Abstract
Chronic pain after peripheral nerve injury is associated with afferent hyperexcitability and upregulation of hyperpolarization-activated, cyclic nucleotide-regulated (HCN)–mediated IH pacemaker currents in sensory neurons. HCN channels thus constitute an attractive target for treating chronic pain. HCN channels are ubiquitously expressed; analgesics targeting HCN1-rich cells in the peripheral nervous system must spare the cardiac pacemaker current (carried mostly by HCN2 and HCN4) and the central nervous system (where all four isoforms are expressed). The alkylphenol general anesthetic propofol (2,6-di-iso-propylphenol) selectively inhibits HCN1 channels versus HCN2–HCN4 and exhibits a modest pharmacokinetic preference for the periphery. Consequently, we hypothesized that propofol, and congeners, should be antihyperalgesic. Alkyl-substituted propofol analogs have different rank-order potencies with respect to HCN1 inhibition, GABAA receptor (GABAA-R) potentiation, and general anesthesia. Thus, 2,6- and 2,4-di-tertbutylphenol (2,6- and 2,4-DTBP, respectively) are more potent HCN1 antagonists than propofol, whereas 2,6- and 2,4-di-sec-butylphenol (2,6- and 2,4-DSBP, respectively) are less potent. In contrast, DSBPs, but not DTBPs, enhance GABAA-R function and are general anesthetics. 2,6-DTBP retained propofol’s selectivity for HCN1 over HCN2–HCN4. In a peripheral nerve ligation model of neuropathic pain, 2,6-DTBP and subhypnotic propofol are antihyperalgesic. The findings are consistent with these alkylphenols exerting analgesia via non-GABAA-R targets and suggest that antagonism of central HCN1 channels may be of limited importance to general anesthesia. Alkylphenols are hydrophobic, and thus potential modifiers of lipid bilayers, but their effects on HCN channels are due to direct drug-channel interactions because they have little bilayer-modifying effect at therapeutic concentrations. The alkylphenol antihyperalgesic target may be HCN1 channels in the damaged peripheral nervous system.
Introduction
Sensitization of the peripheral or central nervous system after injury can produce neuropathic pain, which manifests as allodynia, hyperalgesia, and/or spontaneous pain (Costigan et al., 2009). Neuropathic pain has an all-cause incidence rate of approximately 5% (Costigan et al., 2009) and is often refractory to treatment or treatment has unacceptable side effects (Dray, 2008; Jensen et al., 2009). Neuropathic pain thus represents a significant public health issue, making it important to identify possible therapeutic targets and drugs that selectively act on those targets (Dray, 2008; Costigan et al., 2009).
In animal models of neuropathic pain, hyperexcitability and ectopic activity in primary afferents contribute to peripheral sensitization (Dray, 2008; Costigan et al., 2009; Dib-Hajj et al., 2009). These effects appear to be due, in part, to changes in hyperpolarization-activated, cyclic nucleotide-regulated channel (HCN) channel expression and function. First, in situ hybridization (Kouranova et al., 2008), immunohistochemical (Tu et al., 2004; Jiang et al., 2008; Kouranova et al., 2008), and electrophysiological (Mayer and Westbrook, 1983; Kouranova et al., 2008; Momin et al., 2008) data show that sensory neurons express HCN channels, and that injury causes altered HCN subunit trafficking (Chaplan et al., 2003; Jiang et al., 2008) and enhanced IH current amplitudes (Chaplan et al., 2003; Yao et al., 2003). Second, sensory cell hyperexcitability is inhibited by superfusion with N-ethyl-1,6-dihydro-1,2-dimethyl-6-(methylimino)-N-phenyl-4-pyrimidinamine hydrochloride (ZD7288) (Chaplan et al., 2003; Yao et al., 2003; Jiang et al., 2008), a selective pan-isoform inhibitor of HCN channels, and systemic, but not central, administration of ZD7288 alleviates mechanical allodynia (Chaplan et al., 2003; Lee et al., 2005). Third, HCN1 gene deletion partially blocks development of cold allodynia (Orio et al., 2009), and in a parallel fashion, targeted deletion of HCN2 prevents the development of neuropathic pain in response to chronic nerve constriction (Emery et al., 2011). These observations suggest that the HCN channels underlying IH in peripheral neurons may represent a target for treatment of neuropathic pain (Emery et al., 2012).
Given the primacy of HCN2 and HCN4 in the cardiac current, analgesics that target IH should preferentially inhibit channels composed of, or containing, the HCN1 isoform and should be restricted to the periphery (thereby sparing IH in central neurons, including those that rely on the HCN1 isoform). The intravenous general anesthetic propofol (2,6-di-isopropylphenol) preferentially inhibits HCN1 with respect to HCN2, 3, and 4 (Cacheaux et al., 2005; Chen et al., 2005) and has a modest pharmacokinetic preference for the periphery, that is, plasma levels associated with anesthesia are higher than those in cerebrospinal fluid (CSF) (Table 1). However, its hypnotic properties and its potentiation of type A GABA receptor (GABAA-R) activity (Franks, 2008) limit its routine use as an analgesic, even at low concentrations.
TABLE 1.
Relationship between total and free plasma and CSF propofol concentrations during general anesthesia
| Reference |
Engdahl et al. (1998) |
Dawidowicz et al. (2002) |
Dawidowicz et al. (2003) |
Dawidowicz and Kalitynski (2003) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Propofol dosinga | ||||||||||||
| Bolus (mg/kg) | 2 | — | 2 | — | ||||||||
| Infusion (μg/kg per minute) | 133 × 15 min, then 100 thereafter | — | 200 × 15 min, then 150 × 30 min, then 100 thereafter | — | ||||||||
| Infusion TCI (μg/ml) | — | 4.5–5 × 15 min, then 3.5–4 thereafter | — | 4.5–5 at induction, then 3.5–4 thereafter | ||||||||
| Plasma [propofol] | ||||||||||||
| Total | 2.2 μg/ml | 12.3 μMb | 4.5–5 μg/ml | 25.2–28 μMc | 3.3–4.2 μg/ml | 18.5–23.6 μMc | 6.1 μg/ml | 34.2 μMc | ||||
| Free (measured) | — | — | — | — | — | — | 0.06 μg/ml | 0.34 μMc | ||||
| Free (calculated)c | 0.06 | 0.31 μMb | 0.11–0.13 μg/ml | 0.6–0.7 μMc | 0.08–0.1 μg/ml | 0.4–0.6 μMc | 0.15 μg/ml | 0.86 μMc | ||||
| CSF [propofol] | ||||||||||||
| Total | 36.0 ng/ml | 0.2 μM | 21–29 ng/ml | 0.1–0.16 μM | 51–86 ng/ml | 0.3–0.5 μM | 95.6 ng/ml | 0.54 μM | ||||
| Free | — | — | — | — | — | — | — | — | ||||
| Total | ∼78d | 0.4 μM | ||||||||||
| Free (measured) | ∼24d | 0.1 μM | ||||||||||
TCI, target controlled infusion.
The fixed-rate dosing regimens in Engdahl et al. (1998) and Dawidowicz et al. (2003) yield comparable total propofol plasma levels as the target controlled infusion regimens utilized in Dawidowicz et al. (2002) and Dawidowicz and Kalitynski (2003). Using the same pharmacokinetic model as the Diprofusor software (Dawidowicz et al., 2002), total effect site propofol concentration in Engdahl et al. (1998) is estimated to be 2.75–2.80 μg/ml and 3.25–3.30 μg/ml by Dawidowicz et al. (2003), both of which are in good agreement with the observed values. Our modeling is based on a 30-year-old man, with height of 178 cm and weight of 75 kg.
Data originally provided as mass/ml; molar concentrations calculated using propofol molecular weight = 178.3.
Calculated free [propofol] was determined assuming that the free fraction is 2.5% of the total (Servin et al., 1988).
Different group of subjects than used for measuring plasma and total CSF concentrations. N.B., the total plasma propofol concentration which typically produces loss of consciousness is on the order of 3–5 μg/ml (17–27 μM) (Smith et al., 1994) and references therein).
The 2,6- and 2,4-di-sec-butylphenol analogs of propofol (2,6-DSBP and 2,4-DSBP, respectively) potentiate GABAA-Rs and act as general anesthetics, but the di-tert versions (2,6- and 2,4-DTBP) do neither (James and Glen, 1980; Krasowski et al., 2001). This steric specificity has been interpreted as, and is consistent with, the pharmacological and behavioral effects arising from an association of alkylphenols with a sterically defined pocket on GABAA-Rs (Krasowski et al., 2001).
We therefore examined the relative inhibitory potencies of the 2,6- and 2,4-dibutylphenols on HCN1. DSBPs were less potent than DTBP analogs, with 2,6-DTBP being the most potent inhibitor of HCN1 channels—with little effect on the other HCN isoforms. We also examined propofol (at subhypnotic doses) and 2,6-DTBP in a mouse model of neuropathic pain; both are analgesic. Finally, we tested whether the hydrophobic alkylphenols alter lipid bilayer properties. The butylphenols modestly do this, but not at pharmacologically or behaviorally relevant concentrations; propofol does not alter bilayer properties at the concentrations tested (up to 100 µM).
Materials and Methods
Surgical Procedures and Drug Administration.
With approval from the animal care committee at Columbia University, adult female C57 Black/6J mice (aged 16–24 weeks) were subject to partial sciatic nerve ligation (PNL) (Seltzer et al., 1990) according to institutional and federal guidelines. In brief, after induction of isoflurane (2.5–3%) anesthesia, surgical incisions were made in the upper aspect of both the left and right hind limbs. In the left limb, approximately half of the nerve was ligated using an 8.0 braided silk suture (SofSilk; Coviden, Mansfield, MA). The nerve in the right limb was subject to similar mechanical manipulation but no suture was applied. Mice tended to exhibit protective behavior toward the ipsilateral paw, but their behavior was otherwise normal. Accordingly, and in keeping with the approved protocol, no therapeutic agents other than the indicated experimental test compounds were administered.
To examine 2,6-DTBP bioavailability, surgically naïve mice received dihydroxy-β-cyclodextrin (DHβCD) solubilized 2,6-DTBP (see the Supplemental Methods for details), DHβCD alone or saline by i.p. injection with a dosing schedule as per behavioral testing. Mice were anesthetized with 2.5–3% isoflurane and then exsanguinated by venous puncture 10–60 minutes after receipt of the appropriate final dose. Blood concentration of 2,6-DTBP was determined by gas chromatography (see the Supplemental Methods for details, as well as Supplemental Figs. 1 and 2). To consider the toxicity of a high acute therapeutic dose of 2,6-DTBP, some animals received a single 80 mg/kg bolus. The general condition of all of these animals was followed by qualitative observation of behavior (grooming and exploration) and survival. All animals were sacrificed within 7 days after receipt of a 2,6-DTBP/DHβCD injection.
For electrophysiology experiments, Xenopus oocytes were harvested from frogs anesthetized by immersion in ice-cold pH 7 buffered (sodium phosphate) 0.05% Tricane according to institutional and federal guidelines.
Behavioral Testing.
To monitor the sensitivity of ipsilateral and contralateral hind paws to mechanical and thermal stimuli, mice were subjected to Von Frey fiber and thermal latency stimulus-response analysis using calibrated Von Frey fibers and a timed, tightly focused, variable intensity infrared heat source with an Hargreaves apparatus (IITC Life Science Inc., Woodland Hills, CA) as previously described (Udesky et al., 2005). Briefly, for each test, an animal was separately placed within a 132 cm2 open-topped clear Plexiglas corral on the appropriate surface (a plastic-coated grid with a mesh size of 6 mm or a clear glass thermally regulated platform for the Von Frey and latency analyses, respectively) to which they had been previously acclimated and were allowed to settle for at least 10 minutes. Tests were performed on the plantar surface of both left and right hind paws. In the Von Frey analysis, the probability of paw withdrawal (PW) was obtained by determining how many of 10 trials with a particular fiber resulted in the tested paw being withdrawn. Ipsilateral and contralateral paws were sequentially tested with a single fiber, with fiber strengths of 0.05, 0.4, 0.6, 1.1, 2.5, 3.3, and 4 g tested from weakest to strongest. In the thermal sensitivity analysis, the mean hind paw withdrawal latency (HPWL) was obtained by averaging the latency observed in five separate trials at a particular setting of the heat source. HPWL tests were done with alternating testing of the ipsilateral and contralateral paws. The response to heat source settings of 3 to 30% (in 3% increments, where 100% represents 150 W) was tested in a random order. If the paw was not withdrawn within 30 seconds, the trial was terminated and a latency of 30 seconds was noted.
Von Frey fibers exerting 2.5, 3.3, and 4 g yielded optimal discrimination of mechanical neuropathic hyperalgesia with respect to nociception (see Supplemental Fig. 3 in the Supplemental Results). Thus, these fibers tended to produce withdrawal probabilities greater than 0 in the contralateral paw but less than 1 in the ipsilateral paw (Figs. 1A and 4A; Supplemental Fig. 3) with the ratio of PW,IPSI / PW,CONTRA (where PW,IPSI and PW,CONTRA are probability of withdrawal of the ipsilateral and contralateral paws, respectively) being similar at each stimulus intensity (2.9 ± 0.3, 2.9 ± 0.4, and 2.7 ± 0.2, respectively). Accordingly, we used the mean values of PW ratios over these three stimuli to obtain a single, better defined, value of the ratio for each mouse at each time point and drug condition. To further examine the mechanical response, we performed a Logit transformation (Ln [PW/(1 − PW)]) of the withdrawal probability at each stimulus strength and then determined ES50, the stimulus strength required for a Pw of 0.5 using eq. 1:
| (1) |
Here, b0 and b1 are, respectively, the ordinate intercept and the slope of a regression through the Logit values plotted versus stimulus strength (Fig. 1C).To avoid skewing the data by omitting undefined values (Logit is not defined at a PW of 0 or 1), we assigned such determinations values of 0.01 and 0.99 for the purpose of this transformation.
Fig. 1.
Subhypnotic doses of propofol selectively suppress PNL-induced mechanical hyperalgesia with respect to mechanical nociception. (A and B) PW,IPSI and PW,CONTRA as a function of stimulus fiber strength determined before and after i.p. propofol (A) and PW,IPSI and PW,CONTRA as a function of PW,CONTRA before drug injection (B). Asterisks and crosses indicate PW values statistically different from control PW,IPSI and PW,CONTRA, respectively. In (A), tests are performed separately for each stimulus intensity; in (B), tests are with respect to PW,CONTRA. (C and D) Logit transformation of PW,IPSI and PW,CONTRA in the absence or presence of propofol (C) and the extracted ES50 plotted as a function of drug dose (D). In (C), the color gradations have the same meaning with respect to dose as in (A), the dashed horizontal line is at a PW of 0.1. In (D), asterisks and crosses have the same meaning as in (A), whereas the lines are linear regressions with R2 values of 0.98 for the ipsilateral data and 0.99 for the contralateral data; P ≤ 0.01 for both regressions. Data are from 15 mice (except for the 0.6-g stimulus, which was for 11 mice).
Fig. 4.
2,6-DTBP selectively suppresses PNL-induced mechanical hyperalgesia with respect to mechanical nociception. (A and B) PW,IPSI and PW,CONTRA as a function of stimulus fiber strength determined before and after i.p. 2,6-DTBP (A) and PW,IPSI and PW,CONTRA as a function of PW,CONTRA before drug injection (B). Asterisks and crosses have the same meaning as in Fig. 1. (C and D) Logit transformation of PW,IPSI and PW,CONTRA in the absence or presence of 2,6-DTBP (C) and the extracted ES50 plotted as a function of drug dose (D). In (C), the color gradations have the same meaning with respect to dose as in (A), and the dashed horizontal line is at a PW of 0.1. In (D), asterisks and crosses have the same meaning as in (A), whereas the lines are linear regressions with R2 values of 0.91 for the ipsilateral data and 0.92 for the contralateral data; P ≤ 0.05 for both regressions. Data are from 10 mice.
A heat lamp setting of 15% was optimal for examining thermal hyperalgesia rather than nociception (see Supplemental Fig. 4 in the Supplemental Results). Thus, latencies in both ipsilateral and contralateral paws fell between our fastest and slowest detection thresholds (some 2−3 and 30 seconds, respectively), whereas the ratio HPWLIPSI/HPWLCONTRA (where HPWLIPSI and HPWLCONTRA are hind paw withdrawal latency of the ipsilateral and contralateral paws, respectively) was lowest at 15%. In contrast, 30% stimulation produced a largely, albeit not completely, nociceptive response in injured and uninjured paws, thereby providing data on antinociception.
On the basis of the above observations, we established an objective set of inclusion criteria to identify animals that developed mechanical and thermal neuropathic responses. For an animal to be included in the Von Frey mechanical analysis, control values yielding PW,IPSI/PW,CONTRA >1 and PW,IPSI at 4 g ≥0.3 were required. For inclusion in the HPWL thermal analysis, the control HPWLIPSI/HPWLCONTRA at 15% had to be ≤0.8. The majority of mice that recovered from PNL surgery developed robust and stable mechanical and thermal neuropathic responses.
To examine the influence of alkylphenols and/or vehicle on nocifensive behavior, we constructed cumulative dose-response relationships wherein an aliquot of agent was administered every 60 minutes. After each i.p. injection, animals were allowed to recover for 10 minutes and then their behavior was assayed during the subsequent 50 min. To maximize the sensitivity of our assay while minimizing the duration of the injection/monitoring cycles, and hence of drug clearance, we only examined lamp settings of 15 and 30% and fiber strengths of 0.6, 1.1, 2.5, 3.3, and 4 g during such drug trials. The reported dose of administered agents assumes no clearance of compound during the analysis. Drug trials were performed 7–17 days after PNL surgery (Supplemental Figs. 3 and 4).
Molecular Biology.
cDNA encoding murine HCN1, HCN2, and HCN4 and human HCN3 channels were subcloned into pGH19 (HCN1 and HCN4) or pGHE (HCN2 and HCN3) vectors and amplified in STBL2 cells (Invitrogen Corporation, Grand Island, NY). cRNA was transcribed from NheI (HCN1, HCN3, and HCN4) or SphI (HCN2) linearized DNA using T7 RNA polymerase (Message Machine; Ambion, Houston, TX). We injected 1–50 ng RNA into each Xenopus oocyte.
Electrophysiology.
Recordings were made from Xenopus oocytes 2–5 days after cRNA injection. Cells were maintained in L-15 media without ficoll (Specialty Media, Phillipsburg, NJ) at 17°C until use. Two-microelectrode voltage clamp data, acquired using a Warner Instruments OC-725C amplifier (Hamden, CT), were recorded with Pulse software (HEKA Elektronik, Lambrecht/Pfalz, Germany) after filtering at 2.5 kHz (902 8-pole Bessel filter; Frequency Devices, Haverhill, MA) and digitization at 5 kHz using an ITC-18 interface (Instrutech Corporation, Port Washington, NY).
Microelectrodes were fabricated from 1B120-F4 borosilicate glass (World Precision Instruments, Sarasota, FL) with resistances of 0.1–0.5 MΩ (I passing) and 1–4 MΩ (V sensing) when filled with 3 M KCl. The Ag-AgCl ground wire(s) of the active virtual ground circuit were connected to the bath solution by 3 M KCl-2% agar salt bridges placed downstream of, but close to, the oocyte. Recordings were obtained at room temperature (22–24°C). In all cases, the observed potential was within 1% of the reported command potential. For HCN1 and HCN2, oocytes were bathed in a recording solution of the following (in mM): 107 NaCl, 5 KCl, 2 MgCl2, 10 HEPES-free acid pH 7.4 (NaOH). For the poorly expressing HCN3 and HCN4 channels, the KCl concentration was raised to 25 mM by isoosmolar substitution of NaCl. In all recordings, the holding potential was −30 mV and the tail potential was 0 mV.
To analyze the effects of alkylphenols on HCN channel gating, we constructed isochronal activation curves. In brief, cells were placed in 20-ml glass scintillation vials (3 cells per vial along with 15 ml recording solution that was, where indicated, supplemented with vehicle or compound) and incubated at room temperature on a three-dimensional rotator (Laboratory Line, Melrose Park, IL). After 20 minutes, cells were transferred to a recording chamber continuously perfused with the appropriate drug, vehicle, or control solution. In some experiments, we recorded isochronal activation curves before and after incubation in the presence or absence of drug or vehicle. No cell was exposed to more than one drug or vehicle condition.
Channels were activated by hyperpolarizing voltage steps applied in −10 mV decrements for 5 seconds (HCN1), 30 seconds (HCN2), or 60 seconds (HCN3 and 4). The amplitude of the instantaneous tail currents after each sweep was determined as the difference between the plateau current (observed after the voltage-clamp had settled and the uncompensated linear capacitance decayed but before marked channel closure) and the baseline current (observed after deactivation was complete). These relationships were then fit with a Boltzmann equation (eq. 2):
 |
(2) |
where A1 is the current offset, A2 the maximal amplitude, V the test voltage, V1/2 the activation mid-point, and s the slope factor.
Dose-response data were fit using the Hill equation (eq. 3):
| (3) |
where R is the response, RMAX the maximal response, [A] the alkylphenol concentration, EC50 the ligand concentration that produces a half-maximal response, and n the Hill coefficient.
Fluorescence Quench Measurement of Bilayer Modification by Di-alkylphenols.
Large unilamellar vesicles (LUVs), loaded with the disodium salt of the water-soluble fluorophore 8-aminonaphthalene-1,3,6-trisulfonic acid (ANTS), were made of 1,2-dierucoyl-sn-glycero-3-phosphocholine using a four-part process of sonication, freeze-thaw cycles, extrusion, and elution. The LUVs were doped with the naturally occurring mixture of gramicidins (gA) from Bacillus brevis (gA/lipid mole ratio of approximately 1:1000) and incubated for 20–24 hours at 12.5°C. The fluorescence produced by ANTS is quenched by thallium (Tl+). Tl+ and ANTS cross the bilayer slowly, whereas the bilayer-spanning gA channels are very Tl+ permeable, such that the Tl+ influx rate (the rate of fluorescence quenching) becomes a measure of the number of conducting channels in the LUV membranes. To measure the fluorescence quench rate (at 25°C), the ANTS-loaded LUVs were mixed with the Tl+ quencher using a stopped-flow spectrofluorometer (SX.20; Applied Photophysics, Surrey, UK). The fluorescence quench rate was determined at t = 2 ms using eq. 4 wherein τ0 and β were determined from a fit of the stretched exponential function (eq. 5) to fluorescence recorded between 2 and 100 ms after addition of Tl+.
| (4) |
| (5) |
F0, F(t), and F∞ represent the fluorescence at time zero, time t, and at infinity, respectively (Ingólfsson and Andersen, 2010; Ingólfsson et al., 2010). All experiments were done in triplicate, or more.
Chemicals and Reagents.
NaCl (S7653), KCl (P9333), MgCl2 (M2670), HEPES-free acid (H4034), 2,4-DTBP (137731), 2,6-DTBP (D48400), DHβCD (H107), 2,4-DSBP (S367907), and B. brevis gA (G5002) were from Sigma-Aldrich (St. Louis, MO; catalog numbers as indicated). Propofol and 2,6-DSBP (152830050) were from TCI America (Portland, OR), Sigma Rare Chemicals Library, and Acros Organics (Pittsburgh PA), respectively. ANTS (A350) was from Invitrogen and 1,2-dierucoyl-sn-glycero-3-phosphocholine (850398) was from Avanti Polar Lipids (Alabaster, AL). Stock solutions of alkylphenols were stored at −20°C for no more than 1 week while dilutions in recording solutions were prepared on the day of use. Sterile samples of Diprivan (1% propofol in intralipid, which contains the following: 10% soybean oil, 2.25% glycerol, 1.2% egg lecithin, and 0.005% EDTA-Na2, each as W/V) was obtained from Abraxis Bioscience (Schaumburg, IL) and kept at 4°C.
Data and Statistical Analysis.
Electrophysiological data analysis was performed in PulseFit (HEKA Elektronik) and with custom analysis routines written in IgorPro (Wavemetrics Corporation, Lake Oswego, OR). Behavioral data were compiled and analyzed in Excel (Microsoft Corporation, Redmond, WA). Fluorescence quench data were analyzed using custom routines in Matlab (MathWorks, Natick, MA) and Origin (OriginLab, Northampton, MA) software. Statistical analysis was performed using one-way analysis of variance with post hoc Holm-Sidak analysis (enabled in SigmaStat V3.1; Systat Software, Point Richmond, CA). Unless a reference population is defined, tests were performed across all possible pairs and relevant pairs identified from the matrix post hoc. All data are presented as mean ± S.E.M.; for ES50, the error was determined using the jackknife method. For normalized data, the error around the denominator is that factor’s observed error divided by its observed mean.
Results
Subhypnotic Doses of Propofol Reduce Mechanical and Thermal Hyperalgesia.
Figure 1A shows PW,IPSI and PW,CONTRA in post-PNL mice as a function of stimulus strength (Von Frey fibers ranging from 0.6 to 4 g) and i.p. propofol administration (cumulative dose of propofol ranging from 0 to 60 mg/kg). At these doses, propofol markedly reduced the mechanical hyperalgesia in the ipsilateral paw with only modest disturbance of normal mechanical nociceptive response, as monitored in the contralateral paw. PW,IPSI is higher than PW,CONTRA in the absence of propofol, but the difference is significantly reduced upon administration of 20 mg/kg propofol and, at a dose of 60 mg/kg, the response becomes indistinguishable from the control value of PW,CONTRA. In contrast, 20–60 mg/kg (cumulative dose) propofol had no statistically significant effect on PW,CONTRA, suggesting that in the absence of neuropathy, mechanical nociceptive reflexes are relatively insensitive to propofol. Our findings are consistent with the results of Udesky et al. (2005).
In Fig. 1B, we plot PW,IPSI and PW,CONTRA as a function of the contralateral control value. In each case, the ratios determined across the 2.5- to 4-g stimulus strengths were averaged (see the Materials and Methods). Figure 1B shows that subhypnotic propofol provides an approximately 80% reversal of the neuropathic phenotype (the ratio drops from 3.3 to 1.6, where unity would represent complete amelioration).
Figure 1, C and D, plots the Logit transformation of the data presented in Fig. 1A and the extracted 50% stimulus response value (ES50) as a function of cumulative drug dose, respectively. These data show that subhypnotic propofol reverses the neuropathic phenotype accompanied by a modest nociceptive analgesia. In the absence of propofol, vehicle injection was without effect; ipsilateral ES50 values were 2.4 ± 0.01 and 2.3 ± 0.01 (before and after vehicle, respectively), whereas contralateral values were 4.8 ± 0.1 and 4.6 ± 0.1. No animals lost their righting reflex, consistent with previous demonstrations that >100 mg/kg i.p. propofol is required for obviation of this reflex (Lingamaneni et al., 2001; Udesky et al., 2005). We conclude that selective suppression of neuropathic mechanical hyperalgesia occurs at propofol doses that are 3- to 10-fold lower than those producing hypnosis.
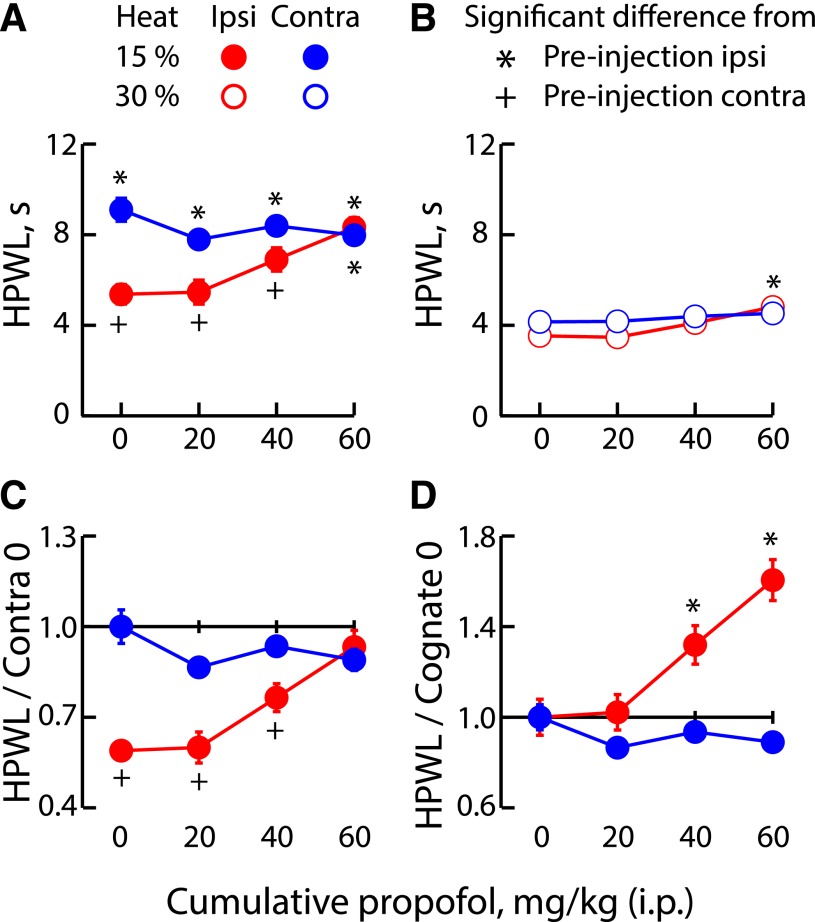
Figure 2 shows the assessment of thermal hyperalgesia analyzed in a manner equivalent to that shown in Fig. 1 for mechanical hyperalgesia. Figure 2, A and B, plots HPWLIPSI and HPWLCONTRA values at stimulus strengths of 15 and 30%, respectively (each as a function of the cumulative i.p. propofol dose), whereas Fig. 2, C and D, shows the latencies as a function of the contralateral control value and their cognate zero drug condition, respectively. As observed with mechanical hyperalgesia, we see that subhypnotic doses of propofol relieve neuropathic thermal hyperalgesia. In the absence of propofol, vehicle injection was without effect; before and after vehicle administration, ipsilateral withdrawal was 2.2 and 2.3 (each ±0.3) times as fast as preinjection contralateral withdrawal.
Fig. 2.
Subhypnotic doses of propofol selectively ameliorate PNL-induced thermal hyperalgesia with respect to thermal nociception. (A and B) HPWL as a function of heat source intensity determined before and after i.p. propofol. (C) HPWLIPSI and HPWLCONTRA as a function of HPWLCONTRA before drug injection. (D) HPWLIPSI and HPWLCONTRA as a function of HPWL observed in the cognate paw before drug injection. Asterisks and crosses indicate HPWL values statistically different from control HPWLIPSI and control HPWLCONTRA, respectively. In (C), statistical tests are with respect to PW,CONTRA; in (D), tests are within paw only. Data are from 10 (30%) or 14 (15%) mice.
2,6-DTBP is a Potent Antagonist of HCN1 Channel Gating.
HCN1 is implicated in the etiology of neuropathic pain and is sensitive to propofol. Because there is selectivity among alkylphenols for GABAA-Rs, we explored whether such selectivity was also true for HCN channels.
Figure 3A shows two-microelectrode voltage clamp recordings from Xenopus oocytes expressing homomeric HCN1 channels (left) and the corresponding tail currents (right) obtained after incubation with either dimethylsulfoxide (DMSO) alone (top) or 10 µM 2,6-DTBP (bottom). In all four panels, the red line highlights the trace recorded with an activation voltage of −65 mV. Qualitative inspection of the data suggests that 2,6-DTBP makes HCN1 channels harder to activate or open, maybe both. The compound slows opening, accelerates closing, and shifts gating to more hyperpolarized potentials. The equilibrium activation curves confirm the latter observation (Fig. 3B). Such findings are qualitatively identical to the effects of propofol (Cacheaux et al., 2005).
Fig. 3.
2,4 and 2,6 tertiary butyl substituted phenols: High potency HCN1 selective pacemaker channel antagonists. (A) HCN1 two-microelectrode voltage clamp records (left) and tail currents (right) obtained after 20- min incubation in either DMSO (top in gray) or 10 µM 2,6-DTBP (bottom in black). Red lines highlight records obtained upon activation at −65 mV. Records are from two separate cells but are from the same donor frog and were acquired on the same day. (B) Tail current activation curves for cells shown in (A). Fits of the Boltzmann function (superimposed lines) yielded values of the V1/2 and slope of –60.6 and 8.7 mV (mean –64.8 ± 1.9 mV and 7.5 ± 0.3 mV; n = 14) versus –90.8 and 9.7 mV (mean –88.3 ± 1.4 mV and 9.6 ± 0.1 mV; n = 6) in the presence of DMSO and 10 µM 2,6-DTBP, respectively. (C) Shift in V1/2 of HCN1 gating as a function of the concentrations of 2,6- (upper panel) or 2,4- (lower panel) dibutylphenols. The solid line (and indicated parameter values) is from a fit of the Hill function to the 2,6-DTBP concentration-response relationship. The dashed lines are the 2,6-DTBP fit line offset by 2-, 15-, and 23-fold for 2,4-DTBP, 2,6-DSBP, and 2,4-DSBP, respectively. Because the V1/2 elicited by 20 µM 2,6-DTBP was similar irrespective of whether the compound was solubilized in DMSO or DHβCD (see Results), these values were pooled here. For DTBPs, the shift in V1/2 was significant at 1 µM and higher; however, for DSBPs, significance was only observed at 20 µM. (D) Shift in V1/2 of gating of HCN1–4 as a function of the indicated concentration of 2,6-DTBP. Asterisks indicate responses statistically different from the paired control.
From recordings such as those shown in Fig. 3, A and B, we constructed a concentration-response relationship for the 2,6-DTBP–induced shift in V1/2 of HCN1 gating (Fig. 3C, top; data fit by the solid line). These results show that 2,6-DTBP is a 5- to 6-fold more potent inhibitor of channel gating than propofol (EC50 of 2.3 µM versus 13 µM), and that the two compounds have similar maximal efficacy (V1/2 of −32 mV versus −45 mV) and apparent stoichiometry (n = 1.4 versus n = 1.1) when the drug effects are determined under equivalent experimental conditions (Lyashchenko et al., 2007). Importantly, 2,6-DTBP had similar efficacy whether it was solubilized in DMSO or DHβCD [V1/2 after 20-minute incubation with 20 µM 2,6-DTBP was −31.9 ± 0.7 mV (n = 6) with DMSO versus −27.7 ± 0.7 mV (n = 6) with DHβCD].
To gain insight into whether 2,6-DTBP alters the behavior of fully activated channels, we determined the maximal current density (as obtained from fits of the Boltzmann equation to equilibrium activation curves) before and after incubation in the presence or absence of 2,6-DTBP or vehicle alone. In all cases, the postincubation current was larger than the preincubation current but this run-up appeared to be modestly blunted by 2,6-DTBP (control: 121% ± 4%, n = 8; DHβCD: 124% ± 7%, n = 7; 20 µM 2,6-DTBP: 108% ± 7%, n = 6; the difference did not reach statistical significance). The run-up in current amplitude may be due to translocation of vesicular channels into the plasma membrane during incubation. In summary, the primary effect of 2,6-DTBP appears similar to that of propofol, which is to stabilize the closed/deactivated state of HCN1 channels with little effect on the conduction path (Lyashchenko et al., 2007).
The Alkylphenol Adduct Sensitivity of HCN1 Channels Contraposes that of GABAA-Rs.
To examine further the nature of the selective alkylphenol association with HCN1 channels, we asked whether coupling of butylphenols to HCN1 channels displayed any dependence on the structure or location of the alkyl adducts attached to the phenol ring. Specifically, we examined the ability of 2,6-DSBP as well as 2,4-DTBP and 2,4-DSBP to inhibit HCN1 channel gating. Figure 3C plots the concentration-response relations for 2,6-DSBP and 2,6-DTBP (top) and 2,4-DSBP and 2,4-DTBP (bottom). In each case, the dashed lines through the respective data represent the fit of the Hill equation to the 2,6-DTBP results, offset by 2-, 15-, and 23-fold (for 2,4-DTBP, 2,6-DSBP, and 2,4-DSBP, respectively). There is a strong preference for the tertiary versus the secondary butyl side chains (by more than 10-fold) and a (weaker) preference for 2,6 over 2,4 substitutions (approximately 2-fold). Importantly, the preference for tertiary butyl groups is the inverse of the steric requirements that modulate alkylphenol effects on GABAA-Rs.
Because we cannot determine the maximal efficacy of the less potent alkylphenols (at the highest concentrations tested, the aqueous solubility limit is exceeded by visual inspection; also see the Supplemental Methods). It is possible that HCN1 channels discriminate between di-sec and di-tert butylphenols more fully than is suggested by comparison of potency alone (Fig. 3).
2,6-DTBP Selectively Antagonizes Gating of HCN1.
Propofol is an efficacious, potent antagonist of HCN1 channel gating with marked selectivity for HCN1 over the other HCN isoforms; 20 µM propofol shifts V1/2 by −45 mV for HCN1 but only −9 mV and 0 mV for HCN2 and HCN4, respectively (Lyashchenko et al., 2007), and −0.6 ± 0.1 mV (n = 6) for HCN3 (unpublished data). Figure 3D shows that 2,6-DTBP retains this selectivity profile, suggesting that 2,6-DTBP could be an analgesic with minimal hypnotic effect.
2,6-DTBP Reverses the Neuropathic Responses to Mechanical and Thermal Insults While Leaving Nociception Largely Intact.
2,6-DTBP does not potentiate GABAA-Rs nor is it a general anesthetic at concentrations equivalent to those that are hypnotic for propofol (James and Glen, 1980; Engdahl et al., 1998; Krasowski et al., 2001; Dawidowicz et al., 2002, 2003; Dawidowicz and Kalitynski 2003). Thus, if potentiation of GABAA-Rs represents a critical aspect of propofol’s efficacy as a neuropathic pain-selective analgesic, then 2,6-DTBP would be expected to be ineffective. In contrast, if HCN1-containing IH channels were involved in propofol’s neuropathic analgesic activity, we would anticipate 2,6-DTBP would retain a propofol-like efficacy (see Discussion for possible alternative targets). Figures 4 and 5 show that 2,6-DTBP ameliorates the behavioral consequences of PNL-induced hyperalgesia with respect to both mechanical (Fig. 4) and thermal insults (Fig. 5) and does so with a preferential retention of nociceptive transmission, a finding that closely mirrors the activity seen with subhypnotic propofol. These therapeutic effects are not due to vehicle. In the absence of 2,6-DTBP, ipsilateral ES50 values were 1.5 ± 0.1 g and 1.3 ± 0.3 g before and after vehicle, whereas contralateral values were 3.7 ± 0.2 g and 4.2 ± 0.6 g; similarly, before and after vehicle administration the ipsilateral withdrawal was 1.8 ± 0.2 and 1.6 ± 0.1 times as fast as preinjection contralateral withdrawal.
Fig. 5.
2,6-DTBP selectively ameliorates PNL-induced thermal hyperalgesia with respect to thermal nociception. (A and B) HPWL as a function of heat source intensity determined before and after i.p. 2,6-DTBP. (C) HPWLIPSI and HPWLCONTRA as a function of HPWLCONTRA before drug injection. (D) HPWLIPSI and HPWLCONTRA as a function of HPWL observed in the cognate paw before drug injection. Asterisks and crosses have the same meaning as in Figure 2. Data are from 10 mice.
Alkylphenol Inhibition of HCN1 Channels is Not Due to Effects on the Lipid Bilayer.
The alkylphenols are hydrophobic, with calculated octanol/water partition coefficients (expressed as cLog P) of approximately 5 (Krasowski et al., 2001), comparable with that of potent bilayer modifiers (Rusinova et al., 2011). General anesthetics have been shown to exert many, but perhaps not all, of their effects via specific interactions with ion channels (Eger et al., 2008), thereby leaving open the possibility that those that are lipophiles (such as the alkylphenols) may also alter embedded membrane protein function via interactions with the lipid bilayer (Lundbaek et al., 2010) (Fig. 6A). We therefore tested whether the alkylphenols alter bilayer properties at the concentrations where they alter HCN channel function. To do so, we examined the ability of propofol and the butylphenols to alter the energetics of gA channel formation (Fig. 6B) using a gA-based fluorescence assay (Ingólfsson and Andersen, 2010; Ingólfsson et al., 2010; Lundbaek et al., 2010).
Fig. 6.
Alkylphenol inhibition of HCN1 channels is not accompanied by effects on the lipid bilayer. (A) Mechanisms by which drugs can inhibit membrane protein (ion channel) function: 1) occluding the pore to block ion movement; 2) binding to sites formed by the channel, to alter function by altering the free energy difference between different channel conformations; 3) binding to selective sites at the channel/bilayer interface, which may alter also the bilayer deformation energy contribution to the free energy difference between different conformations; and 4) nonspecific accumulation at the protein/bilayer interface to alter the local lipid packing as well as 5) partitioning at the bilayer/solution interface to alter lipid bilayer properties, both of which will alter the bilayer contribution to the channel’s conformational equilibrium [modified from Andersen (2008)]. (B) Changes in bilayer properties can be determined by measuring changes in the gA monomer↔dimer equilibrium (Lundbaek et al., 2010) because the ion conducting, bilayer-spanning gA channel’s hydrophobic length is less than the host bilayer’s hydrophobic thickness. gA channel formation thus leads to a local membrane thinning, with an associated energetic cost that varies with changes in the bilayer properties, thus making gA channels suitable probes for changes in bilayer properties. (C) Effect of 2,6-DTBP on the time course of Tl+ quenching of ANTS fluorescence. Normalized fluorescence quench traces displaying the normalized fluorescence time signal over 1 second. Gray dots denote results from all repeats (n > 5 per condition); red lines denote the average of all repeats. The upper two, largely flat and superimposed, traces show fluorescence quenching in the absence of gA and presence of the quencher Tl+ in the absence and presence of 100 µM 2,6-DTBP; the lower four, exponentially decaying and essentially superimposed, traces report the fluorescence time courses observed in gA-containing vesicles incubated with 0, 10, 20, and 100 µM 2,6-DTBP. (D) The first 100 milliseconds of the fluorescence time courses. The dots denote results from a single repeat for each condition; the lines correspond to the stretched exponential fits (2–100 milliseconds) to those repeats. The stippled line marks the 2-milliseconds time point, at which the quenching rate is determined. (E) Fluorescence quench rates in the presence of the indicated concentrations of alkylphenols normalized to the rate determined in the agent’s absence. (F) Log plot showing the EC50 for inhibition of HCN1 by the indicated alkylphenols.
Figure 6, C and D, shows the effect of 2,6-DTBP on the time course of Tl+-induced quenching of the fluorescence emission of ANT encapsulated into gA-doped LUVs. Because Tl+ primarily enters the LUV through membrane-spanning gA channels, changes in the fluorescence quench rate reflect changes in the average number of channels/vesicle. Addition of increasing concentrations of 2,6-DTBP had only a weak effect on either the rate or extent of quenching, meaning that alkylphenols have little effect on the gramicidin monomer↔dimer equilibrium (which varies with changes in lipid bilayer physical properties); this is especially evident when the data here are contrasted with those obtained in vesicles doped with a potent membrane modifying amphiphile, capsaicin (see Supplemental Results and Supplemental Fig. 5).
A modest effect on the quench rate is also observed when vesicles are doped with the other butylphenols but not with propofol (Fig. 6E). Importantly, there is no correspondence between the rank order of potencies by which the five alkylphenols inhibit HCN1 gating (Fig. 6F) and their modest effects on membrane deformation energetics (Fig. 6E).
One caveat with directly comparing the EC50 of channel inhibition with the effect of the alkylphenols on the gA-mediated fluorescence quench rate is the question of the meaning of the added aqueous concentration. Partitioning of compounds with a high cLog P will tend to lower the added aqueous concentration even when the aqueous phase is present in what would normally be considered vast excess. However, the effective lipid/aqueous volumes of our oocyte incubations and our vesicle incubations are similar, indicating that although the aqueous phase “free” concentration of the alkylphenols will be lowered (by approximately 10-fold over the added initial aqueous concentration), this will be similar in the two assays. We conclude that neither 2,6-DTBP nor propofol alter HCN1 channel function through changes in lipid bilayer properties at the concentrations tested, an observation that supports the notion that alkylphenols interact specifically and selectively with HCN1.
Discussion
We tested the hypothesis that IH pacemaker channel antagonists with selectivity for the HCN1 subunit would provide analgesia in a murine model of neuropathic pain, and found that two closely related alkylphenols, the intravenous anesthetic propofol and its nonanesthetic congener, 2,6-DTBP, both inhibit heterologously expressed HCN1 channels and are antihyperalgesic in a mouse model of neuropathic pain. Our results are consistent with selective inhibition of HCN1 channels being the presumptive antihyperalgesic mechanism for this class of compounds. This supposition is supported by data demonstrating that the structurally related clove extract, eugenol (2-methoxy-4-(2-propenyl)phenol), inhibits IH in trigeminal neurons and reverses mechanical allodynia after chronic nerve constriction (Yeon et al., 2011). Recently, 2-ethoxy-N-((1-(4-isopropylpiperazin-1-yl)cyclohexyl)methyl)benzamide, a 1,1-disubstituted cyclohexane that appears to selectively inhibit HCN1 channels, was shown to alleviate nerve injury-induced tactile allodynia in a murine model (McClure et al., 2011), further supporting the idea that specific HCN1 channel blockade might have therapeutic potential in the treatment of neuropathic pain.
Antihyperalgesic Action of Anesthetic and Nonanesthetic Alkylphenols.
Here, we demonstrate that propofol, at subhypnotic doses (Figs. 1 and 2), and 2,6-DTBP (Figs. 4 and 5) profoundly reduce hyperalgesia in a mouse model of neuropathic pain. These observations are consistent with prior results demonstrating that propofol has extended analgesic properties. For example, human subjects who received propofol anesthesia for elective surgical procedures reported less postoperative pain than those anesthetized with the volatile general anesthetics isoflurane (Cheng et al., 2008) or sevoflurane (Tan et al., 2010). In humans, subanesthetic doses of propofol (i.e., 10–25% of the dose required to produce general anesthesia) are associated with a decrease in tibial pressure-induced pain (Briggs et al., 1982), whereas concentrations sufficient to produce mild to moderate sedation are analgesic with respect to acute thermal pain [see Frölich et al. (2005) and Anker-Møller et al. (1991)]. With respect to pathologic pain, and therefore of direct relevance to the present study, intrathecally administered propofol dose-dependently suppresses algesic behavior (paw flinching and shaking) elicited by subcutaneous injection of formalin into the hindpaw in rats (Nishiyama et al., 2004). In addition, subcutaneous injection of propofol suppresses bee venom–induced algesic behaviors in rats when administered ipsilateral, but not contralateral, to the site of venom injection (Sun et al., 2005), suggesting a peripheral, rather than central, site of action.
Potential Sites of Analgesic Action of Anesthetic and Nonanesthetic Alkylphenols.
During general anesthesia, the steady-state plasma concentration of free propofol is on the order of 1 µM (Table 1). Inhibition of neuropathic pain emerges at 3- to 10-fold lower doses than the ED50 for anesthesia (Erenmemisoglu et al., 1993; Lingamaneni et al., 2001), or 0.1 to 0.3 µM. If propofol and 2,6-DTBP partition similarly and proportionally, we further estimate that an analgesic dose of 80 mg/kg 2,6-DTBP (i.p.) would yield a predicted free serum concentration of approximately 0.6 µM. Based on dose-response relations determined from heterologously expressed channels (Fig. 3), such a 2,6-DTBP concentration is anticipated to have a marked effect on HCN1 channel gating whereas that of propofol is on the cusp of the effective dose, although estimates of alkylphenol potency based on inhibition of heterologously expressed channels can be an underestimate by a factor of approximately 10 due to the consequences of partitioning into the lipophilic interior of Xenopus oocytes [see the Results as well as Cacheaux et al. (2005)]. Together, our results are consistent with the alkylphenols exerting their antihyperalgesic action via association with, and inhibition of, HCN1-containing, peripheral IH channels. Given a partial sensitivity of HCN2 to both propofol and 2,6-DTBP, a synergistic contribution of HCN2 inhibition (Ying et al., 2006; Emery et al., 2011) cannot be discounted. A more critical examination of this question will require analysis of analgesia in mice lacking alkylphenol-sensitive HCN isoforms.
If alkylphenols are not exerting their analgesic actions via HCN channels, what are plausible alternative targets? The key molecular targets of propofol-induced hypnosis are the GABAA-Rs (Franks, 2008). However, GABAA-Rs are unlikely to mediate the neuropathic analgesic action of alkylphenols as heterologously expressed (α1β2γ2s subunits) (Krasowski et al., 2001; Ahrens et al., 2009) and native (Xenopus laevis spinal cord neurons) (Krasowski et al., 2001) GABAA-Rs are insensitive to 2,6-DTBP. As for glycine receptors (Gly-Rs), although inhibition of spinal Gly-Rs produces allodynia (Sherman and Loomis, 1994), and both propofol (Pistis et al., 1997; Ahrens et al., 2004) and 2,6-DTBP (Ahrens et al., 2004) enhance the gating of heterologously expressed Gly-Rs, their sensitivity is too low for them to be relevant targets in this regard.
Among voltage-gated channels, NaV1.3, 1.7, 1.8, and 1.9 are implicated as mediators of inflammatory and neuropathic pain (Jarvis et al., 2007; Costigan et al., 2009; Dib-Hajj et al., 2009). Although the alkylphenol sensitivity of these isoforms has not been examined in detail, the Na+ currents of neurohypophysial nerve terminals are inhibited by micromolar concentrations of propofol (Ouyang et al., 2003) and at least one isoform (NaV1.2) is inhibited by 2,6-DTBP albeit at relatively high concentrations (>10 µM) (Haeseler and Leuwer, 2003). Interestingly, an extensive library screen by Abbott Laboratories and Icagen identified a 4-substituted 2,6-dimethoxyphenyl as a NaV antagonist with preference for NaV1.8 (Jarvis et al., 2007). This compound ameliorates some modalities of neuropathic pain at doses comparable with those we show to be efficacious for 2,6-DTBP. Finally, we note that 2,6-DTBP is a presumptive inhibitor of key enzymes in the arachidonic acid cascade (Song et al., 1999). It is unlikely the antihyperalgesic activity of alkylphenols is related to cyclooxygenase inhibition, given that efficacy as an inhibitor of these enzymes is a poor predictor of a compound’s efficacy against neuropathic pain (Vo et al., 2009). However, our results suggest that alkylphenol antihyperalgesic activity may result from a combination of actions, with HCN1 inhibition being a major contributor.
The CSF concentration of propofol required for anesthesia is approximately one-sixth of that in plasma (Table 1). Examination of the extent to which 2,6-DTBP shares this property and exploitation of relative CSF sparing would benefit development of alkylphenols with respect to specifically targeting peripheral sensitization.
Mechanistic Basis for Selective Inhibition of HCN1 Channels by Di-propyl and Di-butyl alkylphenols.
We previously showed that propofol modifies gating of HCN channels in the closed-resting and closed-activated states with little or no pore-blocking effect (Lyashchenko et al., 2007). We now demonstrate that the HCN1 sensitivity to alkylphenols displays a dependence on the steric arrangement of the alkyl adducts. With regard to GABAA-R potentiation, such a steric effect has previously been interpreted as evidence for the existence of a protein pocket of circumscribed dimensions within the channel (Franks and Lieb, 1985; Krasowski et al., 2001). Such phenomena can, however, also arise in a system in which no binding site exists (Ingólfsson and Andersen, 2011), and we examined the ability of alkylphenols to alter lipid bilayer properties. Whereas the butylphenols (but not propofol) modestly altered lipid bilayer properties (as sensed by a membrane-spanning channel), this was seen only at concentrations much higher than those where they altered HCN1 channel function—and there was no apparent rank order in their effect on bilayer properties, and certainly not one that corresponded to the clear rank order effect with respect to inhibition of HCN1 (Fig. 6, E versus F). Propofol was recently shown to occupy a defined pocket in a GABAA-R homolog (Nury et al., 2011); consequently, we hypothesize that a similar site exists within HCN1 albeit with mirror-image dependence (compared with the GABAA-R) on the steric arrangement of the alkylphenol.
Alkylphenol Effects on Lipid Membranes.
Interestingly, although their effect on the energetics of gA channel formation was modest, the trend was for di-butylphenols to reduce the fluorescence quench rate, meaning that they shifted the gA monomer↔dimer equilibrium toward the nonconducting monomers. The usual result is that small bilayer-active molecules shift the monomer↔dimer equilibrium toward the conducting dimers [e.g., capsaicin; see Supplemental Fig. 5 as well as Lundbaek et al. (2010)], although there are exceptions such as the long-chain alcohols (Ingólfsson and Andersen, 2011). The parsimonious interpretation is that the di-butyl-phenols increase acyl chain order and bilayer thickness, although we do not understand why.
We have shown that the closely related anesthetic and nonanesthetic alkylphenols, propofol and 2,6-DTBP, selectively inhibit HCN1 pacemaker channels and provide selective reversal of mechanical and thermal hyperalgesia resulting from peripheral nerve injury in a mouse model. Although these findings do not establish that alkylphenols exert their analgesic action via HCN1 channel inhibition in vivo, they establish that 2,6-DTBP (or derivatives thereof) represent a novel class of analgesic agents that might selectively target chronic neuropathic pain. Our observations also provide a chemical lead for development of peripherally restricted HCN1-selective antagonists based on a widely used, and safe, therapeutic agent. It is interesting to speculate that a suppression of peripheral hyperexcitability by propofol accounts for the lower postsurgical pain that has been reported when it is used to provide anesthesia.
Supplementary Material
Acknowledgments
The authors thank Juliane Stieber and Andreas Ludwig for kindly providing the human HCN3 clone, Steven Siegelbaum’s laboratory for providing the Xenopus oocytes, Dr. Charles Inturissi for his thoughtful comments, and Dr. Kane Pryor for providing the estimated propofol concentrations in footnote a to Table 1.
Abbreviations
- 2,4-DSBP
2,4-di-sec-butylphenol
- 2,4-DTBP
2,4-di-tert-butylphenol
- 2,6-DSBP
2,6-di-sec-butylphenol
- 2,6-DTBP
2,6-di-tert-butylphenol
- ANTS
8-aminonaphthalene-1,3,6-trisulfonic acid
- CSF
cerebrospinal fluid
- DHβCD
dihydroxy-β-cyclodextrin
- DMSO
dimethylsulfoxide
- gA
gramicidins
- GABAA-R
type A GABA receptor
- Gly-R
glycine receptor
- HCN
hyperpolarization-activated, cyclic nucleotide-regulated channel
- HPWL
hind paw withdrawal latency
- HPWLCONTRA
hind paw withdrawal latency of the contralateral paws
- HPWLIPSI
hind paw withdrawal latency of the ipsilateral paws
- LUV
large unilamellar vesicle
- PNL
partial sciatic nerve ligation
- PW
paw withdrawal
- PW,CONTRA
probability of withdrawal of the contralateral paws
- PW,IPSI
probability of withdrawal of the ipsilateral paws
- ZD7288
N-ethyl-1,6-dihydro-1,2-dimethyl-6-(methylimino)-N-phenyl-4-pyrimidinamine hydrochloride
Author Contributions
Participated in research design: Tibbs, Hemmings, Andersen, Flood.
Conducted experiments: Tibbs, Rowley, Sanford, Herold.
Performed data analysis: Tibbs, Sanford, Herold, Proekt, Goldstein.
Wrote or contributed to the writing of the manuscript: Tibbs, Hemmings, Andersen, Goldstein, Flood.
Footnotes
This study was supported in part by the National Institutes of Health Institute of General Medical Sciences [Grants GM021342, GM021342-35S1, and GM58055]; and by the Departments of Anesthesiology of Columbia University and Weill Cornell Medical College. G.R.T., P.A.G., and P.D.F. have applied for a patent to exploit alkylphenols in the treatment of neuropathic pain.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Ahrens J, Haeseler G, Leuwer M, Mohammadi B, Krampfl K, Dengler R, Bufler J. (2004) 2,6 di-tert-butylphenol, a nonanesthetic propofol analog, modulates α1β glycine receptor function in a manner distinct from propofol. Anesth Analg 99:91–96 [DOI] [PubMed] [Google Scholar]
- Ahrens J, Leuwer M, de la Roche J, Foadi N, Krampfl K, Haeseler G. (2009) The non-anaesthetic propofol analogue 2,6-di-tert-butylphenol fails to modulate GABAA receptor function. Pharmacology 83:95–98 [DOI] [PubMed] [Google Scholar]
- Andersen OS. (2008) Perspectives on how to drug an ion channel. J Gen Physiol 131:395–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker-Møller E, Spangsberg N, Arendt-Nielsen L, Schultz P, Kristensen MS, Bjerring P. (1991) Subhypnotic doses of thiopentone and propofol cause analgesia to experimentally induced acute pain. Br J Anaesth 66:185–188 [DOI] [PubMed] [Google Scholar]
- Briggs LP, Dundee JW, Bahar M, Clarke RS. (1982) Comparison of the effect of diisopropyl phenol (ICI 35, 868) and thiopentone on response to somatic pain. Br J Anaesth 54:307–311 [DOI] [PubMed] [Google Scholar]
- Cacheaux LP, Topf N, Tibbs GR, Schaefer UR, Levi R, Harrison NL, Abbott GW, Goldstein PA. (2005) Impairment of hyperpolarization-activated, cyclic nucleotide-gated channel function by the intravenous general anesthetic propofol. J Pharmacol Exp Ther 315:517–525 [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Guo HQ, Lee DH, Luo L, Liu C, Kuei C, Velumian AA, Butler MP, Brown SM, Dubin AE. (2003) Neuronal hyperpolarization-activated pacemaker channels drive neuropathic pain. J Neurosci 23:1169–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Shu S, Bayliss DA. (2005) Suppression of ih contributes to propofol-induced inhibition of mouse cortical pyramidal neurons. J Neurophysiol 94:3872–3883 [DOI] [PubMed] [Google Scholar]
- Cheng SS, Yeh J, Flood P. (2008) Anesthesia matters: patients anesthetized with propofol have less postoperative pain than those anesthetized with isoflurane. Anesth Analg 106:264–269 [DOI] [PubMed] [Google Scholar]
- Costigan M, Scholz J, Woolf CJ. (2009) Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci 32:1–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawidowicz AL, Fijałkowska A, Nestorowicz A, Kalityński R, Trojanowski T. (2003) Cerebrospinal fluid and blood propofol concentration during total intravenous anaesthesia for neurosurgery. Br J Anaesth 90:84–86 [PubMed] [Google Scholar]
- Dawidowicz AL, Kalityński R. (2003) HPLC investigation of free and bound propofol in human plasma and cerebrospinal fluid. Biomed Chromatogr 17:447–452 [DOI] [PubMed] [Google Scholar]
- Dawidowicz AL, Kalityński R, Nestorowicz A, Fijalkowska A. (2002) Changes of propofol concentration in cerebrospinal fluid during continuous infusion. Anesth Analg 95:1282–1284 [DOI] [PubMed] [Google Scholar]
- Dib-Hajj SD, Black JA, Waxman SG. (2009) Voltage-gated sodium channels: therapeutic targets for pain. Pain Med 10:1260–1269 [DOI] [PubMed] [Google Scholar]
- Dray A. (2008) Neuropathic pain: emerging treatments. Br J Anaesth 101:48–58 [DOI] [PubMed] [Google Scholar]
- Eger EI, 2nd, Tang M, Liao M, Laster MJ, Solt K, Flood P, Jenkins A, Raines D, Hendrickx JF, Shafer SL, et al. (2008) Inhaled anesthetics do not combine to produce synergistic effects regarding minimum alveolar anesthetic concentration in rats. Anesth Analg 107:479–485 [DOI] [PubMed] [Google Scholar]
- Emery EC, Young GT, Berrocoso EM, Chen L, McNaughton PA. (2011) HCN2 ion channels play a central role in inflammatory and neuropathic pain. Science 333:1462–1466 [DOI] [PubMed] [Google Scholar]
- Emery EC, Young GT, McNaughton PA. (2012) HCN2 ion channels: an emerging role as the pacemakers of pain. Trends Pharmacol Sci 33:456–463 [DOI] [PubMed] [Google Scholar]
- Engdahl O, Abrahams M, Björnsson A, Vegfors M, Norlander B, Ahlner J, Eintrei C. (1998) Cerebrospinal fluid concentrations of propofol during anaesthesia in humans. Br J Anaesth 81:957–959 [DOI] [PubMed] [Google Scholar]
- Erenmemisoglu A, Madenoglu H, Tekol Y. (1993) Antinociceptive effect of propofol on somatic and visceral pain in subhypnotic doses. Curr Ther Res 53:677–681 [Google Scholar]
- Franks NP. (2008) General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci 9:370–386 [DOI] [PubMed] [Google Scholar]
- Franks NP, Lieb WR. (1985) Mapping of general anaesthetic target sites provides a molecular basis for cutoff effects. Nature 316:349–351 [DOI] [PubMed] [Google Scholar]
- Frölich MA, Price DD, Robinson ME, Shuster JJ, Theriaque DW, Heft MW. (2005) The effect of propofol on thermal pain perception. Anesth Analg 100:481–486 [DOI] [PubMed] [Google Scholar]
- Haeseler G, Leuwer M. (2003) High-affinity block of voltage-operated rat IIA neuronal sodium channels by 2,6 di-tert-butylphenol, a propofol analogue. Eur J Anaesthesiol 20:220–224 [DOI] [PubMed] [Google Scholar]
- Ingólfsson HI, Andersen OS. (2010) Screening for small molecules’ bilayer-modifying potential using a gramicidin-based fluorescence assay. Assay Drug Dev Technol 8:427–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingólfsson HI, Andersen OS. (2011) Alcohol’s effects on lipid bilayer properties. Biophys J 101:847–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingólfsson HI, Sanford RL, Kapoor R, and Andersen OS (2010) Gramicidin-based fluorescence assay; for determining small molecules potential for modifying lipid bilayer properties. J Vis Exp 44:2131. [DOI] [PMC free article] [PubMed]
- James R, Glen JB. (1980) Synthesis, biological evaluation, and preliminary structure-activity considerations of a series of alkylphenols as intravenous anesthetic agents. J Med Chem 23:1350–1357 [DOI] [PubMed] [Google Scholar]
- Jarvis MF, Honore P, Shieh CC, Chapman M, Joshi S, Zhang XF, Kort M, Carroll W, Marron B, Atkinson R, et al. (2007) A-803467, a potent and selective Nav1.8 sodium channel blocker, attenuates neuropathic and inflammatory pain in the rat. Proc Natl Acad Sci USA 104:8520–8525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen TS, Madsen CS, Finnerup NB. (2009) Pharmacology and treatment of neuropathic pains. Curr Opin Neurol 22:467–474 [DOI] [PubMed] [Google Scholar]
- Jiang YQ, Xing GG, Wang SL, Tu HY, Chi YN, Li J, Liu FY, Han JS, Wan Y. (2008) Axonal accumulation of hyperpolarization-activated cyclic nucleotide-gated cation channels contributes to mechanical allodynia after peripheral nerve injury in rat. Pain 137:495–506 [DOI] [PubMed] [Google Scholar]
- Kouranova EV, Strassle BW, Ring RH, Bowlby MR, Vasilyev DV. (2008) Hyperpolarization-activated cyclic nucleotide-gated channel mRNA and protein expression in large versus small diameter dorsal root ganglion neurons: correlation with hyperpolarization-activated current gating. Neuroscience 153:1008–1019 [DOI] [PubMed] [Google Scholar]
- Krasowski MD, Jenkins A, Flood P, Kung AY, Hopfinger AJ, Harrison NL. (2001) General anesthetic potencies of a series of propofol analogs correlate with potency for potentiation of γ-aminobutyric acid (GABA) current at the GABAA receptor but not with lipid solubility. J Pharmacol Exp Ther 297:338–351 [PubMed] [Google Scholar]
- Lee DH, Chang L, Sorkin LS, Chaplan SR. (2005) Hyperpolarization-activated, cation-nonselective, cyclic nucleotide-modulated channel blockade alleviates mechanical allodynia and suppresses ectopic discharge in spinal nerve ligated rats. J Pain 6:417–424 [DOI] [PubMed] [Google Scholar]
- Lingamaneni R, Krasowski MD, Jenkins A, Truong T, Giunta AL, Blackbeer J, MacIver MB, Harrison NL, Hemmings HC., Jr (2001) Anesthetic properties of 4-iodopropofol: implications for mechanisms of anesthesia. Anesthesiology 94:1050–1057 [DOI] [PubMed] [Google Scholar]
- Lundbaek JA, Collingwood SA, Ingólfsson HI, Kapoor R, Andersen OS. (2010) Lipid bilayer regulation of membrane protein function: gramicidin channels as molecular force probes. J R Soc Interface 7:373–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyashchenko AK, Redd KJ, Yang J, Tibbs GR. (2007) Propofol inhibits HCN1 pacemaker channels by selective association with the closed states of the membrane embedded channel core. J Physiol 583:37–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL. (1983) A voltage-clamp analysis of inward (anomalous) rectification in mouse spinal sensory ganglion neurones. J Physiol 340:19–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure KJ, Maher M, Wu N, Chaplan SR, Eckert WA, 3rd, Lee DH, Wickenden AD, Hermann M, Allison B, Hawryluk N, et al. (2011) Discovery of a novel series of selective HCN1 blockers. Bioorg Med Chem Lett 21:5197–5201 [DOI] [PubMed] [Google Scholar]
- Momin A, Cadiou H, Mason A, McNaughton PA. (2008) Role of the hyperpolarization-activated current Ih in somatosensory neurons. J Physiol 586:5911–5929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama T, Matsukawa T, Hanaoka K. (2004) Intrathecal propofol has analgesic effects on inflammation-induced pain in rats. Can J Anaesth 51:899–904 [DOI] [PubMed] [Google Scholar]
- Nury H, Van Renterghem C, Weng Y, Tran A, Baaden M, Dufresne V, Changeux JP, Sonner JM, Delarue M, Corringer PJ. (2011) X-ray structures of general anaesthetics bound to a pentameric ligand-gated ion channel. Nature 469:428–431 [DOI] [PubMed] [Google Scholar]
- Orio P, Madrid R, de la Peña E, Parra A, Meseguer V, Bayliss DA, Belmonte C, Viana F. (2009) Characteristics and physiological role of hyperpolarization activated currents in mouse cold thermoreceptors. J Physiol 587:1961–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang W, Wang G, Hemmings HC., Jr (2003) Isoflurane and propofol inhibit voltage-gated sodium channels in isolated rat neurohypophysial nerve terminals. Mol Pharmacol 64:373–381 [DOI] [PubMed] [Google Scholar]
- Pistis M, Belelli D, Peters JA, Lambert JJ. (1997) The interaction of general anaesthetics with recombinant GABAA and glycine receptors expressed in Xenopus laevis oocytes: a comparative study. Br J Pharmacol 122:1707–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusinova R, Herold KF, Sanford RL, Greathouse DV, Hemmings HC, Jr, Andersen OS. (2011) Thiazolidinedione insulin sensitizers alter lipid bilayer properties and voltage-dependent sodium channel function: implications for drug discovery. J Gen Physiol 138:249–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer Z, Dubner R, Shir Y. (1990) A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain 43:205–218 [DOI] [PubMed] [Google Scholar]
- Servin F, Desmonts JM, Haberer JP, Cockshott ID, Plummer GF, Farinotti R. (1988) Pharmacokinetics and protein binding of propofol in patients with cirrhosis. Anesthesiology 69:887–891 [DOI] [PubMed] [Google Scholar]
- Sherman SE, Loomis CW. (1994) Morphine insensitive allodynia is produced by intrathecal strychnine in the lightly anesthetized rat. Pain 56:17–29 [DOI] [PubMed] [Google Scholar]
- Smith C, McEwan AI, Jhaveri R, Wilkinson M, Goodman D, Smith LR, Canada AT, Glass PS. (1994) The interaction of fentanyl on the Cp50 of propofol for loss of consciousness and skin incision. Anesthesiology 81:820–828, discussion 26A [PubMed] [Google Scholar]
- Song Y, Connor DT, Doubleday R, Sorenson RJ, Sercel AD, Unangst PC, Roth BD, Gilbertsen RB, Chan K, Schrier DJ, et al. (1999) Synthesis, structure-activity relationships, and in vivo evaluations of substituted di-tert-butylphenols as a novel class of potent, selective, and orally active cyclooxygenase-2 inhibitors. 1. Thiazolone and oxazolone series. J Med Chem 42:1151–1160 [DOI] [PubMed] [Google Scholar]
- Sun YY, Li KC, Chen J. (2005) Evidence for peripherally antinociceptive action of propofol in rats: behavioral and spinal neuronal responses to subcutaneous bee venom. Brain Res 1043:231–235 [DOI] [PubMed] [Google Scholar]
- Tan T, Bhinder R, Carey M, Briggs L. (2010) Day-surgery patients anesthetized with propofol have less postoperative pain than those anesthetized with sevoflurane. Anesth Analg 111:83–85 [DOI] [PubMed] [Google Scholar]
- Tu H, Deng L, Sun Q, Yao L, Han JS, Wan Y. (2004) Hyperpolarization-activated, cyclic nucleotide-gated cation channels: roles in the differential electrophysiological properties of rat primary afferent neurons. J Neurosci Res 76:713–722 [DOI] [PubMed] [Google Scholar]
- Udesky JO, Spence NZ, Achiel R, Lee C, Flood P. (2005) The role of nicotinic inhibition in ketamine-induced behavior. Anesth Analg 101:407–411 [DOI] [PubMed] [Google Scholar]
- Vo T, Rice AS, Dworkin RH. (2009) Non-steroidal anti-inflammatory drugs for neuropathic pain: how do we explain continued widespread use? Pain 143:169–171 [DOI] [PubMed] [Google Scholar]
- Yao H, Donnelly DF, Ma C, LaMotte RH. (2003) Upregulation of the hyperpolarization-activated cation current after chronic compression of the dorsal root ganglion. J Neurosci 23:2069–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeon KY, Chung G, Kim YH, Hwang JH, Davies AJ, Park MK, Ahn DK, Kim JS, Jung SJ, Oh SB. (2011) Eugenol reverses mechanical allodynia after peripheral nerve injury by inhibiting hyperpolarization-activated cyclic nucleotide-gated (HCN) channels. Pain 152:2108–2116 [DOI] [PubMed] [Google Scholar]
- Ying SW, Abbas SY, Harrison NL, Goldstein PA. (2006) Propofol block of I(h) contributes to the suppression of neuronal excitability and rhythmic burst firing in thalamocortical neurons. Eur J Neurosci 23:465–480 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.