Abstract
Purpose
With lumbar discectomy for disc herniation, surgeons must choose between limited nucleus removal associated with higher reherniation risk or more aggressive nucleus removal associated with increased back pain and disc degeneration. This trade-off is particularly challenging in patients with large anular defects, which carry the highest risk of reherniation. We examined the effect of an anular closure device on reherniation and clinical outcomes.
Methods
Seventy-five primary discectomy patients had a limited discectomy followed by implantation of an anular closure device and were followed-up to 2 years. Anular defect size and volume of removed nucleus was recorded at surgery. Reherniations were reported, pain and function were monitored throughout, and imaging was performed at annual visits.
Results
The overall symptomatic reherniation rate was 1.4 %, and the asymptomatic reherniation rate was 1.5 % at 12 months and 5.1 % at 24 months. Both rates compare favorably with literature reports which include symptomatic rates ranging between 2 and 18 % (up to 27 % for patients with large anular defects) and an asymptomatic rate of 13 %.
Conclusions
The low reherniation rate in patients at high-risk of reherniation based on anular defect size, despite discectomy being only limited, suggests that an anular closure device may reduce reherniation risk. Clinical outcomes for pain and function at 1 and 2 years post-operatively compared favorably with literature reports. Further study in a randomized controlled trial is required to confirm these results.
Keywords: Discectomy, Reherniation, Recurrent herniation, Anular closure
Introduction
The most frequently performed spinal procedure is lumbar discectomy for disc herniation [1]. While overall reherniation rates between 2 and 18 % following lumbar discectomy have been reported, [2–12] patients with larger anulus defect sizes have been associated with a higher rate of reherniation [3, 13]. Carragee et al. [3] observed that patients whose defects were wider than 6 mm had a 27.3 % rate of documented reherniation versus 4.8 % in the remaining patient cohort. Patients meeting this defect width criterion represented 18 % of the total cohort. McGirt et al. [13] reported an 18 % rate of reherniation requiring reoperation for patients with anular defect sizes greater than 54 mm2 compared to only 4.7 % for patients with defect sizes less than 36 mm2. Patients meeting the defect area criterion represented the upper quartile in that patient population. In addition to defect size, demographic factors such as age [13–15] and BMI [16] have been correlated with reherniation risk in the literature.
Traditionally, aggressive nuclear removal has been proposed to reduce reherniation [2, 6–11, 17, 18], but an increased incidence of persistent back pain has been associated in 19–36 % of patients treated with this aggressive nuclectomy technique [11]. Greater degeneration of the vertebral disc, observed in the form of postoperative anular protrusions/extrusions, endplate degeneration, and loss of disc height, was also reported with aggressive discectomies compared to more limited discectomies [19]. Thus, the surgeon faces a dilemma when operating on the primary herniation patient with poor anular competence (i.e., large defects), and must balance the risk of reherniation against the risk for persistent pain and degeneration of the disc. Significant health care costs have been associated with either outcome [20, 21].
Recently, anular closure devices, such as the Barricaid® (Intrinsic Therapeutics, Inc., Woburn, MA, USA), have been developed to reduce reherniation risk by occluding the defect in the anulus. If such an implant were effective in reducing reherniation, particularly in high-risk patients with large anular defects, the negative outcomes that have been associated with aggressive nucleus removal may also be avoided.
The purpose of this investigation was to evaluate the ability of anular prosthesis to reduce reherniation. Specifically, the objectives were to: (1) assess the rate of both symptomatic and asymptomatic reherniations in primary limited discectomy patients implanted with Barricaid, (2) determine the symptomatic reherniation rate for the subsets of patients with poor anular competence as defined by defect size classifications taken from the literature [3, 13], and (3) investigate whether factors that have been commonly associated with recurrence risk were significantly correlated with reherniation.
Clinical materials and methods
Patient selection
Seventy-five patients were enrolled in two separate, single-arm, prospective clinical trials of the Barricaid in Europe. Ethics committee approval was obtained at each site. All patients provided informed consent. Both studies were monitored by an independent data safety monitoring board (DSMB). The first study (Cohort A) was performed at two sites beginning in April 2008, while the second study (Cohort B) began 1 year later at four different sites. Patients had a confirmed primary lumbar disc herniation with at least 6 weeks of failed conservative treatment prior to surgery. Other inclusion criteria included: (1) Visual Analog Scale (VAS) leg pain of at least 40 out of 100, (2) Oswestry Disability Index (ODI) of at least 40 out of 100, and (3) patient age between 18 and 75 years. Exclusion criteria included spondylolisthesis Grade II or higher; prior surgery at the index level; bone density t-scores <−2.0 for subjects requiring DEXA; and scoliosis of >10°.
Anular closure device
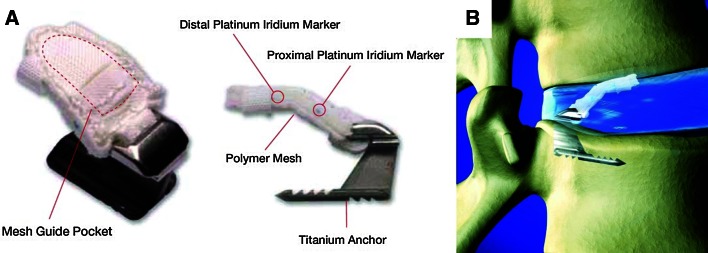
The Barricaid (Intrinsic Therapeutics, Inc., Woburn, MA, USA) anular closure device was designed as an adjunct to lumbar discectomy to block the anular defect and maintain nucleus within the disc space. The device has received CE-marking and is composed of two components: a flexible mesh that prevents migration of the nucleus from within the disc, and a bone anchor which secures the occlusion component to one of the adjacent vertebral bodies (Fig. 1). The anchor is composed of Ti6Al-4V ELI, a typical orthopedic alloy with a long history of use in permanent implants. The mesh is made up of woven polyester (“Dacron”), which has been in use for implantation as cardiovascular graft material in humans for several decades. At the time of the study, the implant was available in one size capable of blocking an anular defect up to 10 mm wide. A minimum disc height of 3 mm pre-operatively is required to implant the device.
Fig. 1.
Images of the Barricaid anular closure device
Surgical technique
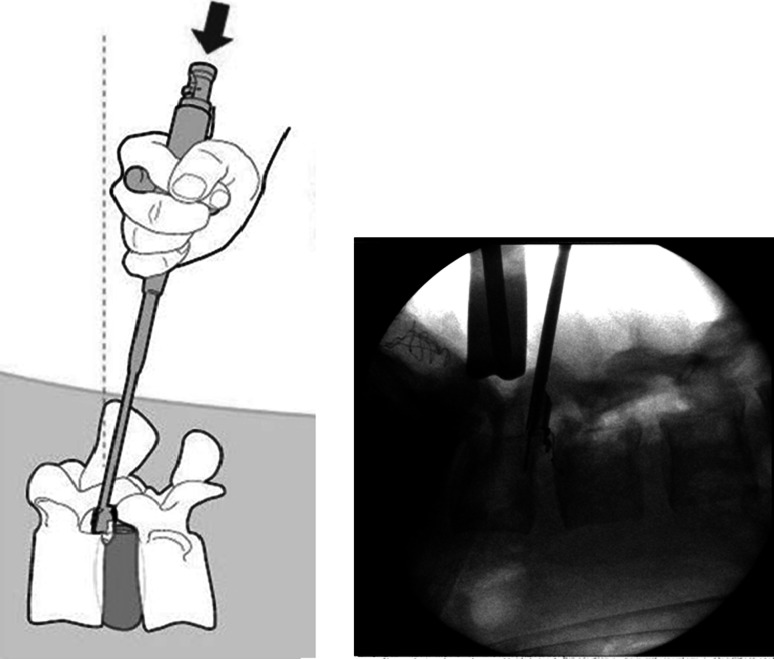
Patients were treated by ten surgeons across the two studies. An open microdiscectomy with limited nucleus removal as described by Spengler [22] was performed. The amount of nucleus removed was recorded. Then, both the height and width of the anular defect were measured using sizing paddles (Intrinsic Therapeutics, Inc., Woburn, MA). Patients whose defects were >6 mm in height or 10 mm in width were excluded from the study, due to limitations in available implant sizes at that time. Patients who met the defect size criteria were implanted with the Barricaid under fluoroscopic guidance, according to the manufacturer’s surgical technique manual and instructions for use. In short, a sizing trial, designed to replicate the size and shape of the loaded delivery tool, was used to confirm access through the lamina and the appropriate angle of approach to the disc. Then the delivery tool was placed in the disc under fluoroscopic control, ensuring that the angle of approach was parallel to the target endplate in the region of implantation. Holding the delivery tool firmly in position, the anchor was hammered into the endplate, thereby simultaneously placing the mesh in position within the disc (Fig. 2).
Fig. 2.
Diagram (left) and intraoperative fluoroscopy image (right) showing insertion of the Barricaid device
Patients were discharged and given post-surgery care instructions per each hospital’s standard regimen following lumbar discectomy, without any additional bracing or other activity restrictions.
Assessment of outcomes
ODI and VAS for leg and back pain were collected pre-operatively and at 6 weeks, 3, 6, 12, and 24 months post-operatively. X-rays were obtained at the same time points (Fig. 3), while computed tomography (CT) and MRI (Fig. 4) were taken pre-operatively and at 12 and 24 months.
Fig. 3.
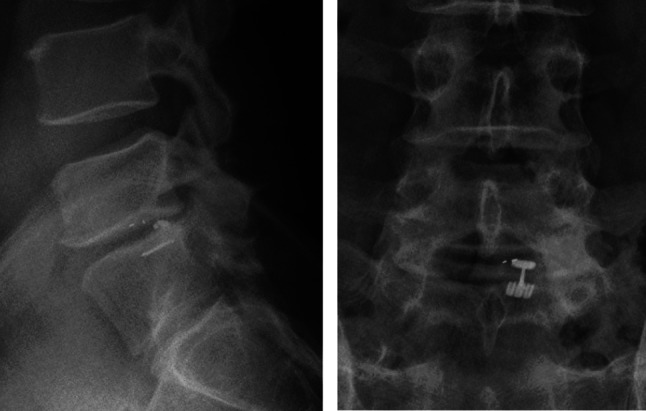
Lateral (left) and antero-posterior (right) lumbar spine X-rays 2 years after implantation of Barricaid anular closure device
Fig. 4.
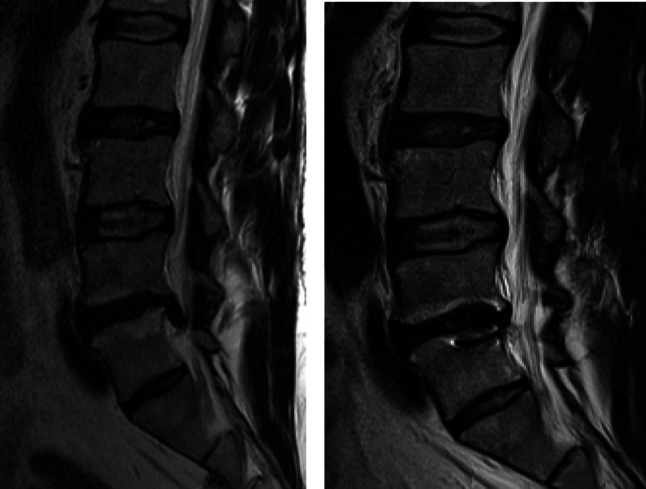
MR images pre-operative (left) and 2 years post-implantation of Barricaid Anular Closure Device (right)
Symptomatic reherniations were reported by the clinical sites as adverse events. In addition, all MRIs were reviewed by an independent radiology lab (Medical Metrics, Inc., Houston, TX) to assess potentially asymptomatic reherniations. Each level was graded according to the recommendations of the Combined Task Forces of the North American Spine Society, American Society of Spine Radiology, and American Society of Neuroradiology [23] as: “None”, “Protrusion”, “Extrusion”, or “Sequestration” (Table 1). Any protrusion, extrusion or sequestration was classified as a herniation.
Table 1.
Reherniation classification scheme according to MRI findings [23]
| Classification | Description |
|---|---|
| 0-None | Absence of reherniation of disc material through the original defect or through another location in the anulus |
| 1-Protrusion | Presence of reherniation of nucleus material through the original defect or through another location in the anulus resulting in a focal “out-pouching” of the disc contour beyond the normal outer limits of the disc (comment <3 mm, 3 to 6 mm or >6 mm) |
| 2-Extrusion | Presence of reherniation of disc material through the original defect or through another location in the anulus such that disc material breaks through the confines of the anulus fibrosus (comment <3 mm, 3 to 6 mm or >6 mm) |
| 3-Sequestration | Presence of reherniation of nuclear material through the original defect or through another location in the anulus such that extruded disc material resides a distance from and no longer contacts the parent disc |
| 4-Indeterminate | Insufficient information to conduct the assessment (please comment) |
Statistical analysis
Pre-operative and intra-operative patient characteristics were compared using the Fisher exact test for categorical variables and an unpaired t test assuming unequal variance for numerical variables. Univariate logistic regressions were used to investigate correlations between patient characteristics and reherniation. Linear regression analyses (numerical variables), unpaired t-tests assuming unequal variance (binary variables), and Kruskal–Wallis tests (categorical variables) were used to investigate correlations between defect size and patient demographics.
Results
Patient characteristics
Thirty patients were enrolled in Cohort A, and 45 patients in Cohort B. In one patient in Cohort A, Barricaid devices were implanted at two levels. Patient characteristics and defect dimension data measured intra-operatively are summarized in Table 2. Over 85 % (65/76) of discs were considered high-risk on the basis of anular defect widths >6 mm. Over 14 % (11/76) of discs were considered high-risk on the basis of anular defect areas >54 mm2. Mean volume of nucleus removed was 1.5 mL.
Table 2.
Patient Characteristics
| Cohort A | Cohort B | Combined | p value | |
|---|---|---|---|---|
| No. of patients | 30 | 45 | 75 | |
| Male:female | 16:14 | 24:21 | 40:35 | 1.000 |
| Age (years) | 38.3 ± 9.5 | 42.3 ± 11.4 | 40.7 ± 10.8 | 0.1075 |
| BMI | 26.8 ± 2.8 | 26.0 ± 4.9 | 26.3 ± 4.2 | 0.3665 |
| Operated level | ||||
| L3–L4 | 0 | 2 | 2 | 0.447 |
| L4–L5 | 19 | 22 | 41 | |
| L5–S1 | 12 | 21 | 33 | |
| Defect size (mm2) | 51.0 (8.3) | 38.6 (10.9) | 44 (12) | 0.000 |
| % with width ≥6 mm | 96.7 % (30 of 31) | 77.8 % (35 of 45) | 85.5 % (65 of 76) | 0.023 |
| % defect ≥54 mm2 | 29.0 % (9 of 31) | 4.4 % (2 of 45) | 14.5 % (11 of 76) | 0.006 |
| Nucleus removed (mL) | 1.3 ± 0.8 | 1.6 ± 1.1 | 1.5 ± 1.0 | 0.2254 |
| Pre-Op VAS ipsilateral leg | 79.8 ± 12.8 | 81.7 ± 13.2a | 80.9 ± 13.0a | 0.5471 |
| Pre-Op ODI | 62.7 ± 13.7 | 60.3 ± 12.7a | 61.3 ± 13.1a | 0.4596 |
| Pre-Op VAS back | 66.3 ± 16.6 | 57.8 ± 27.9a | 61.3 ± 24.1a | 0.1075 |
| Mean of latest follow-up (months) | 23.6 | 15.1a | 18.7a | |
Data are presented as mean ± standard deviation
aTwo patients excluded from analysis due to intra-operative procedural errors. Two patients were lost to follow-up
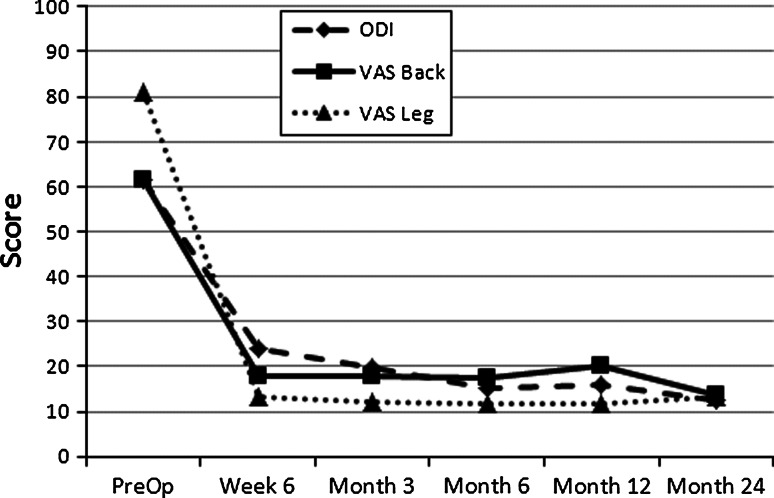
Of the 75 patients, two were excluded from Cohort B for intra-operative procedural errors that resulted in no device being implanted. One patient from Cohort B was a protocol deviation with a pre-operative VAS leg score <40. Yet, this patient was included in the analysis of reherniations. In Cohort A, all 30 patients completed 12-month follow-up, and 29 of 30 (97 %) patients completed 24-month follow-up. In Cohort B, 41 of 43 (95 %) patients completed 1-year exams,1 and to date, 11 patients have completed 2-year follow-up. Pre-operative mean ODI and VAS scores are shown in Table 2, with no significant differences between the two studies. Significant reductions in VAS and ODI were observed at 12 months relative to baseline. Those reductions were maintained at 24 months (Fig. 5).
Fig. 5.
Graph showing Oswestry Disability Index (ODI) and Visual Analogue Scores (VAS) for back pain and ipsilateral leg pain preoperative and at follow-up intervals
Reherniation outcomes
One patient has been lost to follow-up throughout the study. Another patient was lost to follow-up after 6 months. The mean duration of follow-up in the remaining patients was 18.7 months (range 12–24 months, median 24 months). There was one (1.4 %) reported symptomatic reherniation among the 73 followed patients. This reherniation occurred in Cohort B in a patient whose defect measured 4 mm tall and 8 mm wide. The DSMB and operating surgeon independently concluded that the Barricaid had been implanted more anteriorly than described in the surgical technique manual, permitting a nuclear extrusion around the mesh. In this patient, the implant was removed and a posterior lumbar interbody fusion was performed.
MRI accountability was 92 % at 12 months and 93 % at 24 months. The independent MRI review identified a total of two asymptomatic reherniations, yielding asymptomatic reherniation rates of 1.5 % (1/66) at 12 months and 5.1 % (2/39) at 24 months. Each cohort contained one asymptomatic reherniation; both were graded as “Extrusions”.
When defining high-risk patients using anular defect width >6 mm, the rate of symptomatic reherniation was 1.5 % (1 of 65). When defining high-risk patients using anular defect areas >54 mm2, the rate of symptomatic reherniation was zero (0 of 11).
Regression analysis
Symptomatic reherniation risk was not correlated with defect size (p = 0.306), age (p = 0.939), BMI (p = 0.276), or volume of nucleus removed (p = 0.886). Inclusion of asymptomatic reherniations into the analysis did not alter these findings (p > 0.483).
Discussion
The purpose of this study was to evaluate the Barricaid anular closure device’s ability to reduce reherniation risk. The overall symptomatic reherniation rate was 1.4 %, and the asymptomatic reherniation rate was 1.5 % at 12 months and 5.1 % at 24 months. Both rates compare favorably with literature reports which include symptomatic reherniation rates ranging from 2 to 18 % [11] and an asymptomatic rate of 13 % [24].
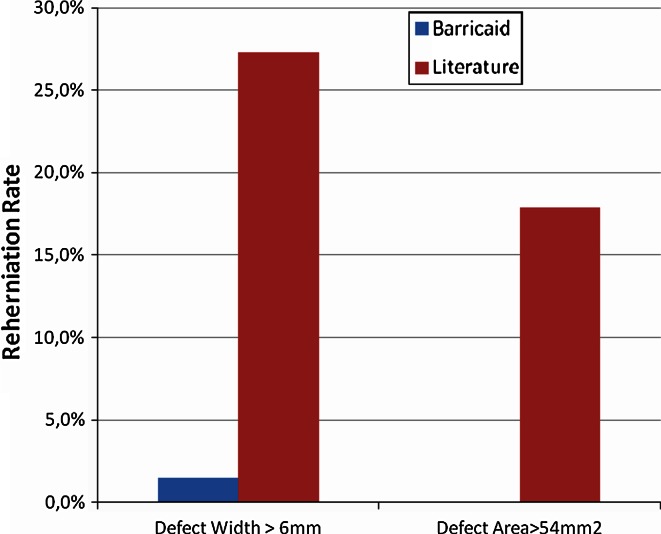
There was a specific focus on determining the reherniation rate in the subset of patients at higher risk for reherniation due to poor anular competence. Larger anular defects have been associated with higher reherniation rates and worse clinical outcomes. Using either of two definitions of poor anular competence, patients implanted with Barricaid exhibited a lower reherniation rate when compared to similar subsets of patients reported in the literature (Fig. 6). When screening for anular defects wider than 6 mm, the symptomatic reherniation rate in patients implanted with the Barricaid was 18 times lower than the 27.3 % (9 of 33) reported by Carragee et al. [3] in a comparable group of patients (Fig. 6). When defining high-risk patients using anular defect areas >54 mm2, the rate of symptomatic reherniation in the present study was zero (0 of 11). For the patient subset satisfying the same anular defect area criterion, McGirt et al. [13] reported an 18 % (5 of 28) rate of symptomatic reherniations requiring reoperation (Fig. 6). It is not clear whether the reherniations in the referenced reports occurred in patients operated on with the assistance of a microscope.
Fig. 6.
Symptomatic reherniation rates in patients with poor anular competence versus literature [3, 13]
The patient population presented here was comparable to previous discectomy studies [2, 3, 13, 17, 18, 25, 26]. The large majority of patients had anular defect widths >6 mm (85.5 %). It is unknown if such a high ratio is representative of the general primary herniation population, as few studies report intraoperative defect sizes. Carragee et al. [3] reported that only 18 % of their patient population had defects wider than 6 mm, as assessed using a Penfield probe as opposed to the designated set of defect measurement instruments that were used in this study. Differences in the study inclusion and exclusion criteria may have contributed to the disparate occurrence of defects that were wider than 6 mm. The defect size contraindication imposed in the present study (i.e., defects were required to be <6 mm in height and 10 mm in width), resulted in only 11 patients (15 %) with anular defects <54 mm2. This small sample size limited the ability to statistically evaluate the performance of the Barricaid in this patient population.
The inclusion of MRI follow-up at 1 and 2 years provided an additional assessment of asymptomatic recurrence after Barricaid implantation. The independent review of these images identified two asymptomatic reherniations, yielding rates of 1.5 % at 12 months and 5.1 % at 24 months. In comparison, Lebow et al. [24] reported an asymptomatic reherniation rate of 13 % (14/108) at 2 years. In a study comparing microdiscectomy and sequestrectomy surgical techniques [19], the researchers also reviewed MRIs and graded the form, size, and location of any canal compromising disc protrusions or extrusions. Protrusions or extrusions that measured >4 mm in size were identified in 66 % of patients in the microdiscectomy group and 68 % of patients in the sequestrectomy group by 2 years. Those rates are much greater than what has been observed in this study, and provide further support that the Barricaid is able to retain the nucleus pulposus within the confines of the disc space.
Literature reviews have concluded that aggressive nucleus removal can reduce reherniation rates, but at the expense of worse clinical outcomes [11, 18]. In a study comparing the outcomes of limited versus aggressive nuclear removal, reherniation rate was halved in the aggressively treated group [2]. However, those patients reported worse back pain and ODI at 12 months. Furthermore, McGirt et al. [13] reported a trend associating increased volume of nucleus removal and decreased reherniation rate. An ideal treatment would combine minimal nucleus removal with low reherniation risk. In this study, patients treated with limited discectomy followed by implantation of an anular closure device exhibited a low overall reherniation rate despite the removal of 25 % less nucleus than the average 2.0 cc that McGirt et al. [13] reported.
Clinical outcomes for pain and function at 1 and 2 years post-operative compared favorably with other reports from the literature [2, 3, 13, 17, 26], suggesting that the device may reduce reherniation risk without affecting the success and safety profile of routine discectomies.
In the present study, over 85 % of patients fit a high-risk profile due to poor anular competence. When faced with this population at an elevated risk of recurrence, the dilemma has been to choose between techniques that have known bad outcomes—aggressive discectomy leading to increased risk of chronic pain and limited discectomy leading to a high-risk of reherniation. Anular closure devices may solve the dilemma by enabling an optimized limited discectomy procedure that minimizes the risk of both failure modes, representing a substantial health care savings to society.
Limitations of the study include the two different patient cohorts and the lack of a control group. The possibility exist that differences in patient demographics or surgical technique between the two cohorts may have influenced the outcomes. Similarly, it is uncertain whether differences between these cohorts and the patients in the literature references, or the discectomy techniques utilized, may have contributed to the differences in outcomes noted. For example, conflicting conclusions have been reported in the literature regarding the effect of tubular versus open discectomy techniques on reherniation [27, 28]. Further evaluation in a randomized, controlled trial is needed to confirm the reduction in reherniation risk using the Barricaid and to assess the percentage of patients who may be at high-risk for reherniation due to poor anular competence.
Conflict of interest
The authors report no conflict of interest. Intrinsic Therapeutics provided support for the clinical research and data analysis.
Footnotes
Revision surgery and device removal was performed on one patient prior to the 12 month time point (see description of symptomatic reherniation). Presentation of clinical outcomes does not include this patient; data from 40 patients in Cohort B were analyzed at 12 months.
References
- 1.Gray DT, Deyo RA, Kreuter W, Mirza SK, Heagerty PJ, Comstock BA, Chan L. Population-based trends in volumes and rates of ambulatory lumbar spine surgery. Spine. 2006;31:1957–1963. doi: 10.1097/01.brs.0000229148.63418.c1. [DOI] [PubMed] [Google Scholar]
- 2.Carragee EJ, Spinnickie AO, Alamin TF, Paragioudakis S. A prospective controlled study of limited versus subtotal posterior discectomy: short-term outcomes in patients with herniated lumbar intervertebral discs and large posterior anular defect. Spine. 2006;31:653–657. doi: 10.1097/01.brs.0000203714.76250.68. [DOI] [PubMed] [Google Scholar]
- 3.Carragee EJ, Han MY, Suen PW, Kim D. Clinical outcomes after lumbar discectomy for sciatica: the effects of fragment type and anular competence. J Bone Joint Surg Am. 2003;85-A:102–108. [PubMed] [Google Scholar]
- 4.Findlay GF, Hall BI, Musa BS, Oliveira MD, Fear SC. A 10-year follow-up of the outcome of lumbar microdiscectomy. Spine. 1998;23:1168–1171. doi: 10.1097/00007632-199805150-00019. [DOI] [PubMed] [Google Scholar]
- 5.Fountas KN, Kapsalaki EZ, Feltes CH, Smisson HF, III, Johnston KW, Vogel RL, Robinson JS., Jr Correlation of the amount of disc removed in a lumbar microdiscectomy with long-term outcome. Spine. 2004;29:2521–2524. doi: 10.1097/01.brs.0000145413.79277.d0. [DOI] [PubMed] [Google Scholar]
- 6.Kowalski JM, Olsewski JM, Simmons ED., Jr Results of intervertebral diskectomy without fusion at L4–5 versus L5–S1. J Spinal Disord. 1995;8:457–463. doi: 10.1097/00002517-199512000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Loupasis GA, Stamos K, Katonis PG, Sapkas G, Korres DS, Hartofilakidis G. Seven- to 20-year outcome of lumbar discectomy. Spine. 1999;24:2313–2317. doi: 10.1097/00007632-199911150-00005. [DOI] [PubMed] [Google Scholar]
- 8.Mariconda M, Galasso O, Secondulfo V, Rotonda GD, Milano C. Minimum 25-year outcome and functional assessment of lumbar discectomy. Spine. 2006;31:2593–2599. doi: 10.1097/01.brs.0000240726.26096.be. [DOI] [PubMed] [Google Scholar]
- 9.Thome C, Barth M, Scharf J, Schmiedek P. Outcome after lumbar sequestrectomy compared with microdiscectomy: a prospective randomized study. J Neurosurg Spine. 2005;2:271–278. doi: 10.3171/spi.2005.2.3.0271. [DOI] [PubMed] [Google Scholar]
- 10.Tureyen K. One-level one-sided lumbar disc surgery with and without microscopic assistance: 1-year outcome in 114 consecutive patients. J Neurosurg. 2003;99:247–250. doi: 10.3171/spi.2003.99.3.0247. [DOI] [PubMed] [Google Scholar]
- 11.Watters WC, III, McGirt MJ. An evidence-based review of the literature on the consequences of conservative versus aggressive discectomy for the treatment of primary disc herniation with radiculopathy. Spine J. 2009;9:240–257. doi: 10.1016/j.spinee.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Yorimitsu E, Chiba K, Toyama Y, Hirabayashi K. Long-term outcomes of standard discectomy for lumbar disc herniation: a follow-up study of more than 10 years. Spine. 2001;26:652–657. doi: 10.1097/00007632-200103150-00019. [DOI] [PubMed] [Google Scholar]
- 13.McGirt MJ, Eustacchio S, Varga P, Vilendecic M, Trummer M, Gorensek M, Ledic D, Carragee EJ. A prospective cohort study of close interval computed tomography and magnetic resonance imaging after primary lumbar discectomy: factors associated with recurrent disc herniation and disc height loss. Spine. 2009;34:2044–2051. doi: 10.1097/BRS.0b013e3181b34a9a. [DOI] [PubMed] [Google Scholar]
- 14.Keskimaki I, Seitsalo S, Osterman H, Rissanen P. Reoperations after lumbar disc surgery: a population-based study of regional and interspecialty variations. Spine. 2000;25:1500–1508. doi: 10.1097/00007632-200006150-00008. [DOI] [PubMed] [Google Scholar]
- 15.Martin BI, Mirza SK, Flum DR, Wickizer TM, Heagerty PJ, Lenkoski AF, Deyo RA. Repeat surgery after lumbar decompression for herniated disc: the quality implications of hospital and surgeon variation. Spine J. 2012;12:89–97. doi: 10.1016/j.spinee.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moliterno JA, Knopman J, Parikh K, Cohan JN, Huang QD, Aaker GD, Grivoyannis AD, Patel AR, Hartl R, Boockvar JA. Results and risk factors for recurrence following single-level tubular lumbar microdiscectomy. J Neurosurg Spine. 2010;12:680–686. doi: 10.3171/2009.12.SPINE08843. [DOI] [PubMed] [Google Scholar]
- 17.Barth M, Weiss C, Thome C. Two-year outcome after lumbar microdiscectomy versus microscopic sequestrectomy: part 1: evaluation of clinical outcome. Spine. 2008;33:265–272. doi: 10.1097/BRS.0b013e318162018c. [DOI] [PubMed] [Google Scholar]
- 18.McGirt MJ, Ambrossi GL, Datoo G, Sciubba DM, Witham TF, Wolinsky JP, Gokaslan ZL, Bydon A. Recurrent disc herniation and long-term back pain after primary lumbar discectomy: review of outcomes reported for limited versus aggressive disc removal. Neurosurgery. 2009;64:338–344. doi: 10.1227/01.NEU.0000337574.58662.E2. [DOI] [PubMed] [Google Scholar]
- 19.Barth M, Diepers M, Weiss C, Thome C. Two-year outcome after lumbar microdiscectomy versus microscopic sequestrectomy: part 2: radiographic evaluation and correlation with clinical outcome. Spine. 2008;33:273–279. doi: 10.1097/BRS.0b013e31816201a6. [DOI] [PubMed] [Google Scholar]
- 20.Ambrossi GL, McGirt MJ, Sciubba DM, Witham TF, Wolinsky JP, Gokaslan ZL, Long DM. Recurrent lumbar disc herniation after single-level lumbar discectomy: incidence and health care cost analysis. Neurosurgery. 2009;65:574–578. doi: 10.1227/01.NEU.0000350224.36213.F9. [DOI] [PubMed] [Google Scholar]
- 21.Parker SL, Xu R, McGirt MJ, Witham TF, Long DM, Bydon A. Long-term back pain after a single-level discectomy for radiculopathy: incidence and health care cost analysis. J Neurosurg Spine. 2010;12:178–182. doi: 10.3171/2009.9.SPINE09410. [DOI] [PubMed] [Google Scholar]
- 22.Spengler DM. Lumbar discectomy. Results with limited disc excision and selective foraminotomy. Spine. 1982;7:604–607. doi: 10.1097/00007632-198211000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Fardon DF, Milette PC. Nomenclature and classification of lumbar disc pathology: recommendations of the combined task forces of the North American Spine Society, American Society of Spine Radiology, and American Society of Neuroradiology. Spine. 2001;26:E93–E113. doi: 10.1097/00007632-200103010-00006. [DOI] [PubMed] [Google Scholar]
- 24.Lebow RL, Adogwa O, Parker SL, Sharma A, Cheng J, McGirt MJ. Asymptomatic same-site recurrent disc herniation after lumbar discectomy: results of a prospective longitudinal study with 2-year serial imaging. Spine. 2011;36:2147–2151. doi: 10.1097/BRS.0b013e3182054595. [DOI] [PubMed] [Google Scholar]
- 25.Weber H. Lumbar disc herniation. A controlled, prospective study with ten years of observation. Spine. 1983;8:131–140. doi: 10.1097/00007632-198303000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Weinstein JN, Lurie JD, Tosteson TD, Tosteson AN, Blood EA, Abdu WA, Herkowitz H, Hilibrand A, Albert T, Fischgrund J. Surgical versus nonoperative treatment for lumbar disc herniation: four-year results for the Spine Patient Outcomes Research Trial (SPORT) Spine. 2008;33:2789–2800. doi: 10.1097/BRS.0b013e31818ed8f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arts MP, Brand R, van den Akker ME, Koes BW, Bartels RH, Peul WC. Tubular diskectomy vs conventional microdiskectomy for sciatica: a randomized controlled trial. JAMA. 2009;302:149–158. doi: 10.1001/jama.2009.972. [DOI] [PubMed] [Google Scholar]
- 28.Teli M, Lovi A, Brayda-Bruno M, Zagra A, Corriero A, Giudici F, Minoia L. Higher risk of dural tears and recurrent herniation with lumbar micro-endoscopic discectomy. Eur Spine J. 2010;19:443–450. doi: 10.1007/s00586-010-1290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]