Abstract
This study examined the effect of long-term control of hyperglycemia with a new sodium glucose cotransporter 2 inhibitor, luseogliflozin, given alone or in combination with lisinopril on the progression of renal injury in the T2DN rat model of type 2 diabetic nephropathy. Chronic treatment with luseogliflozin (10 mg/kg/day) produced a sustained increase in glucose excretion and normalized blood glucose and glycosylated hemoglobin levels to the same level as seen in the rats treated with insulin. It had no effect on blood pressure. In contrast, lisinopril (10 mg/kg/day) reduced mean blood pressure from 140 to 113 mmHg. Combination therapy significantly reduced blood pressure more than that seen in the rats treated with lisinopril. T2DN rats treated with vehicle exhibited progressive proteinuria, a decline in glomerular filtration rate (GFR), focal glomerulosclerosis, renal fibrosis, and tubular necrosis. Control of hyperglycemia with luseogliflozin prevented the fall in GFR and reduced the degree of glomerular injury, renal fibrosis, and tubular necrosis. In contrast, control of hyperglycemia with insulin had no effect on the progression of renal disease in T2DN rats. Reducing blood pressure with lisinopril prevented the fall in GFR and reduced proteinuria and the degree of glomerular injury and tubular necrosis. Combination therapy reduced the degree of glomerular injury, renal fibrosis, and tubular necrosis to a greater extent than administration of either drug alone. These results suggest that control of hyperglycemia with luseogliflozin slows the progression of diabetic nephropathy more than that seen with insulin, and combination therapy is more renoprotective than administration of either compound alone.
Introduction
Chronic kidney disease (CKD) is a major health problem in the United States. Approximately 26 million Americans have CKD, and the associated Medicare costs for treatment exceeds 34 billion dollars a year (US Renal Data System, 2012). The CKD program now consumes 16% of the Medicare budget and the number of patients and associated costs have increased markedly over the last decade. Diabetes is the major cause of end-stage renal disease in the United States (Remuzzi et al., 2006). It promotes the development of proteinuria, focal glomerulosclerosis, and tubulointerstitial fibrosis and reduces glomerular filtration rate (GFR). Current antidiabetic therapies to control hyperglycemia or administration of angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers to control blood pressure slow but do not prevent the progression of diabetic nephropathy (Ohkubo et al., 1995; Ueda et al., 2003; Casas et al., 2005; Mann et al., 2008). Thus, there is a need to explore alternative diabetic therapies that might be more renoprotective.
Recently, a new class of sodium glucose cotransporter 2 (SGLT2) inhibitors that block glucose reabsorption in the proximal tubule have been developed for the treatment of diabetes. Normally, the SGLT2 transporter in the proximal tubule reabsorbs 90% of the filtered load of glucose. The expression of SGLT2 and glucose reabsorption in the proximal tubule are markedly elevated in diabetic animals, and upregulation of the reabsorption of the filtered load of glucose plays an important role in maintaining the hyperglycemic state (Vestri et al., 2001; Rahmoune et al., 2005; Tabatabai et al., 2009). Thus, selective SGLT2 inhibitors have been considered as potential new therapeutic targets for the treatment of diabetes. Indeed, several SGLT2 inhibitors have been reported to be effective antidiabetic agents in animal models and more recently in clinical trials (Han et al., 2008; Bailey et al., 2010; Kakinuma et al., 2010; Yamamoto et al., 2011; Suzuki et al., 2012; Tahara et al., 2012). However, the potential long-term effects of these agents on renal hemodynamics and the progression of renal injury in diabetic animal models or in patients with diabetes have not been determined.
On one hand, blockade of SGLT2-mediated sodium and glucose cotransport in the proximal tubule might elevate the delivery of sodium to the macula densa to activate tubuloglomerular feedback, constrict the afferent arteriole, and lower GFR (Thomson et al., 2012). This could have either positive or negative effects on the progression of renal injury depending on whether the kidney is exposed to hyperfiltration in the early stages of the disease or whether GFR is compromised in patients with more advance kidney disease. On the other hand, improved control of hyperglycemia and a reduction in blood pressure secondary to a diuretic effect of the SGLT2 inhibitor might slow the progression of diabetic nephropathy.
To address these issues, the present studies explored the long-term effects of a new SGLT2 inhibitor, luseogliflozin, given alone and in combination with the ACE inhibitor lisinopril on the development of renal injury in T2DN rats, a recently described type 2 model of diabetic nephropathy (Nobrega et al., 2004, 2009; Williams et al., 2011).
Materials and Methods
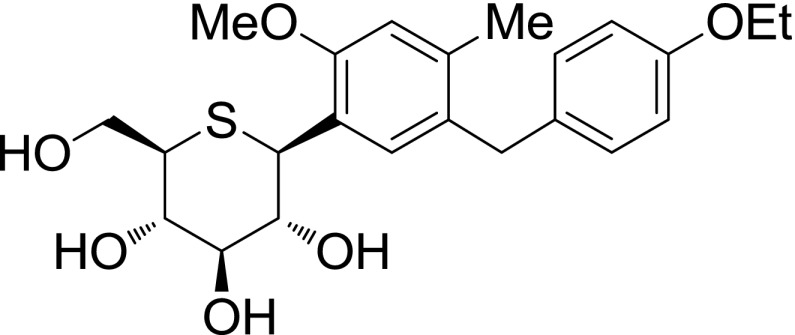
Luseogliflozin [TS-071: (1S)- 1,5-anhydro-1-[5-(4-ethoxybenzyl)-2-methoxy-4-methylphenyl]-1-thio-d-glucitol] is a new SGLT2 inhibitor (Kakinuma et al., 2010) that was synthesized by Medical Chemistry Laboratories in Taisho Pharmaceutical Co., Ltd. The structure of this compound is presented in Fig. 1.
Fig. 1.
Structure of luseogliflozin.
Lisinopril was purchased from Cayman Chemical (Ann Arbor, MI). Linplant is a long-acting implant that releases a constant daily dose of human recombinant insulin. It was purchased from LinShin Canada (Toronto, ON, Canada). Ketamine was purchased from Phoenix Pharmaceutical (St. Joseph, MO). Thiobutabarbital (Inactin) was purchased from Sigma-Aldrich (St. Louis, MO).
The present studies were performed using 14-month-old male T2DN rats that were obtained from inbred colonies maintained at the University of Mississippi Medical Center. The T2DN rat is a recently described genetic model of type 2 diabetic nephropathy that was derived by introducing alleles from the Fawn Hooded Hypertensive rat that are susceptible to renal injury into the genetic background of the Goto-Kakizaki rat that develops type 2 diabetes but are resistant to the development of renal disease (Nobrega et al., 2009). Previous studies have indicated that T2DN rats develop diabetes by 3 months of age (Nobrega et al., 2004) but unlike the original Goto-Kakizaki parental strain, T2DN rats develop diabetic nephropathy characterized by thickening of glomerular and tubular basement membranes, progressive proteinuria, renal interstitial fibrosis, and focal glomerulosclerosis (Nobrega et al., 2004; Williams et al., 2011) by 12 months of age. The rats in this study had free access to food and water throughout the study, and the protocols were approved by the Animal Care Committee of the University of Mississippi Medical Center.
Effects of Luseogliflozin Versus Lisinopril or Insulin Therapy on Blood Glucose Levels, Proteinuria, and Blood Pressure in T2DN Rats.
These experiments were performed on 14-month-old male T2DN rats that were randomly assigned to five treatment groups. Ten rats were assigned to each group in an attempt to obtain complete data sets from 8 rats per group because any rats that showed any signs of distress or failed to eat or groom properly over the course of the study were killed and the incomplete data from these animals were excluded from the analysis. Group 1 served as the vehicle-treated control group and received Harlan Teklad 8640 powdered rat chow containing 1% NaCl (Harlan Laboratories, Madison, WI) and free access to drinking water through the study. Group 2 received the selective SGLT2 inhibitor luseogliflozin in the rat chow at a concentration of 0.02%, which was sufficient to deliver an average daily dose of 10 mg/kg. Group 3 received the ACE inhibitor lisinopril in the drinking water to deliver an average daily dose of 10 mg/kg. Group 4 received luseogliflozin in the rat chow and lisinopril in the drinking water at average daily doses of 10 mg/kg, respectively (combination therapy). Group 5 was implanted with a single Linplant implant subcutaneously that delivers a constant dose of human recombinant insulin as a rate of ∼2 U/day/implant for up to 40 days. In preliminary studies, we found that this was the minimum dose that could restore normoglycemia in T2DN rats. The rats received a second implant if the nonfasting blood glucose level rose above 200 mg/dl. In addition, a group of untreated 14-month-old T2DN rats were studied to serve as a baseline control group for the assessment of renal hemodynamics and renal injury. Blood samples were collected monthly from the tail vein for measurement of blood glucose levels, glycosylated hemoglobin (HbA1c) levels, and plasma insulin and luseogliflozin concentrations. These samples were collected with ad libitum feeding 3 hours into the lights-on cycle between 9 and 10 AM. Twenty-four-hour urine samples were also collected via metabolic cages to study the time course of changes in the excretion of glucose, protein, and nephrin. Urinary total protein concentration was determined colorimetrically using the Bradford method (Bio-Rad Laboratories, Hercules, CA). Urinary glucose concentration was determined using the glucose oxidase-peroxide method (Cayman Chemical, Ann Arbor, MI). Urinary nephrin concentration was determined by enzyme-linked immunosorbent assay (Exocell, Philadelphia, PA). Plasma glucose and HbA1c levels were measured using glucometer (Bayer HealthCare, Mishawaka, IN) and a rapid HbA1c device (Bayer HealthCare, Sunnyvale, CA). Blood pressure was measured using a tail-cuff device (Hatteras Instruments, Cary, NC). Plasma insulin concentration was measured using a rat insulin enzyme-linked immunosorbent assay kit (Mercodia, Uppsala, Sweden), and the cross reactivity of the antibody with human insulin is 167%. Plasma luseogliflozin concentration was measured by LC/MS/MS.
Renal Clearance Study.
At the end of the chronic study, six rats from each treatment group were randomly chosen for study of renal hemodynamics. These animals were anesthetized with ketamine (20 mg/rat i.m.) and Inactin (50 mg/kg i.p.) and placed on a warming table to maintain body temperature at 37°C. The trachea was cannulated with PE-240 tubing to facilitate breathing, and catheters were placed in the right femoral artery and vein for measurement of mean arterial pressure (MAP) and for intravenous infusions. The rats received an intravenous infusion of 0.9% NaCl solution containing 2% bovine serum albumin and 2 mg/ml fluorescein isothiocyanate-labeled inulin at a rate of 6 ml/h throughout the experiment for measurement of GFR. An ultrasound flow probe (Transonic System, Ithaca, NY) was placed on the renal artery to measure renal blood flow (RBF). After surgery and a 30-minute equilibration period, urine and plasma samples were collected during a 30-minute clearance period. Plasma and urine inulin concentration were measured using a fluorescent microplate reader (BioTek Instruments, Winooski, VT) to calculate GFR. Renal vascular resistance (RVR) was calculated as the ratio of MAP to RBF.
Histology.
At the end of the renal clearance studies, the kidneys of the rats were collected and weighed. The left kidney was fixed in 10% buffered formalin solution. Sections were prepared and stained with Masson’s trichrome stain to evaluate the degree of glomerular injury and renal fibrosis, and the degree of tubular necrosis was assessed by measuring the percentage of area occupied by red-stained protein casts. Thirty glomeruli per section were scored in a blinded fashion on a 0–4+ scale, with 0 representing a normal glomerulus, 1+ representing loss of 1–25% of glomerular capillary area, 2+ representing a 26–50% loss, 3+ representing a 51–75% loss, and 4+ representing >75% loss of the capillaries in the glomerular tuft. Glomerular diameters were also measured. Images were captured using a Nikon Eclipse 55i microscope equipped with a Nikon DS-Fi1 color camera (Nikon Instruments Inc., Melville, NY), and the degree of renal fibrosis was assessed by measuring the percentage of blue staining of collagen and fibronectin in the section using the NIS-Elements D 3.0 software (Nikon Instruments Inc.). The percentage of red-stained protein casts, area, and the diameter of glomeruli were also determined using the same software.
Statistics.
Mean values ± S.E. are presented. The significance of difference in mean values between and within groups was determined using an analysis of variance followed by a Holm-Sidak test for preplanned comparisons using the SigmaPlot 11 software (Systat Software, San Jose, CA).
Results
Drug Intake and Plasma Luseogliflozin Concentration.
A summary of the intake of luseogliflozin and lisinopril and plasma luseogliflozin concentrations in T2DN rats are presented in Table 1. The daily intake of luseogliflozin averaged approximately 10 mg/kg in luseogliflozin and combination therapy treatment groups. Lisinopril intake also averaged approximately 10 mg/kg in lisinopril and combination therapy treatment groups. Plasma concentration of luseogliflozin averaged approximately 50 ng/ml in the rats treated with luseogliflozin or in the rats that received combination therapy.
TABLE 1.
Daily intake of luseogliflozin or lisinopril and plasma concentration of luseogliflozin in T2DN rats
| Group | 1-Month | 2-Month | 3-Month | Average |
|---|---|---|---|---|
| Luseogliflozin Intake (mg/kg) | ||||
| Luseogliflozin | 15.5 ± 1.6 | 14.2 ± 1.0 | 14.6 ± 0.5 | 14.8 ± 0.9 |
| Combination | 13.9 ± 1.0 | 13.0 ± 0.4 | 13.6 ± 1.0 | 13.5 ± 0.4 |
| Lisinopril Intake (mg/kg) | ||||
| Lisinopril | 12.4 ± 1.1 | 9.8 ± 0.8 | 9.5 ± 1.3 | 10.5 ± 0.9 |
| Combination | 14.1 ± 0.3 | 9.2 ± 0.3 | 8.6 ± 0.7 | 10.7 ± 0.3 |
| Plasma Luseogliflozin Concentration (ng/ml) | ||||
| Luseogliflozin | 65.9 ± 4.9 | 50.0 ± 10.1 | 56.1 ± 13.5 | 57.3 ± 7.8 |
| Combination | 61.2 ± 7.3 | 49.5 ± 6.1 | 31.0 ± 3.7 | 47.2 ± 4.5 |
Effects of the Various Treatments on Body Weight and Food Intake.
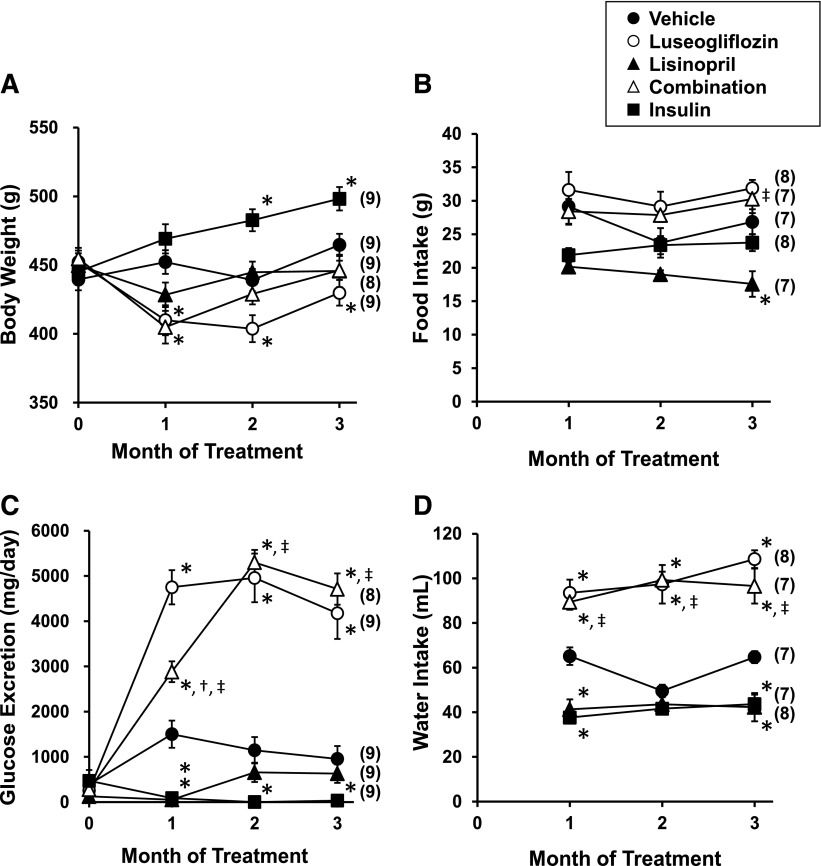
The results of these experiments are presented in Fig. 2, A and B. Body weight was relatively stable in the rats treated with vehicle or lisinopril. Body weight decreased significantly in rats treated with luseogliflozin despite the fact that food intake was greater in this group compared with the rats treated with vehicle. In contrast, body weight increased significantly in the rats treated with insulin relative to vehicle-treated rats in the absence of changes in food intake.
Fig. 2.
Effects of luseogliflozin, lisinopril, combination therapy, and insulin on body weight (A), daily food intake (B), urinary glucose excretion (C), and daily water intake (D) in T2DN rats. Numbers in parentheses indicate the number of rats studied per group. *Indicates a significant difference from the corresponding value in vehicle-treated rats. †Indicates a significant difference from the corresponding value in luseogliflozin-treated rats. ‡Indicates a significant difference from the corresponding value in lisinopril-treated rats.
Effects of the Various Treatments on Glucose Excretion and Water Intake.
The results of these experiments are presented in Fig. 2, C and D. Urinary glucose excretion increased significantly in T2DN rats treated with luseogliflozin compared with vehicle-treated rats. In contrast, treatment of the rats with insulin reduced glucose excretion. Chronic treatment of the rats with lisinopril produced a transient reduction in glucose excretion that was associated with an initial reduction in food intake and body weight. Combination therapy increased urinary glucose excretion to the same extent as that seen in the T2DN rats given luseogliflozin alone. Daily water intake was elevated in the rats treated with luseogliflozin or combination therapy compared with the rats treated with vehicle. In contrast, chronic treatment of T2DN rats with lisinopril or insulin reduced daily water intake compared with the rats treated with vehicle.
Effects of the Various Treatments on Blood Glucose Levels.
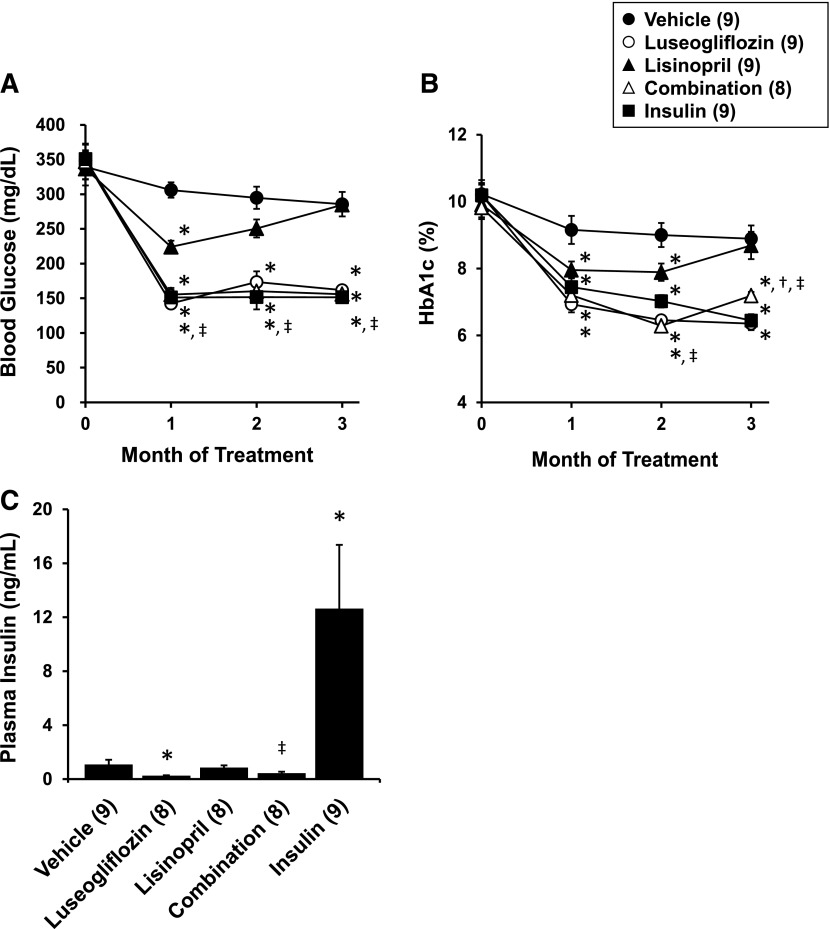
The effects of the various treatments on nonfasting blood glucose levels and HbA1c levels are presented in Fig. 3, A and B. All of the T2DN rats were diabetic at the beginning of the study, with blood glucose levels averaging approximately 350 mg/dl and HbA1c levels of 10%. Chronic treatment of luseogliflozin or the Linplant implant effectively reduced blood glucose levels and HbA1c levels into the normoglycemic range. Similar results were seen in the T2DN rats receiving combination therapy. Lisinopril produced a transient reduction in blood glucose and HbA1c levels during the first few weeks of treatment that was associated with a reduction in food intake and loss of weight.
Fig. 3.
Effects of luseogliflozin, lisinopril, combination therapy, and insulin on nonfasting blood glucose levels (A), HbA1c levels (B), and plasma insulin levels (C) in T2DN rats. Numbers in parentheses indicate the number of rats studied per group. *Indicates a significant difference from the corresponding value in vehicle-treated rats. †Indicates a significant difference from the corresponding value in luseogliflozin-treated rats. ‡Indicates a significant difference from the corresponding value in lisinopril-treated rats.
Effects of the Various Treatments on Plasma Insulin Levels.
The effects of the various treatments on plasma insulin levels measured at the end of experiment are presented in Fig. 3C. Chronic treatment with the Linplant implant raised plasma insulin levels 10-fold. Lisinopril had no significant effect on circulating insulin levels. In contrast, plasma insulin levels were significantly reduced in rats treated with luseogliflozin compared with the levels measured in vehicle-treated rats.
Effects of the Various Treatments on Blood Pressure.
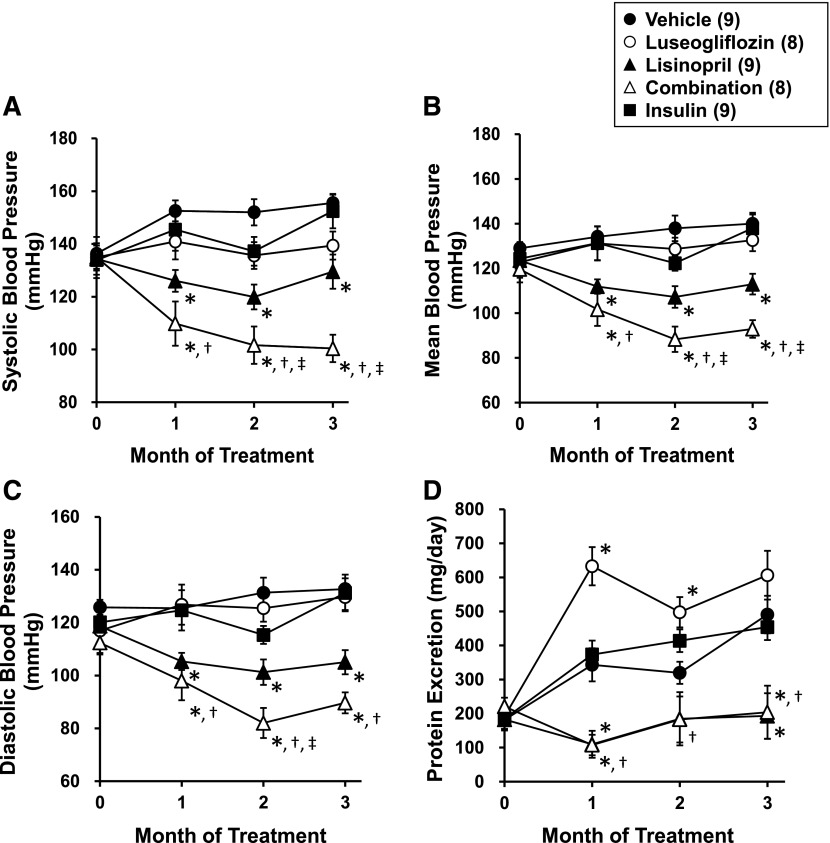
The effects of the various treatments on blood pressure are presented in Fig. 4, A–C. Chronic treatment of the rats with luseogliflozin or insulin had no effect on blood pressure. In contrast, chronic treatment of the rats with lisinopril significantly reduced blood pressure. Combination therapy significantly reduced blood pressure to a greater extent than that seen in T2DN rats treated with luseogliflozin or lisinopril alone.
Fig. 4.
Effects of luseogliflozin, lisinopril, combination therapy, and insulin on systolic blood pressure (A), mean blood pressure (B), diastolic blood pressure (C), and urinary protein excretion (D) in T2DN rats. Numbers in parentheses indicate the number of rats studied per group. *Indicates a significant difference from the corresponding value in vehicle-treated rats. †Indicates a significant difference from the corresponding value in luseogliflozin-treated rats. ‡Indicates a significant difference from the corresponding value in lisinopril-treated rats.
Effects of the Various Treatments on Proteinuria.
The effects of the various treatments on proteinuria are presented in Fig. 4D. T2DN rats exhibited a progressive increase in proteinuria. Control of hyperglycemia with insulin did not reduce proteinuria in T2DN rats. Chronic treatment of the rats with luseogliflozin increased proteinuria during the first 2 months of treatment, but the levels returned to those seen in the vehicle-treated rats by the end of the experiment. In contrast, the lowering blood pressure with lisinopril or combination therapy significantly reduced proteinuria throughout the study.
Effects of the Various Treatments on Renal Hemodynamics.
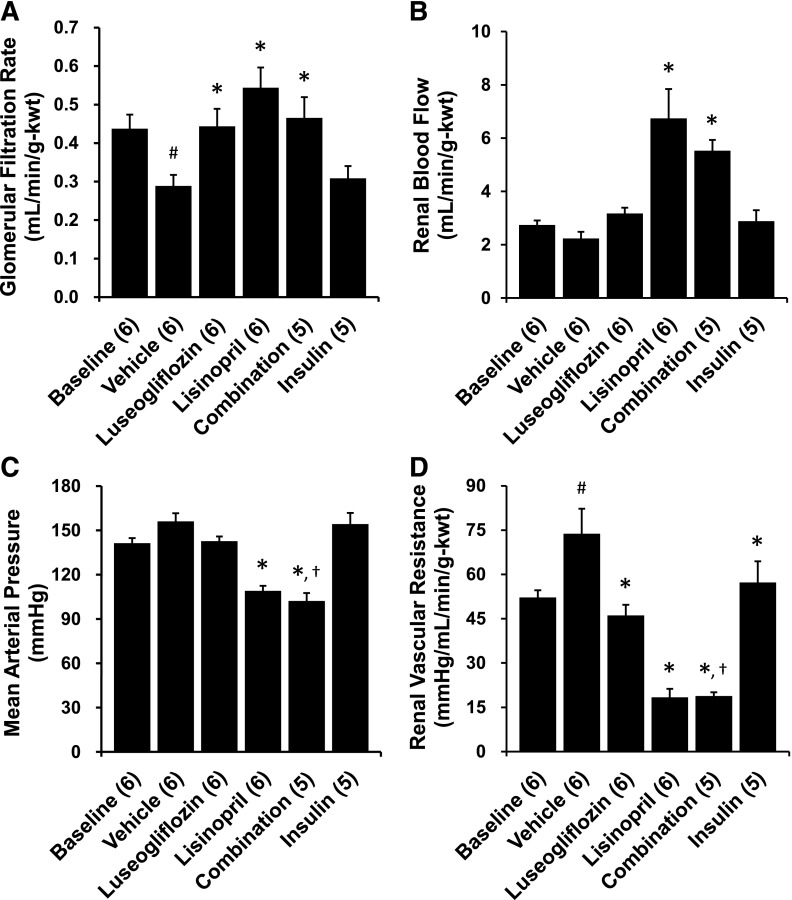
The effects of the various treatments on renal hemodynamics are presented in Fig. 5. GFR declined significantly and RVR increased in 17-month-old T2DN rats treated with vehicle compared with the levels measured in 14-month-old baseline control T2DN rats. MAP decreased significantly in the rats treated with lisinopril, whereas luseogliflozin or insulin had no effect on MAP. Lisinopril increased RBF and prevented the decline in GFR in comparison with the values seen in rats treated with vehicle. Similarly, chronic treatment of the rats with luseogliflozin also prevented the decline in GFR relative to T2DN rats treated with vehicle. Chronic treatment with luseogliflozin, lisinopril, and insulin significantly reduced RVR compared with the levels seen in T2DN rats treated with vehicle. Combination therapy also increased RBF, prevented the decline in GFR, and reduced MAP and RVR compared with the levels seen in the rats treated with vehicle.
Fig. 5.
Effects of 3-month chronic treatment with luseogliflozin, lisinopril, combination therapy, and insulin on glomerular filtration rate (A), renal blood flow (B), mean arterial pressure (C), and renal vascular resistance (D) in 17-month-old T2DN rats. Numbers in parentheses indicate the number of rats studied per group. kwt, Kidney weight. #Indicates a significant difference from the corresponding values in 14-month-old T2DN rats. *Indicates a significant difference from the corresponding value in vehicle-treated rats. †Indicates a significant difference from the corresponding value in luseogliflozin-treated rats.
Renoprotective Effects of the Various Treatments.
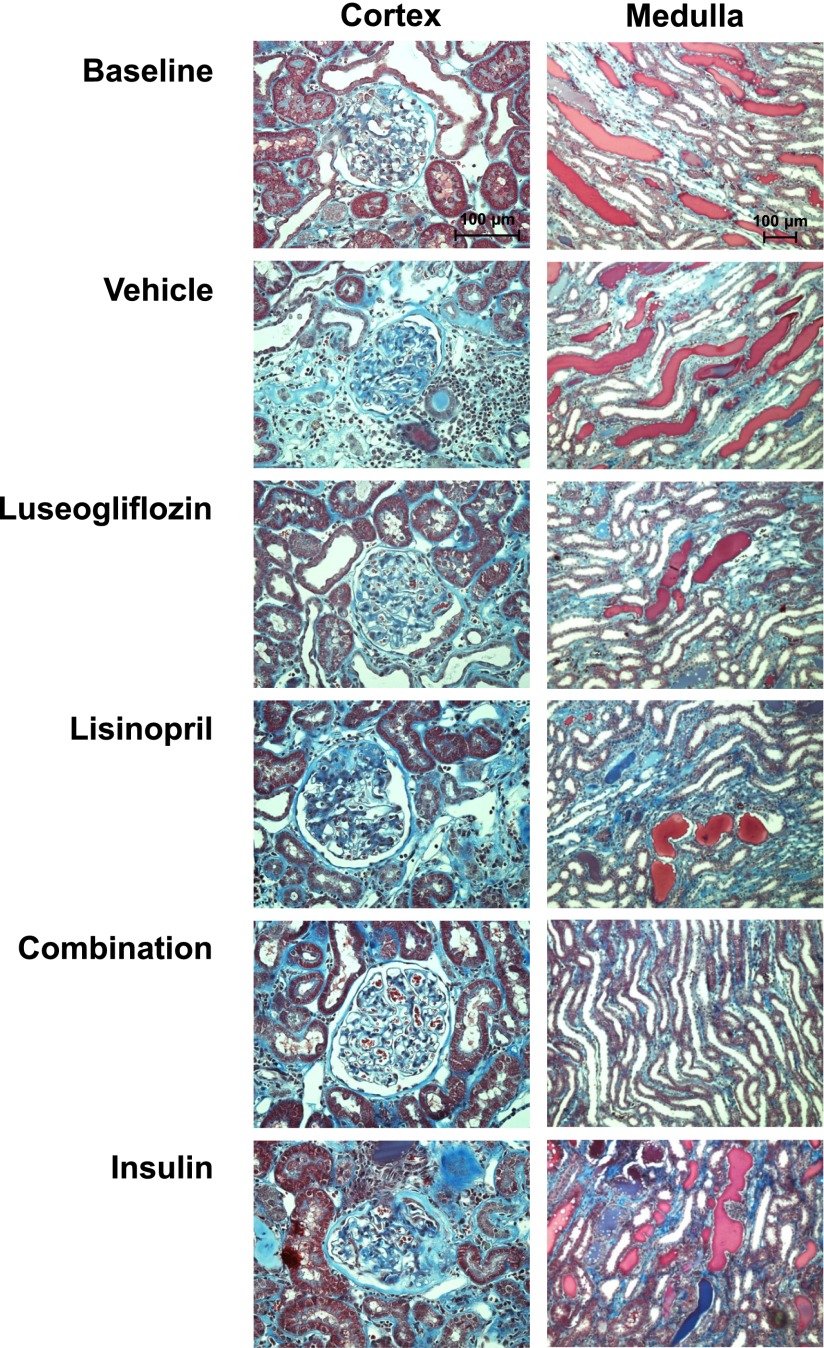
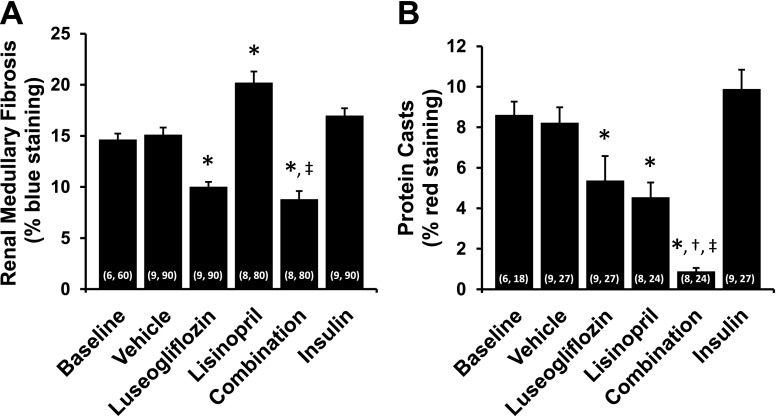
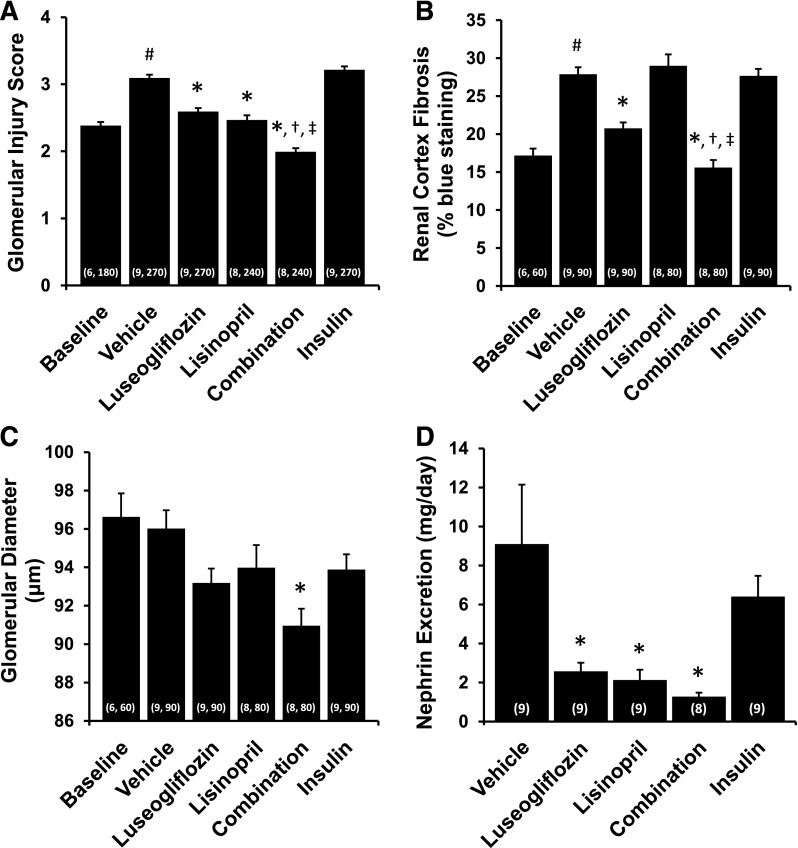
The effects of the various treatments on the degree of renal injury are presented in Figs. 6–8. The kidneys of T2DN rats treated with vehicle exhibited severe glomerulosclerosis, renal fibrosis, and tubular necrosis. Chronic treatment of the rats with luseogliflozin significantly reduced the degree of glomerular injury, renal fibrosis, and protein casts, which is an index of tubular necrosis. In contrast, control of hyperglycemia in T2DN rats treated with insulin had no significant effect on the degree of glomerular injury, renal fibrosis, or the formation of protein casts. Chronic treatment with lisinopril significantly reduced glomerular injury and the formation of protein casts but had no effect on the degree of renal fibrosis. Combination therapy significantly reduced the degree of glomerular injury, renal fibrosis, and the formation of protein casts more effectively than that seen in T2DN rats treated with luseogliflozin or lisinopril alone. Combination therapy also reduced mean glomerular diameter compared with the value seen in rats treated with vehicle. Chronic treatment with luseogliflozin, lisinopril, or combination therapy reduced urinary nephrin excretion. In contrast, chronic treatment of insulin had no effect on the excretion of nephrin.
Fig. 6.
Representative microphotographs of the renal cortex (200×) and medulla (100×) stained with Masson’s trichrome in 17-month-old T2DN rats treated with vehicle, luseogliflozin, lisinopril, combination therapy, and insulin.
Fig. 8.
Effects of 3-month chronic treatment with luseogliflozin, lisinopril, combination therapy, and insulin on renal outer medullary fibrosis (blue staining of collagen and fibronectin) (A) and red-stained tubular protein casts an index of tubular necrosis (B) in 17-month-old T2DN rats. Numbers in parentheses indicate the number of areas scored and the number of rats studied per group. *Indicates a significant difference from the corresponding value in vehicle-treated rats. †Indicates a significant difference from the corresponding value in luseogliflozin-treated rats. ‡Indicates a significant difference from the corresponding value in lisinopril-treated rats.
Fig. 7.
Effects of 3-month chronic treatment with luseogliflozin, lisinopril, combination therapy, and insulin on glomerular injury score (A), renal cortex fibrosis (B), glomerular diameter (C), and urinary nephrin excretion (D) in 17-month-old T2DN rats. Numbers in parentheses indicate the number of glomeruli or areas scored and the number of rats studied per group. #Indicates a significant difference from the corresponding values in 14-month-old T2DN rats. *Indicates a significant difference from the corresponding value in vehicle-treated rats. †Indicates a significant difference from the corresponding value in luseogliflozin-treated rats. ‡Indicates a significant difference from the corresponding value in lisinopril-treated rats.
Discussion
Recently, several new SGLT2 inhibitors that block glucose reabsorption in the proximal tubule have been developed for the treatment of diabetes. One of the compounds in this class is luseogliflozin. It has an IC50 value of 2.3 nmol/l for inhibition of SGLT2 and exhibits a 1765-fold selectivity for inhibition of SGLT2 over SGLT1 (Yamamoto et al., 2011). It has also been reported to effectively control hyperglycemia in both type 1 and 2 rat models of diabetes (Yamamoto et al., 2011). However, the potential long-term effects of luseogliflozin, or any other SGLT2 inhibitor, on renal hemodynamics and the progression of renal injury in animal models of diabetes have not been determined. Thus, the present study examined the effects of luseogliflozin on the development of renal injury in the T2DN rat model of type 2 diabetic nephropathy.
Plasma concentrations of luseogliflozin averaged approximately 50 ng/ml in the rats treated with luseogliflozin. This corresponds to an unbound plasma concentration of 5 nmol/l (95% protein bound), which exceeds the IC50 by twofold. Chronic treatment of the rats with luseogliflozin reduced body weight by 10% although food intake increased. This likely reflects the large increase in glucose excretion and the associated loss of calories.
Luseogliflozin given alone, or in combination with lisinopril, produced an effective long-term control of blood glucose and HbA1c levels in T2DN rats. The antihyperglycemic actions of luseogliflozin were equivalent to that seen in a group of rats that received a slow-release insulin implant. However, the insulin implant increased plasma insulin levels by 10-fold, whereas luseogliflozin reduced plasma insulin levels. This likely reflects the effective control of hyperglycemia in the luseogliflozin-treated animals that lessen the stimulus for insulin secretion. Part of the very high insulin levels in the rats given the Linplant implant may be related to the fact that human insulin delivered by this implant crossreacts more strongly (167%) with the antibody used in the insulin assay. Nevertheless, the insulin levels were elevated at least fivefold in the rats receiving the Linplant implant.
The sustained increase in glucose excretion seen in the rats treated with luseogliflozin is unexpected. One would assume that blockade of the SGLT2 would increase glucose excretion in hyperglycemic T2DN rats but as the degree of hyperglycemia was normalized glucose excretion would decrease. Although the exact reason for the sustained increase in glucose excretion remains to be determined, one might speculate that perhaps the SGLT2 inhibitor facilitates the rapid excretion of the glucose after each meal and that this leads to the sustained increase in glucose excretion.
Consistent with the results of previous studies (Nobrega et al., 2004; Williams et al., 2011), the T2DN rats exhibited a progressive decline in GFR and increased proteinuria and developed severe glomerulosclerosis, renal interstitial fibrosis, and the formation of protein casts— an index of tubular necrosis. Control of hyperglycemia with luseogliflozin prevented the fall in GFR and significantly reduced the degree of glomerular injury, renal interstitial fibrosis, and tubular necrosis in comparison with the degree of injury observed in T2DN rats treated with vehicle. However, luseogliflozin produced a transient increase in proteinuria. We do not think that the transient increase in proteinuria in rats treated with luseogliflozin is because of an increase in the filtered load of protein because recent studies have indicated that SGLT2 inhibitors activate tubuloglomerular feedback response and reduce rather than increase GFR in diabetic animals (Thomson et al., 2012). One possibility that remains to be determined is that blockade of the sodium and glucose reabsorption in the proximal tubule may reduce reuptake of filtered protein secondary to an increase in flow rate reduced contact time. This could lead to an increase in protein excretion in the absence of glomerular injury and fit with the present observations that luseogliflozin significantly reduced the degree of glomerular injury and renal fibrosis.
Interestingly, the degree of renal protection produced by luseogliflozin was greater than that seen in rats treated with insulin. Although insulin effectively controlled hyperglycemia, it did not reduce proteinuria or prevent the fall in GFR in T2DN rats. More importantly, it had no effect on the degree of glomerular injury and renal interstitial fibrosis or the formation of protein casts. The reason for the failure of insulin to reduce renal injury in this model remains to be determined. It may be attributable the fact that we studied 1-year-old animals, and perhaps controlling blood glucose levels in these older T2DN rats with preexisting renal injury cannot slow the progression of the disease. The other possibility is that the plasma insulin levels had to be elevated approximately 10-fold to control the hyperglycemia in the T2DN rats that are insulin resistant. In this regard, elevated levels of insulin have been reported to increase the expression of TGF-β1 and the production of matrix in cultured mesangial cells (Anderson et al., 1996) and exacerbate glomerular and renal interstitial fibrosis in diabetic animals (Sarafidis and Ruilope, 2006). In contrast to the results obtained in the insulin-treated animals, controlling hyperglycemia with luseogliflozin was renoprotective. Again the reason for the difference remains to be determined. One possibility is that luseogliflozin lowers plasma insulin levels, and this may reduce the proliferative actions of insulin on the kidney. In addition, chronic inhibition SGLT2 with luseogliflozin reduces proximal reabsorption of sodium and shifts the burden for salt balance from the early proximal tubule to the distal nephron (Thomson et al., 2012). This may activate tubuloglomerular feedback to mitigate hyperfiltration, and the reduction in glomerular capillary pressure might reduce the development of glomerular injury. This idea fits with the present finding that chronic treatment of luseogliflozin reduced the degree of glomerular sclerosis and fibrosis and prevented the fall in GFR seen in T2DN rats over the course of the study. Moreover, elevated glucose concentrations have been reported to increase oxidative stress and stimulate the synthesis of collagen and fibronectin by proximal tubular cells (Ziyadeh et al., 1990; Phillips et al., 1997; Jones et al., 1999). Thus, it may be possible that chronic inhibition of glucose reuptake with luseogliflozin may suppress the progression of tubular injury and renal interstitial fibrosis in diabetic animals.
We also compared the degree of renal protection produced by luseogliflozin with that seen in rats treated with the ACE inhibitor lisinopril, which is the standard of care to reduce renal injury in diabetic patients. Long-term blockade of the renin angiotensin system with lisinopril reduced blood pressure in our T2DN rats. This fits with the idea that the renin angiotensin system is activated in diabetic animals secondary to volume depletion secondary to glucosuria. Lisinopril also reduced proteinuria and increased RBF and GFR relative to vehicle-treated animals. It also reduced the degree of glomerular injury and the formation of protein casts, an index of tubular necrosis, but it had no effect on the degree of renal interstitial fibrosis. The mechanisms of the renoprotective effects of blocking the renin angiotensin system in the T2DN rats remain to be determined. Part of the effect is likely attributable to the fall in systemic pressure and a reduction in efferent arteriolar tone, because we found that filtration fraction was reduced. Both of these effects lower glomerular capillary pressure and the filtration of protein. Blockade of the renin angiotensin system also reduces oxidative stress and the stimulatory effects of angiotensin II on the expression of a variety of growth factors and cytokines.
Blood glucose and HbA1c levels were effectively controlled and blood pressure was reduced in the rats that received combination therapy more than that seen in animals treated with either agent alone. The greater fall in blood pressure likely reflects combined effects of the volume loss associated with the diuretic effect of luseogliflozin combined with blockade of the renin angiotensin system with lisinopril. Moreover, the degree of glomerular injury, renal fibrosis, and the formation of protein casts was lower in the rats receiving combination therapy than that seen in the animals treated with luseogliflozin or lisinopril alone. This probably is attributable to the effective dual control of both hyperglycemia and blood pressure in the animals receiving combination therapy.
Urinary nephrin excretion has been reported to increase and the glomerular expression of nephrin decrease in diabetic animals (Bonnet et al., 2001; Saleh et al., 2011). Chronic treatment with luseogliflozin or lisinopril reduced urinary nephrin excretion in the present study. This indicates that luseogliflozin and lisinopril may reduce damage to the podocytes and the loss of glomerular filtration barrier function in T2DN rats.
These results indicate that chronic administration of a selective SGLT2 inhibitor luseogliflozin and the ACE inhibitor lisinopril slow the progression of renal injury in a rat model of type 2 diabetes. Combination therapy of luseogliflozin and lisinopril is more effective at lowering blood pressure and is more renoprotective than administration of either agent alone. These results suggest that SGLT2 inhibitors are an attractive therapeutic option for the treatment of diabetes and when used in combination with inhibitors of the renin angiotensin system may effectively reduce the renal complications associated with diabetes.
Acknowledgments
The authors thank Dr. Takekazu Kubo and Sota Kato for assistance and helpful comments on this manuscript, and Chris Purser and Dr. Rodney Baker for the liquid chromatography-tandem mass spectrometry analysis of plasma luseogliflozin concentrations.
Abbreviations
- ACE
angiotensin converting enzyme
- CKD
chronic kidney disease
- GFR
glomerular filtration rate
- HbA1c
glycosylated hemoglobin
- MAP
mean arterial pressure
- RBF
renal blood flow
- RVR
renal vascular resistance
- SGLT
sodium glucose cotransporter
Authorship Contributions
Participated in research design: Kojima, Williams, Takahashi, Miyata, Roman.
Conducted experiments: Kojima, Williams.
Performed data analysis: Kojima, Williams, Roman.
Wrote or contributed to the writing of the manuscript: Kojima, Williams, Roman.
Footnotes
This research was funded in part by the National Institutes of Health [Grant HL36279]; and funds provided by a contract research agreement from Taisho Pharmaceutical Co., Ltd., Saitama, Japan, to evaluate the renal effects of Luseogliflozin.
A portion of this work was previously presented: Kojima N and Roman RJ (2012) Renoprotective effects of SGLT2 inhibitor, Luseogliflozin, on the progression of diabetic nephropathy in T2DN rats. 2012 High Blood Pressure Research Scientific Sessions; 2012 Sept 19; Washington, DC; and the abstract was published in Hypertension 2012; 60: A221.
References
- Anderson PW, Zhang XY, Tian J, Correale JD, Xi XP, Yang D, Graf K, Law RE, Hsueh WA. (1996) Insulin and angiotensin II are additive in stimulating TGF-beta 1 and matrix mRNAs in mesangial cells. Kidney Int 50:745–753 [DOI] [PubMed] [Google Scholar]
- Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. (2010) Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet 375:2223–2233 [DOI] [PubMed] [Google Scholar]
- Bonnet F, Cooper ME, Kawachi H, Allen TJ, Boner G, Cao Z. (2001) Irbesartan normalises the deficiency in glomerular nephrin expression in a model of diabetes and hypertension. Diabetologia 44:874–877 [DOI] [PubMed] [Google Scholar]
- Casas JP, Chua W, Loukogeorgakis S, Vallance P, Smeeth L, Hingorani AD, MacAllister RJ. (2005) Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta-analysis. Lancet 366:2026–2033 [DOI] [PubMed] [Google Scholar]
- Han S, Hagan DL, Taylor JR, Xin L, Meng W, Biller SA, Wetterau JR, Washburn WN, Whaley JM. (2008) Dapagliflozin, a selective SGLT2 inhibitor, improves glucose homeostasis in normal and diabetic rats. Diabetes 57:1723–1729 [DOI] [PubMed] [Google Scholar]
- Jones SC, Saunders HJ, Pollock CA. (1999) High glucose increases growth and collagen synthesis in cultured human tubulointerstitial cells. Diabet Med 16:932–938 [DOI] [PubMed] [Google Scholar]
- Kakinuma H, Oi T, Hashimoto-Tsuchiya Y, Arai M, Kawakita Y, Fukasawa Y, Iida I, Hagima N, Takeuchi H, Chino Y, et al. (2010) (1S)-1,5-anhydro-1-[5-(4-ethoxybenzyl)-2-methoxy-4-methylphenyl]-1-thio-D-glucitol (TS-071) is a potent, selective sodium-dependent glucose cotransporter 2 (SGLT2) inhibitor for type 2 diabetes treatment. J Med Chem 53:3247–3261 [DOI] [PubMed] [Google Scholar]
- Kojima N, Williams JM, Takahasi T, Miyata N, Roman RJ. (2012) Renoprotective effects of SGLT2 inhibitor, Luseogliflozin, on the progression of diabetic nephropathy in T2DN rats (Abstract). Abstracts of High Blood Pressure Research 2012 Scientific Sessions, Sept 19–22, 2012, Washington, DC. Abstract 221, page 91. [Google Scholar]
- Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, Wang X, Maggioni A, Budaj A, Chaithiraphan S, et al. ONTARGET investigators (2008) Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 372:547–553 [DOI] [PubMed] [Google Scholar]
- Nobrega MA, Fleming S, Roman RJ, Shiozawa M, Schlick N, Lazar J, Jacob HJ. (2004) Initial characterization of a rat model of diabetic nephropathy. Diabetes 53:735–742 [DOI] [PubMed] [Google Scholar]
- Nobrega MA, Solberg Woods LC, Fleming S, Jacob HJ. (2009) Distinct genetic regulation of progression of diabetes and renal disease in the Goto-Kakizaki rat. Physiol Genomics 39:38–46 [DOI] [PubMed] [Google Scholar]
- Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, Kojima Y, Furuyoshi N, Shichiri M. (1995) Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract 28:103–117 [DOI] [PubMed] [Google Scholar]
- Phillips AO, Steadman R, Morrisey K, Martin J, Eynstone L, Williams JD. (1997) Exposure of human renal proximal tubular cells to glucose leads to accumulation of type IV collagen and fibronectin by decreased degradation. Kidney Int 52:973–984 [DOI] [PubMed] [Google Scholar]
- Rahmoune H, Thompson PW, Ward JM, Smith CD, Hong G, Brown J. (2005) Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes 54:3427–3434 [DOI] [PubMed] [Google Scholar]
- Remuzzi G, Macia M, Ruggenenti P. (2006) Prevention and treatment of diabetic renal disease in type 2 diabetes: the BENEDICT study. J Am Soc Nephrol 17(4, Suppl 2)S90–S97 [DOI] [PubMed] [Google Scholar]
- Saleh MA, Boesen EI, Pollock JS, Savin VJ, Pollock DM. (2011) Endothelin receptor A-specific stimulation of glomerular inflammation and injury in a streptozotocin-induced rat model of diabetes. Diabetologia 54:979–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarafidis PA, Ruilope LM. (2006) Insulin resistance, hyperinsulinemia, and renal injury: mechanisms and implications. Am J Nephrol 26:232–244 [DOI] [PubMed] [Google Scholar]
- Suzuki M, Honda K, Fukazawa M, Ozawa K, Hagita H, Kawai T, Takeda M, Yata T, Kawai M, Fukuzawa T, et al. (2012) Tofogliflozin, a potent and highly specific sodium/glucose cotransporter 2 inhibitor, improves glycemic control in diabetic rats and mice. J Pharmacol Exp Ther 341:692–701 [DOI] [PubMed] [Google Scholar]
- Tabatabai NM, Sharma M, Blumenthal SS, Petering DH. (2009) Enhanced expressions of sodium-glucose cotransporters in the kidneys of diabetic Zucker rats. Diabetes Res Clin Pract 83:e27–e30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara A, Kurosaki E, Yokono M, Yamajuku D, Kihara R, Hayashizaki Y, Takasu T, Imamura M, Qun L, Tomiyama H, et al. (2012) Antidiabetic effects of SGLT2-selective inhibitor ipragliflozin in streptozotocin-nicotinamide-induced mildly diabetic mice. J Pharmacol Sci 120:36–44 [DOI] [PubMed] [Google Scholar]
- Thomson SC, Rieg T, Miracle C, Mansoury H, Whaley J, Vallon V, Singh P. (2012) Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am J Physiol Regul Integr Comp Physiol 302:R75–R83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H, Ishimura E, Shoji T, Emoto M, Morioka T, Matsumoto N, Fukumoto S, Miki T, Inaba M, Nishizawa Y. (2003) Factors affecting progression of renal failure in patients with type 2 diabetes. Diabetes Care 26:1530–1534 [DOI] [PubMed] [Google Scholar]
- US Renal Data System (2012) 2011 USRDS Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD [Google Scholar]
- Vestri S, Okamoto MM, de Freitas HS, Aparecida Dos Santos R, Nunes MT, Morimatsu M, Heimann JC, Machado UF. (2001) Changes in sodium or glucose filtration rate modulate expression of glucose transporters in renal proximal tubular cells of rat. J Membr Biol 182:105–112 [DOI] [PubMed] [Google Scholar]
- Williams JM, Zhang J, North P, Lacy S, Yakes M, Dahly-Vernon A, Roman RJ. (2011) Evaluation of metalloprotease inhibitors on hypertension and diabetic nephropathy. Am J Physiol Renal Physiol 300:F983–F998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Uchida S, Kitano K, Fukuhara N, Okumura-Kitajima L, Gunji E, Kozakai A, Tomoike H, Kojima N, Asami J, et al. (2011) TS-071 is a novel, potent and selective renal sodium-glucose cotransporter 2 (SGLT2) inhibitor with anti-hyperglycaemic activity. Br J Pharmacol 164:181–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziyadeh FN, Snipes ER, Watanabe M, Alvarez RJ, Goldfarb S, Haverty TP. (1990) High glucose induces cell hypertrophy and stimulates collagen gene transcription in proximal tubule. Am J Physiol 259:F704–F714 [DOI] [PubMed] [Google Scholar]