering—a composite of annulus fibrosus and nucleus pulposus—make the tissue engineering approach more appealing.
Conclusion
Despite the promising results in disc tissue engineering, there is still much work to be done regarding the clinical application.
Keywords: Tissue engineering, Annulus fibrosus, Scaffolds, Intervertebral disc degeneration
Introduction
Intervertebral disc (IVD) degeneration is a common musculoskeletal disease and progresses to disc herniation, spinal canal stenosis, and degenerative spondylolisthesis. The precise etiology of disc degeneration is still far from delineated. Multiple factors have been found involved in the initiation and progression of the disease including aging, loading changes, poor nutrient supply, and hereditary factors [1–7]. IVD degeneration is characterized by changes of cellular microenvironment, loss of proteoglycan, loss of disc height, tears of annulus fibrosus (AF) tissue, spinal stenosis, herniated discs, neoinnervation, hypermobility, and inflammation [8–11]. At present, all treatments for degenerative disc disease are limited to treating the symptoms of the condition. Due to its avascular and aneural structure, the intervertebral disc has little ability to regenerate. When nucleotomy (a surgical treatment for disc herniation) is performed, only minimal regeneration of the annulus is observed, making degeneration of the disc an inevitable consequence of this procedure. Repair of the herniated or degenerative disc or annulus with appropriate analogs would therefore represent an ideal application for tissue engineering technology. A number of artificial disc prostheses have been designed and used in hospitals [12–17]. While certainly a major advancement in the treatment of this condition, artificial discs composed of metal alloys or plastics are inherently limited in their biocompatibility and do not recapitulate native disc tissue. Therefore, both nucleus pulposus (NP) and AF tissue engineering have been investigated. Herein we will review the current advances in annulus tissue engineering. We will address three major components of AF regeneration: cells, growth factor treatments, and scaffolds.
Current attempts to regenerate the intervertebral disc
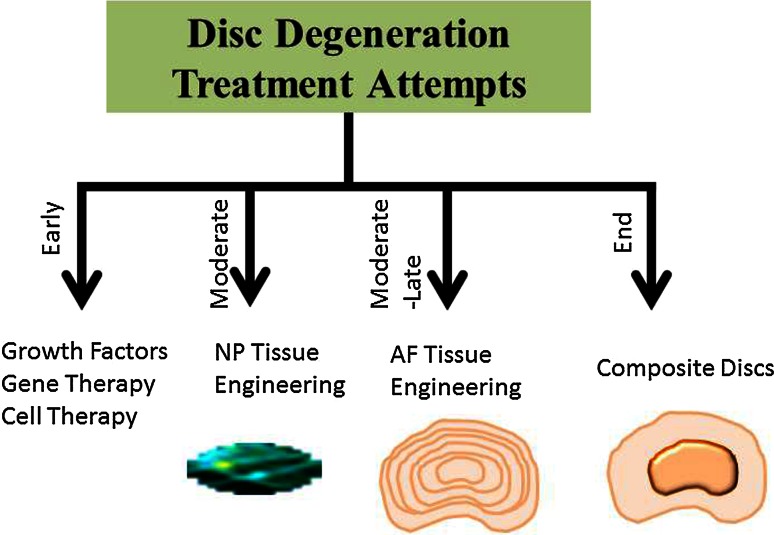
One of the hallmarks of the disc degeneration is the loss of matrices. Surgical procedures are attempting to relieve symptoms rather than restore the native structure and function. Thus the ideal strategy for disc regeneration is to restore the function and integrity of the disc by using biomaterials, native matrices, growth factors, and cells that producing matrices. The complex biological and mechanical environment of IVD makes the synthesis of an artificial IVD a difficult task. At the early stage of disc degeneration, cell therapy and biological methods—injection of growth factors and enzymatic inhibitors—can be used to augment anabolic metabolism (Fig. 1). However, the half-life of growth factors is short and limits their use in treating disc degeneration. The hydrated proteoglycans in the NP are essential to maintain the osmotic pressure and therefore have a major effect on the load bearing properties of the disc [18]. Thus, NP tissue is more vulnerable than AF towards the environmental changes [19, 20] that suggesting NP tissue engineering is important for regeneration of the functional disc. Considerable effort has been devoted to restoring NP function using growth factors, biomaterial, and cells. The effectiveness of various hydrogel-based scaffolds including natural hydrophilic biomolecules or synthetic polymers for NP engineering or repair has been reported. A variety of biomaterials have been used for fabricating scaffolds in NP tissue engineering, such as chitosan/hydroxybutyl chitosan, alginate, collagen/atelocollagen, gelatin, hyaluronic acid, calcium polyphosphate, poly-D-L-lactide (PDLA), demineralized bone matrix (DBM), small intestine submucosa (SIS), carboxymethylcellulose, and PGA–hyaluronan [21–27]. In addition to the native or synthetic biomaterials, injectable scaffold has also been used as a carrier to deliver NP cells or mesenchyme stem cells to facilitate restoring disc structure and retarding further disc degeneration [25, 28–30]. However, without a functional AF tissue to resist intra-disc pressure, disc regeneration is deemed to fail, which will be discussed in detail in the current review. The purpose of AF tissue engineering is to mend the injured AF to prevent disc herniation and to retard further AF degeneration. Current approaches for AF tissue regeneration combine cells (disc cells or stem cells) and native/synthetic biodegradable scaffolds with different porosities and fiber orientations to resemble the native extracellular matrix component and arrangement. Recently, biological repair of the whole disc with nucleus and annulus composite tissues has become of interest for severe disc degeneration. Bowles et al. [31] constructed a biphasic whole IVD using collagen I for an AF and alginate for the NP. Another study [32] that used electrospinning fabricated a bi-phasic IVD using porcine chondrocytes seeded poly(caprolactone) (PCL) and agarose as the AF and NP tissues, respectively. Recent studies on total disc tissue engineering have attained positive results in small animal models, while the challenges on preclinical and clinical studies remain unknown.
Fig. 1.
Scheme showing the current attempts for disc degeneration treatment
Biology of annulus fibrosus
The AF is originated from mesenchymal cells during embryogenesis, and is composed of highly oriented concentric lamellae sheets that surround the nucleus. It has a much higher collagen and lower water content when compared to the nucleus [33]. These lamella sheets are filled with proteoglycans, interspersed with elastin fibers [34], and provide the tremendous axial load strength. The outer lamellae are majorly type I collagen, as toward to the nucleus, type II collagen increases and type I collagen decreases. The inner AF mainly produces type II collagen. Other collagen types such as III, V, VI, IX and XI were also detected in the inner AF. The inner AF consists of greater amounts of glycosaminoglycans as compared with the outer region. Large proteoglycans in AF are aggrecan and versican [35–39], and small proteoglycans are biglycan, decorin, fibromodulin, and lumican [40–42].
What are AF cells
All cells in the AF are derived from the mesenchyme. However, they have different morphologies and compositions of extracellular matrices [23, 43–45]. The outer AF cells are fibrous and ligamentous while the inner AF cells are spherical shaped fibrocartilaginous. Recent studies showed that the expression of type V collagen and tenomodulin was higher in AF cells than in NP and articular chondrocytes, which suggested that they might serve as AF markers [46, 47]. A recent study demonstrated that human AF cells exhibited altered gene profiles among different grades of disc degeneration [48]. Due to interspecies and age variation, however, there is no specific marker to distinguish the AF cells from other cells.
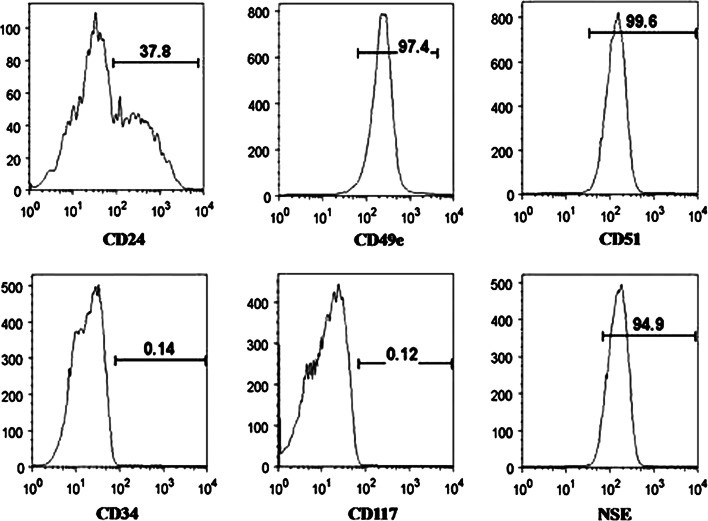
By flow cytometry sorting, we found that some human inner AF cells expressed stem cell surface antigens similar to that of adipose and bone marrow derived stem cells, i.e., CD29, CD49e, CD51, CD73, CD90, CD105, CD166, and CD184 (Fig. 2). In addition, the neuronal stem cell markers, nestin and neuron-specific enolase, were also detected in human inner AF cells. A subpopulation of human inner AF cells was able to differentiate to different cell lineages upon appropriate culture condition: adipocytes, osteoblasts, chondrocytes, neurons, and endothelial cells. These results indicated that a portion of inner AF cells have mesenchymal stem cell (MSC) characterization [49]. In another study, Risbud et al. [50] showed evidence of skeletal progenitor cells in the degenerated human intervertebral disc capable of differentiation into adipose cells, osteoblasts, and chondrocytes. All these findings suggest that AF tissue contains multipotential stem cells.
Fig. 2.
Flow cytometry showing annulus fibrosus cells express stem cell and neuronal cell markers. The horizontal axis represents the parameter’s signal value in channel numbers, and the vertical axis represents the number of events per channel number. The number in each panel shows the percentage of cells expressing the specific cell marker
AF cells amplification for tissue regeneration
Studies showed that AF cells cultured in monolayer or three-dimensional matrix can proliferate and produce proteoglycan and collagen [51, 52], which provided the foundation of AF cells in annulus tissue engineering. While compared with a 3D culture, a monolayer culture of AF cells produce less proteoglycan and type II collagen. One of the key elements of cell based therapy or tissue engineering is to amplify a large number of cells. Since the successful cultivation of porcine AF cells in alginate bead [53], many approaches have been used for expanding AF cells in vitro [52, 54, 55]. We have shown that a rotating bioreactor stimulates the anabolic and decreases the catabolic metabolisms of human AF cells. The proliferation of AF cells was greatly enhanced in the rotating bioreactor culture condition [56], which makes the large amplification of AF cells possible in tissue engineering application.
AF cells response to different stimuli
By the immunohistochemistry method, expressions of TGFβRII, BMPRII, FGFR3 and IGFRI growth factor receptors have been detected in human IVDs. There were no significant differences among expressions of four receptors in non-degenerate and degenerate biopsies [57]. This observation suggests that these growth factor receptors play a role in normal disc homeostasis and that the administration of growth factors to the degenerate human IVD would stimulate matrix production. Not surprised, TGFβ -1 and -2, bFGF and PDGF have been highlighted in herniated intervertebral disc tissue [58]. A study also showed that AF cells produce greater quantities of IL-6, IL-8, PGE2, PGF2α, and VEGF when co-cultured with macrophages [59]. Both IL-1 and IL-4 are involved in the response of human AF cells derived from non-degenerative tissue to the cyclic tensile strain [60]. Recently Hegewald et al. [61] showed that isolated human AF cells were in response to serum and chemokine migratory effects and expressed chemokine receptors. Of the five tested cytokines (CXCL7, CXCL10, CXCL12, CCL25, and XCL1), CXCL10, a potent attractant for mesenchymal stem cells, and XCL1 recruited the AF cells [61].
Both NP and AF cells from older donors show a decreased production of matrix enriched in aggrecan, but this phenomenon can be overcome by gene therapy or exposure to different stimuli [62]. In in vitro culture conditions, a variety of growth factors have been found stimulating matrix production of AF cells (Table 1): TGF-β [52, 54, 63], osteogenic protein-1 [64–66], BMP12 [67], GDF-5 [68–70], IGF-1 [54, 71–73], PDGF, FGF [74, 75], BMP2 [76, 77], BMP13 and the transcription factor Sox9 [78]. Since GDF5 knockout mice display a degenerated intervertebral disc with disrupted lamellar architecture of the AF and a shrunken and disorganized NP [79], we investigated whether GDF5 gene therapy could reverse the degenerative process [68, 80]. GDF5 protein treatment augmented anabolic metabolism of disc cells from either GDF5 deficient mice or wild-type mice. Intra-disc injection experiments also showed that the administration of GDF5 promotes inner AF cells migrating to injured NP area and presenting chondrogenic phenotype [81].
Table 1.
Different growth factors stimulate matrix production of AF cells
| Growth factor | Results | References |
|---|---|---|
| TGF-β | TGF-β elevated the expression of matrix genes, preserved the expression of TGF-beta receptors, and decreased aggrecan turnover in AF and NP cells. | [52, 54, 63] |
| Osteogenic Protein -1 | Osteogenic protein-1 increases proteoglycan and collagen contents in both NP and AF cells | [64–66] |
| BMP-12 | Adenovirus mediated BMP-12 significantly increased matrix protein synthesis and DNA content of human AF and NP cells in pellet culture | [67] |
| BMP-2 | BMP-2 up-regulates the expression Col I, type II, and aggrecan in AF cells | [66, 76, 77] |
| IGF-1 | IGF-1 stimulates sGAG, type I and II collagen expression in AF cells | [54, 71–73] |
| Sox9 and BMP-13 | BMPs and Sox9 increase proteoglycan and collagen expression in bovine AF cells | [78] |
| GDF-5 | GDF-5 augments anabolic metabolism of disc cells | [58, 68–70, 80, 81] |
| FGF and PDGF | Both FGF and PDGF stimulate the proliferation of bovine AF and NP cells | [74, 75] |
| Link N peptide | Link N peptide increases matrix production in disc cells | [82–84] |
Growth factors are not the only molecules that stimulate AF cell proliferation, in a serum free culture system, the amino terminal peptide of link protein (DHLSDNYTLDHDRAIH) (link N) acts directly on disc cells to stimulate matrix production, which involves increased accumulation of proteoglycan, and type II and IX collagens both in vitro and in vivo [82–84]. Link N is generated by the cleavage of human link protein by stromelysins 1 and 2, gelatinase A and B, and collagenase between His(16) and Ile(17).
All these studies demonstrated that AF cells reacted to environment cues and were involved in tissue modification in response to injury.
Scaffolds for AF tissue engineering
A number of scaffolds have been used for AF tissue engineering in laboratory settings (Table 2). These scaffolds include porous silks, alginate/chitosan, a demineralized bone matrix, electrospun poly(ε-caprolactone) (PCL) fibers, hyaluronic acid/nanofibrous, collagen/glycosaminoglycan, and polyglycolic acid mesh. Scaffolds can be summarized into two categories: single unit (oriented or non-oriented to simulate the organized lamellae) and biphasic to simulate inner and outer layer of AF.
Table 2.
Biomaterials used for AF tissue engineering
| AF tissue engineering scaffold | Biomaterials | Reference |
|---|---|---|
| Single unit without fiber orientation | Atelocollagen honeycomb | [85, 86] |
| Collagen-glycosaminoglycan | [87] | |
| Alginate/Chitosan | [88] | |
| Poly(1,8-octanediol malate) | [89] | |
| Poly-D-L-lactide/Bioglass | [90, 91] | |
| Silk | [92, 93] | |
| Genipin crosslinked fibrin | [94] | |
| Single unit with fiber orientation | Aligned alginate/chitosan | [88] |
| Polycarbonate polyurethane linked with dihydroxyl oligomer | [95, 96] | |
| Polycaprolactone | [97, 98] | |
| Poly-L-Lactide | [99] | |
| Silk fibers/chondroitin sulphate | [100] | |
| Collagen fibrils | [101] | |
| Biphasic scaffold | Poly (polycaprolactone triol malate)/DBM | [102] |
| Composite scaffold | Poly-L-Lactide as AF and hyaluronic acid hydrogel as NP | [24] |
| Polyglycolic Acid/poly-l-lactic as AF and alginate hydrogel as NP | [103] | |
| Silk scaffold as AF and silicon as NP or hyaluronic acid as NP | [104, 105] | |
| Polycaprolactone fibers as AF and agarose gel as NP | [32] | |
| Collagen gel as AF and Polyethylene/alginate as NP | [31, 106] |
Single unit without fiber orientation
Using an ACHMS scaffold (atelocollagen honeycomb-shaped scaffolds sealed with a membrane), a Japanese group showed that rabbit AF cells grew and maintained phenotype in the scaffold and produced type II collagen and proteoglycan [85]. In a lucana disc model, after laser vaporization of the NP of rabbit intervertebral discs, the honeycomb shaped ACHMS scaffold was implanted. The study showed that AF cells exhibited a proliferation activity, resulting in the production of hyaline-like cartilage similar to the original AF tissue. This prevented the narrowing of the disc space up to 12 postoperative weeks [86].
Adult canine AF cells were able to adhere and proliferate on a collagen–glycosaminoglycan (GAG) scaffold, a material that serves as an analog of extracellular matrix [87]. In another study, alginate or alginate/chitosan was fabricated for AF cells culture using a wet-spinning and lyophilization technique. The alginate/chitosan hybrid scaffold exhibited a slower degradation rate, maintained the growth of canine AF cells, and produced specific extracellular matrix molecules [88].
Using a direct one step polycondensation method, we were able to create a malic acid-based polyester poly(1,8-octanediol malate) (POM) film and support the proliferation of rat AF cells [89]. Adjusting post-polymerization time can control the tensile strength of POM: the tensile strength increased from 7.32 to 25.6 MPa at 9 days after polymerization. In contrast, the elongation decreased over the polymerization time from 14.34 to 3.86 MPa from day 3 to day 9. When rat AF cells were cultured on the POM scaffold, the cells penetrated into the scaffold as visualized by SEM, and expressed higher amount of proteoglycan and collagen II (Fig. 3).
Fig. 3.
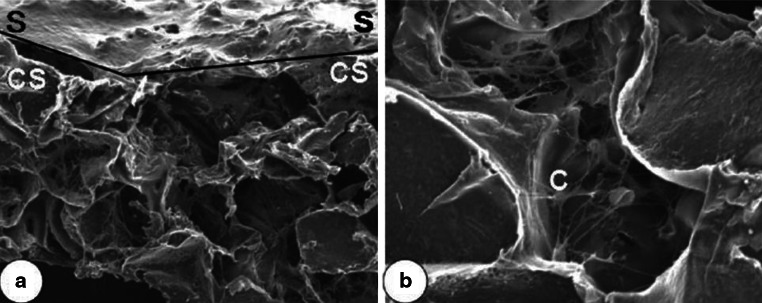
SEM images of rat AF cells cultured on the POM scaffold a 70× and b 200× for 3 weeks. S scaffold surface, CS scaffold cross-section, C cells [89]
Due to the specific position of intervertebral disc, the deformability is a critical biomechanical property for the AF tissue. The mechanical tests revealed that POM has an excellent deformability: the compressive stress, young’s modulus, and tensile strength markedly increased as the polymerization time increased. There was no permanent deformation after 500 press-loading and release cycles with 30 % maximum strain. When implanted into mice back subcutaneous pocket, there were no inflammation responses were found [89].
Similarly, studies showed that Poly-D-L-Lactide (PDLLA) foams incorporated with different percentages (0, 5 and 30 wt %) of bioglass particles supported bovine AF cell growth and matrix production [90, 91]. The PDLLA and bioglass scaffold, prepared with the thermally induced phase separation process, has a highly porous and foam like structure. The authors claimed that the pore size, mechanical properties, and degradation rates of the PDLLA foams can be controlled by varying the concentration, temperature, and solvent of the PDLLA. Bioactive glasses on the other hand determined mechanics, bioactivity and degradation kinetics of the foams. AF cells were also able to grow on porous silk scaffolds. Modified silk scaffold with arginine-glycineaspartate RGD has been shown to enhance the attachment of other cell types, change the cell morphology and matrix components but did not enhance the cell adhesion [92, 93].
Recently, a genipin crosslinked fibrin gel was used to support the human AF cell growth and adhesion. The authors showed that mechanical modulus of genipin crosslinked fibrin gels can be created similar to native annular tissue. These gels can be used for small AF defects or as an adhesive to augment large annulus repair [94].
Single unit with oriented fiber
The highly organized fibril architecture of AF provides IVD the capacity to resistant tensile and shear stress. Therefore in the design of scaffolds, the fiber alignment is a critical component. Studies have attempted to address the aligned and anisotropic nature of the AF such as collagen gel, aligned PCL fibers, alginate/chitosan, polycarbonate polyurethane (PU), and PPCLM concentric sheets.
With a wet-spinning and lyophilization technique, Shao et al. [88] created an aligned alginate/chitosan scaffold. Collagen gels with varying structure and heterogeneity were used to create circumferentially fibers. AF cells orient themselves along the aligned scaffolds and deposit matrix components that contribute to construct mechanics under loading conditions relevant to the in vivo environment.
Polycarbonate polyurethane (PU) was modified by chemically linking with an anionic dihydroxyl oligomer (ADO). The polymeric materials were fabricated into nanoscale fibrous scaffolds using electrospinning. PU nanofibrous scaffolds in the absence or presence of different amounts of ADO were similar in appearance. Increasing the material surface’s polar character of the scaffolds resulted in a positive enhancement of AF cell attachment [95]. Both the tensile strength and initial modulus of aligned scaffolds were higher than the random fiber scaffold. The soluble and non-soluble degradation products were found to be non-toxic to bovine AF cells grown in vitro [96].
Other electrospun nanofibrous scaffolds such as polycaprolactone were fabricated in random, aligned, and round-end configurations [97]. A modified electrospinning technique was utilized to generate aligned nanofibrous polymer scaffolds for engineering the basic functional unit of the AF, a single lamella [98]. Uniaxial tension was tested and demonstrated a nonlinear dependence of modulus on fiber angle similar to the nonlinearity and anisotropy of native AF. Culturing bovine AF cells onto a bioactive scaffold with poly-L-lactide incorporated with TGF-β, one study showed that the synthesis of GAG and collagen had markedly increased in the growth factor group compared to the control scaffold alone group [99]. Silk fibers crosslinking with chondroitin sulphate have also been shown to support human chondrocytes re-differentiation [100]. Although not specifically for AF tissue engineering, using molecular crowding and confinement techniques, Saeidi et al. has recently reported producing highly organized arrays of collagen fibrils. In addition, fibrils are organized in multi-layer structures similar to the structure of the collagen fibrils in the extracellular matrices of native tissues, which has potential for AF tissue engineering [101].
Biphasic scaffold
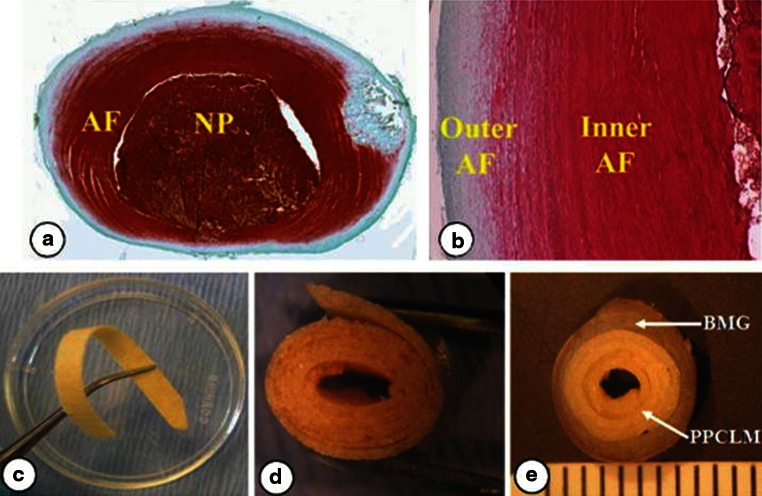
AF is a biphasic structure: an outer layer enriched in collagen I, and an inner layer with more collagen II. Therefore, we constructed a biphasic IVD with a ring-shaped demineralized bone matrix (DBM) as an outer AF and poly (polycaprolactone triol malate) (PPCLM) orientated concentric sheets seeded with chondrocytes as an inner AF [102] (Fig. 4). The DBM was extracted from cortical bone that mimicked the type I collagen structure and fibril property of the outer AF. The resulting PPCLM/DBM biphasic scaffold had excellent elasticity, with no permanent deformation after at least 100 press-loading and release cycles. The compressive stress of the DBM/PPCLM scaffold was significantly higher than that of pure PPCLM, with the incorporation of DBM enhancing the compressive strength of the PPCLM scaffold from 0.21 to 1.26 MPa. The gelatinous pulpous of the IVD absorbs and transmits compressive loads into the tensile stretch at the periphery of the AF. In the strain–stress curve, the stress increased linearly with the strain. The tensile stress of the DBM/PPCLM scaffold was 3.37 MPa, which was much higher than that of the pure PPCLM scaffold at 0.06 MPa for three sheets, and approaching that of rabbit AF at 6.95 MPa.
Fig. 4.
a Horizontal section and b vertical section of normal rabbit IVD stained with Safranin-O. The outer layer (reduced red staining) and inner layer (abundant red staining) of AF can clearly be seen. c The elastic biomaterial PPCLM orientated in concentric sheets (d) and inserted into a BMG ring to mimic the structure of inner and outer AF (e), respectively [102]
Total disc construct
Recently, the tissue engineering field has focused on creating composite tissue engineered total disc replacement, which consists of an inner NP surrounded with AF tissue. The strategy is to resemble the native structure and mechanical property of the intervertebral disc. These works provided advancement in the rational approach to the production of hierarchical and functional scaffolds.
Human mesenchymal stem cells were seeded into a hyaluronic acid hydrogel center and enveloped with a poly(L-lactic acid) nanofibrous scaffold to mimic the native disc structure. Nesti et al. [24] showed that seeded cells exhibited a chondrocyte phenotype. Using a non-woven mesh of polyglycolic acid (PGA) and solvent-cast polylactic acid (PLA) as an outer ‘AF’ ring seeded with AF cells and an alginate hydrogel to serve as the ‘NP’ core, Mizuno et al. created a scaffold in the cylindrical shape of the IVD. The AF and NP cells maintained their phenotypes and integrated over time in culture. When in vitro disc composites were subcutaneously implanted into athymic mice, the implants formed distinct AF and NP tissue as indicated by the expression of extracellular matrix and mechanical properties. By 16 weeks, the biochemical composition and mechanical properties of tissue-engineered intervertebral discs were similar to that of native tissue [103].
By loading rabbit BMSCs (bone marrow stem cells) hyper-confluent cell sheets on a silk scaffold, and a silicon NP substitute, See et al. showed that the cells are viable, and produced proteoglycan and type I, II collagen after 4 weeks in vitro culture. The ratio of collagen type I to collagen type II within the extracellular matrix of the BMSC sheets also decreased significantly over the period of the culture. The type of collagen found within the BMSC cell sheets were initially predominantly collagen types I. However, following a 4 week culture period within the assembly, collagen type II deposition became more pronounced within the extracellular matrix [104].
In another study, circumferentially orientated polycaprolactone fibers seeding with porcine chondrocytes were used to mimic the AF tissue, and the cell-agarose gel in the center was used to copy NP. They showed that the fibril alignment was formed, the chondrocytes were well distributed around the boundary of NP and AF, and the composite scaffolds had strong mechanical properties than that of agarose gel alone [32]. A different group created the AF/NP composite constructs with silk protein as an AF material and fibrin/hyaluronic acid gel as a NP tissue, and the composites were cultured in vitro up to 6 weeks [105].
Bowless et al. made the composite IVD constructs by using collagen gels seeded with ovine AF cells surrounding with either polyethylene or alginate in the center. By using the contraction feature of cell seeded collagen gel and controlling the boundary conditions, the authors were able to create aligned circumferential fibril structures, and found that more alignment occurred in annular-shaped 1 mg/mL gels compared with 2.5 mg/mL gels [106]. Later, they implanted the composite constructs into the athymic rat caudal spine, and demonstrated that up to 6 months the engineered IVD maintained similar IVD shapes, disc heights, levels of collagen and aggrecan to the native disc. The scaffold integrated well with the host body. More importantly, the engineered disc had a similar axial load capacity to the native disc. This finding provides the direct evidence that tissue engineering is possible, at least in a small animal model [31].
Conclusions
Despite the promising results in disc tissue engineering, there is still much work to be done regarding the clinical application. Although the combined NP/AF concept seems promising, it might be questionable whether the technique is effective to be used in repairing larger annulus defects. Novel strategies for delivery and fixation may be required. In addition, a suitable cell source for the reengineering of the “whole disc” is still challenging.
Acknowledgments
We apologize for not citing all of the important contributions to this field due to space limitations. We deeply appreciate Mrs. Janet Stack and Mr. Phillip Scott for their editing assistance. We thank the financial support from AO International and AONA Young Investigator Award.
Conflict of interest
None.
References
- 1.Freemont TJ, LeMaitre C, Watkins A, Hoyland JA. Degeneration of intervertebral discs: current understanding of cellular and molecular events, and implications for novel therapies. Expert Rev Mol Med. 2001;2001:1–10. doi: 10.1017/S1462399401002885. [DOI] [PubMed] [Google Scholar]
- 2.Adams MA, Freeman BJ, Morrison HP, Nelson IW, Dolan P. Mechanical initiation of intervertebral disc degeneration. Spine (Phila Pa 1976) 2000;25:1625–1636. doi: 10.1097/00007632-200007010-00005. [DOI] [PubMed] [Google Scholar]
- 3.Mannion AF, Adams MA, Dolan P. Sudden and unexpected loading generates high forces on the lumbar spine. Spine (Phila Pa 1976) 2000;25:842–852. doi: 10.1097/00007632-200004010-00013. [DOI] [PubMed] [Google Scholar]
- 4.Horner HA, Urban JP. Volvo award winner in basic science studies: effect of nutrient supply on the viability of cells from the nucleus pulposus of the intervertebral disc. Spine (Phila Pa 1976) 2001;26:2543–2549. doi: 10.1097/00007632-200112010-00006. [DOI] [PubMed] [Google Scholar]
- 5.Brown MD, Malinin TI, Davis PB. A roentgenographic evaluation of frozen allografts versus autografts in anterior cervical spine fusions. Clin Orthop Relat Res. 1976;119:231–236. [PubMed] [Google Scholar]
- 6.Bibby SR, Urban JP. Effect of nutrient deprivation on the viability of intervertebral disc cells. Eur Spine J. 2004;13:695–701. doi: 10.1007/s00586-003-0616-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang X, Li X. Nucleus pulposus tissue engineering: a brief review. Eur Spine J. 2009;18:1564–1572. doi: 10.1007/s00586-009-1092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine (Phila Pa 1976) 1995;20:1307–1314. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- 9.Haefeli M, Elfering A, Kilian R, Min K, Boos N. Nonoperative treatment for adolescent idiopathic scoliosis: A 10- to 60-year follow-up with special reference to health-related quality of life. Spine (Phila Pa 1976) 2006;31:355–366. doi: 10.1097/01.brs.0000197664.02098.09. [DOI] [PubMed] [Google Scholar]
- 10.Anderson DG, Tannoury C. Molecular pathogenic factors in symptomatic disc degeneration. Spine J. 2005;5:260S–266S. doi: 10.1016/j.spinee.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Anderson DG, Albert TJ, Fraser JK, Risbud M, Wuisman P, Meisel HJ, Tannoury C, Shapiro I, Vaccaro AR. Cellular therapy for disc degeneration. Spine (Phila Pa 1976) 2005;30:S14–S19. doi: 10.1097/01.brs.0000175174.50235.ba. [DOI] [PubMed] [Google Scholar]
- 12.Kurtz SM, Devine JN. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials. 2007;28:4845–4869. doi: 10.1016/j.biomaterials.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurtz SM, Ong KL, Schmier J, Mowat F, Saleh K, Dybvik E, Karrholm J, Garellick G, Havelin LI, Furnes O, Malchau H, Lau E. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(Suppl 3):144–151. doi: 10.2106/JBJS.G.00587. [DOI] [PubMed] [Google Scholar]
- 14.Kurtz SM, van Ooij A, Ross R, de Waal Malefijt J, Peloza J, Ciccarelli L, Villarraga ML. Polyethylene wear and rim fracture in total disc arthroplasty. Spine J. 2007;7:12–21. doi: 10.1016/j.spinee.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Punt IM, Visser VM, van Rhijn LW, Kurtz SM, Antonis J, Schurink GW, van Ooij A. Complications and reoperations of the SB charite lumbar disc prosthesis: experience in 75 patients. Eur Spine J. 2008;17:36–43. doi: 10.1007/s00586-007-0506-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serhan HA, Dooris AP, Parsons ML, Ares PJ, Gabriel SM. In vitro wear assessment of the charite artificial disc according to ASTM recommendations. Spine (Phila Pa 1976) 2006;31:1900–1910. doi: 10.1097/01.brs.0000228716.60863.ab. [DOI] [PubMed] [Google Scholar]
- 17.Slivka MA, Spenciner DB, Seim HB, 3rd, Welch WC, Serhan HA, Turner AS. High rate of fusion in sheep cervical spines following anterior interbody surgery with absorbable and nonabsorbable implant devices. Spine (Phila Pa 1976) 2006;31:2772–2777. doi: 10.1097/01.brs.0000245935.69927.a1. [DOI] [PubMed] [Google Scholar]
- 18.Lyons G, Eisenstein SM, Sweet MB. Biochemical changes in intervertebral disc degeneration. Biochim Biophys Acta. 1981;673:443–453. doi: 10.1016/0304-4165(81)90476-1. [DOI] [PubMed] [Google Scholar]
- 19.An HS, Masuda K, Cs-Szabo G, Zhang Y, Chee A, Andersson GB, Im HJ, Thonar EJ, Kwon YM. Biologic repair and regeneration of the intervertebral disk. J Am Acad Orthop Surg. 2011;19:450–452. [PubMed] [Google Scholar]
- 20.Cs-Szabo G, Ragasa-San Juan D, Turumella V, Masuda K, Thonar EJ, An HS. Changes in mRNA and protein levels of proteoglycans of the anulus fibrosus and nucleus pulposus during intervertebral disc degeneration. Spine (Phila Pa 1976) 2002;27:2212–2219. doi: 10.1097/00007632-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 21.O’Halloran DM, Pandit AS. Tissue-engineering approach to regenerating the intervertebral disc. Tissue Eng. 2007;13:1927–1954. doi: 10.1089/ten.2005.0608. [DOI] [PubMed] [Google Scholar]
- 22.Chan SC, Gantenbein-Ritter B. Intervertebral disc regeneration or repair with biomaterials and stem cell therapy–feasible or fiction? Swiss Med Wkly. 2012;142:w13598. doi: 10.4414/smw.2012.13598. [DOI] [PubMed] [Google Scholar]
- 23.Alini M, Roughley PJ, Antoniou J, Stoll T, Aebi M. A biological approach to treating disc degeneration: not for today, but maybe for tomorrow. Eur Spine J. 2002;11(Suppl 2):S215–S220. doi: 10.1007/s00586-002-0485-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nesti LJ, Li WJ, Shanti RM, Jiang YJ, Jackson W, Freedman BA, Kuklo TR, Giuliani JR, Tuan RS. Intervertebral disc tissue engineering using a novel hyaluronic acid-nanofibrous scaffold (HANFS) amalgam. Tissue Eng Part A. 2008;14:1527–1537. doi: 10.1089/ten.tea.2008.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woiciechowsky C, Abbushi A, Zenclussen ML, Casalis P, Kruger JP, Freymann U, Endres M, Kaps C (2012) Regeneration of nucleus pulposus tissue in an ovine intervertebral disc degeneration model by cell-free resorbable polymer scaffolds. J Tissue Eng Regen Med [Epub ahead of print] [DOI] [PubMed]
- 26.Pei M, Shoukry M, Li J, Daffner SD, France JC, Emery SE. Modulation of in vitro microenvironment facilitates synovium-derived stem cell-based nucleus pulposus tissue regeneration. Spine (Phila Pa 1976) 2012;37:1538–1547. doi: 10.1097/BRS.0b013e31825150bf. [DOI] [PubMed] [Google Scholar]
- 27.Gupta MS, Cooper ES, Nicoll SB. Transforming growth factor-beta 3 stimulates cartilage matrix elaboration by human marrow-derived stromal cells encapsulated in photocrosslinked carboxymethylcellulose hydrogels: potential for nucleus pulposus replacement. Tissue Eng Part A. 2011;17:2903–2910. doi: 10.1089/ten.tea.2011.0152. [DOI] [PubMed] [Google Scholar]
- 28.Collin EC, Grad S, Zeugolis DI, Vinatier CS, Clouet JR, Guicheux JJ, Weiss P, Alini M, Pandit AS. An injectable vehicle for nucleus pulposus cell-based therapy. Biomaterials. 2011;32:2862–2870. doi: 10.1016/j.biomaterials.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 29.Mortisen D, Peroglio M, Alini M, Eglin D. Tailoring thermoreversible hyaluronan hydrogels by “click” chemistry and RAFT polymerization for cell and drug therapy. Biomacromolecules. 2010;11:1261–1272. doi: 10.1021/bm100046n. [DOI] [PubMed] [Google Scholar]
- 30.Peroglio M, Grad S, Mortisen D, Sprecher CM, Illien-Junger S, Alini M, Eglin D. Injectable thermoreversible hyaluronan-based hydrogels for nucleus pulposus cell encapsulation. Eur Spine J. 2012;21(Suppl 6):S839–S849. doi: 10.1007/s00586-011-1976-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bowles RD, Gebhard HH, Hartl R, Bonassar LJ. Tissue-engineered intervertebral discs produce new matrix, maintain disc height, and restore biomechanical function to the rodent spine. Proc Natl Acad Sci USA. 2011;108:13106–13111. doi: 10.1073/pnas.1107094108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lazebnik M, Singh M, Glatt P, Friis LA, Berkland CJ, Detamore MS. Biomimetic method for combining the nucleus pulposus and annulus fibrosus for intervertebral disc tissue engineering. J Tissue Eng Regen Med. 2011;5:e179–e187. doi: 10.1002/term.412. [DOI] [PubMed] [Google Scholar]
- 33.Schollmeier G, Lahr-Eigen R, Lewandrowski KU. Observations on fiber-forming collagens in the anulus fibrosus. Spine (Phila Pa 1976) 2000;25:2736–2741. doi: 10.1097/00007632-200011010-00004. [DOI] [PubMed] [Google Scholar]
- 34.Yu J, Tirlapur U, Fairbank J, Handford P, Roberts S, Winlove CP, Cui Z, Urban J. Microfibrils, elastin fibres and collagen fibres in the human intervertebral disc and bovine tail disc. J Anat. 2007;210:460–471. doi: 10.1111/j.1469-7580.2007.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adams P, Eyre DR, Muir H. Biochemical aspects of development and ageing of human lumbar intervertebral discs. Rheumatol Rehabil. 1977;16:22–29. doi: 10.1093/rheumatology/16.1.22. [DOI] [PubMed] [Google Scholar]
- 36.Hayes AJ, Benjamin M, Ralphs JR. Extracellular matrix in development of the intervertebral disc. Matrix Biol. 2001;20:107–121. doi: 10.1016/S0945-053X(01)00125-1. [DOI] [PubMed] [Google Scholar]
- 37.Hayes AJ, Smith SM, Gibson MA, Melrose J. Comparative immunolocalisation of the elastin fibre associated proteins fibrillin-1, LTBP2 and MAGP-1 with components of the collagenous and proteoglycan matrix of the foetal human IVD. Spine (Phila Pa 1976) 2011;36(21):E1365–E1372. doi: 10.1097/BRS.0b013e31821fd23e. [DOI] [PubMed] [Google Scholar]
- 38.Eyre DR, Muir H. Types I and II collagens in intervertebral disc. interchanging radial distributions in annulus fibrosus. Biochem J. 1976;157:267–270. doi: 10.1042/bj1570267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rufai A, Benjamin M, Ralphs JR. The development of fibrocartilage in the rat intervertebral disc. Anat Embryol (Berl) 1995;192:53–62. doi: 10.1007/BF00186991. [DOI] [PubMed] [Google Scholar]
- 40.Hayes AJ, Isaacs MD, Hughes C, Caterson B, Ralphs JR. Collagen fibrillogenesis in the development of the annulus fibrosus of the intervertebral disc. Eur Cell Mater. 2011;22:226–241. doi: 10.22203/ecm.v022a18. [DOI] [PubMed] [Google Scholar]
- 41.Singh K, Masuda K, Thonar EJ, An HS, Cs-Szabo G. Age-related changes in the extracellular matrix of nucleus pulposus and anulus fibrosus of human intervertebral disc. Spine (Phila Pa 1976) 2009;34:10–16. doi: 10.1097/BRS.0b013e31818e5ddd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pattappa G, Li Z, Peroglio M, Wismer N, Alini M, Grad S. Diversity of intervertebral disc cells: phenotype and function. J Anat. 2012;221(6):480–496. doi: 10.1111/j.1469-7580.2012.01521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bruehlmann SB, Rattner JB, Matyas JR, Duncan NA. Regional variations in the cellular matrix of the annulus fibrosus of the intervertebral disc. J Anat. 2002;201:159–171. doi: 10.1046/j.1469-7580.2002.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Errington RJ, Puustjarvi K, White IR, Roberts S, Urban JP. Characterisation of cytoplasm-filled processes in cells of the intervertebral disc. J Anat. 1998;192(Pt 3):369–378. doi: 10.1046/j.1469-7580.1998.19230369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roughley P, Hoemann C, DesRosiers E, Mwale F, Antoniou J, Alini M. The potential of chitosan-based gels containing intervertebral disc cells for nucleus pulposus supplementation. Biomaterials. 2006;27:388–396. doi: 10.1016/j.biomaterials.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 46.Clouet J, Grimandi G, Pot-Vaucel M, Masson M, Fellah HB, Guigand L, Cherel Y, Bord E, Rannou F, Weiss P, Guicheux J, Vinatier C. Identification of phenotypic discriminating markers for intervertebral disc cells and articular chondrocytes. Rheumatology (Oxford) 2009;48:1447–1450. doi: 10.1093/rheumatology/kep262. [DOI] [PubMed] [Google Scholar]
- 47.Minogue BM, Richardson SM, Zeef LA, Freemont AJ, Hoyland JA. Transcriptional profiling of bovine intervertebral disc cells: implications for identification of normal and degenerate human intervertebral disc cell phenotypes. Arthritis Res Ther. 2010;12:R22. doi: 10.1186/ar2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gruber HE, Hoelscher GL, Hanley EN., Jr Annulus cells from more degenerated human discs show modified gene expression in 3D culture compared with expression in cells from healthier discs. Spine J. 2010;10:721–727. doi: 10.1016/j.spinee.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 49.Feng G, Yang X, Shang H, Marks IW, Shen FH, Katz A, Arlet V, Laurencin CT, Li X. Multipotential differentiation of human anulus fibrosus cells: an in vitro study. J Bone Joint Surg Am. 2010;92:675–685. doi: 10.2106/JBJS.H.01672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Risbud MV, Guttapalli A, Tsai TT, Lee JY, Danielson KG, Vaccaro AR, Albert TJ, Gazit Z, Gazit D, Shapiro IM. Evidence for skeletal progenitor cells in the degenerate human intervertebral disc. Spine (Phila Pa 1976) 2007;32:2537–2544. doi: 10.1097/BRS.0b013e318158dea6. [DOI] [PubMed] [Google Scholar]
- 51.Poiraudeau S, Monteiro I, Anract P, Blanchard O, Revel M, Corvol MT. Phenotypic characteristics of rabbit intervertebral disc cells. Comparison with cartilage cells from the same animals. Spine (Phila Pa 1976) 1999;24:837–844. doi: 10.1097/00007632-199905010-00002. [DOI] [PubMed] [Google Scholar]
- 52.Gruber HE, Fisher EC, Jr, Desai B, Stasky AA, Hoelscher G, Hanley EN., Jr Human intervertebral disc cells from the annulus: three-dimensional culture in agarose or alginate and responsiveness to TGF-beta1. Exp Cell Res. 1997;235:13–21. doi: 10.1006/excr.1997.3647. [DOI] [PubMed] [Google Scholar]
- 53.Maldonado BA, Oegema TR., Jr Initial characterization of the metabolism of intervertebral disc cells encapsulated in microspheres. J Orthop Res. 1992;10:677–690. doi: 10.1002/jor.1100100510. [DOI] [PubMed] [Google Scholar]
- 54.Gruber HE, Leslie K, Ingram J, Hoelscher G, Norton HJ, Hanley EN., Jr Colony formation and matrix production by human anulus cells: modulation in three-dimensional culture. Spine (Phila Pa 1976) 2004;29:E267–E274. doi: 10.1097/01.BRS.0000129029.10036.64. [DOI] [PubMed] [Google Scholar]
- 55.Mizuno H, Roy AK, Vacanti CA, Kojima K, Ueda M, Bonassar LJ. Tissue-engineered composites of anulus fibrosus and nucleus pulposus for intervertebral disc replacement. Spine (Phila Pa 1976) 2004;29:1290–1297. doi: 10.1097/01.BRS.0000128264.46510.27. [DOI] [PubMed] [Google Scholar]
- 56.Yang X, Wang D, Hao J, Gong M, Arlet V, Balian G, Shen FH, Li XJ. Enhancement of matrix production and cell proliferation in human annulus cells under bioreactor culture. Tissue Eng Part A. 2011;17:1595–1603. doi: 10.1089/ten.tea.2010.0449. [DOI] [PubMed] [Google Scholar]
- 57.Le Maitre CL, Richardson SM, Baird P, Freemont AJ, Hoyland JA. Expression of receptors for putative anabolic growth factors in human intervertebral disc: implications for repair and regeneration of the disc. J Pathol. 2005;207:445–452. doi: 10.1002/path.1862. [DOI] [PubMed] [Google Scholar]
- 58.Tolonen J, Gronblad M, Vanharanta H, Virri J, Guyer RD, Rytomaa T, Karaharju EO. Growth factor expression in degenerated intervertebral disc tissue. An immunohistochemical analysis of transforming growth factor beta, fibroblast growth factor and platelet-derived growth factor. Eur Spine J. 2006;15:588–596. doi: 10.1007/s00586-005-0930-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim JH, Studer RK, Vo NV, Sowa GA, Kang JD. p38 MAPK inhibition selectively mitigates inflammatory mediators and VEGF production in AF cells co-cultured with activated macrophage-like THP-1 cells. Osteoarthritis Cartilage. 2009;17:1662–1669. doi: 10.1016/j.joca.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 60.Gilbert HT, Hoyland JA, Freemont AJ, Millward-Sadler SJ. The involvement of interleukin-1 and interleukin-4 in the response of human annulus fibrosus cells to cyclic tensile strain: an altered mechanotransduction pathway with degeneration. Arthritis Res Ther. 2011;13:R8. doi: 10.1186/ar3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hegewald AA, Neumann K, Kalwitz G, Freymann U, Endres M, Schmieder K, Kaps C, Thome C. The chemokines CXCL10 and XCL1 recruit human annulus fibrosus cells. Spine (Phila Pa 1976) 2011;37(2):101–107. doi: 10.1097/BRS.0b013e318210ed55. [DOI] [PubMed] [Google Scholar]
- 62.Thonar E, An H, Masuda K. Compartmentalization of the matrix formed by nucleus pulposus and annulus fibrosus cells in alginate gel. Biochem Soc Trans. 2002;30:874–878. doi: 10.1042/BST0300874. [DOI] [PubMed] [Google Scholar]
- 63.Thompson JP, Oegema TR, Jr, Bradford DS. Stimulation of mature canine intervertebral disc by growth factors. Spine (Phila Pa 1976) 1991;16:253–260. doi: 10.1097/00007632-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 64.Masuda K, Takegami K, An H, Kumano F, Chiba K, Andersson GB, Schmid T, Thonar E. Recombinant osteogenic protein-1 upregulates extracellular matrix metabolism by rabbit annulus fibrosus and nucleus pulposus cells cultured in alginate beads. J Orthop Res. 2003;21:922–930. doi: 10.1016/S0736-0266(03)00037-8. [DOI] [PubMed] [Google Scholar]
- 65.Masuda K, An HS. Prevention of disc degeneration with growth factors. Eur Spine J. 2006;15(Suppl 3):S422–S432. doi: 10.1007/s00586-006-0149-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takegami K, An HS, Kumano F, Chiba K, Thonar EJ, Singh K, Masuda K. Osteogenic protein-1 is most effective in stimulating nucleus pulposus and annulus fibrosus cells to repair their matrix after chondroitinase ABC-induced in vitro chemonucleolysis. Spine J. 2005;5:231–238. doi: 10.1016/j.spinee.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 67.Gilbertson L, Ahn SH, Teng PN, Studer RK, Niyibizi C, Kang JD. The effects of recombinant human bone morphogenetic protein-2, recombinant human bone morphogenetic protein-12, and adenoviral bone morphogenetic protein-12 on matrix synthesis in human annulus fibrosis and nucleus pulposus cells. Spine J. 2008;8:449–456. doi: 10.1016/j.spinee.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 68.Cui M, Wan Y, Anderson DG, Shen FH, Leo BM, Laurencin CT, Balian G, Li X. Mouse growth and differentiation factor-5 protein and DNA therapy potentiates intervertebral disc cell aggregation and chondrogenic gene expression. Spine J. 2008;8:287–295. doi: 10.1016/j.spinee.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 69.Shen FH, Zeng Q, Lv Q, Choi L, Balian G, Li X, Laurencin CT. Osteogenic differentiation of adipose-derived stromal cells treated with GDF-5 cultured on a novel three-dimensional sintered microsphere matrix. Spine J. 2006;6:615–623. doi: 10.1016/j.spinee.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 70.Walsh AJ, Bradford DS, Lotz JC. In vivo growth factor treatment of degenerated intervertebral discs. Spine (Phila Pa 1976) 2004;29:156–163. doi: 10.1097/01.BRS.0000107231.67854.9F. [DOI] [PubMed] [Google Scholar]
- 71.Hayes AJ, Ralphs JR. The response of foetal annulus fibrosus cells to growth factors: modulation of matrix synthesis by TGF-beta1 and IGF-1. Histochem Cell Biol. 2011;136:163–175. doi: 10.1007/s00418-011-0835-x. [DOI] [PubMed] [Google Scholar]
- 72.Masuda K, An HS. Growth factors and the intervertebral disc. Spine J. 2004;4:330S–340S. doi: 10.1016/j.spinee.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 73.Osada R, Ohshima H, Ishihara H, Yudoh K, Sakai K, Matsui H, Tsuji H. Autocrine/paracrine mechanism of insulin-like growth factor-1 secretion, and the effect of insulin-like growth factor-1 on proteoglycan synthesis in bovine intervertebral discs. J Orthop Res. 1996;14:690–699. doi: 10.1002/jor.1100140503. [DOI] [PubMed] [Google Scholar]
- 74.Pratsinis H, Kletsas D. PDGF, bFGF and IGF-I stimulate the proliferation of intervertebral disc cells in vitro via the activation of the ERK and akt signaling pathways. Eur Spine J. 2007;16:1858–1866. doi: 10.1007/s00586-007-0408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chujo T, An HS, Akeda K, Miyamoto K, Muehleman C, Attawia M, Andersson G, Masuda K. Effects of growth differentiation factor-5 on the intervertebral disc–in vitro bovine study and in vivo rabbit disc degeneration model study. Spine (Phila Pa 1976) 2006;31:2909–2917. doi: 10.1097/01.brs.0000248428.22823.86. [DOI] [PubMed] [Google Scholar]
- 76.Sobajima S, Vadala G, Shimer A, Kim JS, Gilbertson LG, Kang JD. Feasibility of a stem cell therapy for intervertebral disc degeneration. Spine J. 2008;8:888–896. doi: 10.1016/j.spinee.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 77.Tim Yoon S, Su Kim K, Li J, Soo Park J, Akamaru T, Elmer WA, Hutton WC. The effect of bone morphogenetic protein-2 on rat intervertebral disc cells in vitro. Spine (Phila Pa 1976) 2003;28:1773–1780. doi: 10.1097/01.BRS.0000083204.44190.34. [DOI] [PubMed] [Google Scholar]
- 78.Zhang Y, Anderson DG, Phillips FM, Thonar EJ, He TC, Pietryla D, An HS. Comparative effects of bone morphogenetic proteins and Sox9 overexpression on matrix accumulation by bovine anulus fibrosus cells: implications for anular repair. Spine (Phila Pa 1976) 2007;32:2515–2520. doi: 10.1097/BRS.0b013e318158cc09. [DOI] [PubMed] [Google Scholar]
- 79.Li X, Leo BM, Beck G, Balian G, Anderson GD. Collagen and proteoglycan abnormalities in the GDF-5-deficient mice and molecular changes when treating disk cells with recombinant growth factor. Spine (Phila Pa 1976) 2004;29:2229–2234. doi: 10.1097/01.brs.0000142427.82605.fb. [DOI] [PubMed] [Google Scholar]
- 80.Feng G, Wan Y, Balian G, Laurencin CT, Li X. Adenovirus-mediated expression of growth and differentiation factor-5 promotes chondrogenesis of adipose stem cells. Growth Factors. 2008;26:132–142. doi: 10.1080/08977190802105917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liang H, Ma SY, Feng G, Shen FH, Joshua Li X. Therapeutic effects of adenovirus-mediated growth and differentiation factor-5 in a mice disc degeneration model induced by annulus needle puncture. Spine J. 2010;10:32–41. doi: 10.1016/j.spinee.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mwale F, Demers CN, Petit A, Roughley P, Poole AR, Steffen T, Aebi M, Antoniou J. A synthetic peptide of link protein stimulates the biosynthesis of collagens II, IX and proteoglycan by cells of the intervertebral disc. J Cell Biochem. 2003;88:1202–1213. doi: 10.1002/jcb.10479. [DOI] [PubMed] [Google Scholar]
- 83.Mwale F, Masuda K, Pichika R, Epure LM, Yoshikawa T, Hemmad A, Roughley PJ, Antoniou J. The efficacy of link N as a mediator of repair in a rabbit model of intervertebral disc degeneration. Arthritis Res Ther. 2011;13:R120. doi: 10.1186/ar3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Petit A, Yao G, Rowas SA, Gawri R, Epure L, Antoniou J, Mwale F. Effect of synthetic link N peptide on the expression of type I and type II collagens in human intervertebral disc cells. Tissue Eng Part A. 2011;17:899–904. doi: 10.1089/ten.tea.2010.0494. [DOI] [PubMed] [Google Scholar]
- 85.Sato M, Kikuchi M, Ishihara M, Ishihara M, Asazuma T, Kikuchi T, Masuoka K, Hattori H, Fujikawa K. Tissue engineering of the intervertebral disc with cultured annulus fibrosus cells using atelocollagen honeycomb-shaped scaffold with a membrane seal (ACHMS scaffold) Med Biol Eng Comput. 2003;41:365–371. doi: 10.1007/BF02348444. [DOI] [PubMed] [Google Scholar]
- 86.Sato M, Asazuma T, Ishihara M, Ishihara M, Kikuchi T, Kikuchi M, Fujikawa K. An experimental study of the regeneration of the intervertebral disc with an allograft of cultured annulus fibrosus cells using a tissue-engineering method. Spine (Phila Pa 1976) 2003;28:548–553. doi: 10.1097/01.BRS.0000049909.09102.60. [DOI] [PubMed] [Google Scholar]
- 87.Saad L, Spector M. Effects of collagen type on the behavior of adult canine annulus fibrosus cells in collagen-glycosaminoglycan scaffolds. J Biomed Mater Res A. 2004;71:233–241. doi: 10.1002/jbm.a.30150. [DOI] [PubMed] [Google Scholar]
- 88.Shao X, Hunter CJ. Developing an alginate/chitosan hybrid fiber scaffold for annulus fibrosus cells. J Biomed Mater Res A. 2007;82:701–710. doi: 10.1002/jbm.a.31030. [DOI] [PubMed] [Google Scholar]
- 89.Wan Y, Feng G, Shen FH, Balian G, Laurencin CT, Li X. Novel biodegradable poly(1,8-octanediol malate) for annulus fibrosus regeneration. Macromol Biosci. 2007;7:1217–1224. doi: 10.1002/mabi.200700053. [DOI] [PubMed] [Google Scholar]
- 90.Helen W, Gough JE. Cell viability, proliferation and extracellular matrix production of human annulus fibrosus cells cultured within PDLLA/Bioglass composite foam scaffolds in vitro. Acta Biomater. 2008;4:230–243. doi: 10.1016/j.actbio.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 91.Helen W, Merry CL, Blaker JJ, Gough JE. Three-dimensional culture of annulus fibrosus cells within PDLLA/Bioglass composite foam scaffolds: assessment of cell attachment, proliferation and extracellular matrix production. Biomaterials. 2007;28:2010–2020. doi: 10.1016/j.biomaterials.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 92.Chang G, Kim HJ, Vunjak-Novakovic G, Kaplan DL, Kandel R. Enhancing annulus fibrosus tissue formation in porous silk scaffolds. J Biomed Mater Res A. 2010;92:43–51. doi: 10.1002/jbm.a.32326. [DOI] [PubMed] [Google Scholar]
- 93.Chang G, Kim HJ, Kaplan D, Vunjak-Novakovic G, Kandel RA. Porous silk scaffolds can be used for tissue engineering annulus fibrosus. Eur Spine J. 2007;16:1848–1857. doi: 10.1007/s00586-007-0364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schek RM, Michalek AJ, Iatridis JC. Genipin-crosslinked fibrin hydrogels as a potential adhesive to augment intervertebral disc annulus repair. Eur Cell Mater. 2011;21:373–383. doi: 10.22203/ecm.v021a28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang L, Kandel RA, Chang G, Santerre JP. Polar surface chemistry of nanofibrous polyurethane scaffold affects annulus fibrosus cell attachment and early matrix accumulation. J Biomed Mater Res A. 2009;91:1089–1099. doi: 10.1002/jbm.a.32331. [DOI] [PubMed] [Google Scholar]
- 96.Yeganegi M, Kandel RA, Santerre JP. Characterization of a biodegradable electrospun polyurethane nanofiber scaffold: mechanical properties and cytotoxicity. Acta Biomater. 2010;6:3847–3855. doi: 10.1016/j.actbio.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 97.Koepsell L, Zhang L, Neufeld D, Fong H, Deng Y. Electrospun nanofibrous polycaprolactone scaffolds for tissue engineering of annulus fibrosus. Macromol Biosci. 2011;11:391–399. doi: 10.1002/mabi.201000352. [DOI] [PubMed] [Google Scholar]
- 98.Nerurkar NL, Elliott DM, Mauck RL. Mechanics of oriented electrospun nanofibrous scaffolds for annulus fibrosus tissue engineering. J Orthop Res. 2007;25:1018–1028. doi: 10.1002/jor.20384. [DOI] [PubMed] [Google Scholar]
- 99.Vadala G, Mozetic P, Rainer A, Centola M, Loppini M, Trombetta M, Denaro V. Bioactive electrospun scaffold for annulus fibrosus repair and regeneration. Eur Spine J. 2012;21(Suppl 1):S20–S26. doi: 10.1007/s00586-012-2235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bhattacharjee M, Miot S, Gorecka A, Singha K, Loparic M, Dickinson S, Das A, Bhavesh NS, Ray AR, Martin I, Ghosh S. Oriented lamellar silk fibrous scaffolds to drive cartilage matrix orientation: towards annulus fibrosus tissue engineering. Acta Biomater. 2012;8:3313–3325. doi: 10.1016/j.actbio.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 101.Saeidi N, Karmelek KP, Paten JA, Zareian R, Dimasi E, Ruberti JW. Molecular crowding of collagen: a pathway to produce highly-organized collagenous structures. Biomaterials. 2012;33:7366–7374. doi: 10.1016/j.biomaterials.2012.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wan Y, Feng G, Shen FH, Laurencin CT, Li X. Biphasic scaffold for annulus fibrosus tissue regeneration. Biomaterials. 2008;29:643–652. doi: 10.1016/j.biomaterials.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 103.Mizuno H, Roy AK, Zaporojan V, Vacanti CA, Ueda M, Bonassar LJ. Biomechanical and biochemical characterization of composite tissue-engineered intervertebral discs. Biomaterials. 2006;27:362–370. doi: 10.1016/j.biomaterials.2005.06.042. [DOI] [PubMed] [Google Scholar]
- 104.See EY, Toh SL, Goh JC (2012) Simulated intervertebral disc-like assembly using bone marrow-derived mesenchymal stem cell sheets and silk scaffolds for annulus fibrosus regeneration. J Tissue Eng Regen Med 6:528–535 [DOI] [PubMed]
- 105.Park SH, Gil ES, Cho H, Mandal BB, Tien LW, Min BH, Kaplan DL. Intervertebral disk tissue engineering using biphasic silk composite scaffolds. Tissue Eng Part A. 2012;18:447–458. doi: 10.1089/ten.tea.2011.0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bowles RD, Gebhard HH, Dyke JP, Ballon DJ, Tomasino A, Cunningham ME, Hartl R, Bonassar LJ. Image-based tissue engineering of a total intervertebral disc implant for restoration of function to the rat lumbar spine. NMR Biomed. 2011;25(3):443–451. doi: 10.1002/nbm.1651. [DOI] [PubMed] [Google Scholar]