Abstract
Cisplatin (cDDP) is an anticancer drug used in a number of malignancies, including testicular, ovarian, cervical, bladder, lung, head, and neck cancers. Its use is limited by the development of resistance, often rationalized via effects on cellular uptake. It has been claimed that human copper transporter 1 (hCTR1), the human high-affinity copper transporter, is the major entry pathway for cDDP and related drugs via a mechanism that mimics copper. This is an unexpected property of hCTR1, a highly selective copper (I) transporter. We compared the uptake rates of copper with cDDP (and several analogs) into human embryonic kidney 293 cells overexpressing wild-type or mutant hCTR1, mouse embryonic fibroblasts that do or do not express CTR1, and human ovarian tumor cells that are sensitive or resistant to cDDP. We have also compared the effects of extracellular copper, which causes regulatory endocytosis of hCTR1, to those of cDDP. We confirm the correlation between higher hCTR1 levels and higher platinum drug uptake in tumor cells sensitive to the drug. However, we show that hCTR1 is not the major entry route of platinum drugs, and that the copper transporter is not internalized in response to extracellular drug. Our data suggest the major entry pathway for platinum drugs is not saturable at relevant concentrations and not protein-mediated. Clinical trials have been initiated that depend upon regulating membrane levels of hCTR1. If reduced drug uptake is a major factor in resistance, hCTR1 is unlikely to be a productive target in attempts to enhance efficacy, although the proteins involved in copper homeostasis may play a role.
Introduction
Platinum drugs, including cisplatin (dichloro-diamino-platinum, or cDDP), have been used since the 1970s as chemotherapeutic agents for a variety of malignancies, including testicular, ovarian, cervical, bladder, lung, head, and neck cancers (Loehrer and Einhorn, 1984; Zumaeta et al., 2001). One major limitation of cDDP usage is the rapid development of resistance (Giaccone, 2000). cDDP, along with its later developed analogs, acts by forming DNA crosslinks. Platinum crosslinks inhibit DNA replication and halt cell division. The DNA repair system is activated, and once repair is unsuccessful, apoptosis is activated (Andrews and Howell, 1990; Kelland, 2007). There has been considerable interest in understanding the cellular entry mechanism of platinum drugs, as their effectiveness depends on the amount delivered to the tumor cell.
Copper is an essential micronutrient playing a critical role as a cofactor in a number of cellular enzymes, including superoxide dismutase and cytochrome c oxidase, among others. Initial studies on the entry of cDDP into yeast cells indicated that entry correlated with the expression of copper transporter 1 (CTR1), the copper transporter in the yeast plasma membrane (Ishida et al., 2002; Lin et al., 2002). The same correlation was seen with human CTR1 (hCTR1) in some tumor cells (Song et al., 2004; Zisowsky et al., 2007). Several subsequent studies confirmed this correlation and led to the suggestion that hCTR1, the major high-affinity copper transporter, also mediates platinum drug uptake (Pabla et al., 2009; Larson et al., 2009, 2010b; Chen et al., 2012; Kalayda et al., 2012).
Elevated copper in the extracellular media causes an internalization of the transporter, with an associated decrease in copper entry, a process that is reversed when copper loads return to normal. This acute regulatory response protects against excessive copper accumulation (Molloy and Kaplan, 2009). It had earlier been reported that copper-induced internalization of hCTR1 results in degradation of the transporter (Petris et al., 2003). Such internalization and degradation have also been reported following cDDP addition to human ovarian carcinoma cells (Holzer and Howell, 2006). Copper homeostasis in mammalian cells utilizes hCTR1 for entry and ATP7A and ATP7B (copper-activated ATPases) for mediating its exit (Kaplan and Lutsenko, 2009). These ATPases are also responsible for delivering copper to proteins in the secretory pathway. Samimi et al. (2004) showed that Me32a human fibroblasts that expressed neither ATP7A nor ATP7B were more sensitive to cDDP, copper, and carboplatin than cells transfected with vectors expressing ATP7A or ATP7B. Thus, transporters involved in copper homeostasis were again associated with cDDP efficacy. A recent clinical trial assumed that increases in membrane levels of hCTR1 would lead to enhanced platinum drug entry and increased drug effectiveness (Fu et al., 2012).
Although there is experimental evidence that the proteins involved in copper homeostasis, the uptake and efflux transporters and cellular copper chaperones, may interact with platinum-containing drugs, most of the evidence that identifies hCTR1 as the major cDDP entry pathway relies upon correlative relationships rather than demonstrated causality (Rabik and Dolan, 2007). It is surprising that a highly selective transporter, such as hCTR1, that has been shown to transport only silver and copper (Cu) (I) ions would also be capable of mediating cDDP transport. cDDP has a diameter of 9.57A (Hilder and James, 2007), whereas Cu (I) in aqueous solution has a diameter of approximately 3.6 A (Blumberger et al., 2004). cDDP is more than two times larger than copper and therefore highly unlikely to fit within the narrow pore of hCTR1, which has an estimated diameter at the entrance of less than 8A (De Feo et al., 2007).
We have carried out a detailed analysis of the relationship between hCTR1, copper uptake, and cDDP (and several analogs) uptake in human embryonic kidney (HEK) cells that overexpress hCTR1, in mouse embryonic fibroblasts (mefs) that do or do not express CTR1, and in human ovarian tumor cells that are sensitive or resistant to cDDP. We have compared the responses to copper of hCTR1 in these cells to cDDP, and examined the effects of varied hCTR1 levels on copper and cDDP entry rates and the consequences of altering hCTR1 expression on the rates of copper and platinum drug entry. We provide evidence that the major entry pathway into human cells for platinum-containing anticancer drugs is not hCTR1, and is most likely not protein-mediated. Our data have considerable significance for future approaches to improve platinum-drug efficacy.
Materials and Methods
Cell Culture.
Cells were maintained in a humidified incubator at 37°C under 5% CO2 atmosphere and passaged every 3–5 days. HEK293 Flp-In T-Rex (Invitrogen, Carlsbad, CA), Mef +/+ and −/− cells (a gift from Dr. Dennis Thiele, Duke University) and human ovarian tumor cells (A2780) and cisplatin-resistant A2780 (A2780CP) cells (Fox Chase Cancer Center, Jenkintown, PA) were grown in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Norcross, GA) and 25 mM Hepes (Gibco).
HEK293 Flp-In cells containing tetracycline-regulated N-terminal FLAG–tagged hCTR1 were created as previously described (Maryon et al., 2007). The cells were transfected with hCTR1 construct using Lipofectamine 2000 (Invitrogen). Transfected cells were selected in 12 μg/ml blastocidin S (Research Products International Corp., Mount Prospect, IL) and 400 μg/ml hygromycin (Invitrogen). Resistant colonies were pooled and tested for tetracycline-regulated expression. Cells were cultured in media containing 1 μg/ml tetracycline for 48 hours before harvesting. Mutants of hCTR1 were created as previously described (E. Maryon et al., submitted manuscript).
Biotinylation of Surface hCTR1.
Biotinylation of cells were carried out using the cell-impermeable, thiol-cleavable Sulfo-NHS-SS-biotin (Pierce, Rockford, IL) reagent to label cell surface proteins at 4°C, as described previously (Molloy and Kaplan, 2009). Samples were separated by SDS-PAGE, and hCTR1 was detected using either an anti-hCTR1 or anti-FLAG antibody by Western blot analysis, as described later.
SDS-PAGE and Western Blot Analysis.
Protein samples were separated using protocol described in our earlier studies (Zimnicka et al., 2007). For detection of hCTR1 protein, the following primary antibodies were used: rabbit anti–C-terminal hCTR1 antibody at 1:4000 dilution (Maryon et al., 2007) or mouse anti-FLAG antibody (GenScript, Piscataway, NJ) followed by anti-rabbit IgG horseradish peroxidase conjugate (GE Healthcare, Waukesha, WI) or anti-mouse horseradish peroxidase (Thermo Scientific, Waltham, MA). Western blot signals were obtained using SuperSignal West reagents (Pierce), and the intensity of the Western signals was measured using chemiluminescent imaging with the ChemiDoc XRS (Bio-Rad, Hercules, CA) and quantitated using Quantity One version 2.6.2 software (Bio-Rad). Loading controls were either the α subunit of the Na,K-ATPase or β-catenin, as indicated in the figures. It should be pointed out that commercially available antibodies against hCTR1 have proven to be of limited value for following hCTR1 in Western blot analysis. Several of these show either multiple bands or bands at approximately 25 kDa, the mass of the unglycosylated precursor, but not 35 kD, the mature product. Any antibody used against hCTR1, which has poor antigenicity, needs to be validated against recombinant proteins and in cells that do and do not express hCTR1 along with effects of deglycosylation on apparent mass.
64Cu Uptake.
The day before the assay, cells were plated at 0.6 × 106 cells/ml into each well of 3 × 4 tissue culture plate in DMEM containing 10% fetal bovine serum, and incubated at 37°C. The following day, fresh medium containing 64Cu (Isotrace Technologies Inc., Fallon, MO) was added and was incubated for 1 hour at 37°C. Copper uptake rates were then measured as previously described (Zimnicka et al., 2011). All transport determinations were carried out in triplicate. The amount of protein per well was determined after radioisotope decay, and the copper uptake per well was calculated as pmol of Cu/mg of protein/h; the average of triplicate wells was determined for each treatment.
Platinum Drug Uptake.
One day prior to the assay, cells were plated at 10.8 × 106 cells/ml in 10-cm tissue culture plates in DMEM containing 10% fetal bovine serum and incubated at 37°C. The day of the assay, the medium was aspirated, and cells were incubated in cisplatin, carboplatin, transplatin, or oxaliplatin dissolved in DMEM containing 10% fetal bovine serum for 1 hour at 37°C. The uptake was stopped by the addition of stop buffer (Zimnicka et al., 2011). The cells were washed an additional two times with stop buffer. Cells were collected and resuspended in 70% nitric acid for 3 hours. Afterward, the cells were diluted to 14% nitric acid by the addition of 1 ml of water, mixed, centrifuged, and the supernatant was submitted for platinum content by inductively coupled plasma mass spectrometry at the Chemical Analysis Laboratory in Athens, GA. The results were expressed as pmol of Pt/milligram of protein/h.
Drugs.
Cisplatin (Enzo Life Sciences, Farmingdale, NY), transplatin (Sigma-Aldrich, St. Louis, MO), carboplatin (TCI, Tokyo, Japan), and oxaliplatin (Enzo Life Sciences) solutions were freshly prepared for each experiment. Cisplatin and transplatin were dissolved in 100% dimethylsulfoxide; carboplatin and oxaliplatin were dissolved in water.
Cell Fractionation.
Fractionation of cells was carried out using a five-step sucrose gradient as previously described (Zimnicka et al., 2007).
Small Interfering RNA-Mediated Knockdown of hCTR1.
A2780 cells were transiently transfected with small interfering (siRNA) plasmids from Ambion (Life Technologies, Carlsbad, CA) using Lipofectamine RNAiMAX transfection reagent (Invitrogen) according to the manufacturer’s instructions. The cells were cultured to 50% confluence and incubated with either siRNA duplexes against CTR1 or siRNA negative control in Opti-MEM medium (Invitrogen). The following day, fresh DMEM was added. Human CTR1 knockdown was a predesigned sequence. The forward target sequence used was as follows: 5′-GCCUAUGACCUUCUACUUUtt-3′; the reverse target sequence was as follows: 5′-AAAGUAGAAGGUCAUAGGCat-3′.
Statistical Analysis.
Data are shown as the mean ± S.D. Statistical analysis was performed using Student’s t test, and P < 0.05 was considered statically significant. The data represent means of at least three independent experiments, and values are expressed as percentages of total cell surface hCTR1, to which no copper or cisplatin was added.
Results
HEK Cells Overexpressing hCTR1.
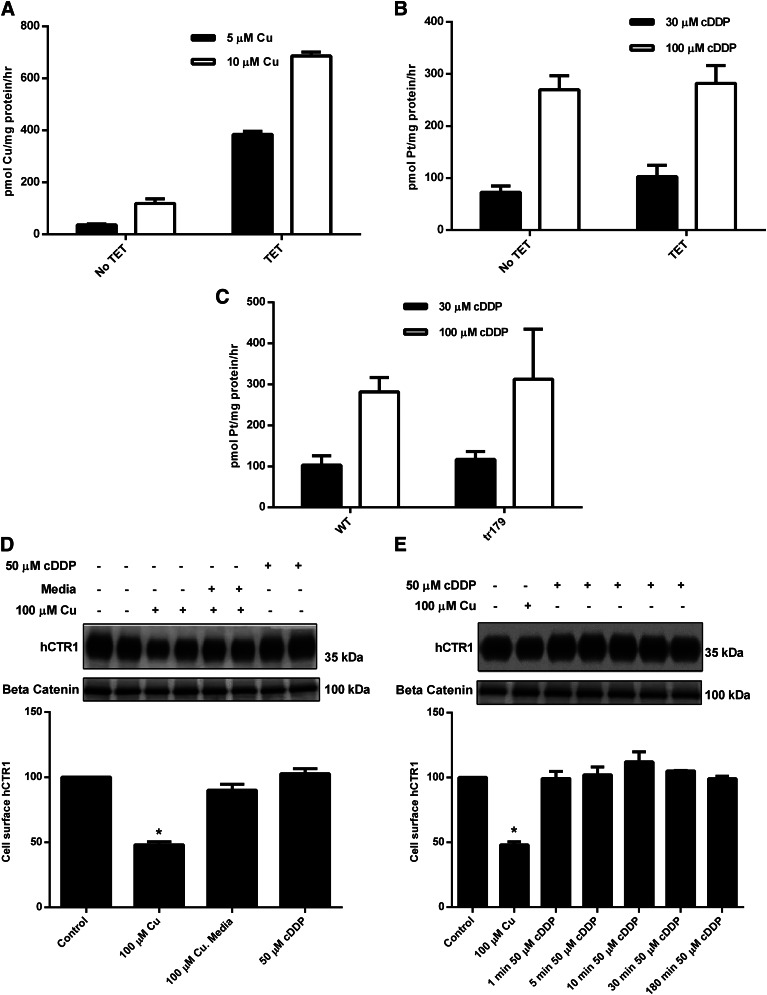
We have previously developed a line of HEK cells that express hCTR1, the human high-affinity copper transporter at the flip-in recombinase site, under the influence of a tetracycline-sensitive promoter (Maryon et al., 2007). When the initial rate of 64Cu uptake into these cells, grown in the absence or presence of tetracycline, is measured, cells grown in the presence of the antibiotic show a higher (approximately 8–10-fold; see Fig. 1A) rate. However, when the rate of cDDP entry is determined in these two sets of cells, there is no difference in the rate of uptake of the platinum drug (Fig. 1B). These data suggest that elevated rates of copper entry correlate with an increase in the expression level of hCTR1, whereas the rate of cDDP entry is not altered by overexpression of hCTR1. The increase in hCTR1 expression has been previously documented (Zimnicka et al., 2011).
Fig. 1.
Copper uptake, cDDP uptake, and cell surface expression of hCTR1 in HEK293 cells. HEK 293 cells overexpressing hCTR1 were induced with tetracycline (TET) 48 hours prior to the experiment. (A) 64Cu uptake was measured in cells incubated with 5 or 10 µM copper for 30 minutes in media. (B and C) cDDP uptake was measured in cells incubated with 30 or 100 µM cDDP for 5 hours. (D) Tetracycline-induced cells were incubated with 100 µM copper for 30 minutes, and then washed three times with media and placed back in media for 30 minutes, or incubated with 50 µM cDDP for 30 minutes. (E) Tetracycline-induced HEK cells were incubated with 100 µM copper for 1 hour, or 50 µM cDDP for 1, 5, 10, 30, or 180 minutes. (D and E) Cells were biotinylated, and the biotinylated protein was analyzed on Western blots. hCTR1 protein was detected using an anti-FLAG antibody, and the protein levels were normalized to β-catenin, loading control. WT, wild type. *Statistical analysis was performed using Student's t test, and P < 0.05 was considered statically significant.
If hCTR1 is important for the entry of cDDP, and copper and cDDP interact in a similar fashion with hCTR1, it would be expected that cells expressing mutants of hCTR1 that show elevated rates of copper entry would also show enhanced cDDP entry rates. We have recently discovered that truncations at the intracellular C-terminus of hCTR1 produce hCTR1 mutants with greatly enhanced (approximately 10-fold) Vmax values for copper entry (E. Maryon et al., submitted manuscript). The entry of copper into cells expressing the tr179 (truncation at amino acid 179) mutant that lacks the final 11 amino acid residues of hCTR1 has a much higher rate (approximately 15-fold) than cells expressing wild-type hCTR1. However, the rates of cDDP entry of these two sets of cells are identical (Fig. 1C) and the same as cells not overexpressing hCTR1 (Fig. 1B). Thus, it is likely that cDDP is not transported by hCTR1 in the same manner as copper in these cells.
It has previously been shown that, when cells are exposed to elevated copper levels, one mechanism to protect the cells against copper toxicity is a lowering of the entry rate. This is accomplished by a copper-dependent endocytosis of hCTR1 (Petris et al., 2003; Molloy and Kaplan, 2009) and associated decrease in copper entry (Molloy and Kaplan, 2009). This phenomenon is illustrated in the data of Fig. 1D, where cell-surface biotinylation has been used to determine the plasma membrane level of hCTR1. Clearly, the amount of hCTR1 in the HEK plasma membranes falls when the cells are incubated with excess copper; and when copper is removed, the transporter returns to the surface (Fig. 1D). In these same cells, there is no internalization of the transporter in response to high levels of extracellular cDDP (Fig. 1D). This is in contrast to results reported in human ovarian carcinoma cells (Holzer et al., 2004a; Holzer and Howell, 2006) and in agreement with previous studies in HEK293 cells (Guo et al., 2004; Pabla et al., 2009) and renal proximal tubules cells, as well as studies performed in mice (Pabla et al., 2009). Previous studies have suggested that only a brief exposure (5–15 minutes) to cDDP causes rapid internalization and degradation of hCTR1 (Holzer et al., 2004a; Holzer and Howell, 2006; Larson et al., 2009). We exposed tetracycline-induced HEK cells to 50 µM cDDP for 1, 5, 10, 30, and 180 minutes. No reduction of surface hCTR1 (i.e., internalization) was evident (Fig. 1E). We also exposed cells to 3 µM cDDP for the same times, with the same results (data not shown). These results suggest cDDP does not cause internalization of hCTR1, and thus cellular entry of cDDP is unlikely to occur via the endocytosis of hCTR1.
Mouse Embryonic Fibroblasts.
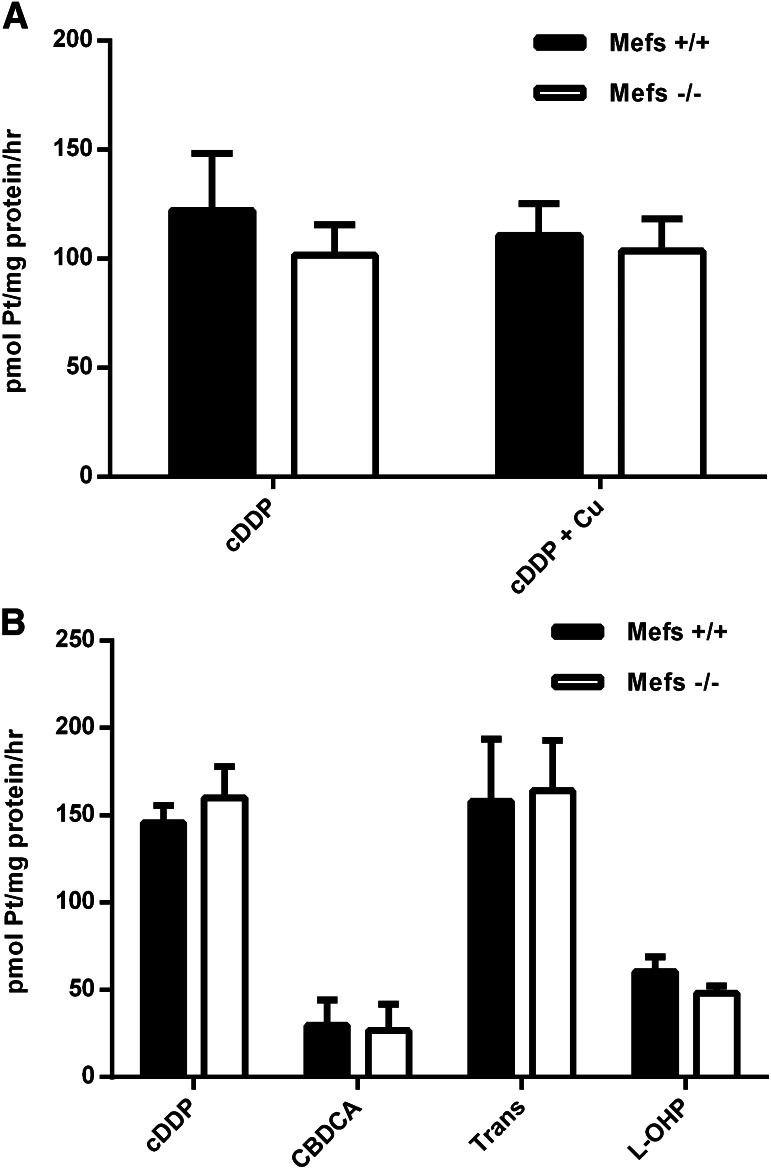
When the rate of cDDP entry into cells that do not express CTR1 (mefs −/−) are compared with similar cells that endogenously express the copper transporter (mefs +/+), it is apparent that neither the presence nor the absence of CTR1 has an influence on the rate of cDDP entry (see Fig. 2A). Furthermore, the presence of copper in the medium did not inhibit the rate of cDDP entry (Fig. 2A). It has previously been shown that the copper entry rate into the −/− mefs is approximately 25–30% of the +/+ cells via an unidentified pathway (Eisses and Kaplan, 2002; Lee et al., 2002; Zimnicka et al., 2011). Thus it seems unlikely that CTR1 in these cells plays a significant role in cDDP entry. We have also examined the entry rates of several analogs of cDDP that are in clinical use. The absence or presence of CTR1 does not alter their rates of uptake (Fig. 2B).
Fig. 2.
Platinum uptake rates in Mefs. (A) Mefs (+/+) and (−/−) were incubated with 30 µM cDDP or 30 µM cDDP and 100 µM copper for 5 hours. (B) Mefs (+/+) and (−/−) were incubated with 30 µM cDDP, carboplatin (CBDCA), transplatin (Trans), or oxaliplatin (L-OHP) for 5 hours.
It is apparent from these studies that CTR1 does not play a major role in mediating platinum drug entry into these model cells. However, the most relevant issue is whether hCTR1 plays a role in cDDP entry into tumor cells. We have extended our studies on cDDP entry to tumor cells of relevance to these issues.
Ovarian Tumor Cell Lines.
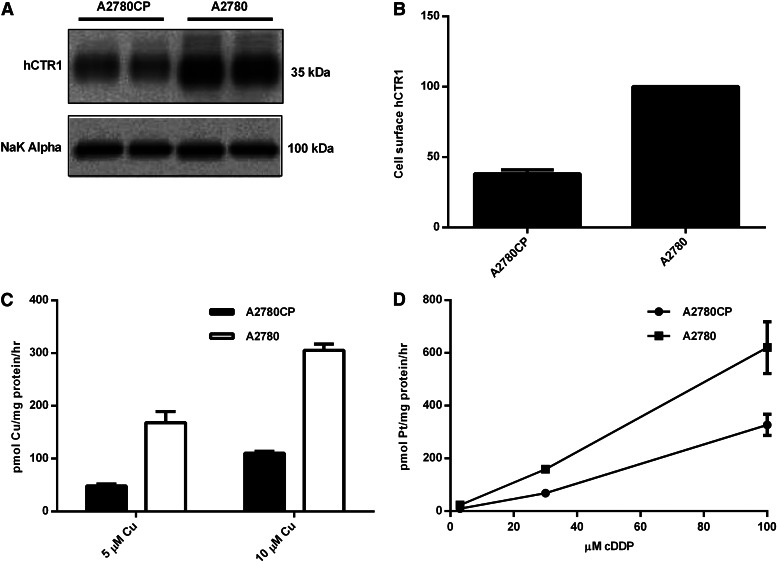
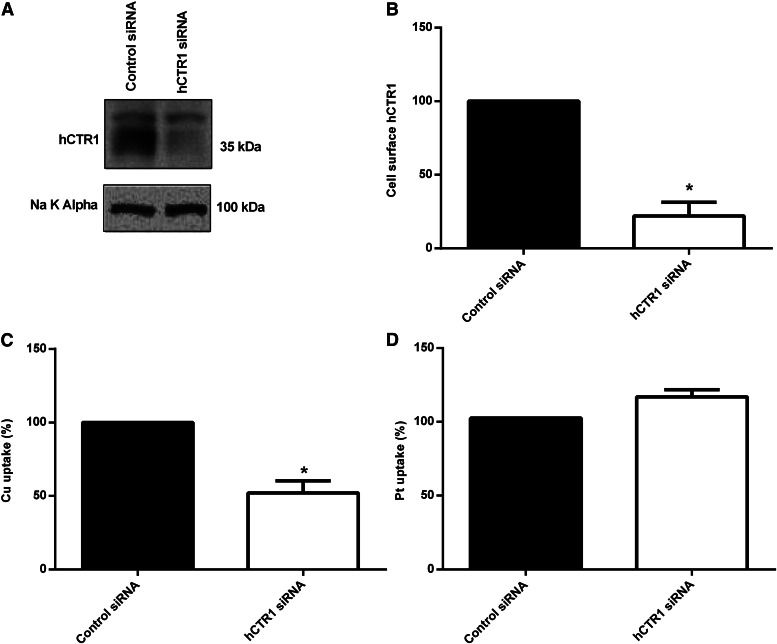
Two ovarian cancer cell lines were examined, A2780 and A2780CP, that were sensitive or resistant to cDDP, respectively (see Materials and Methods). It is clear that there is a significant difference in the expression levels of hCTR1 in the plasma membrane of these cells. The sensitive cells have a higher level of hCTR1 than the resistant cells (approximately 2-fold; see Fig. 3, A and B). This agrees with previous correlations that led to the suggestion that hCTR1 played a major role in cDDP entry, as the resistant line would take up less cDDP than sensitive tumor cells. Rates of copper transport were measured, and resistant tumor cells had a 2–3-fold lower rate of copper uptake when compared with sensitive tumor cells (Fig. 3C), in keeping with their lower expression of hCTR1. When cDDP entry rates are compared in the two cell lines, the resistant cells take up cDDP at a significantly slower rate (Fig. 3D), which correlates with the hCTR1 expression levels.
Fig. 3.
hCTR1 protein expression, copper uptake, and cDDP uptake in human ovarian carcinoma cells. (A) cDDP-resistant (A2780CP) and cDDP-sensitive (A2780) cells were fractionated, and plasma membranes were obtained and analyzed on Western blots. hCTR1 was detected using an anti-CTR1 antibody (rabbit). The Na+,K+-ATPase α subunit was the loading control. (B) Biotinylated hCTR1 protein was quantified using Quality One software (Bio-Rad), and the protein levels were normalized to the Na+,K+-ATPase α subunit. (C) 64Cu uptake was measured in cells incubated with 5 or 10 µM copper for 30 minutes in media. (D) cDDP uptake was measured in cells incubated with 3, 30, or 100 µM cDDP for 5 hours in media.
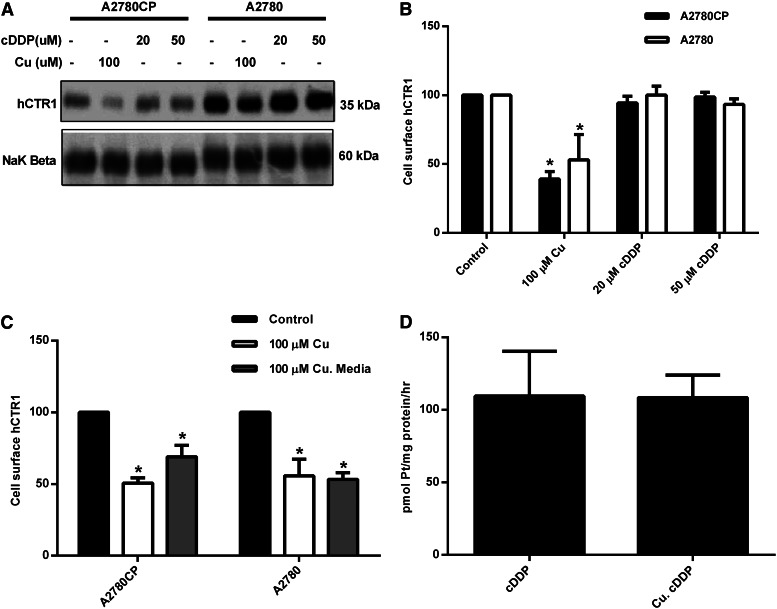
Exposure of the tumor cells to excess copper causes an internalization of plasma membrane hCTR1 (see Fig. 4, A and B) as in HEK 293 cells (Fig. 1, D and E). Exposure of the tumor cells to cDDP (20 or 50 µM) did not cause internalization of the transporter, just as had been observed in the HEK cells, emphasizing that hCTR1 does not respond in the same manner in tumor cells or in normal cells to excess cDDP and to excess copper. However, in contrast to the HEK cells, removal of copper from the external medium did not result in the return of hCTR1 to the plasma membrane of the ovarian tumor cells (Fig. 4C), as is observed in the acute regulatory mechanism seen in HEK cells. This enabled a further experiment to be performed in this cell system. If hCTR1 is internalized following exposure to elevated copper (∼50%), and if hCTR1 is responsible for cDDP entry, then cDDP entry should be inhibited by a prior Cu treatment of the ovarian cells. This protocol was applied to the ovarian cells. It can be seen that, although about 50% of the plasma membrane hCTR1 is internalized following copper treatment, cDDP uptake is unaffected (Fig. 4D).
Fig. 4.
Surface expression of hCTR1 and the effects of copper and cDDP. (A) cDDP-resistant (A2780CP) and cDDP-sensitive (A2780) cells were incubated with 100 µM copper, 20 µM cDDP, or 50 µM cDDP for 1 hour. (C) Cells were incubated with 100 µM copper for 30 minutes or 100 µM Cu and then washed three times with media and placed back in media for 30 minutes. (A and C) Cells were biotinylated, and the biotinylated protein was analyzed on Western blots using Quality One software. hCTR1 protein was detected using an anti-hCTR1(rabbit) antibody. The Na+,K+-ATPase β subunit was used as the loading control. (B) Western blot quantitation of (A). (D) A2780 cells were incubated with 100 µM copper for 1 hour, rinsed three times with media, and then placed back in fresh media with 30 µM cDDP for 3 hours. *Statistical analysis was performed using Student's t test, and P < 0.05 was considered statically significant.
In an experiment to directly alter hCTR1 expression in tumor cells, we used siRNA of hCTR1 in the A2780 ovarian carcinoma cells. Figure 5, A and B, shows we achieved ∼80% knockdown of plasma membrane hCTR1. In the cells transiently transfected with hCTR1 siRNA, the rate of copper transport was reduced by ∼50% (Fig. 5C). However, cDDP uptake was not altered (Fig. 5D).
Fig. 5.
hCTR1 knockdown in A2780 cells. (A) cDDP-sensitive (A2780) cells were transiently transfected with siRNA duplexes against CTR1 or siRNA negative control in Opti-MEM medium. Cells were biotinylated, and the biotinylated protein was analyzed on Western blots. hCTR1 protein was detected using an anti-CTR1 antibody (rabbit). (B) Biotinylated hCTR1 protein was quantified using Quality One software (Bio-Rad), and the protein levels were normalized to the Na+,K+-ATPase α subunit. After transient transfection, cells were incubated with 5 µM copper for 1 hour (C) or 30 µM cDDP for 3 hours (D). In (C), Cu uptake is expressed as a percentage of control (control rate 129.01 pmol of Cu/mg of protein/h), and in (D), cDDP uptake is expressed as a percentage of control (control rate 92.05 pmol of Pt/mg of protein/h). *Statistical analysis was performed using Student's t test, and P < 0.05 was considered statically significant.
The Mechanism of cDDP Entry.
In earlier studies in Ehrlich ascites tumor cells, based on the use of nonspecific chemical reagents, it was suggested that cDDP entry might occur by a non–protein-mediated pathway (Gale et al., 1973), the most likely being a partition-diffusion mechanism, where an uncharged or lipophilic molecule partitions into the membrane and diffuses down its concentration gradient to emerge into the cytosol. One of the characteristics of such a mechanism is that it would be nonsaturating (since a limited number of sites are not involved). Furthermore, interactions between proteins and substrates are expected to show some stereospecific discrimination. This is often seen where D- but not L-isomers are recognized by enzymes or transporters (Narawa et al., 2007). We have tested both of these expectations.
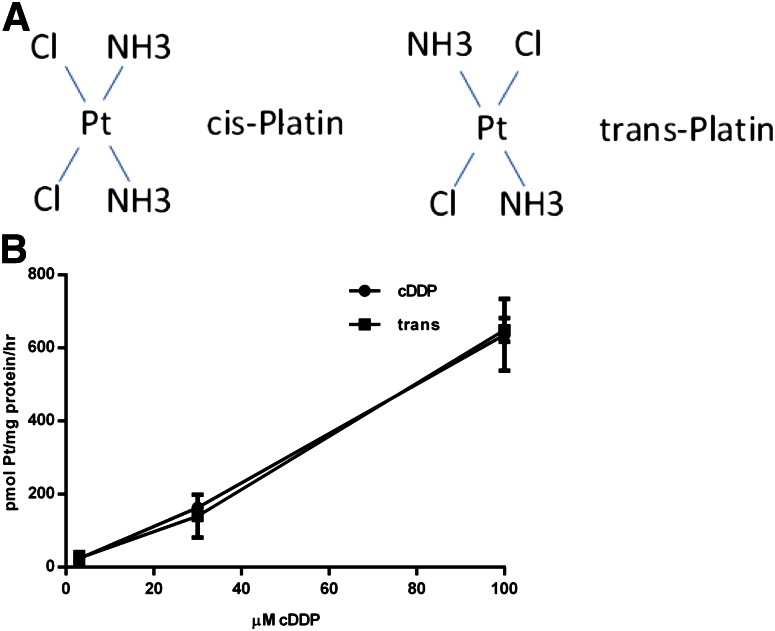
In Fig. 6B (see also Fig. 3D), the concentration dependence of cDDP uptake into ovarian tumor cells has been examined. Over a wide range of concentrations, uptake is approximately linear, and there is no sign of saturation. Although D- and L-isomers of cDDP are not available, the essential elements of such enantiomers are retained by comparison of cDDP (or cisplatin) and the nontherapeutic analog, transplatin (Fig. 6A). The concentration dependence values of the uptakes of these two analogs are identical (Fig. 6B).
Fig. 6.
Cisplatin versus transplatin (trans) cDDP uptake. (A) The chemical structure of cisplatin and transplatin are shown. (B) cDDP- sensitive (A2780) cells were incubated with 3, 30, or 100 μM cDDP or transplatin for 5 hours. Platinum content was measured by inductively coupled plasma mass spectrometry and is shown as pmol of Pt/mg of protein/h.
Discussion
Correlations between hCTR1 expression and drug entry in yeast and tumor cells (Ishida et al., 2002; Lin et al., 2002; Song et al., 2004) led to the idea that the copper transporter mediated cDDP entry. The basis for clinical trials (Fu et al., 2012) of agents that might influence responsiveness to cDDP is that cDDP is transported similar to copper, via a pore formed by the transporter (Larson et al., 2010b). It was also suggested that extracellular cDDP, similar to copper, causes internalization of hCTR1 (Holzer et al., 2004a; Holzer and Howell, 2006). Thus, hCTR1 would be downregulated in response to cDDP and further uptake would be reduced, leading to resistance. Our studies provide strong evidence that, although the proteins involved in copper homeostasis influence cellular cDDP levels, hCTR1 does not play a major role in cDDP entry. The most probable entry mechanism is via a non–protein-mediated pathway.
cDDP Uptake in Normal Eukaryotic Cells.
hCTR1 does not play a major role in cDDP uptake in HEK and mef cells based on the following: 1) overexpression of hCTR1 increases copper entry, but not cDDP (Fig. 1, A and B); 2) cells lacking CTR1 (−/− mefs) show the same rates of cDDP entry as cells (+/+ mefs) with hCTR1 (Fig. 2, A and B); and 3) cells overexpressing hyperactive hCTR1 mutants have rates of cDDP uptake identical to cells not overexpressing hCTR1 (Fig. 1C).
Previous studies in mefs, HEK cells, and yeast yielded varied conclusions. In HEK cells, no increase in cytotoxicity to cDDP was seen when hCTR1 was overexpressed (Rabik et al., 2009). Other studies in HEK and other renal cells claimed that an siRNA-induced decrease in hCTR1 resulted in a decrease in Pt uptake (Pabla et al., 2009). However, questions remain on the antibody (Ab) used to quantitate hCTR1 (see Internalization of hCTR1 in Response to cDDP). In previous studies using mefs, in contrast to our results, higher uptake of Pt was seen in cells expressing hCTR1 than in −/− cells (Larson et al., 2009, 2010a,b). These studies make several interesting points: most of the overexpressed hCTR1 protein was not delivered to the plasma membrane, there is a lack of correlation between cDDP uptake and cytotoxicity, truncation of the first 45 amino acids of hCTR1 does not inhibit cDDP uptake (Larson et al., 2010b), and replacement of Met residues 150 and 154 (Larson et al., 2010a) increases cDDP uptake and decreases Cu uptake. The authors conclude that if hCTR1 is responsible for cDDP uptake, its interactions with cDDP are different from those with Cu. Cytotoxic effects seen in those studies are observed following very brief (15 seconds or 5 minutes) exposures to cDDP. It is difficult to conclude that surface-binding phenomena are separated from uptake. The results of the present work suggest that if hCTR1 does interact with cDDP, it can play only a minor role, if any, in its cellular accumulation. Studies in yeast were the first to show the correlation between copper transporter and cDDP entry (Ishida et al., 2002). However, yeast CTR1 and hCTR1 have only 39% identity, and yeast protein is considerably larger. cDDP transport was characterized in CTR1-expressing strains, and Km values of 129 and 140 µM were reported for copper and cDDP, respectively (Lin et al., 2002). The values for copper are considerably higher than the 3–5 µM reported for hCTR1 (Eisses and Kaplan, 2002; Zimnicka et al., 2007; Liang et al., 2009) and 5 µM for yeast CTR1 reported previously (Dancis et al., 1994).
cDDP Uptake in Tumor Cells.
Our observations of cDDP entry into tumor cells replicate findings in normal cells: 1) copper treatment which lowers surface hCTR1 does not affect cDDP entry (Fig. 4, C and D); 2) lowering hCTR1, by 80% using siRNA (Fig. 5, A and B), reduces copper entry (Fig. 5C), but not cDDP uptake (Fig. 5D); and 3) exposure to cDDP causes an absence of internalization of hCTR1 (Fig. 4, A and B).
In ovarian carcinoma cells (A2780), Holzer et al. (2004b) achieved 20-fold overexpression of hCTR1 and only 30–50% increase in cDDP accumulation. They also reported cDDP treatment of cells decreased hCTR1 and copper uptake. Subsequently, they confirmed these observations and, interestingly, noted that increased cDDP accumulation was not associated with increased cytotoxicity (Holzer et al., 2004b). In contrast, it was recently reported on the same cells, in agreement with our findings, that increased membrane levels of hCTR1 had no effect on cDDP accumulation, and cDDP treatment did not decrease hCTR1 levels (Kalayda et al., 2012). Similarly, in cervix squamous carcinoma cells (A431), hCTR1 overexpression (3–4-fold) had no effect on cDDP accumulation (Beretta et al., 2004). In tumor cells, the concentration dependence of cDDP uptake has been reported. In agreement with our results in A2780 cells, uptake is linear up to 100 µM cDDP in 2008 cells (Andrews et al., 1988), in Ehrlich ascites cells (Gale et al., 1973), and in A2780 and HeLa cells (Zisowsky et al., 2007). In small-cell lung carcinoma cells, a Km of approximately 15 µM for cDDP uptake was reported (Liang et al., 2009), a value that is considerably lower (at least an order of magnitude) than reported in other cells.
The Entry Mechanism of cDDP.
It seems likely that most of the cDDP entry into tumor cells is mediated via a non–protein-mediated pathway. This is supported by our observations that, over a wide range of concentrations, no saturation of uptake is seen (Figs. 3D and 6B), and that the cisplatin and transplatin isomers of cDDP have identical uptake rates (Fig. 6B). Our data support entry occurring predominantly via solubility diffusion through the membrane (Gately and Howell, 1993). Other candidates have been proposed (OCT2, Na+,K+-ATPase, and SLC family members) (Koepsell and Endou, 2004; Ahmed et al., 2009; Filipski et al., 2009). Our observations suggest they are not primary players.
Internalization of hCTR1 in Response to cDDP.
Study of hCTR1 has been hampered by limitations of many of the available Abs. For example, a very interesting study of the effects of mutations on cDDP uptake in SCLCs (small-cell lung cancer) (Liang et al., 2009) shows an apparent mass of 25 kDa for both the wild-type and the N15Q mutant. It has been shown that N15Q reduces the mass of endogenous protein by about 10 kDa (Eisses and Kaplan, 2002). hCTR1 can appear as multiple bands above 35 kDa, due to the presence of stable monomers and dimers (Eisses and Kaplan, 2002; Maryon et al., 2007), which complicates analysis. These issues make it important that Abs used show the appropriate behavior in Western blots, such as shifts to lower mass on enzymatic deglycosylation (or with a nonglycosylated mutant), do not appear in cells that do not express hCTR1 or disappear on downregulation with siRNA. In HEK cells, we do not observe any hCTR1 internalization with cDDP, although extracellular copper causes internalization. This confirms previous findings in HEK cells with cDDP using epitope-tagged hCTR1 and an anti-Myc Ab (Guo et al., 2004). cDDP has been claimed to cause internalization and degradation in A2780 cells (Holzer et al., 2004b), but has been recently disputed (Kalayda et al., 2012), in agreement with the present work. Similarly, in yeast, relocalization of CTR1 following cDDP treatment was not observed (Sinani et al., 2007).
Platinum Drugs and Copper Homeostasis.
cDDP interacts with several proteins in copper homeostasis. Increased expression of ATP7A and ATP7B has been associated with cDDP resistance (Komatsu et al., 2000; Katano et al., 2002; Rabik and Dolan, 2007). cDDP also binds to the copper chaperone Atox1, destabilizing Atox1 and resulting in unfolding and aggregation. Atox1 was suggested as a potential candidate for cDDP resistance (Palm et al., 2011). Further work is needed to clarify the interactions between platinum drugs and proteins in copper homeostasis. Such interactions are significant, as lowering cell copper alters tumor cell sensitivity to platinum drugs (Ishida et al., 2010). The observations by Holzer et al. (2004b) of separate cytotoxic effects of cDDP from uptake suggest that a more complete understanding of the fate of cDDP following its initial interactions with the cell surface would be of great value. Inhibition of proteasomal activity enhances delivery of cDDP in ovarian carcinoma cells, and promises significant clinical potential, but its basis is also not yet clear (Jandial et al., 2009). The recent observation that copper is involved in growth factor kinase cycling in tumor cells introduces a new complexity (Tsai et al., 2012), and recent studies suggest important interactions between ATP7A, hCTR1, Atox1, and the platelet-derived growth factor receptor in cell activation (Ashino et al., 2010).
The present studies focus attention on the proteins involved in copper homeostasis as important contributors to the complexity of the cellular pharmacology of the widely used Pt drugs. It seems likely that interactions among the proteins involved in copper homeostasis play a major role in deciding the fate and effectiveness of these drugs, following cellular entry through a pathway that is not related to the entry mechanisms used by copper.
Acknowledgments
The authors thank Dennis Thiele (Duke University) for the mouse embryonic fibroblasts.
Abbreviations
- A2780
human ovarian tumor cells
- A2780CP
cisplatin-resistant human ovarian tumor cells
- Ab
antibody
- cDDP
cisplatin
- CTR1
copper transporter 1
- Cu
copper
- DMEM
Dulbecco’s modified Eagle’s medium
- hCTR1
human copper transporter 1
- HEK
human embryonic kidney cells
- mefs
mouse embryonic fibroblasts
- Pt
platinum
- siRNA
small interfering RNA
Authorship Contributions
Participated in research design: Ivy, Kaplan.
Performed data analysis: Ivy, Kaplan.
Wrote or contributed to the writing of the manuscript: Ivy, Kaplan.
Footnotes
This work was supported by the National Institutes of Health National Institute of General Medical Sciences [Grant P01 GM 067166] (to J.H.K.).
This research was presented at the following meeting: Ivy KD and Kaplan JH (2012) Mechanism of cellular uptake of Pt drugs: the role of hCTR1. 2012 American Association for Cancer Research Annual Meeting; 2012 March 31–April 4; Chicago, IL.
References
- Ahmed Z, Deyama Y, Yoshimura Y, Suzuki K. (2009) Cisplatin sensitivity of oral squamous carcinoma cells is regulated by Na+,K+-ATPase activity rather than copper-transporting P-type ATPases, ATP7A and ATP7B. Cancer Chemother Pharmacol 63:643–650 [DOI] [PubMed] [Google Scholar]
- Andrews PA, Howell SB. (1990) Cellular pharmacology of cisplatin: perspectives on mechanisms of acquired resistance. Cancer Cells 2:35–43 [PubMed] [Google Scholar]
- Andrews PA, Velury S, Mann SC, Howell SB. (1988) cis-Diamminedichloroplatinum(II) accumulation in sensitive and resistant human ovarian carcinoma cells. Cancer Res 48:68–73 [PubMed] [Google Scholar]
- Ashino T, Sudhahar V, Urao N, Oshikawa J, Chen GF, Wang H, Huo Y, Finney L, Vogt S, McKinney RD, et al. (2010) Unexpected role of the copper transporter ATP7A in PDGF-induced vascular smooth muscle cell migration. Circ Res 107:787–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beretta GL, Gatti L, Tinelli S, Corna E, Colangelo D, Zunino F, Perego P. (2004) Cellular pharmacology of cisplatin in relation to the expression of human copper transporter CTR1 in different pairs of cisplatin-sensitive and -resistant cells. Biochem Pharmacol 68:283–291 [DOI] [PubMed] [Google Scholar]
- Blumberger J, Bernasconi L, Tavernelli I, Vuilleumier R, Sprik M. (2004) Electronic structure and solvation of copper and silver ions: a theoretical picture of a model aqueous redox reaction. J Am Chem Soc 126:3928–3938 [DOI] [PubMed] [Google Scholar]
- Chen HH, Yan JJ, Chen WC, Kuo MT, Lai YH, Lai WW, Liu HS, Su WC. (2012) Predictive and prognostic value of human copper transporter 1 (hCtr1) in patients with stage III non-small-cell lung cancer receiving first-line platinum-based doublet chemotherapy. Lung Cancer 75:228–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancis A, Yuan DS, Haile D, Askwith C, Eide D, Moehle C, Kaplan J, Klausner RD. (1994) Molecular characterization of a copper transport protein in S. cerevisiae: an unexpected role for copper in iron transport. Cell 76:393–402 [DOI] [PubMed] [Google Scholar]
- De Feo CJ, Aller SG, Unger VM. (2007) A structural perspective on copper uptake in eukaryotes. Biometals 20:705–716 [DOI] [PubMed] [Google Scholar]
- Eisses JF, Kaplan JH. (2002) Molecular characterization of hCTR1, the human copper uptake protein. J Biol Chem 277:29162–29171 [DOI] [PubMed] [Google Scholar]
- Filipski KK, Mathijssen RH, Mikkelsen TS, Schinkel AH, Sparreboom A. (2009) Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin Pharmacol Ther 86:396–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S, Naing A, Fu C, Kuo MT, Kurzrock R. (2012) Overcoming platinum resistance through the use of a copper-lowering agent. Mol Cancer Ther 11:1221–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale GR, Morris CR, Atkins LM, Smith AB. (1973) Binding of an antitumor platinum compound to cells as influenced by physical factors and pharmacologically active agents. Cancer Res 33:813–818 [PubMed] [Google Scholar]
- Gately DP, Howell SB. (1993) Cellular accumulation of the anticancer agent cisplatin: a review. Br J Cancer 67:1171–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaccone G. (2000) Clinical perspectives on platinum resistance. Drugs 59 (Suppl 4):9–17; discussion 37–18. [DOI] [PubMed]
- Guo Y, Smith K, Petris MJ. (2004) Cisplatin stabilizes a multimeric complex of the human Ctr1 copper transporter: requirement for the extracellular methionine-rich clusters. J Biol Chem 279:46393–46399 [DOI] [PubMed] [Google Scholar]
- Hilder T, Hill J. (2007) Modelling the encapsulation of the anticancer drug cisplatin into carbon nanotubes. Nanotechnology 18:275704 [Google Scholar]
- Holzer AK, Howell SB. (2006) The internalization and degradation of human copper transporter 1 following cisplatin exposure. Cancer Res 66:10944–10952 [DOI] [PubMed] [Google Scholar]
- Holzer AK, Katano K, Klomp LW, Howell SB. (2004a) Cisplatin rapidly down-regulates its own influx transporter hCTR1 in cultured human ovarian carcinoma cells. Clin Cancer Res 10:6744–6749 [DOI] [PubMed] [Google Scholar]
- Holzer AK, Samimi G, Katano K, Naerdemann W, Lin X, Safaei R, Howell SB. (2004b) The copper influx transporter human copper transport protein 1 regulates the uptake of cisplatin in human ovarian carcinoma cells. Mol Pharmacol 66:817–823 [DOI] [PubMed] [Google Scholar]
- Ishida S, Lee J, Thiele DJ, Herskowitz I. (2002) Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc Natl Acad Sci USA 99:14298–14302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida S, McCormick F, Smith-McCune K, Hanahan D. (2010) Enhancing tumor-specific uptake of the anticancer drug cisplatin with a copper chelator. Cancer Cell 17:574–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jandial DD, Farshchi-Heydari S, Larson CA, Elliott GI, Wrasidlo WJ, Howell SB. (2009) Enhanced delivery of cisplatin to intraperitoneal ovarian carcinomas mediated by the effects of bortezomib on the human copper transporter 1. Clin Cancer Res 15:553–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalayda GV, Wagner CH, Jaehde U. (2012) Relevance of copper transporter 1 for cisplatin resistance in human ovarian carcinoma cells. J Inorg Biochem 116:1–10 [DOI] [PubMed] [Google Scholar]
- Kaplan JH, Lutsenko S. (2009) Copper transport in mammalian cells: special care for a metal with special needs. J Biol Chem 284:25461–25465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katano K, Kondo A, Safaei R, Holzer A, Samimi G, Mishima M, Kuo YM, Rochdi M, Howell SB. (2002) Acquisition of resistance to cisplatin is accompanied by changes in the cellular pharmacology of copper. Cancer Res 62:6559–6565 [PubMed] [Google Scholar]
- Kelland L. (2007) The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer 7:573–584 [DOI] [PubMed] [Google Scholar]
- Koepsell H, Endou H. (2004) The SLC22 drug transporter family. Pflugers Arch 447:666–676 [DOI] [PubMed] [Google Scholar]
- Komatsu M, Sumizawa T, Mutoh M, Chen ZS, Terada K, Furukawa T, Yang XL, Gao H, Miura N, Sugiyama T, et al. (2000) Copper-transporting P-type adenosine triphosphatase (ATP7B) is associated with cisplatin resistance. Cancer Res 60:1312–1316 [PubMed] [Google Scholar]
- Larson CA, Adams PL, Blair BG, Safaei R, Howell SB. (2010a) The role of the methionines and histidines in the transmembrane domain of mammalian copper transporter 1 in the cellular accumulation of cisplatin. Mol Pharmacol 78:333–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson CA, Adams PL, Jandial DD, Blair BG, Safaei R, Howell SB. (2010b) The role of the N-terminus of mammalian copper transporter 1 in the cellular accumulation of cisplatin. Biochem Pharmacol 80:448–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson CA, Blair BG, Safaei R, Howell SB. (2009) The role of the mammalian copper transporter 1 in the cellular accumulation of platinum-based drugs. Mol Pharmacol 75:324–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Petris MJ, Thiele DJ. (2002) Characterization of mouse embryonic cells deficient in the ctr1 high affinity copper transporter. Identification of a Ctr1-independent copper transport system. J Biol Chem 277:40253–40259 [DOI] [PubMed] [Google Scholar]
- Liang ZD, Stockton D, Savaraj N, Tien Kuo M. (2009) Mechanistic comparison of human high-affinity copper transporter 1-mediated transport between copper ion and cisplatin. Mol Pharmacol 76:843–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Okuda T, Holzer A, Howell SB. (2002) The copper transporter CTR1 regulates cisplatin uptake in Saccharomyces cerevisiae. Mol Pharmacol 62:1154–1159 [DOI] [PubMed] [Google Scholar]
- Loehrer PJ, Einhorn LH. (1984) Drugs five years later. Cisplatin. Ann Intern Med 100:704–713 [DOI] [PubMed] [Google Scholar]
- Maryon EB, Molloy SA, Kaplan JH. (2007) O-linked glycosylation at threonine 27 protects the copper transporter hCTR1 from proteolytic cleavage in mammalian cells. J Biol Chem 282:20376–20387 [DOI] [PubMed] [Google Scholar]
- Molloy SA, Kaplan JH. (2009) Copper-dependent recycling of hCTR1, the human high affinity copper transporter. J Biol Chem 284:29704–29713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narawa T, Tsuda Y, Itoh T. (2007) Chiral recognition of amethopterin enantiomers by the reduced folate carrier in Caco-2 cells. Drug Metab Pharmacokinet 22:33–40 [DOI] [PubMed] [Google Scholar]
- Pabla N, Murphy RF, Liu K, Dong Z. (2009) The copper transporter Ctr1 contributes to cisplatin uptake by renal tubular cells during cisplatin nephrotoxicity. Am J Physiol Renal Physiol 296:F505–F511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm ME, Weise CF, Lundin C, Wingsle G, Nygren Y, Björn E, Naredi P, Wolf-Watz M, Wittung-Stafshede P. (2011) Cisplatin binds human copper chaperone Atox1 and promotes unfolding in vitro. Proc Natl Acad Sci USA 108:6951–6956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petris MJ, Smith K, Lee J, Thiele DJ. (2003) Copper-stimulated endocytosis and degradation of the human copper transporter, hCtr1. J Biol Chem 278:9639–9646 [DOI] [PubMed] [Google Scholar]
- Rabik CA, Dolan ME. (2007) Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev 33:9–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabik CA, Maryon EB, Kasza K, Shafer JT, Bartnik CM, Dolan ME. (2009) Role of copper transporters in resistance to platinating agents. Cancer Chemother Pharmacol 64:133–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samimi G, Katano K, Holzer AK, Safaei R, Howell SB. (2004) Modulation of the cellular pharmacology of cisplatin and its analogs by the copper exporters ATP7A and ATP7B. Mol Pharmacol 66:25–32 [DOI] [PubMed] [Google Scholar]
- Sinani D, Adle DJ, Kim H, Lee J. (2007) Distinct mechanisms for Ctr1-mediated copper and cisplatin transport. J Biol Chem 282:26775–26785 [DOI] [PubMed] [Google Scholar]
- Song IS, Savaraj N, Siddik ZH, Liu P, Wei Y, Wu CJ, Kuo MT. (2004) Role of human copper transporter Ctr1 in the transport of platinum-based antitumor agents in cisplatin-sensitive and cisplatin-resistant cells. Mol Cancer Ther 3:1543–1549 [PubMed] [Google Scholar]
- Tsai CY, Finley JC, Ali SS, Patel HH, Howell SB. (2012) Copper influx transporter 1 is required for FGF, PDGF and EGF-induced MAPK signaling. Biochem Pharmacol 84:1007–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimnicka AM, Ivy K, Kaplan JH. (2011) Acquisition of dietary copper: a role for anion transporters in intestinal apical copper uptake. Am J Physiol Cell Physiol 300:C588–C599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimnicka AM, Maryon EB, Kaplan JH. (2007) Human copper transporter hCTR1 mediates basolateral uptake of copper into enterocytes: implications for copper homeostasis. J Biol Chem 282:26471–26480 [DOI] [PubMed] [Google Scholar]
- Zisowsky J, Koegel S, Leyers S, Devarakonda K, Kassack MU, Osmak M, Jaehde U. (2007) Relevance of drug uptake and efflux for cisplatin sensitivity of tumor cells. Biochem Pharmacol 73:298–307 [DOI] [PubMed] [Google Scholar]
- Zumaeta E, Gonzalez Griego A, Ferrandiz J, Villanueva A, Soto V, Almeida R, Gonzalez VE, Gonzalez G, Lugo MG, Ramirez V, et al. (2001) Predicted duration of protective anti-HBs antigens in Peruvian health care workers after six years of vaccination. Rev Gastroenterol Peru 21:276–281 (in Spanish)b [PubMed] [Google Scholar]