Abstract
Purpose
The purpose of this study is to present a new endoscopic procedure, aiming to achieve the success rate equivalent to microsurgical discectomy, while addressing the drawbacks and limitations of other percutaneous techniques.
Methods
A series of 43 patients with uncontained lumbar disc herniation underwent surgery with irrigation endoscopic discectomy (IED). The endoscope and instruments are placed directly over the surface of the lamina through two posterior skin portals 5 mm each without any muscle retraction or dilatation. Pump irrigation is used for the opening of a potential working space. The rest of the procedure is performed endoscopically like the standard microsurgical discectomy.
Results
Outcome according to modified Macnab criteria was excellent in 78 %, good in 17 %, and poor in 5 % of patients. VAS for leg pain dropped from 78 preoperatively to 7, and the Oswestry Low-Back Pain Disability Questionnaire dropped from 76 to 19. The mean time for postoperative ambulation was 4 h, hospital stay was 8 h, and for return to work was 7 days.
Conclusions
Preliminary clinical experience with IED shows it to be as effective as microsurgical discectomy, and in comparison to other percutaneous procedures addressing noncontained herniations, a reduction in the cost, technical difficulty and surgical invasiveness has been demonstrated.
Keywords: Irrigation endoscopic discectomy, Endoscopic discectomy, Minimally invasive spine surgery, Lumbar discectomy, Percutaneous discectomy
Introduction
In the history of lumbar disc surgery there have been numerous attempts to achieve a percutaneous procedure to decrease the postoperative pain, the hospital stay and the recovery time while in the mean time being as successful as the gold standard open microsurgical discectomy [1].
The percutaneous techniques could be broadly classified into two groups. In the former, a posterolateral route approaching the disc outside the spinal canal is utilized [2–8]. In a multicenter study, the reported success rate of these procedures was only 55 %, and the presence of sequestrated fragments, lateral recess or canal stenosis remained a contraindication [9].
On the contrast is the endoscopic procedure using the posterior interlaminar access to the spinal canal which is the microendoscopic discectomy (MED). The technique relies on sequential muscle dilatation followed by the introduction of a 1.8 cm tubular retractor for the 90° angled endoscope and instruments. Success rate comparable to microdiscectomy without any limitation in case selection was achieved [10–12]. Relative reduction in the surgical morbidity in the MED in comparison to microsurgical discectomy has been shown in several studies [13–17]. However, the relatively steep learning curve and the technical problems associated with MED remained a point of concern [18–20].
The purpose of this study is to describe the irrigation endoscopic discectomy (IED); a novel posterior endoscopic procedure aiming to achieve the success rate equivalent to microsurgical discectomy and MED without any limitations in case selection, while adding the following advantages to percutaneous procedures: (1) further reduction in the surgical invasiveness as the endoscope and instruments are directly placed over the surface of the lamina without any muscle stripping or dilatation through two skin portals 5 mm each, (2) cost reduction and widespread use due to the use of ordinary arthroscopic and spine instruments without need for special endoscopic sets, (3) free movement and angulation of the surgical tool and the endoscope independent of each other as they are not restricted by the confines of a common working portal which results in marked reduction in the technical difficulties, (4) the use of saline irrigation abolishes the problem of repeatedly cleaning the endoscopic lens of accumulated fog or blood.
Materials and methods
Patient characteristics
This is a prospective clinical study of a series of 43 consecutive patients with symptomatic lumbar disc herniation who underwent the irrigation endoscopic discectomy. Patients’ characteristics are listed in Table 1. Selection criteria: patients having unremitting sciatica, with or without back pain, and/or a neurological deficit that correlated with appropriate level and side of neural compression as revealed on imaging. Morbid obesity was not considered a contraindication. Exclusion criteria were patients with predominant back pain and other spinal degenerative conditions such as central stenosis, discogenic back pain or instability and L1–L2 level.
Table 1.
Patients’ characteristics in the study
| Age | 27–56 years (mean: 38) |
| Male to female ratio | 25:18 |
| Morbid obesity (males more than 340 lbs; females more than 225 lbs) | 6 |
| Noncontained herniation | 43 |
| Lateral recess/foraminal stenosis | 17 |
| Level | |
| L2–L3 | 4 |
| L3–L4 | 8 |
| L4–L5 | 17 |
| L5–S1 | 14 |
Instruments
The standard surgical arthroscopic facilities used are shown in Figs. 1 and 2, in addition to the ordinary spine instruments in Table 2.
Fig. 1.

Standard arthroscopic facilities: A video monitor, B xenon light source device, C video integrator device, D arthroscopic pump, E arthroscopic shaver device
Fig. 2.

Standard arthroscopic facilities: A arthroscope cannula 5 mm in diameter with a port for pump irrigation, B arthroscope with a view angle 30°; length 18 cm; outer diameter 4 mm, C abrader extension for arthroscopic shaver; size 4.5, D burr extension for arthroscopic shaver; size 5.5, E camera head, F arthroscopic shaver handle
Table 2.
Spine instruments
| Kerrison and pituitary rongeurs size 3 and 4 with a shaft length 18 cm |
| Curettes, angled and straight size 0, shaft diameter 5 mm and length 18 cm |
| Penfield dissectors 5 mm wide 18 cm long |
| Nerve root canal probe size 2 |
| Blade handle 5 mm wide, 18 cm long |
| Periosteal elevator 5 mm wide, 18 cm long |
Room setup and patient positioning
The operative room should be of adequate size to accommodate the fluoroscopy unit, and the video equipment for the endoscope. The procedure is performed under general anesthesia. The patient is positioned prone with the abdomen free and the spine flexed to open the interlaminar space. The author prefers to use a Wilson frame, but these goals can be achieved by other methods.
Surgical technique
To evaluate the learning curve and the surgical efficacy and complications of this new technique, all cases were operated by a single surgeon.
Endoscopic portals placement
Under image intensification a spinal needle is inserted in the paraspinal muscles 1 cm parallel to the midline to localize the desired surgical level. After level confirmation the needle is removed and two portals 5 mm in diameter are formed using a number 15 surgical blade with a 5 mm handle so that it could be advanced to pierce the fascia which is markedly deep in obese patients. The portals are 1 cm lateral to the midline, with the first directly overlying the intervertebral disc and is used for the introduction of the arthroscope and the second is 3–4 cm caudal to the former and is used for the surgical instruments (Fig. 3). However, switching the portals through the operation could be done according to the surgeon’s preference. The distance between both portals allows the surgeon to perform the triangulation technique with complete freedom of the surgical tool. The 5 mm periosteal elevator is then introduced through the paraspinal muscles without any dissection till it is docked over the lamina then it is used to sweep the overlying soft tissues.
Fig. 3.
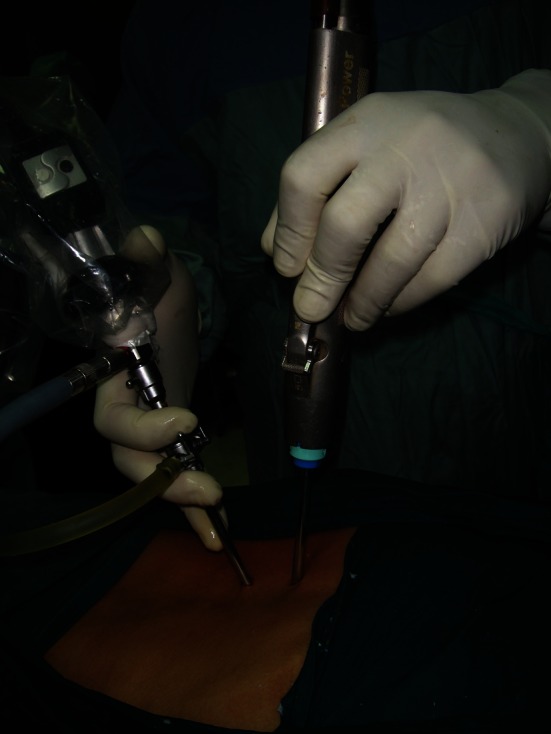
Intraoperative image showing the endoscope and arthroscopic shaver introduced through two separate portals
Insertion of the endoscope and preparation of the surgical field
The endoscopic cannula and trochar are introduced through the first portal till they are docked over the superior lamina. The pump irrigation fluid is initiated and the trochar is removed to allow the blood to be washed out followed by endoscope introduction through the cannula. The pump pressure adjustment ranges from 30 to 60 mmHg according to the patient’s weight with high pressures required in obese patients. After identification of the lamina the endoscopic orientation is adjusted by rotating the camera to obtain a field of vision matching that of open surgery. This is aided by introducing the abrader through the second portal and using its position as a guide to identify the medial, lateral, cephalic and caudal points in the field. Ideally the facet joint (lateral anatomy) should lie in the bottom of the screen, the base of spinous process (medial anatomy) in top of screen, superior lamina on the left in case of left sided herniation and on the right in case of right sided herniation with the inferior lamina on the opposite side. The shaver with the abrader function is then used to clean any remnants of soft tissue or muscles over the lamina and ligamentum flavum (Fig. 4).
Fig. 4.

Arthroscopic abrader cleaning the soft tissue; white arrows point to the superior lamina and base of spinous process, and red arrows to the interlaminar space
Laminotomy/medial facetectomy/ligamentum flavum removal
An angled curette is used to detach the superficial layer of the ligamentum flavum from the inferior edge of the superior lamina. A kerrison rongeour is used to perform a hemilaminotomy (Fig. 5) till the superior edge of the deep part of the ligamentum flavum is freed (Fig. 6). Using the curette, the plane between the ligament and the dura is identified, ensuring that it is free from adhesions and the ligament is peeled down in a caudal direction. This followed by the removal of the ligamentum flavum using the kerrison rongeour (Fig. 7a, b). The use of pump irrigation allows the control of any epidural bleeding in addition to the maintenance of the potential working space.
Fig. 5.

Kerrison removing the inferior edge of superior lamina and medial half of the facet; red arrow points to the superior lamina and the white arrow to the medial facetectomy site
Fig. 6.

Laminotomy site; blue arrows point to the superior free edge of the ligamentum flavum, yellow arrow to the epidural space, black arrow to the superior lamina and green arrow to the medial facetectomy site
Fig. 7.

aBlack arrow point to the kerrison rongeour removing the ligamentum flavum, blue arrows to the ligamentum flavum, red arrows to the dural sac and green arrow to the shoulder of the nerve root. b After removal of the ligamentum flavum with the red arrows pointing to the dural sac and the green arrow to the nerve root
Nerve root identification/disc removal
After nerve root identification adjacent to the dural sac, the author prefers to undercut the facet down to the medial wall of the pedicle. This allows for the disc work to be conducted without any nerve root retraction in addition to achieving lateral recess decompression. If an annulotomy is required it could be performed using a sheathed microknife. This is followed by discectomy, and the removal of any free fragments and foraminotomy if required (Fig. 8a, b).
Fig. 8.
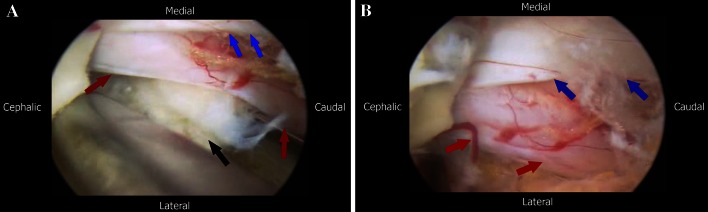
a Removal of the herniated fragment; black arrow points to the disc fragment, red arrows point to the nerve root and the blue arrows to the lateral edge of the dural sac. b Nerve root and dural sac after disc fragment removal; red arrows show the nerve root after relief of compression and the blue arrows point to the dura
Closure
The endoscope and instruments are removed, and any remaining fluid is discharged by manually squeezing the skin around each portal (Fig. 9), followed by wound closure using a single stitch.
Fig. 9.
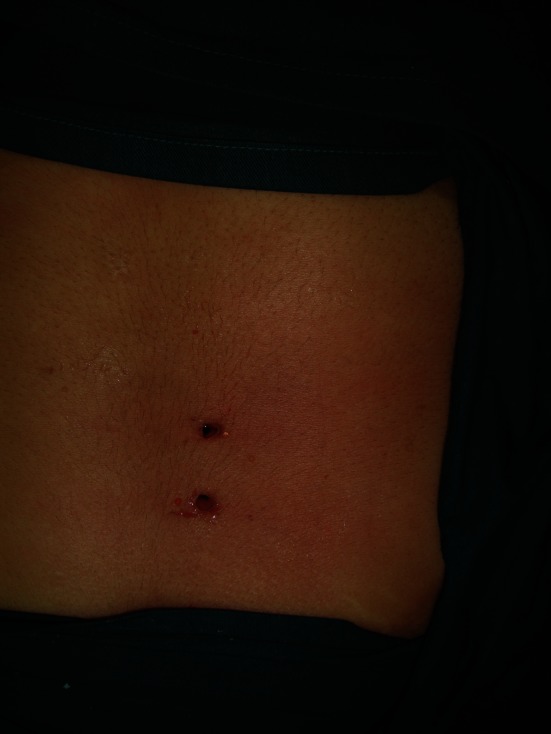
Picture showing postoperative wound before closure
Follow up
Following the immediate postoperative neurological examination, patients were evaluated at time of discharge, and at 10 days, 1, 3, 6, 12 and 24 months postoperative. Outcome was evaluated using the VAS for back and leg pain [21], the Oswestry Low-Back Pain Disability Questionnaire (ODI) [22] and the modified Macnab criteria [23].
Technical remarks and pitfalls
(1) Once the epidural space is reached, the pump pressure should not exceed 40 mmHg and the surgical procedure should be interrupted after 60 min for a period of 3 min, to relief nerve root compression caused by the pump irrigation and subsequent possibility of neuropraxia. (2) The irrigation fluid used is isotonic saline to avoid tissue oedema.
Statistical analysis tests
Data were statistically described in terms of mean ± standard deviation (±SD), or frequencies (number of cases) and percentages when appropriate. Comparison of numerical variables between the study groups was done using Student t test for independent samples. Within group comparison of numerical variables was done using general linear model with repeated measures with posthoc multiple two-group comparisons. p values <0.05 were considered statistically significant. All statistical calculations were done using computer programs SPSS (Statistical Package for the Social Science; SPSS Inc., Chicago, IL, USA) version 15 for Microsoft Windows.
Results
All patients were contacted by phone 10 and 2 days prior to the date of follow-up examination and filling of the questionnaire which was performed at the clinic. The results of 41 patients are presented, while the remaining two cases did not respond to any attempt of contact which was conducted on personal basis and to their closest relative.
Significant reduction in the operative time was achieved with case proficiency which dropped from a mean of 93 min in the first ten cases to 60 min in the next 31 cases (p < 0.001). There were two cases of small dural tears; however, the external pressure applied by the pump irrigation acted as a tamponade against CSF leak and the tear was sealed within 5 min with a small bleb. One patient experienced transient urine retention. There was one case of recurrent disc herniation and one case of persistent severe back pain. There were no other complications.
Immediate postoperative evaluation revealed that all patients had substantial relief of their radicular pain. With the exception of one case who complained of significant back pain, all patients were able to sit and to ambulate with minimal discomfort within 4 h following surgery. The mean time for hospital stay was 8 h, and that for return-to-work was 7 days.
At 24 months postoperatively, patients’ outcome using modified Macnab criteria revealed that 32 patients (78 %) had excellent outcome, 7 patients (17 %) had good outcome, and 2 patients (5 %) had poor outcome.
The mean outcome of the 41 cases revealed significant improvement of the VAS for leg pain from 78 to 7 (p < 0.001) and that for back pain from 24 to 9 (p < 0.001) and the Oswestry score from 76 to 19 (p < 0.001). One case of recurrent disc herniation underwent re-surgery by the same technique. One case of marked back pain was treated with posterior instrumentation and fusion. We believe that this was due to poor patient selection.
Discussion
IED has proven to be safe and effective for performing minimally invasive percutaneous lumbar discectomy. Sequestrated fragments and associated lateral recess or foraminal stenosis was easily addressed. Meanwhile postoperative back pain was negligible with patients ambulating 4 h postoperatively with minimal discomfort, and time for return to work averaged 7 days.
The technique offered the following advantages: (1) further reduction in surgical morbidity due to absence of any muscle retraction or dilatation; this is especially evident in patients with morbid obesity (Fig. 10a, b) and at a double level where only additional 5 mm portals are required; (2) cost reduction and the possibility of widespread application as a special endoscope or instruments are not required; (3) free movement of the endoscope with the ability to obtain a panoramic view on endoscopic retraction, and zooming in on its advancement; (4) superior image quality with no problem related to fog accumulation or the need to repeatedly clean the blood from the endoscopic lens; (5) the absence of a common working portal for the endoscope and instruments, allowed for independent movement and angulation of the surgical tool being unrestricted by the endoscope which markedly reduces the procedure’s difficulty; (6) pump irrigation under pressure served in opening a potential working space, furthermore the pressure created, acted as a tamponade against epidural bleeding; and (7) relatively easy learning curve once the surgeon gets acquainted to the triangulation technique which is evident from the difference between the operative time in the first ten cases and the remaining study patients.
Fig. 10.
a Picture of a morbidly obese patient with L4–L5 lumbar disc herniation. b Picture showing the 18 cm long endoscope and pituitary rongeur placed entirely in the patients back during discectomy
On the other hand the disadvantages include the need for training on basic arthroscopic triangulation technique, the need for the interruption of the surgical procedure at timely intervals following opening the epidural space to avoid any possible neuropraxia from prolonged nerve root compression.
Study limitations include that this is not a multicentered study and there was no randomized control group; however, there is solid literature providing historical controls of the gold standard microsurgical discectomy and the posterior endoscopic technique (microendoscopic discectomy), as well as the author’s previous experience with both techniques.
Study strengths include that this is a prospective study and all cases were operated by a single surgeon for careful evaluation of the learning curve and the technical difficulties. All patients were followed up to 2 years, with loss of follow-up of only two cases. Validated clinical scoring systems were used to analyze the outcome, with emphasis on the rate of recovery and the time for return to work.
At the conclusion it should be borne in mind that though the results are encouraging, however, this is just a preliminary report. Larger patient series and randomized control studies are needed to evaluate fully the role of IED in the future of minimally invasive low back surgery.
Acknowledgments
There was no external funding for this study.
Conflict of interest
None.
References
- 1.Perez-Cruet MJ, Fessler RG, Perin NI. Review: complications of minimally invasive spinal surgery. Neurosurgery. 2002;51(Suppl 5):S26–S36. [PubMed] [Google Scholar]
- 2.Smith L. Enzyme dissolution of the nucleus pulposus in humans. JAMA. 1964;265:137–140. doi: 10.1001/jama.1964.03060150061016. [DOI] [PubMed] [Google Scholar]
- 3.Hijikata S, Yamgishi M, Nakayama T, Oomon K. Percutaneous discectomy: a new treatment method for lumbar disc herniation. J Toden Hosp. 1975;5:5–13. [Google Scholar]
- 4.Onik G, Helms CA, Ginsberg L. Percutaneous lumbar discectomy using a new aspiration probe. AJR Am J Roentgenol. 1985;144:1137–1140. doi: 10.2214/ajr.144.6.1137. [DOI] [PubMed] [Google Scholar]
- 5.Schreiber A, Suezawa Y. Transdiscoscopic percutaneous nucleotomy in disc herniation. Orthop Rev. 1986;15:35–38. [PubMed] [Google Scholar]
- 6.Choy DSJ, Case RB, Fielding W. Percutaneous laser nucleolysis of lumbar discs. N Engl J Med. 1987;317:771–772. doi: 10.1056/NEJM198709173171217. [DOI] [PubMed] [Google Scholar]
- 7.Mathews HH. Transforaminal endoscopic microdiscectomy. Neurosurg Clin N Am. 1996;7:59–63. [PubMed] [Google Scholar]
- 8.Lew SM, Mehalic TF, Fagone KL. Transforaminal percutaneous endoscopic discectomy in the treatment of far-lateral and foraminal lumbar disc herniations. J Neurosurg. 2001;94:216–220. doi: 10.3171/spi.2001.94.2.0216. [DOI] [PubMed] [Google Scholar]
- 9.Kahanovitz N, Viola K, Goldstein T, Dawson EA. Multicenter analysis of percutaneous discectomy. Spine. 1990;15:713–715. doi: 10.1097/00007632-199007000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Perez-Cruet MJ, Foley KT, Isaacs RE, et al. Microendoscopic lumbar discectomy: technical note. Neurosurgery. 2002;51(Suppl 2):S129–S136. [PubMed] [Google Scholar]
- 11.Foley KT, Smith MM. Microendoscopic discectomy. Tech Neurosurg. 1997;3:301–307. [Google Scholar]
- 12.Brayda-Bruno M, Cinnella P. Posterior endoscopic discectomy (and other procedures) Eur Spine J Suppl. 2000;7:S024–S029. doi: 10.1007/PL00010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Constantin S, Elefterios T, Joyti S. Microendoscopic discectomy compared with standard microsurgical discectomy for treatment of uncontained or large contained disc herniations. Neurosurgery. 2005;57(4):357–360. doi: 10.1227/01.neu.00000176650.71193.f5. [DOI] [PubMed] [Google Scholar]
- 14.Orlando R, Asdrubal F, Osmar A. Comparison of open discectomy with microendoscopic discectomy in lumbar disc herniations: results of a randomized controlled trial. Neurosurgery. 2007;61(3):545–549. doi: 10.1227/01.NEU.0000290901.00320.F5. [DOI] [PubMed] [Google Scholar]
- 15.Xiaotao W, Suyang Z, Zubin M, Hui C. Microendoscopic discectomy for lumbar disc herniation: surgical technique and outcome in 873 consecutive cases. Spine. 2006;31(23):2689–2694. doi: 10.1097/01.brs.0000244615.43199.07. [DOI] [PubMed] [Google Scholar]
- 16.Schick U, Döhnert J, Richter A, et al. Microendoscopic lumbar discectomy versus open surgery: an intraoperative EMG study. Eur Spine J. 2002;11(1):20–26. doi: 10.1007/s005860100315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jen Huang T, Wei Hsu R, Yao Li Y, et al. Less systemic cytokine response in patients following microendoscopic versus open lumbar discectomy. J Orthop Res. 2005;23(2):406–411. doi: 10.1016/j.orthres.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Nakagawa H, Kamimura M, Uchiyama S, et al. Microendoscopic discectomy (MED) for lumbar disc prolapsed. J Clin Neurosci. 2003;10(2):231–235. doi: 10.1016/S0967-5868(02)00337-5. [DOI] [PubMed] [Google Scholar]
- 19.Adrian N. Assessment of the learning curve for lumbar microendoscopic discectomy. Neurosurgery. 2005;56(4):755–762. doi: 10.1227/01.NEU.0000156470.79032.7B. [DOI] [PubMed] [Google Scholar]
- 20.Maroon JC. Current concepts in minimally invasive discectomy. Neurosurgery. 2002;51(Suppl 5):S137–S145. [PubMed] [Google Scholar]
- 21.Daltroy LH, Cats-Baril WL, Katz JN, et al. The North American spine society (NASS) lumbar spine outcome instrument: reliability and validity tests. Spine. 1996;21:741–749. doi: 10.1097/00007632-199603150-00017. [DOI] [PubMed] [Google Scholar]
- 22.Fairbank J, Pynsent P. The Oswestry Disability Index. Spine. 2000;25:2940–2953. doi: 10.1097/00007632-200011150-00017. [DOI] [PubMed] [Google Scholar]
- 23.Macnab I. Negative disc exploration. an analysis of the causes of nerve-root involvement in sixty-eight patients. J Bone Jt Surg Am. 1971;53:891–903. [PubMed] [Google Scholar]



