Abstract
The classification feast/famine regulatory proteins (FFRPs) encompasses archaeal DNA-binding proteins with Escherichia coli transcription factors, the leucine-responsive regulatory protein and the asparagine synthase C gene product. In this paper, we describe two forms of the archaeal FFRP FL11 (pot0434017), both assembled from dimers. When crystallized, a helical cylinder is formed with six dimers per turn. In contrast, in solution, disks are formed, most likely consisting of four dimers each; an observation by cryoelectron microscopy. Whereas each dimer binds a 13-bp sequence, different forms will discriminate between promoters, based on the numbers of repeating 13-bp sequences, and types of linkers inserted between them, which are either of 7-8 or ≈18 bp. The amino acid sequences of these FFRPs are designed to form the same type of 3D structures, and the transition between their assembly forms is regulated by interaction with small molecules. These considerations lead us to propose a possible mechanism for regulating a number of genes by varying assembly forms and by combining different FFRPs into these assemblies, responding to environmental changes.
Transcription regulation in archaea appears to be a chimera, with general transcription factors being eukaryotic and candidates for regulating specific genes being eubacterial (1). The best characterized of the latter, so far, are archaeal homologs of the Escherichia coli leucine-responsive regulatory protein (Lrp) and the asparagine synthase C gene product (AsnC) (2, 3). In an archeal genome, up to 16 proteins in this group are coded (2, 4), whereas in the genome of the eubacterium Pseudomonas aerginosa, eight such proteins have been identified (2, 4, 5).
In this paper, continuing the practice (2, 4-8), homologs of Lrp and AsnC are referred to as the feast/famine regulatory proteins (FFRPs). The term feast/famine regulation was coined by Calvo and Matthews (9) to summarize the general function of Lrp. Sensing a high concentration of leucine in rich nutrition, E. coli shifts its metabolism to a more heterotrophic mode, activates absorption of nutrients, accelerates cell replication, and changes its pathogenicity. For this adaptation, Lrp regulates transcription of a number of genes in various ways (9, 10).
The principle of feast/famine regulation also applies to AsnC and the last E. coli FFRP, YbaO. Depending on the concentration of asparagine, AsnC down-regulates biosynthesis of this amino acid (11). Also, part of the replication origin is positioned inside an operon regulated by AsnC (12). YbaO resembles another FFRP, the glutamate uptake regulatory protein (13), which regulates uptake of glutamate by another eubacterium (14).
Many FFRPs, including Lrp, have ≈160 amino acid residues; i.e., full length (FL)-FFRPs. Some other eubacterial and archaeal FFRPs have only ≈80 amino acid residues, as we reported (2, 4, 15, 16), corresponding to the C-terminal halves of FL-FFRPs, i.e., demi (DM)-FFRPs. These FFRPs do not bind DNA, unlike their counterpart.
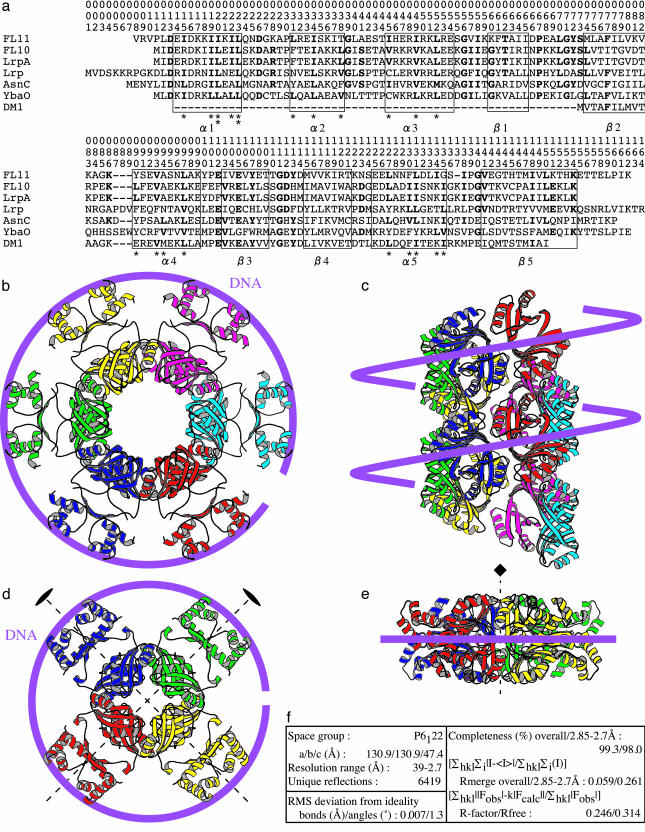
The presence of homologs of Lrp and AsnC in archaea was reported by Kyrpides and Ouzounis (17). In vivo function of these proteins remains unknown, because current genetic tools are not effectively applicable to archaea. The hyperthermophilic archaeon Pyrococcus sp. OT3 has 11 FL- and 3 DM-FFRPs (2, 4). One of the DM-FFRPs, DM1 (previously referred to as pot1216151), has been crystallized by our group (15, 16). The crystal structure of a FL-FFRP from Pyrococcus furiosus has been reported by another group (18), LrpA, which is an ortholog of FL10 (pot0377090) from Pyrococcus OT3 (Fig. 1a). In these crystals, four dimers each assemble into a disk (Fig. 1 d and e). To our knowledge, no eubacterial FFRP has been crystallized, nor has a DNA complex of any FFRP.
Fig. 1.
The amino acid sequences (a) and the crystal structures of FL11 (b and c) and LrpA (d and e; PDB ID code 1I1G and ref. 18), and the statistics (f) of the determination of the FL11 structure. A systematic numbering, 001-164, for the positions in the proteins are shown in a. A DM-FFRP, DM1 (pot1216151), lacksα1-3 and β1, starting from β2. Inside the α-helical regions, Ile, Leu, and Val, forming the hydrophobic phases (asterisks), are highlighted in bold. Outside theα-helices, residues frequent at individual positions are bold. Secondary structural elements found in FL11 are indicated. Dimers are differentiated by colors in the ribbon diagrams of top (b and d) and side (c and e) views. Possible directions of the DNA helix axes, when interacting, are traced. In c, 12 dimers only are shown, with deletion of their N-terminal halves for a better presentation of the assembly of the C-terminal halves. These two halves are connected by nonrigid polypeptides. A pair of twofold symmetry axes (d) and a fourfold symmetry axis (e) are shown.
Recently, we have crystallized another FL-FFRP from Pyrococcus OT3, FL11 (pot0434017) (6). In the 3D structure described in this paper, each pair of monomers dimerize into a structure similar to that of LrpA. However, FL11 dimers assemble into a quite different form. The transition between these forms and its potential roles for gene regulation are the focus of this paper.
Materials and Methods
Crystallization of FL11. Nucleotides coding the histidine tag were added upstream of gene fl11, and the modified gene was introduced into the E. coli strain BL21 (DE3) by using the pET28a plasmid. The expressed protein was purified, after heat treatment at 75°C for 30 min, by affinity chromatography using an Ni-nitrilotriacetic acid column (Pharmacia) and a 20- to 1,000-mM gradient of imidazole, followed by gel filtration with Sephacryl S-300 (Pharmacia). The protein was concentrated by using ultrafiltration to ≈20 mg/ml in 50 mM sodium phosphate buffer (pH 8.0) containing 300 mM NaCl. Crystals (0.4 × 0.4 × 0.2 mm) were obtained by vapor diffusion, using the sitting drop method at 20°C in ≈3 days: 4 μl of the protein solution was mixed with the same volume of 0.1 M Tris·HCl buffer (pH 8.5) containing 1.5 M ammonium sulfate and 12% (vol/vol) glycerol.
Structure Determination. The crystals were frozen by using nitrogen gas, and were kept at 100 K, while diffraction was recorded by using a synchrotron x-ray source (1.0 Å at the Photon Factory BL18B, Tsukuba, Japan) and an area detector (Quantum 4R, Area Detector Systems, Poway, CA). The reflections were merged and processed by using the ccp4 package (19).
The 3D structure was determined by molecular replacement (20). The N-terminal half of the initial model (positions 12-73 in Fig. 1a) was made by using the coordinates of LrpA (PDB ID code 1I1G and ref. 18) as a template, and the C-terminal half (positions 74-152) by using the coordinates of a high-resolution crystal structure of DM1 (H.K. and M.S., unpublished work). By using the program amore (21), the C-terminal half model was placed into the asymmetric unit. Then, the N-terminal half was placed nearby, guided by the geometry of the two halves in LrpA.
While monitoring the R factor, the positions of the two halves were adjusted independently, and their connectivity was repaired by improving the whole structure by using automated (22) and manual (23) methods. These processes were iterated, while nine more residues at the C terminus were modeled stepwise, and 50 water molecules were added. The coordinates of the final model (positions 12-157), with an R factor of 24.6% at a resolution of 2.7 Å (see Fig. 1f for other statistics), were deposited in the Protein Data Bank (PDB ID code 1RI7).
Cryo-Electron Microscopy. Protein solutions were prepared as follows: 34 μg/ml FL11 in 10 mM Tris·HCl buffer (pH 7.5) containing 133 mM KCl and 40 μg/ml DM1, were expressed and purified as described (15), in 50 mM Tris·HCl buffer (pH 7.0) containing 200 mM NaCl. The DNA-FL11 complex was prepared by adding a 91-bp DNA fragment (positions 434483-434573 in the Pyrococcus OT3 genome), made by the PCR (24), to the protein solution, yielding a concentration of 42 μg/ml. The molar ratio of the DNA fragment to the protein monomer was 1:2.9.
Each solution (4 μl) placed on a grid, was stained by using 16% ammonium molybdate and frozen in liquid ethane to the amorphous ice state (25). The grid was maintained at a liquid nitrogen temperature by a holder (CT3500, Oxford) while an electron microscope (-10 to +10 eV, Tecnai F20, FEI) was operated at 200 KeV. Zero-loss electrons selected by an energy filter (GIF200, Gatan) were focused with slight defocusing to enhance the contrast (26, 27). Images were recorded by using a charge-coupled device camera (794IF, Gatan) and were processed with the software package eman (28). In these images, DM1 was negatively stained as expected (29). FL11 and the DNA were positively stained, possibly due to the staining time of ≈90 sec, which is longer than that used for DM1, 30 sec or less.
Electrophoretic Mobility-Shift Assay. The 91-bp DNA fragment and FL11 were mixed in 10 mM Tris·HCl buffer (pH 7.5) containing 133 mM KCl, yielding a set of solutions; 15 μl each, containing various concentrations of the protein and 42 μg/ml DNA. These were incubated for 30 min at 20°C. After adding the loading buffer (6× Loading dye, Toyobo, Osaka), 3 μl of each was subjected to electrophoresis by using a 3% agarose (Nussieve GTG, BMA) gel buffered by 40 mM Tris acetate (pH 8.0) containing 1 mM EDTA. The gel was stained by ethidium bromide.
Results and Discussion
Cylinder Formation by FL11. Dimers of FL11 are assembled into cylinders with a right-handed helicity, continuing from one end of the crystal to the other (Fig. 1 b and c). The structural unit is a dimer, as was in the structures of another FL-FFRP, LrpA (18), and DM1 (15). Each monomer forms an asymmetric unit, and there are six dimers in each helical turn of 47.4 Å.
The C-terminal halves (strands β2-5, helices α4, 5) form the multimeric core, from which N-terminal halves (α1-3 and β1) protrude (Fig. 1b), fulfilling requirements for forming DNA-binding domains (DBDs). A set of three α-helices are combined in each N domain in the same way as in many other DBDs (31): a helix-turn-helix combination plus one more helix shielding an inner hydrophobic core. Positions of Lrp 13, 34, 40, 41, 44, 46, 48, 61, 65, and 70 (Fig. 1a), genetically identified as involved in DNA recognition (30), are all found inside this domain.
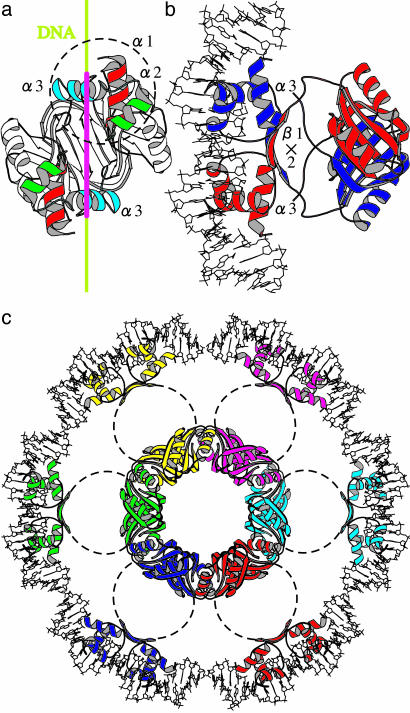
In such a trihelical DBD, it is the third helix that frequently recognizes DNA (31). In a dimer, the relative geometry of a pair of α3 is fixed by interaction between two β1 (Fig. 2b): the separation is < 34 Å; i.e., the helical pitch of the standard DNA, and the two helices are not totally parallel. Thus, a dimer will bind DNA around two sites separated by ≈8 bp.
Fig. 2.
Steps toward modeling of the DNA-FL11 complex. (a) A ribbon diagram of an FL11 dimer, viewed from a hypothetically bound DNA. The 34-Å scale is shown along the DNA axis in crimson. The three α-helices in one DBD are circled. (b) Another view of a shown with a crystal structure of 22-bp DNA (PDB ID code 1CGP and ref. 41). Monomers are differentiated by colors. (c) A possible geometry of a set of DBDs in the FL11 cylinder after interaction with DNA. The linkers connecting pairs of domains are stretched: their conformations are not specified. Dimers are differentiated by colors.
Disk and Dimeric Forms of FL11. In solution FL11 predominantly behaves as a dimer. The molecular weight measured by gel filtration was ≈30 kDa, a value not so different from twice the real molecular mass, 18,914 Da. Also, the sedimentation coefficient of the protein is consistent with this idea (F. Arisaka, unpublished work). In solution, no fibrous structure was observed by cryo-electron microscopy. Instead, globules much smaller than 100 Å were observed (see the background of Fig. 3f).
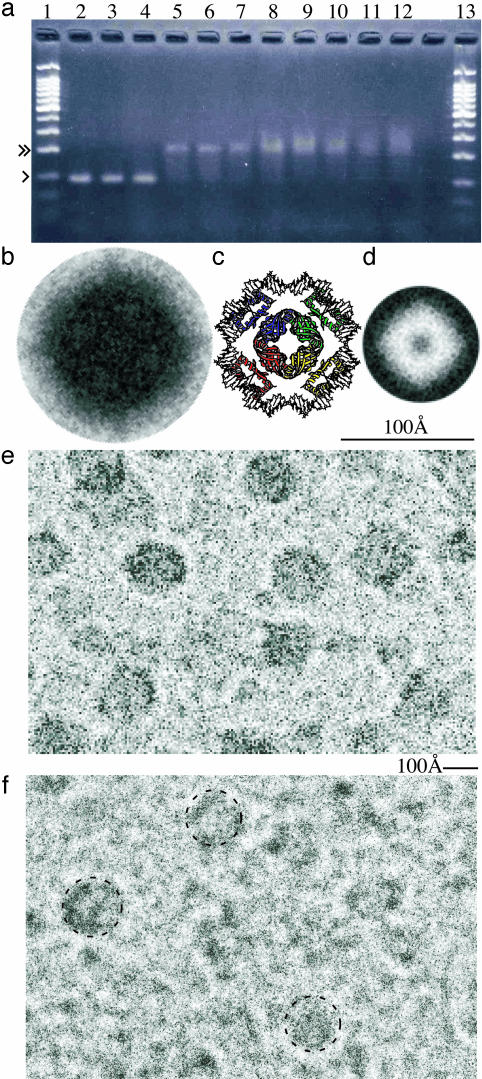
Fig. 3.
DNA binding by FL11. (a) An electrophoretic mobility-shift assay using an agarose gel. Lanes 1 and 13: reference 100-bp ladders, with the 100- and 200-bp bands marked with single and double arrows, respectively, for lane 1. The molar ratio of the 91-bp DNA and the FL11 monomer are 1:0 (lane 2), 1:1.5 (lane 3), 1:3 (lane 4), 1:4.5 (lane 5), 1:6 (lane 6), 1:7.5 (lane 7), 1:9 (lane 8), 1:10.5 (lane 9), 1:12 (lane 10), 1:13.5 (lane11), and 1:15 (lane 12). (b) A processed electron microscopic image of the DNA-FL11 complex. Eleven original images, most of which are shown in e, were symmetrized by assuming a fourfold axis (see Fig. 1e), and averaged with rotation, yielding the best correlation with each other. Note that b- d are shown at the same scale. (c) A model of DNA complex of an FFRP disk, made by using the structure of LrpA (Fig. 1d). Each DBD pair interacts with DNA essentially in the same way as in Fig. 2b. The conformation of the FFRP linker is not specified. (d) An electron microscopic image of the DM-FFRP DM1 obtained by processing, in the same way as in b, nine originals negatively stained. (e and f) Parts of electron micrographs (magnification of ×135,000) of FL11 in the presence e and absence f of the 91-bp DNA, shown at the same scale. In f, disk-like densities are circled.
A small population, however, was larger (≈150 Å), showing some substructures (circled in Fig. 3f). These sizes are similar to the diameter of the disk assembly of another FL-FFRP, LrpA (Fig. 1d), but are larger than the negatively stained DM1, which is ≈72 Å (Fig. 3d).
DNA Binding by the FL11 Disk. E. coli Lrp and AsnC autoregulate transcription of their genes (11, 32). The electric mobility of a 91-bp DNA fragment, containing the promoter region of gene fl11, when an increasing amount of FL11 was added (Fig. 3a), changed close to that of a 200-bp DNA, suggesting predominant formation of a single type of complex (Fig. 3a). This DNA is large enough to circle once around the FL11 disk, but is unable to wind around many turns of the cylinder. The binding is tight with a constant of 108 to 109 mol-1, and is cooperative; the binding ratio is 1 (90-bp DNA) to 0.5-1 (FL11 octamer). As is expected, when a mixture was subjected to cryo-electron microscopy, dense and smoothly circular objects with the diameters of ≈150 Å predominated (Fig. 3e).
Consensus sequences of DNA bound by dimers of various FFRPs have the same form [NANBNCNDNE]N3[NENDNCNBNA] (2), whereas NA, for example, is the base complementary to NA, N is often a T base, and NE is either A or T (Fig. 4a). In the 91-bp promoter of gene fl11, four repeats of ≈21 bases; i.e., 13 bases similar to [ATAAA]ATT[TTTAT] followed by nonconserved ≈8 bases, were identified (Fig. 4b).
Fig. 4.
Binding sites of FFRP dimers. (a) Consensus sequences of DNA recognized by dimers of various FFRPs. Those of FL11 and YbaO are newly deduced. The rest of the sequences were adopted from refs. 2 and 11. Runs of T bases at the centers, extending toward either ends, are underlined. The sequences for Lrp and Ptr1, 2 are essentially the same as those published by other groups (42, 43). Binding by LysM extends to each end by two bases (37), which might be recognized by the protein outside the recognition helix. Some of the sequences were deduced from small numbers of examples and may therefore need modifications in the future (11). The sequence for LrpA was deduced by assuming 8-bp insertion between dimer-binding sites. If the correct number is 7, the consensus will change (2). (b) Binding sites of FL11 dimers, predicted upstream of gene fl11. Runs of T or A bases at around the centers are underlined. Bases outside the central three positions are boxed, when they are the same as those in consensus.
Modeling of a Complex. In the 13-bp consensus sequence of FL11 binding sites, two centers of 5 bp, inversely related, are separated by 8 bp, as is expected from the crystal structure of a dimer (Fig. 2b). In fact, the overall “13 + ≈8” arrangement satisfies requirements for forming a complex with the FL11 disk (Fig. 3c). To bind around the disk, the four sites contacting individual protein dimers need to be positioned on the same side along the DNA: the centers of neighboring sites need to be bridged by a multiple of ≈10.5 bp. If this number is ≈21 (i.e., 13 + ≈8), the helix axis of ≈84 bp will circle with a diameter of ≈90 Å (Fig. 3c). Considering the distribution and the van der Waals radii of phosphate groups around the helix axis, the size of the complex will not be so different from the observed 150 Å.
On the protein side, to follow the DNA superstructure, the diameter for arranging the DBDs needs to decrease by ≈10% from what is found in the LrpA crystal (Fig. 1d). This adjustment can be made (Fig. 3c) if the linker connecting the two domains in each FFRP is flexible, as is expected from the crystal structures (Fig. 1 b and d).
The DNA bound to the disk would, in vivo, be part of the continuous genomic DNA. Thus, the two ends of the DNA circling the disk do not connect to each other, but are separated by one superhelical turn. Biochemical experiments (33) have shown that such a DNA turn induced by another FFRP disk is right-handed; i.e., the same handedness of DBDs on the surface of the FL11 cylinder (Fig. 1c): a disk-to-cylinder transition can take place as smoothly as in a similar transition observed with the tobacco mosaic virus (34).
Discrimination Between Promoters by the Same FFRPs. Upstream of an orthologous gene, pf1443271, of P. furiosus, not four, but 11 dimer-binding sites appear to repeat. Some of them are close to ideal; e.g., [ATTAA]TTG[TTTAT], but not more than two such ideal sequences repeat sequentially. Thus, the protein might bind this promoter not by forming a disk but by forming a cylinder. Similarly, four imperfect repeats might be good enough for interaction with a disk, but might not suit a dimer, particularly if the best half-sites are separated into different dimer-binding sites: the same FFRP in different forms can have different selectivity for promoters.
In addition to the ≈21-bp periodicity, another periodicity of ≈31 bp (i.e., a 13-bp dimer-binding site plus an ≈18-bp linker) frequently occur in promoters recognized by various FFRPs (2, 7). When assembled into a disk, to follow the ≈31-bp periodicity, the linker connecting the two domains in each FFRP will need to stretch, producing a larger diameter, which is in contrast to its proposed shortening for the ≈21-bp periodicity (Fig. 3c). Linkers of FFRPs in a cylinder would already need to stretch to accommodate the ≈21-bp periodicity (Fig. 2c), and further stretching to follow the ≈31-bp periodicity appears to be difficult. Thus, different numbers of DBDs combined in one or more helical turns will discriminate between promoters.
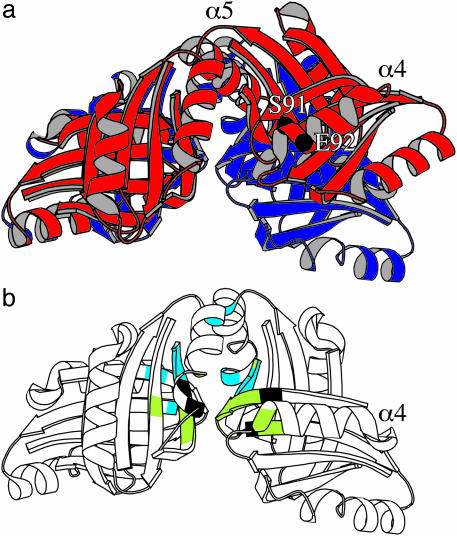
Selection of Forms of FFRPs by Ligands. The cleft formed between FL11 dimers will open on the transition from a disk to a helical turn (Fig. 5a). The hinge of this movement is positioned near the N termini of a pair of α5 helices. This movement will necessarily change the orientation of α4 of the dimer opening (Fig. 5, compare a with b), so that Ser-91 and Glu-92 therein will form hydrogen bonds, as in the cylindrical crystal, with Glu-92 and Ser-91, respectively, of α4 in another monomer. The two α4 helices contact at the interface between the two helical turns in the same phase, thereby stabilizing the cylinder.
Fig. 5.
Clefts formed between pairs of dimers in assembly domains. (a) The pairs of FL11 (red) overlaid on DM1 (dark blue) by best correlating particular halves (left). The positions of Cα atoms of Ser-91 and Glu-92 are indicated. (b) The pairs of DM1 with highlighting positions (green) facing a ligand absorbed from E. coli cells (5), and positions (light blue) genetically identified as important for leucine-binding by Lrp (30). Positions identified for both are black.
This transition can be prevented by “locking” the moving dimer at its edge. In a refined structure of the disk assembly of DM1, the density of an unidentified ligand, absorbed by the protein from E. coli cells after expression, is found in two of the four clefts (H.K. and M.S., unpublished work). Indeed, the ligands stabilizing this disk are positioned near amino acid residues, 79, 90, 108-115, 131, 132, 136, and 144-150 (5), all facing the cleft (highlighted in green or black in Fig. 5b). Similarly, by adding glutamine, formation of a higher order assembly by FL11 (an apparent molecular mass of ≈400 kDa in gel filtration) in solution was observed in some experiments. Further transition to a cylinder after crystallization, on the other hand, was prevented by glutamine.
Interaction with leucine, however, disassembles Lrp, probably to dimers (9, 10, 35, 36). Mutations at positions 108, 114, 124, 136, 147, 148, and 149 affected the leucine-dependence of regulation by Lrp (30). Around the cleft, they are slightly shifted toward the hinge (highlighted in light blue or black in Fig. 5b), suggesting destabilization of the hinge by the interaction.
Regulations by many other FFRPs are affected by different amino acids: AsnC by asparagine (11), LysM by lysine (37), the glutamate uptake regulatory protein by glutamate (14), PutR by proline (38), and MdeR by methionine (39). These ligands might diffuse into the FFRP assemblies through the holes formed at their centers (Fig. 1 b and d).
Possible Combinations of Different FFRPs into the Same Assemblies. The role of DM-FFRPs remains unexplained, because they have no DBD. Synthesizing these by spending many amino acids only to buffer the concentrations of single amino acids is possible but unlikely. A more likely possibility is that DM-FFRPs form assemblies in combination with FL-FFRPs. Some archaeal operons are composed of FL- and DM-FFRPs, and they might be such pairs. Heterogeneous formation might occur between FL-FFRPs as well. The assembly domains in dimers of the three FFRPs crystallized are very similar: the rms deviation between sets of Cα coordinates are 1.18-1.83 Å.
Depending on particular FFRPs combined into an assembly, the number and binding specificity of DBDs will vary. Also, amino acid residues facing the cleft between assembly domains will change, necessarily differentiating the partner ligands. In this way, a small number of variations of FFRPs can create a wide variation of assemblies, each selectively stabilized by a ligand signaling a particular change inside or outside the organism.
As a reviewer has noted, the expected diameter of DNA binding around an FFRP disk approximately equals the diameter of the eukaryotic nucleosome. However, the FFRP disk is thinner, and in the nucleosome, the DNA forms two superhelical turns of ≈80 bp each (40). In some sense, the cylindrical form of FL11 is comparable with a higher-order structure formed by nucleosomes. However, assemblies of FFRPs are different from the eukaryotic chromatin in that they have high DNA-binding specificity and the ability to interact with ligands. These last properties would be required, if, as we propose here, archaeal FFRPs are involved, in combination, in regulating transcription of a large number of genes in response to environmental changes.
Acknowledgments
This work was supported by the Core Research for Evolutionary Science and Technology program of the Japan Science and Technology Agency and by the Mitsubishi Foundation.
Abbreviations: AsnC, asparagine synthase C gene product; DBD, DNA-binding domain; FFRP, feast/famine regulatory protein; FL-FFRP, full-length FFRP; DM-FFRP, demi-FFRP; Lrp, leucine-responsive regulatory protein.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 1RI7).
References
- 1.Kyrpides, N. C. & Ouzounis, C. A. (1999) Proc. Natl. Acad. Sci. USA 96, 8545-8550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suzuki, M. (2003) Proc. Japan Acad. Ser. B 79, 274-289. [Google Scholar]
- 3.Brinkman, A. B., Ettema, T. J. G., de Vos, W. M. & van der Oost, J. (2003) Mol. Microbiol. 48, 287-294. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki, M., Amano, N. & Koike, H. (2003) Proc. Japan Acad. Ser. B 79, 92-98. [Google Scholar]
- 5.Suzuki, M., Koike, H. & Aramaki, H. (2003) Proc. Japan Acad. Ser. B 79, 242-247. [Google Scholar]
- 6.Koike, H., Sakuma, M., Mikami, A., Amano, N. & Suzuki, M. (2003) Proc. Japan Acad. Ser. B 79, 63-69. [Google Scholar]
- 7.Suzuki, M. (2003) Proc. Japan Acad. Ser. B 79, 213-222. [Google Scholar]
- 8.Suzuki, M. & Koike, H. (2003) Proc. Japan Acad. Ser. B 79, 114-119. [Google Scholar]
- 9.Calvo, J. M. & Matthews, R. G. (1994) Microbiol. Rev. 58, 466-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newman, E. B. & Lin, R. (1995) Annu. Rev. Microbiol. 49, 747-775. [DOI] [PubMed] [Google Scholar]
- 11.Kölling, R. & Lother, H. (1985) J. Bacteriol. 164, 310-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Wind, N., de Jong, M., Meijer, M. & Stuitje, A. R. (1985) Nucleic Acids Res. 13, 8797-8811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng, Q. & Summers, A. O. (1997) Mol. Microbiol. 24, 231-232. [PubMed] [Google Scholar]
- 14.Peekhaus, N., Tolner, B. P., Poolman, B. & Krämer, R. (1995) J. Bacteriol. 177, 5140-5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kudo, N., Allen, M. D., Koike, H., Katsuya, Y. & Suzuki, M. (2001) Acta Crystallogr. D 57, 469-471. [DOI] [PubMed] [Google Scholar]
- 16.Kudo, N. (2001) Ph.D. thesis (Univ. of Tokyo, Tokyo).
- 17.Kyrpides, N. C. & Ouzounis, C. A. (1995) Trends Biochem. Sci. 20, 140-141. [DOI] [PubMed] [Google Scholar]
- 18.Leonard, P. M., Smits, S. H. J., Sedelnikova, S. E., Brinkman, A. B., de Vos, W. M., van der Oost, J., Rice, D. W. & Rafferty, J. B. (2001) EMBO J. 20, 990-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collaborative Computational Project 4 (1994) Acta Crystallogr. D 50, 760-776.15299374 [Google Scholar]
- 20.Rossman, M. G. & Blow, D. M. (1962) Acta Crystallogr. 15, 24-31. [Google Scholar]
- 21.Navaza, J. (1994) Acta Crystallogr. A 50, 157-163. [Google Scholar]
- 22.Brünger, A. T., Adams, P. D., Clore, G. M., DeLano, W. L., Gros, P., Grosse-Kunstleve, R. W., Jiang, J.-S., Kuszewski, J., Nilges, M., Pannu, N. S., et al. (1998) Acta Crystallogr. D 54, 905-921. [DOI] [PubMed] [Google Scholar]
- 23.Jones, T. A., Zhou, J.-Y., Cowan, S. W. & Kjeldgaard, M. (1991) Acta Crystallogr. A 47, 110-119. [DOI] [PubMed] [Google Scholar]
- 24.Saiki, R. K., Gelfand, D. H., Stoffel, S., Scharf, S. J., Higuchi, R., Horn, G. T., Mullis, K. B. & Erlich, H. A. (1988) Science 239, 487-491. [DOI] [PubMed] [Google Scholar]
- 25.Adrian, M., Dubochet, J., Fuller, S. D. & Harris, J. R. (1998) Micron 29, 145-160. [DOI] [PubMed] [Google Scholar]
- 26.Klug, A. (1979) Chemica Scripta. 14, 245-256. [Google Scholar]
- 27.Erickson, H. P. & Klug, A. (1971) Philos. Trans. R. Soc. London B 261, 105-118. [Google Scholar]
- 28.Ludtke, S. J., Baldwin, P. R. & Chiu, W. (1999) J. Struct. Biol. 128, 82-97. [DOI] [PubMed] [Google Scholar]
- 29.Brenner, S. & Horne, R. W. (1959) Biochim. Biophys. Acta 34, 60-71. [DOI] [PubMed] [Google Scholar]
- 30.Platko, J. V. & Calvo, J. M. (1993) J. Bacteriol. 175, 1110-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki, M. & Brenner, S. E. (1995) FEBS Lett. 372, 215-221. [DOI] [PubMed] [Google Scholar]
- 32.Wang, Q., Wu, J., Friedberg, D., Plakto, J. V. & Calvo, J. M. (1994) J. Bacteriol. 176, 1831-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beloin, C., Jeussei, J., Révet, B., Mirambeau, G., Le Hégarat, F. & Le Cam, E. (2003) J. Biol. Chem. 278, 5333-5342. [DOI] [PubMed] [Google Scholar]
- 34.Durham, A. C., Finch, J. T. & Klug, A. (1971) Nat. New Biol. 229, 37-42. [DOI] [PubMed] [Google Scholar]
- 35.Marasco, R., Varcamonti, M., La Cara, F., Ricca, E., de Felice, M. & Sacco, M. (1994) J. Bacteriol. 176, 5197-5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen, S., Rosner, M. H. & Calvo, J. M. (2001) J. Mol. Biol. 312, 625-635. [DOI] [PubMed] [Google Scholar]
- 37.Brinkman, A. B., Bell, S. D., Lebbink, R. J., de Vos, W. M. & van der Oost, J. (2002) J. Biol. Chem. 277, 29537-29549. [DOI] [PubMed] [Google Scholar]
- 38.Cho, K. & Winans, S. C. (1996) Mol. Microbiol. 22, 1025-1033. [DOI] [PubMed] [Google Scholar]
- 39.Inoue, H., Inagaki, K., Eriguchi, S., Tamura, T., Esaki, N., Soda, K. & Tanaka, H. (1997) J. Bacteriol. 179, 3956-3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richmond, T. J., Finch, J. T., Rushton, B., Rhodes, D. & Klug, A. (1984) Nature 311, 532-537. [DOI] [PubMed] [Google Scholar]
- 41.Schulz, S. C., Shields, G. C. & Steitz, T. A. (1991) Science 253, 1001-1007. [DOI] [PubMed] [Google Scholar]
- 42.Cui, Y., Wang, Q., Stormo, G. D. & Calvo, J. M. (1995) J. Bacteriol. 177, 4872-4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ouhammouch, M. & Geiduschek, E. P. (2001) EMBO J. 20, 146-156. [DOI] [PMC free article] [PubMed] [Google Scholar]