Abstract
The monoacylglycerol lipase (MAGL) inhibitor 4-nitrophenyl 4-(dibenzo[d][1,3]dioxol-5-yl(hydroxy)methyl)piperidine-1-carboxylate (JZL184) produces antinociceptive and anti-inflammatory effects. However, repeated administration of high-dose JZL184 (40 mg/kg) causes dependence, antinociceptive tolerance, cross-tolerance to the pharmacological effects of cannabinoid receptor agonists, and cannabinoid receptor type 1 (CB1) downregulation and desensitization. This functional CB1 receptor tolerance poses a hurdle in the development of MAGL inhibitors for therapeutic use. Consequently, the present study tested whether repeated administration of low-dose JZL184 maintains its antinociceptive actions in the chronic constriction injury of the sciatic nerve neuropathic pain model and protective effects in a model of nonsteroidal anti-inflammatory drug–induced gastric hemorrhages. Mice given daily injections of high-dose JZL184 (≥16 mg/kg) for 6 days displayed decreased CB1 receptor density and function in the brain, as assessed in [3H]SR141716A binding and CP55,940 [(−)-cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl) cyclohexanol]-stimulated guanosine 5′-O-(3-[35S]thio)triphosphate binding assays, respectively. In contrast, normal CB1 receptor expression and function were maintained following repeated administration of low-dose JZL184 (≤8 mg/kg). Likewise, the antinociceptive and gastroprotective effects of high-dose JZL184 underwent tolerance following repeated administration, but these effects were maintained following repeated low-dose JZL184 treatment. Consistent with these observations, repeated high-dose JZL184, but not repeated low-dose JZL184, elicited cross-tolerance to the common pharmacological effects of Δ9-tetrahydrocannabinol. This same pattern of effects was found in a rimonabant [(5-(4-chlorophenyl)-1-(2,4-dichloro-phenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide)]-precipitated withdrawal model of cannabinoid dependence. Taken together, these results indicate that prolonged, partial MAGL inhibition maintains potentially beneficial antinociceptive and anti-inflammatory effects, without producing functional CB1 receptor tachyphylaxis/tolerance or cannabinoid dependence.
Introduction
Cannabinoids represent a diverse array of compounds that bind to and activate cannabinoid receptors, and include cannabis-derived chemicals, synthetic ligands, and the endogenous ligands (i.e., endocannabinoids) N-arachidonoylethanolamine (anandamide; AEA) and 2-arachidonoylglycerol (2-AG). It is well established that elevation of either AEA or 2-AG, via inhibition of their respective catabolic enzymes, fatty acid amide hydrolase (FAAH) or monoacylglycerol lipase (MAGL), produces analgesic and anti-inflammatory effects in vivo (Schlosburg et al., 2009b). For example, FAAH inhibition attenuates neuropathic pain (Jhaveri et al., 2006; Russo et al., 2007; Kinsey et al., 2009; Clapper et al., 2010), inflammatory pain (Booker et al., 2011), collagen-induced arthritis (Kinsey et al., 2011a), and gastric ulceration (Naidu et al., 2009; Sasso et al., 2012), as well as elicits anxiolytic-like behavioral effects (Patel and Hillard, 2006; Naidu et al., 2007) in rodents. Similarly, the selective MAGL inhibitor 4-nitrophenyl 4-(dibenzo[d][1,3]dioxol-5-yl(hydroxy)methyl)piperidine-1-carboxylate (JZL184) attenuates nociception in neuropathic (Kinsey et al., 2009) and inflammatory (Ghosh et al., 2012) pain models. JZL184 also decreases gastric hemorrhaging (Kinsey et al., 2011b) and produces anxiolytic-like behavior in mice (Kinsey et al., 2011c) and rats (Sciolino et al., 2011).
Despite the potential beneficial effects of MAGL inhibition, repeated administration of high-dose JZL184 produces tolerance to its antinociceptive effects, cannabinoid receptor type 1 (CB1) downregulation, CB1 receptor desensitization, and decrements in endocannabinoid-dependent synaptic plasticity (Schlosburg et al., 2010). Moreover, whereas the synthetic pan-cannabinoid receptor agonist WIN55,212-2 attenuates neuropathic pain (Rahn et al., 2007), mice treated repeatedly with JZL184 displayed cross-tolerance to the antinociceptive effects of WIN55,212-2 in neuropathic and thermal pain models (Schlosburg et al., 2010).
JZL184 has anti-inflammatory effects in a model of gastric hemorrhagic lesions caused by the nonsteroidal anti-inflammatory drug (NSAID) diclofenac ( 2-(2,6-dichloranilino) phenylacetic acid) sodium (Kinsey et al., 2011b). However, the gastroprotective effects of JZL184 persisted after repeated administration. A key distinction between these two reports is that 4 mg/kg JZL184 was used in the gastric hemorrhage study (Kinsey et al., 2011b), but 40 mg/kg JZL184 was used in the investigation by Schlosburg and colleagues (2010). Similarly, the antinociceptive effects of low-dose JZL184 persisted after repeated administration in the acetic acid-induced abdominal stretching model of visceral pain (Busquets-Garcia et al., 2011) and carrageenan-induced inflammatory pain (Ghosh et al., 2012). However, direct dose-response comparisons have not been performed to test whether repeated administration of low-dose JZL184 leads to CB1 receptor functional tolerance.
The present study tested whether CB1 receptor functional tolerance induced by repeated administration of JZL184 is dose-dependent. Accordingly, five objectives were incorporated to test this hypothesis. First, the impact of repeated JZL184 (1.6–40 mg/kg) administration on CB1 receptor expression and function in the brain was evaluated using [3H]SR141716A (5-(4-chlorophenyl)-1-(2,4-dichloro-phenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide) binding and CP55, 940-stimulated guanosine 5′-O-(3-[35S]thio)triphosphate ([35S]GTPγS) binding. Second, whole brain levels of AEA, 2-arachidonoylglycerol (2-AG), and other relevant lipids were quantified following repeated high-dose JZL184 treatment. Third, we tested the gastroprotective and antiallodynic effects of low-dose and high-dose administration of JZL184 following repeated administration in two whole animal function assays: the nonsteroidal anti-inflammatory drug (NSAID)–induced gastric hemorrhage model and the chronic constriction injury (CCI) neuropathic pain model. Whereas previous research has found that the antiallodynic actions of high-dose JZL184 undergo tolerance following repeated administration in the CCI model, and low-dose JZL184 given acutely or repeatedly blocks the gastric inflammatory effects of the NSAID diclofenac sodium in mice (Kinsey et al., 2011b), the consequences of other doses in these assays have yet to be assessed. Thus, JZL184 (4–40 mg/kg) was administered acutely or repeatedly, followed by gastric hemorrhage induction by the nonsteroidal anti-inflammatory drug diclofenac. A similar schedule was used to test the antiallodynic effects of repeated low-dose and high-dose JZL184 administration in mice subjected to CCI. Fourth, cross-tolerance to the cataleptic, antinociceptive, and hypothermic effects of Δ9-tetrahydrocannabinol (THC; (−)-(6aS,10aS)-6,6,9-trimethyl-3-pentyl-6a,7,8,10a-tetrahydro-6H-benzo[c]chromen-1-ol) were assessed in mice treated repeatedly with low or high JZL184 doses. Previous research demonstrated cross-tolerance between plant-derived, synthetic, and endocannabinoids (Fan et al., 1994; Schlosburg et al., 2010), which further indicates that CB1 receptors can be functionally compromised after repeated administration of cannabinoid receptor agonists. Finally, repeated high-dose JZL184 treatment produces physical dependence similar in magnitude to a moderate THC dosing regimen, as assessed in the rimonabant-precipitated withdrawal assay (Schlosburg et al., 2010). Thus, the fifth objective of this study was to assess somatic cannabinoid withdrawal signs in mice repeatedly administered low- or high-dose JZL184. Here, we report that repeated low-dose JZL184 maintains effectiveness in protecting against NSAID-induced ulcers and reducing neuropathic pain, without the occurrence of CB1 receptor functional tolerance or cannabinoid dependence.
Materials and Methods
Animals.
Subjects were male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME), which were approximately 10 weeks of age at the beginning of the study. Mice were given free access to food and water and were housed in a temperature- (20–22°C) and humidity-controlled, Association for Assessment and Accreditation of Laboratory Animal Care–approved facility. Mice weighed approximately 25 g, and were housed five per cage and maintained on a 12:12 light:dark cycle (lights on at 6:00 AM EST). Mice were randomly assigned to each treatment group. All experiments were approved by the Institutional Animal Care and Use Committee at Virginia Commonwealth University.
Drugs.
JZL184 was synthesized in the Cravatt laboratory as described previously (Long et al., 2009a), diclofenac sodium was purchased from Sigma-Aldrich (St. Louis, MO), and THC and rimonabant were obtained from the National Institute on Drug Abuse (Bethesda, MD). All drugs were dissolved in a vehicle consisting of equal parts ethanol and Alkamuls-620 (Sanofi-Aventis, Bridgewater, NJ), and diluted in 18 parts normal saline. All compounds were administered at a volume of 10 µl/g body mass i.p., with the exception of diclofenac, which was administered per oral (p.o.) route. All solutions were warmed to room temperature prior to administration.
Repeated Dosing Regimen.
For 5 consecutive days, mice were weighed and administered JZL184 (1.6–40 mg/kg) or vehicle, every 24 hours. On day 6, each mouse was given its respective injection of JZL184 or vehicle, followed 2 hours later by behavioral assessment or tissue collection as described previously (Long et al., 2009b; Schlosburg et al., 2010). To address the effects of acute JZL184 treatment, a separate group of mice was treated repeatedly with vehicle, and then administered JZL184 on the test day.
Preparation of Mouse Brain Membrane Proteomes.
Brains from mice were Dounce homogenized in phosphate-buffered saline, pH 7.5, and then underwent a low-speed spin (1400g, 5 minutes) to remove debris. The supernatant was then subjected to centrifugation (64,000g, 45 minutes) to provide the cytosolic fraction in the supernatant and the membrane fraction as a pellet. The pellet was washed and resuspended in phosphate-buffered saline buffer by sonication. Total protein concentration in each fraction was determined using a protein assay kit (Bio-Rad Laboratories, Hercules, CA). Samples were stored at –80°C until use.
Measurement of Brain Lipids.
Following decapitation, brains were rapidly removed, frozen on dry ice, and stored at −80°C. On the day of processing, the preweighed tissues were homogenized with 1.4 ml of chloroform: methanol (2:1 v/v containing 0.0348 g/ml phenylmethylsulfonyl fluoride) after the addition of internal standards to each sample [2 pmol AEA-d8, 1 nmol 2-AG-d8, 3.3 nmol N-palmitoylethanolamine (PEA)-d4, 3 nmol N-oleoylethanolamine (OEA)-d4, and 1 nmol arachidonic acid (AA)-d8]. Homogenates were mixed with 0.3 ml of 0.73% w/v NaCl, vortexed, and centrifuged for 10 minutes at 3220g (4°C). The aqueous phase and debris were collected and extracted again twice with 0.8 ml of chloroform. The organic phases from the three extractions were pooled, and the organic solvents were evaporated under nitrogen gas. Dried samples were reconstituted with 0.1 ml of chloroform and mixed with 1 ml of cold acetone. The mixtures were centrifuged for 5 minutes at 1811g and 4°C to precipitate protein. The upper layer of each sample was collected and evaporated under nitrogen. Dried samples were reconstituted with 0.1 ml of methanol and placed in autosample vials for analysis.
Liquid chromatography-tandem mass spectrometry was used to quantify AEA, 2-AG, PEA, OEA, and AA. The mobile phase consisted of methanol (90:10): 0.1% ammonium acetate and 0.1% formic acid. The column used was a Discovery HS C18, 4.6 × 15 cm, 3 μm (Sigma-Aldrich). Ions were analyzed in multiple reaction monitoring mode, and the following transitions were monitored in positive mode: 348 > 62 and 348 > 91 for AEA, 356 > 62 for AEA-d8, 379 > 287 and 279 > 269 for 2-AG, 387 > 96 for 2AG-d8, 300 > 62 and 300 > 283 for PEA, 304 > 62 for PEA-d4, 326 > 62 and 326 > 309 for OEA, and 330 > 66 for OEA-d4; the following transitions were monitored in negative mode: 303 > 259 and 303 > 59 for AA and 311 > 267 for AA-d8.
A calibration curve was constructed for each assay based on linear regression using the peak area ratios of the calibrators. The extracted standard curves ranged from 0.039 to 40 pmol for AEA, from 0.0625 to 64 nmol for 2-AG, from 0.156 to 0.5 nmol for PEA and OEA, and from 0.1 to 32 nmol for AA.
Agonist-Stimulated [35S]GTPγS Binding.
Mice were injected with JZL184 or vehicle for 5 consecutive days, and after 24 hours were humanely euthanized. The brains were removed, hemisected along the midsagital plane, flash-frozen in liquid nitrogen, and stored at −80°C until the assay for GTPγS, as described previously (Sim-Selley et al., 2006). One half of each brain was placed in 15 ml of cold TME [tris(hydroxymethyl)aminomethane, 10 mM MgCl2, 1 mM EDTA] membrane buffer (50 mM Tris-HCl, 3 mM MgCl2, 1 mM EGTA, pH 7.4) and homogenized. The other half of each brain was processed for radioligand binding, as described later. The samples were then centrifuged at 50,000g for 10 minutes at 5°C. The supernatant was removed and samples were resuspended in 15 ml of TME membrane buffer. Centrifugation was repeated, the pellet resuspended in TME buffer, and the protein concentration determined. Membranes then were pretreated with adenosine deaminase (10 mU/ml) for 15 minutes at 30°C. Membrane protein (10 μg) was incubated in TME/Na (assay buffer with 100 mM NaCl) with 0.1% bovine serum albumin (BSA), 30 μM GDP, 0.1 nM [35S]GTPγS, and varying concentrations of CP55,940 (0.01–10 μM) for 2 hours at 30°C. Nonspecific binding was determined using 20 μM unlabeled GTPγS. Basal binding was determined in the absence of agonist. The incubation was terminated by rapid filtration through glass fiber filters (Grade GF/B) and three washes with ice-cold Tris-HCl (pH 7.4). Liquid scintillation spectrophotometry was used to evaluate bound radioactivity after the extraction of filters in Budget-Solve (Research Products InternationaI Corp., Mount Prospect, IL) scintillation fluid.
[3H]SR141716A Binding.
Half of each brain from mice treated with JZL184 or vehicle for 5 consecutive days, as described earlier, was placed in 15 ml of cold TME membrane buffer (50 mM Tris-HCl, 3 mM MgCl2, 1 mM EGTA, pH 7.4) and homogenized, as described earlier. Saturation analysis was performed by incubating 30 μg of membrane protein with 0.2–3 nM [3H]SR141716A (rimonabant; CB1 receptor antagonist) in assay buffer A + BSA (0.5 per g) with 2 μM of unlabeled rimonabant for 90 minutes at 30°C to determine nonspecific binding. Total binding was determined in the absence of unlabeled rimonabant. The reactions were terminated using vacuum filtration through a Whatman GF/B glass fiber filter (Sigma-Aldrich) presoaked in Tris buffer containing 5 g of L-1 BSA (Tris-BSA) and three washes with 4°C Tris-BSA. Bound radioactivity was evaluated by liquid scintillation spectrophotometry at 45% efficiency after extraction in Budget-Solve scintillation fluid.
Neuropathic Pain Model.
Mice were subjected to CCI of the sciatic nerve, as described previously (Shimoyama et al., 2002). Under isoflurane anesthesia, the right hind leg was shaved and the area wabbed with Betadine solution (Purdue Product, L.P., Stamford, CT), followed by ethanol. A small incision was made in the skin posterior to the femur, the muscle was separated, and the sciatic nerve was isolated and ligated twice with a 5-0 (1.0 metric) black silk braided suture (Surgical Specialties Corporation, Reading, PA). The surrounding muscle and skin were then sutured with 6-0 nylon. Mice were placed in a heated cage to recover from anesthesia before being returned to the vivarium.
Mice were tested for allodynia prior to, and 10 days after, CCI surgery to establish baseline levels of allodynia. The mice were treated for 5 consecutive days with JZL184 (4 or 40 mg/kg i.p.) or vehicle, and tested 2 hours after administration on the sixth day. Cross-tolerance to THC was tested 24 hours later (i.e., day 7). On each test day, mice were weighed and individually placed in ventilated plastic cylinders on an aluminum mesh table and allowed to acclimate to the apparatus for 60 minutes prior to testing, as described previously (Kinsey et al., 2009). For the acetone-induced cold allodynia test, 10 µl of acetone (99% high-performance liquid chromatography grade; Fisher Scientific, Pittsburgh, PA) was projected via air burst, using a 100-µl pipette (Rainin Instruments, Oakland, CA), onto the plantar surface of each hind paw (Choi et al., 1994; Decosterd and Woolf, 2000). Total time lifting or clutching each paw was recorded, with a maximum cutoff time of 20 seconds (Decosterd and Woolf, 2000). Approximately 45 minutes later, mechanical allodynia was assessed with von Frey filaments (North Coast Medical, Morgan Hill, CA), using the “up-down” method (Chaplan et al., 1994; Kinsey et al., 2009). The plantar surface of each hind paw was stimulated five times with each filament (0.16–6.0 g), at a frequency of roughly 2 Hz, starting with the 0.6 g filament. Positive responses consisted of paw clutching, lifting, shaking, or licking. Three or more positive responses per filament were coded as a threshold response. Once a threshold response was detected, sequentially lower weight filaments were used to assess minimum paw withdrawal threshold. The experimenter was blinded with respect to treatment conditions in these experiments.
Gastric Hemorrhage Assay.
Gastric lesions were induced based on published methods (Sanchez et al., 2002; Kinsey et al., 2011b). Briefly, mice were food deprived (with free access to water) for 22 hours, injected with JZL184 (4 or 40 mg/kg i.p.) or vehicle, and then, at 24 hours, administered diclofenac (100 mg/kg p.o.) or vehicle and returned to the home cage. Mice were administered JZL184 or vehicle for 5 consecutive days prior to the test day (a total of 6 consecutive days of JZL184 treatment). The acute effects of vehicle, 4, 16, and 40 mg/kg JZL184 on diclofenac-induced gastric ulcers are reprinted from Kinsey et al. (2011b). However, both the acute and repeated JZL184 experiments were conducted within the same time frame. At 30 hours, the mice were humanely euthanized via rapid cervical dislocation, and stomachs were harvested and opened along the greater curvature, rinsed with distilled water, and photographed on a backlit stage using a Canon EOS Rebel XS digital camera with a Canon 250D close-up lens (Adorama Inc., New York, NY). The length of each hemorrhage was marked with a 1-pixel-wide line and compared with the reference, such that the total hemorrhage score in millimeters for each stomach = pixels (hemorrhage) / pixels (Liu et al., 1998; Kinsey et al., 2011c). Images were scored by an experimenter who was blinded to the treatment conditions.
Cannabimimetic Behavior Assessments.
Mice were acclimated to the test room for at least 1 hour prior to testing for catalepsy, antinociception, and hypothermia (Wiley and Martin, 2003). The mice were treated for 5 consecutive days with JZL184 (4 or 40 mg/kg, i.p.) or vehicle, and cross-tolerance to THC was tested on the sixth day. To reduce the total number of animals required for these experiments, a within-subjects cumulative dosing design was used. Baseline rectal temperature and nociception were assessed, and then the mice received an increased dose of THC (i.p.) every 40 minutes, and were tested 30 minutes after each subsequent injection. Thus, the testing battery was completed in less than 4 hours (Falenski et al., 2010). Mice were tested for catalepsy, then nociception, and finally rectal temperature. Catalepsy was measured using the bar test, in which both forelimbs of each mouse were placed on a horizontal bar during a 60-second test. Hypothermia was quantified by change in rectal body temperature from baseline. Antinociception was measured via the tail immersion test, in which the distal 2 cm of the tail was immersed in 52.0°C water, with latency to withdraw the tail as the dependent variable. A maximum cutoff of 20 seconds was used to minimize possible tissue damage.
Rimonabant Precipitated Cannabinoid Withdrawal.
Mice were given an i.p. injection of JZL184 (4, 8, 16, or 40 mg/kg) every 24 hours for 6 days. On day 6, subjects were given their final injection of JZL184, placed in the test chamber for 0.5 hour to acclimate, given rimonabant (10 mg/kg i.p.), and immediately returned to the test chamber for 1-hour testing. Behavior was recorded using a Fire-i digital camera (Unibrain, San Ramon, CA), and the videos were recorded using the ANY-maze video tracking software (Stoelting Co., Wood Dale, IL). Videos were sampled at alternate 5-minute intervals starting at 5 minutes post rimonabant injection (i.e., 5–10 minutes, 15–20 minutes, etc.). The recorded videos were randomized and scored by an observer, who was blinded to treatment. Behavioral signs common to cannabinoid withdrawal in mice, including front paw tremors (i.e., paw flutters and twitches) and head twitches (i.e., rotational shakes of the head along the horizontal, usually referred to as “wet dog shakes” in rats), as well as rimonabant-induced hind leg scratching were quantified as described previously (Schlosburg et al., 2009b; Falenski et al., 2010). All behaviors were counted as new incidences when separated by at least 1 second or interrupted by any other normal behavior of the mouse. Previous reliability studies from our laboratory revealed inter-rater reliability correlations (r > 0.90) for each measure. The test chamber was composed of contrasting white acrylic (20 × 20 cm), with a clear acrylic front panel and a mirrored back panel. The chambers were enclosed in sound-attenuating cabinets that contained an indirect filtered light-emitting diode light source and fans for air circulation and white noise. Chambers were cleaned with water in between each test.
Data Analysis.
Data are reported as the mean ± S.E.M. and were analyzed using analysis of variance (ANOVA), followed by Dunnett’s post-hoc test, comparing each dose to vehicle control groups, with the exception of the liquid chromatography-tandem mass spectrometry data. These data were analyzed using one-way ANOVA, and planned comparisons were made between drug treatments and vehicle, consisting of Bonferroni corrected t tests. [3H]SR141716A and [35S]GTPγS binding data were fit by nonlinear regression analysis to obtain Bmax and KD or Emax and EC50, values, respectively. The pharmacological effects of THC were analyzed using a mixed-design ANOVA (JZL184 pretreatment was the between-subjects variable, and THC dose was the within-subjects variable) followed by Dunnett’s test to compare each JZL184 pretreatment with vehicle at individual THC doses. Differences of P < 0.05 were considered statistically significant.
Results
CB1 Receptor Activity and Expression Are Maintained Following Repeated Administration of Low-Dose JZL184.
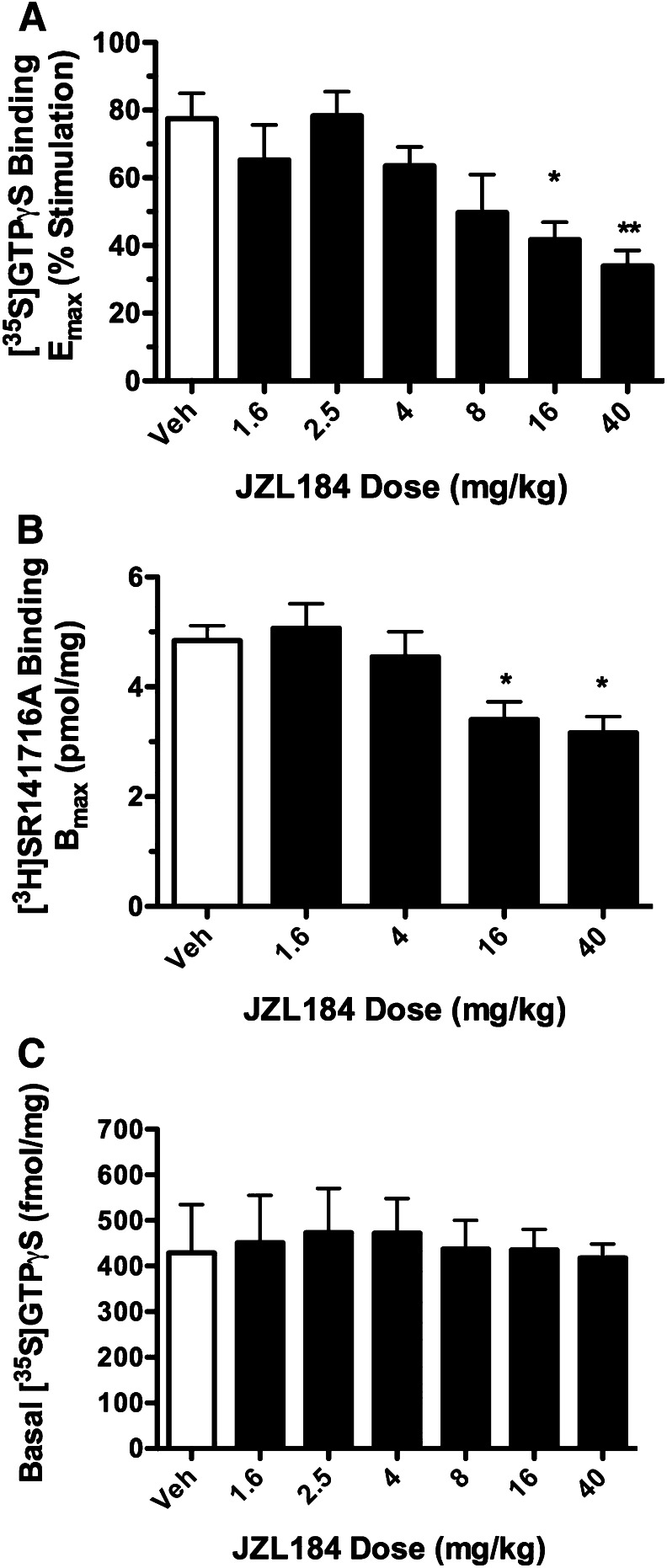
To compare the effects of repeated administration of low versus high doses of JZL184 on CB1 receptor activity in the brain, mice were injected with varying doses over a 25-fold range (1.6–40 mg/kg JZL184 i.p.) or vehicle for 5 consecutive days. CB1 receptor–mediated G-protein activation was examined using concentration-effect curves of CP55,940–stimulated [35S]GTPγS binding in membranes prepared from mouse brain homogenates. As shown in Fig. 1A, repeated dosing with 16 and 40 mg/kg JZL184 produced, respectively, 46 and 56% reductions in maximal CP55,940-stimulated G-protein activity (Emax), with no effect of either JZL184 dose on CP55,940 EC50 values (Table 1). In contrast, low JZL184 doses (1.6, 4, or 8 mg/kg) did not significantly affect CP55,940 Emax (Fig. 1A) or EC50 values. To determine whether effects of JZL184 on CB1 receptor–mediated G-protein activation were associated with decreased CB1 receptor levels, saturation binding analysis with the CB1-selective antagonist [3H]SR141716A ([3H]rimonabant) was conducted in mice repeatedly administered JZL184 (1.6, 4, 16, or 40 mg/kg) or vehicle, as described earlier. Repeated dosing with 16 and 40 mg/kg JZL184 reduced [3H]SR141716A Bmax values by 30 and 35%, respectively. However, repeated administration of low doses of JZL184 did not significantly alter [3H]SR141716A Bmax values (Fig. 1B). Basal [35S]GTPγS binding was not affected by repeated treatment with any dose of JZL184 (P = 0.998; Fig. 1C). Moreover, [3H]SR141716A KD values were unaffected by repeated treatment with any of the JZL184 doses tested.
Fig. 1.
CB1 receptor activity and expression are attenuated following high-dose JZL184, but are maintained following repeated administration of low-dose JZL184. Mice were treated for 5 days with vehicle or JZL184 (i.p.), euthanized on day 6, and then brain homogenates were used to test CP55,940-stimulated [35S]GTPγS binding (A) and membrane-specific CB1 receptor binding by the antagonist [3H]SR141716A (B). (C) Baseline [35S]GTPγS binding did not differ by JZL184 treatment. Whereas repeated treatment with JZL184 doses ≥16 mg/kg attenuated CB1 receptor binding and activity, lower doses of JZL184 did not produce this adaptation. Data presented as the mean ± S.E.M. (n = 5–6). *P < 0.05; **P < 0.01 versus vehicle (Veh).
TABLE 1.
Curve-fit values from CP55,940-stimulated [35S]GTPγS binding and [3H]SR141716A saturation binding
Values represent the mean ± S.E.M. and were derived from nonlinear fitting of the data shown in Fig. 1.
| JZL-184 Dose | CP55,940-Stimulated [35S]GTPγS Binding |
[3H]SR141716A Binding |
||
|---|---|---|---|---|
| Emax | EC50 | Bmax | KD | |
| % Stim. | nM | pmol/mg | nM | |
| Vehicle (mg/kg) | 74.3 ± 8.2 | 6.2 ± 1.0 | 4.84 ± 0.26 | 1.03 ± 0.10 |
| 1.6 | 65.3 ± 10.3 | 7.9 ± 2.0 | 5.07 ± 0.44 | 1.10 ± 0.09 |
| 2.5 | 78.3 ± 7.1 | 5.8 ± 0.7 | N.D. | N.D. |
| 4 | 64.4 ± 5.7 | 8.6 ± 1.7 | 4.55 ± 0.45 | 1.23 ± 0.14 |
| 8 | 49.7 ± 11.2 | 5.8 ± 1.3 | N.D. | N.D. |
| 16 | 41.7 ± 5.1* | 7.6 ± 0.8 | 3.40 ± 0.32* | 1.11 ± 0.14 |
| 40 | 33.9 ± 4.6** | 8.3 ± 1.7 | 3.16 ± 0.29* | 1.17 ± 0.16 |
N.D., not determined; % Stim., % stimulation.
P < 0.05; **P < 0.01 versus vehicle-treated (Dunnett’s post-hoc test).
Brain Endocannabinoid Levels and Hydrolysis Are Differentially Affected by Repeated Low Versus High Dosing of JZL184.
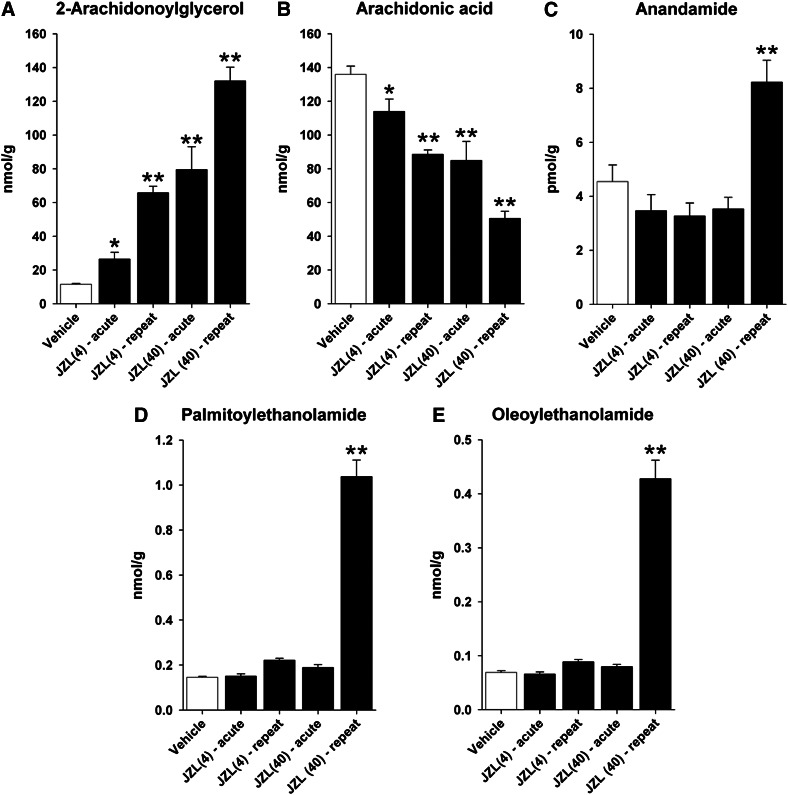
JZL184 significantly increased brain levels of 2-AG [F(4,25) = 40.8; P < 0.0001; Fig. 2A]. Planned comparisons revealed that all treatments significantly increased brain 2-AG levels (4 mg/kg: acute = 2.3-fold, repeated = 5.7-fold; 40 mg/kg: acute = 6.9-fold, repeated = 11.4-fold increase). Similarly, JZL184 significantly decreased brain levels of AA [F(4,25) = 22.8; P < 0.0001; Fig. 2B]. Significant effects of JZL184 treatment were found for AEA [F(4,25) = 12.1; P < 0.0001; Fig. 2C], PEA [F(4,25) = 132.3; P < 0.0001; Fig. 2D], and OEA [F(4,25) = 132.3; P < 0.0001; Fig. 2E]. Planned comparisons revealed that these increases in brain levels of fatty acid derivatives were driven by repeated administration of 40 mg/kg JZL184, as previously reported (Schlosburg et al., 2010).
Fig. 2.
Brain endocannabinoid, arachidonic acid, and fatty acid derivative levels are differentially affected by acute versus repeated administration and low versus high doses of JZL184. Acute and repeated low- (4 mg/kg) and high-dose (40 mg/kg) JZL184 significantly increased brain levels of 2-AG (A) and decreased brain AA levels (B). (C–E) Repeated administration of high-dose JZL184 increased levels of AEA, PEA, and OEA, whereas acute high-dose as well as acute or repeated low-dose JZL184 had no effect. Data presented as the mean ± S.E.M. (n = 6). *P < 0.05; **P < 0.01 versus vehicle.
Repeated Low-Dose JZL184 Administration Maintains Antiallodynic Effects in the CCI Model of Neuropathic Pain.
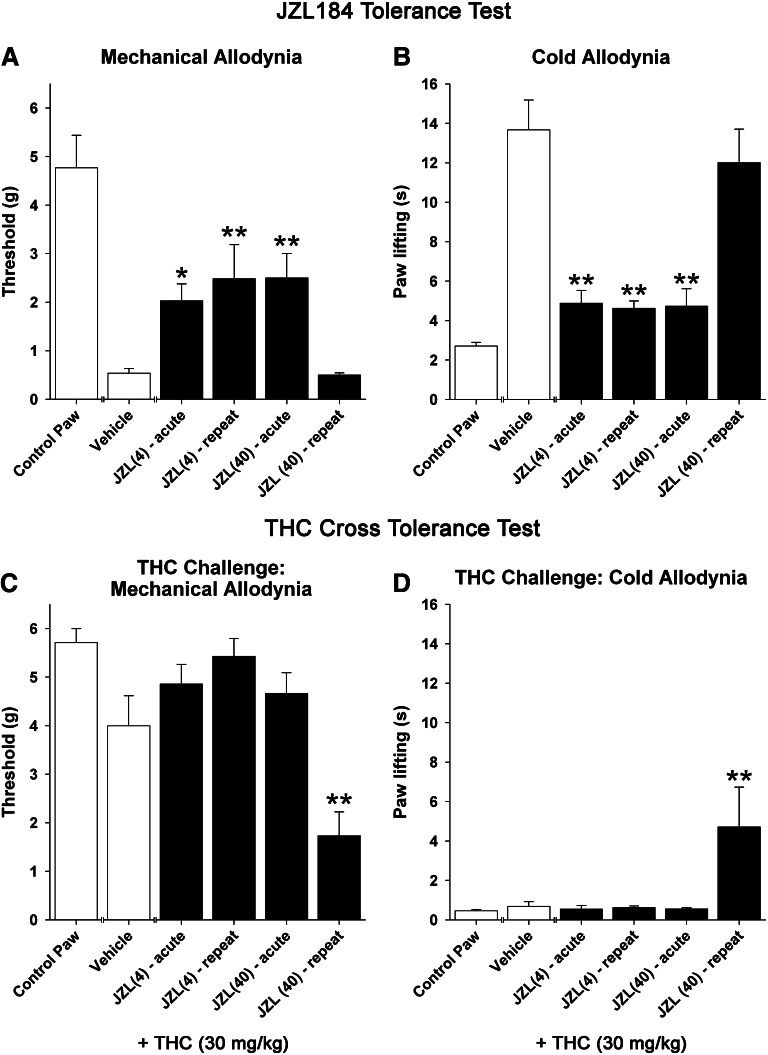
Acute JZL184 caused a significant reduction in cold and mechanical allodynia at 4 mg/kg and higher (Fig. 3). In separate groups of mice, acute and repeated JZL184 treatment significantly attenuated cold allodynia [F(4,29) = 16.9; P < 0.0001; Fig. 3A] and mechanical allodynia [F(4,29) = 6.0; P < 0.01; Fig. 3B]. Post-hoc analysis revealed that daily administration of JZL184 (4 mg/kg) for 6 days remained effective in reducing both cold and mechanical allodynia, whereas the antiallodynic effects of high-dose JZL184 (40 mg/kg) underwent tolerance, as this group did not differ from the vehicle group. Baseline allodynia values did not differ between groups prior to JZL184 treatment in either the acetone (pre-CCI: P = 0.95; post-CCI: P = 0.84) or von Frey (pre-CCI: P = 0.20; post-CCI: P = 0.96) assays. Drug treatment had no effect on either measure in the contralateral, control paws.
Fig. 3.
Repeated low-dose JZL184 administration continues to reduce nociceptive behavior in the CCI of the sciatic nerve model of neuropathic pain, but these antinociceptive effects undergo tolerance following repeated high-dose JZL184. Acute or repeated JZL184 treatment significantly attenuated mechanical allodynia (A) and cold allodynia (B). Repeated, 6-day administration of JZL184 (4 mg/kg) remained effective at reducing both cold and mechanical allodynia, whereas JZL184 (40 mg/kg) did not differ from vehicle treatment. (C and D) Twenty-four hours later, mice given repeated high-dose JZL184 displayed cross-tolerance to THC (30 mg/kg i.p.), whereas THC retained its effectiveness in mice treated repeatedly with low-dose JZL184. Control paw represents contralateral paws of vehicle-treated mice. Data presented as the mean ± S.E.M. (n = 6–7). *P < 0.05; **P < 0.01 versus vehicle.
Twenty-four hours after tolerance testing, mice were assessed for cross-tolerance to THC (30 mg/kg). Significant effects were found for both cold allodynia [F(4,29) = 4.51; P < 0.01; Fig. 3C] and mechanical allodynia [F(4,29) = 10.4; P < 0.0001; Fig. 3D]. THC completely reversed cold and mechanical allodynic responses in each group, except the mice treated repeatedly with high-dose JZL184 (40 mg/kg), which were resistant to the antiallodynic effects of high-dose THC.
Repeated Low-Dose JZL184 Blocks NSAID-Induced Gastric Hemorrhages.
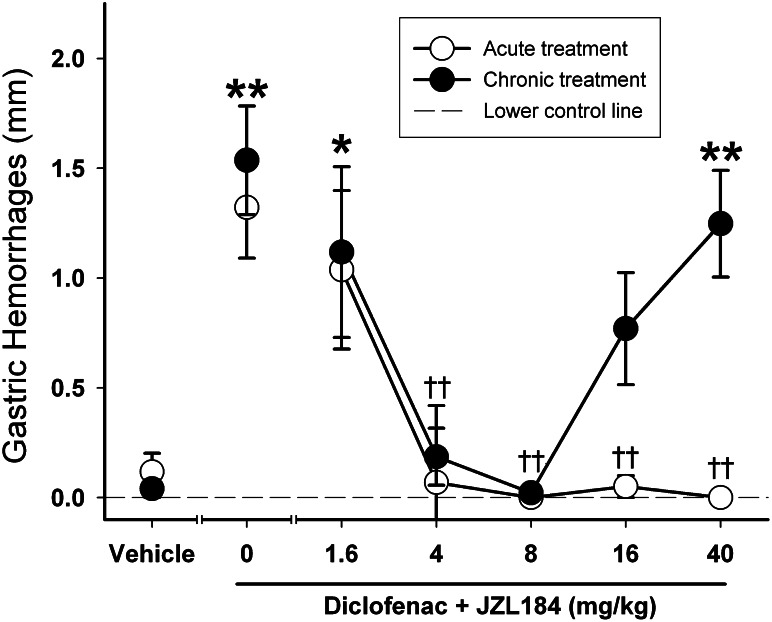
We previously reported that acute JZL184 treatment significantly reduced diclofenac-induced gastric hemorrhages [F(6,61) = 12.8; P < 0.0001; data reproduced in Fig. 4] at 4, 16, or 40 mg/kg (Kinsey et al., 2011b). Repeated JZL184 treatment significantly reduced diclofenac-induced gastric hemorrhages in fasted mice [F(6,51) = 7.9; P < 0.0001]. Post-hoc analysis revealed that the antihemorrhagic effects of repeated JZL184 persisted after repeated administration of 4 or 8 mg/kg, but was ineffective after repeated administration of the high doses of 16 or 40 mg/kg.
Fig. 4.
JZL184 significantly reduced gastric hemorrhages induced by diclofenac sodium (100 mg/kg p.o.) in fasted mice. Repeated low-dose JZL184 treatment significantly reduced NSAID-induced gastric hemorrhages, but this gastroprotective effect underwent tolerance with high-dose JZL184. Acute data depicting vehicle, 4, 16, and 40 mg/kg JZL184 are reprinted from Kinsey et al. (2011b). Data presented as the mean ± S.E.M. (n = 8–12). *P < 0.05; **P < 0.01 versus vehicle; ††P < 0.01 versus diclofenac treatment.
Repeated Administration of High-Dose, but Not Low-Dose, JZL184 Leads to THC Cross-Tolerance.
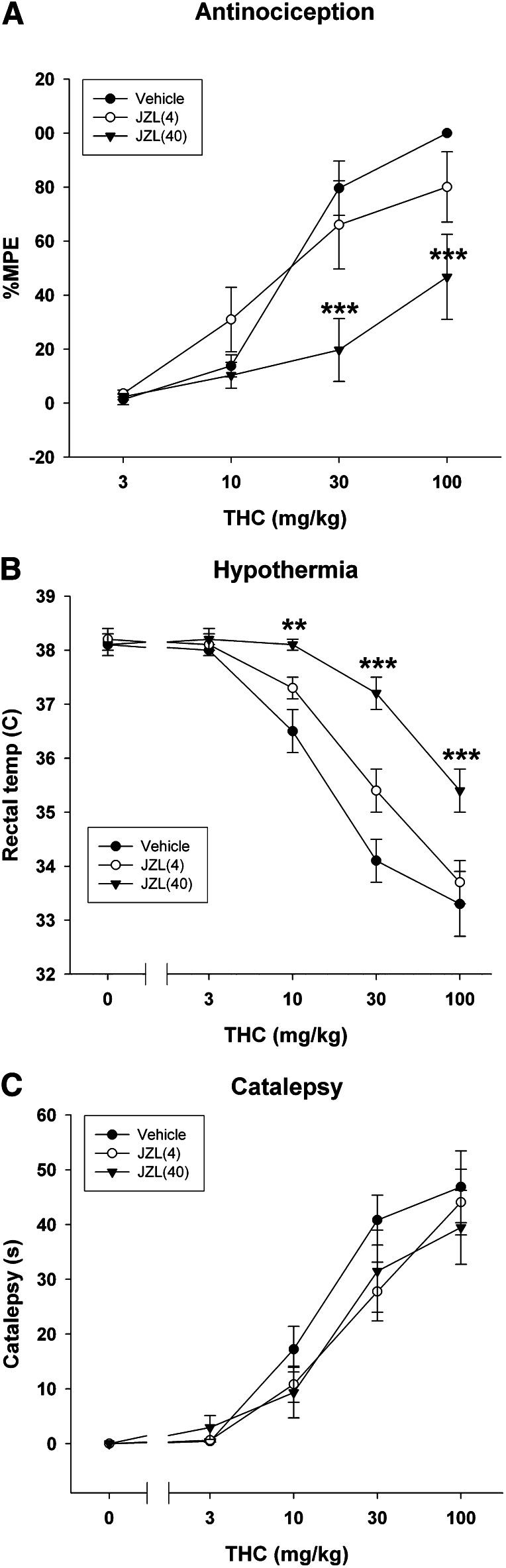
Cross-tolerance to THC after prolonged MAGL inhibition was measured using three tests sensitive to the pharmacological effects of THC. THC significantly increased tail withdrawal latencies in the tail immersion test [F(3,60) = 51.9; P < 0.0001; Fig. 5A], although this antinociceptive effect was significantly attenuated in mice repeatedly administered 40 mg/kg JZL184. Similarly, THC caused a significant decrease in body temperature [F(4,84) = 93.2; P < 0.001; Fig. 5B], which was significantly attenuated in mice repeatedly administered 40 mg/kg JZL184. However, THC elicited catalepsy irrespective of repeated JZL184 treatment [F(4,84) = 75.48; P < 0.0001; Fig. 5C].
Fig. 5.
THC-sensitive effects are maintained after repeated low-dose JZL184 administration, but cross-tolerance to THC develops after repeated high-dose JZL184 administration. Mice were administered daily injections of JZL184 (4 or 40 mg/kg i.p.) or vehicle for 5 days. On the sixth day, mice were injected with increasing doses of THC, and then analgesia (A), hypothermia (B), and catalepsy (C) were tested. Data presented as the mean ± S.E.M. (n = 6–7). **P < 0.01; ***P < 0.001 versus vehicle. MPE, maximal possible effect.
Precipitated Somatic Cannabinoid Withdrawal Signs Occur after Repeated High-Dose, but Not Low-Dose, JZL184 Administration.
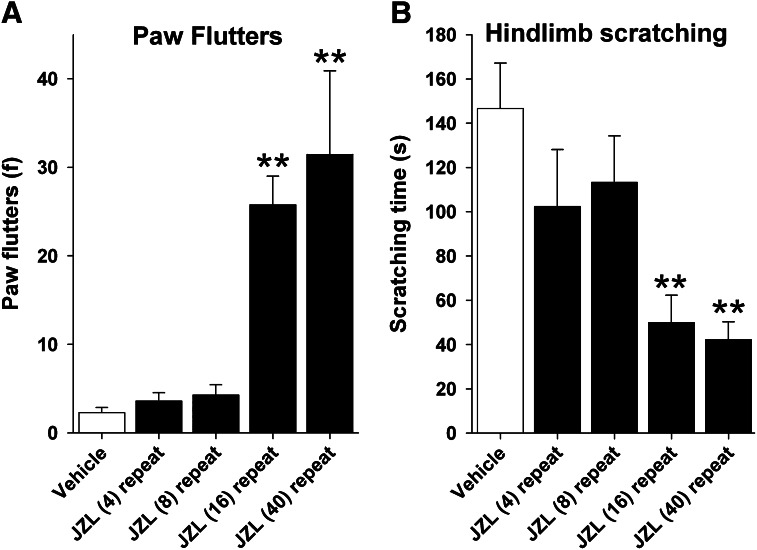
Rimonabant challenge produced a significant increase in paw flutters in mice given repeated injections of high-dose JZL184 [F(4,42) = 7.55; P < 0.0001; Fig. 6A]. Similarly, rimonabant-induced hind limb scratching was significantly decreased by repeated JZL184 treatment [F(4,42) = 6.01; P < 0.001; Fig. 6B]. Post-hoc analyses revealed that these differences were evident in mice repeatedly administered 16 or 40 mg/kg JZL184. No significant effects of JZL184 treatment on head twitches were found (mean = 3.6; P > 0.84).
Fig. 6.
Repeated high-dose JZL184, but not low-dose JZL184, results in cannabinoid dependence. Mice were treated (i.p.) with JZL184 every 24 hours for 6 days, and then rimonabant-induced somatic cannabinoid withdrawal signs (i.e., paw flutters) (A) and rimonabant-induced scratching (B) were quantified on the 6th day. Data presented as the mean ± S.E.M. (n = 6–7). **P < 0.01 versus vehicle.
Discussion
The present studies were designed to test the hypothesis that CB1 receptor functional tolerance occurs following repeated administration of high-dose JZL184 (i.e., 40 mg/kg), but not after repeated administration of low-dose JZL184 (i.e., 4 mg/kg). Here, we report that the effectiveness of JZL184 following repeated administration in the CCI and diclofenac-induced ulcer models was related to dose. Specifically, JZL184 maintained its antiallodynic and gastroprotective actions following repeated administration of low-dose JZL184, but these effects underwent tolerance following repeated administration of high doses. Acute doses as low as 4 mg/kg attenuated CCI-induced allodynia and NSAID-induced gastric hemorrhages, which persisted after repeated administration. In agreement with previous reports (Schlosburg et al., 2010; Kinsey et al., 2011b; Ghosh et al., 2012), the behavioral and anti-inflammatory effects of JZL184 (≥16 mg/kg) underwent tolerance. The present study also systematically investigated the consequences of repeated administration of a broad dose range of JZL184 (1.6–40 mg/kg) on CB1 receptor expression and function in the brain. CB1 receptor binding and CP55,940-stimulated [35S]GTPγS binding were decreased by high, but not low, doses of JZL184. Additionally, mice treated repeatedly with high-dose JZL184 demonstrated cross-tolerance to the antiallodynic, analgesic, and hypothermic effects of THC. However, prolonged treatment with low-dose JZL184 did not lead to cross-tolerance. Finally, cannabinoid somatic withdrawal symptoms were induced in mice treated repeatedly with high (i.e., 16 or 40 mg/kg), but not low (i.e., 4 or 8 mg/kg), doses of JZL184.
The plethora of pharmacological effects of THC, including static ataxia, reduced locomotor activity, hypothermia, and antinociception, are well known to undergo tolerance following repeated drug administration, as well as cross-tolerance to synthetic cannabinoids (Dewey et al., 1972; Fan et al., 1994). Both pharmacokinetic and pharmacodynamic adaptations have been found following repeated THC administration (Lazenka et al., 2012). With respect to drug absorption, repeated administration of THC to dogs led to decreased levels of THC equivalents in the brain as compared with animals receiving an acute THC injection (Martin et al., 1976). In addition, a considerable body of evidence indicates that subacute THC causes changes in receptor signaling, including CB1 receptor downregulation and functional tolerance in discrete brain areas (Breivogel et al., 1999; Sim-Selley, 2003), as well as cross-tolerance to synthetic agonists (Fan et al., 1994). Indeed, cross-tolerance to THC-induced catalepsy was not observed following repeated administration of high-dose JZL184 in the present study. Schlosburg et al. (2010) similarly reported a lack of cross-tolerance to the cataleptic effects of WIN55,212-2 in mice treated repeatedly with 40 mg/kg JZL184. Thus, the observations of decreased antinociceptive and anti-inflammatory effects of JZL184 following repeated high-dose administration are likely due to decreases in expression and functional activity of CB1 receptors in key circuits mediating these actions.
The observation that repeated rimonabant administration prevents CB1 receptor functional tolerance produced by repeated high-dose JZL184 treatment (Schlosburg et al., 2010) indicates that this phenomenon is mediated by increased levels of endocannabinoids acting at CB1 receptors. JZL184 elevated 2-AG brain levels as a function of both dose and repeated treatment. However, these effects cannot be attributed exclusively to 2-AG. In addition to blocking MAGL, JZL184 inhibits FAAH, which leads to increased brain AEA levels following repeated high-dose treatment. Thus, it is unclear whether elevated brain AEA levels contribute to the CB1 receptor functional tolerance produced by repeated high-dose JZL184 administration. Likewise, repeated high-dose JZL184 increased PEA and OEA levels, which are agonists for peroxisome proliferator-activated receptor α receptors and have anti-inflammatory effects in vivo (Lo Verme et al., 2005). However, increasing brain levels of AEA and other fatty acid amides does not appear to be a requisite for this phenomenon. Specifically, MAGL(−/−) mice show a decrease in CB1 receptor function, despite expressing wild-type levels of AEA, OEA, and PEA (Schlosburg et al., 2010). Additionally, FAAH(−/−) mice or wild-type mice given repeated injections of a FAAH inhibitor show increased brain levels of AEA and other fatty acid amides, but have normal 2-AG levels and show normal CB1 receptor function (Schlosburg et al., 2010).
JZL184 increases 2-AG brain levels while concomitantly reducing brain levels of arachidonic acid (Nomura et al., 2011). Arachidonic acid is required for the synthesis of prostaglandins, which are involved in a plethora of physiologic regulatory processes, including inflammation. Indeed, acute high-dose JZL184 (40 mg/kg i.p.) attenuates mitogen-stimulated brain levels of the proinflammatory cytokines interleukin-1α (IL-1α), IL-1β, IL-6, and tumor necrosis factor α (TNF-α) through a cannabinoid receptor independent mechanism of action (Nomura et al., 2011). Likewise, Kerr et al. (2012) reported that acute administration of JZL184 in rats led to reduced levels of arachidonic acid in the frontal cortex, although not in the spleen. Consistent with the observation that JZL184 is considerably less potent in inhibiting MAGL in rats than in mice (Long et al., 2009c), no changes in 2-AG concentrations were detected in the frontal cortex or spleen. Nonetheless, JZL184 significantly attenuated lipopolysaccharide-induced increases in TNF-α and IL-10 in plasma, effects that were differentially attenuated by CB1 and cannabinoid receptor type 2 antagonists. Thus, the consequences of repeated JZL184 administration on NSAID-induced gastric ulceration or altered neuroinflammation and nociception resulting from sciatic nerve injury may not only be limited to alterations of cannabinoid receptor function, but also could depend on changes in arachidonic acid metabolites.
Repeated administration of high-dose JZL184 produced cross-tolerance to the hypothermic and antinociceptive effects of THC. Although locomotor activity was not quantified in the present study, THC (30 mg/kg) produced marked reductions in activity and responsiveness to external stimuli in each group, except mice that received repeated injections of 40 mg/kg JZL184. In contrast, no cross-tolerance to THC-induced catalepsy was observed. This pattern of results is similar as that previously reported for JZL184 cross-tolerance to WIN55,212-2 (Schlosburg et al., 2010), and may be indicative of differential degrees of tolerance across brain regions. Indeed, repeated administration of JZL184 (40 mg/kg) causes region-specific downregulation and desensitization of CB1 receptors in the hippocampus, cingulate cortex, somatosensory cortex, and periaqueductal gray of mouse brain, but no significant alterations in the caudate putamen, globus pallidus, hypothalamus, substantia nigra, or cerebellum (Schlosburg et al., 2010). Similarly, repeated THC administration causes regionally dependent reductions in CB1 receptor agonist–stimulated [35S]GTPγS binding, including large decreases in the hippocampus, cingulate cortex, periaqueductal gray, and cerebellum, while the striatum is resistant to these adaptive changes (McKinney et al., 2008).
A consequence of repeated administration of cannabinoid receptor agonists is dependence. Rimonabant challenge to THC-dependent mice precipitates somatic withdrawal signs (Lichtman et al., 2001; Schlosburg et al., 2009b). Congruent with previous results, rimonabant precipitated cannabinoid withdrawal signs in mice given repeated administration of high-dose JZL184 (i.e., 16 or 40 mg/kg), but not low-dose JZL184 (i.e., 4 or 8 mg/kg). A common side effect of rimonabant is scratching behavior (Schlosburg et al., 2009a). Although rimonabant-induced scratching was significantly suppressed in mice repeatedly treated with high-dose JZL184, this response was preserved in mice treated with low doses, which still elicited antiallodynic and anti-inflammatory effects. Thus, low-dose JZL184 continues to produce antinociceptive and gastroprotective effects after repeated administration, without concomitant CB1 receptor–mediated tolerance or dependence.
The MAGL inhibitor JZL184 reliably reduces nociceptive behavior in inflammatory and neuropathic models of pain (Kinsey et al., 2009; Booker et al., 2011; Ghosh et al., 2012), blocks NSAID-induced gastric inflammation (Kinsey et al., 2011b), and attenuates anxiety-like behaviors (Kinsey et al., 2011c; Sciolino et al., 2011). However, a critical barrier in the development of MAGL inhibitors as potential therapeutic treatments for a range of ailments is the occurrence of CB1 receptor functional tolerance and dependence following repeated administration (Schlosburg et al., 2010). The results of the present studies indicate that the previously reported behavioral and cellular effects of tolerance to high-dose JZL184 can be avoided by decreasing the dose. These findings reveal that JZL184 can partially reverse mechanical and cold allodynia at substantially lower doses than previously reported, and can be administered repeatedly to increase brain 2-AG levels, without altering CB1 receptor function or causing dependence. Collectively, these data indicate that partial inhibition of MAGL represents a potential therapeutic strategy for the treatment of neuropathic pain, NSAID-induced gastric ulcerations, and other ailments.
Acknowledgments
The authors thank Justin Poklis, Scott O’Neal, Irina Beletskaya, and Carlotta Jackson for their technical assistance.
Abbreviations
- 2-AG
2-arachidonoylglycerol
- AA
arachidonic acid
- AEA
anandamide, (N-arachidonoylethanolamine)
- 2-AG
2-arachidonoylglycerol
- ANOVA
analysis of variance
- BSA
bovine serum albumin
- CB1
cannabinoid receptor type 1
- CCI
chronic constriction injury
- CP55,940
2-[(1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl) cyclohexyl]-5-(2-methyloctan-2-yl)phenol
- diclofenac sodium
2-(2,6-dichloranilino) phenylacetic acid
- FAAH
fatty acid amide hydrolase
- [35S]GTPγS
guanosine 5′-O-(3-[35S]thio)triphosphate
- IL-1
interleukin-1
- JZL184
4-nitrophenyl 4-(dibenzo[d][1,3]dioxol-5-yl(hydroxy)methyl)piperidine-1-carboxylate
- MAGL
monoacylglycerol lipase
- NSAID
nonsteroidal anti-inflammatory drug
- OEA
N-oleoylethanolamine
- PEA
N-palmitoylethanolamine
- SR141716A
rimonabant (5-(4-chlorophenyl)-1-(2,4-dichloro-phenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide)
- THC
Δ9-tetrahydrocannabinol, (−)-(6aS,10aS)-6,6,9-trimethyl-3-pentyl-6a,7,8,10a-tetrahydro-6H-benzo[c]chromen-1-ol
Authorship Contributions
Participated in research design: Kinsey, Wise, Ramesh, Selley, Cravatt, Lichtman.
Conducted experiments: Kinsey, Wise, Ramesh, Abdullah.
Performed data analysis: Kinsey, Wise, Selley, Lichtman.
Wrote or contributed to the writing of the manuscript: Kinsey, Wise, Abdullah, Selley, Cravatt, Lichtman.
Footnotes
This work was supported by the National Institutes of Health [Grants T32DA007027, P01DA009789, P01DA017259, P50DA005274, R01DA030404, and R01DA015197]; and a Toni Rosenberg Fellowship.
B.F.C. and A.H.L. serve on the advisory board of Abide Therapeutics. A.H.L. also serves as a consultant for Ironwood Pharmaceuticals.
References
- Booker L, Kinsey SG, Abdullah RA, Blankman JL, Long JZ, Ezzili C, Boger DL, Cravatt BF, Lichtman AH. (2011) The fatty acid amide hydrolase (FAAH) inhibitor PF-3845 acts in the nervous system to reverse LPS-induced tactile allodynia in mice. Br J Pharmacol 165:2485–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Vogt LJ, Sim-Selley LJ. (1999) Chronic delta9-tetrahydrocannabinol treatment produces a time-dependent loss of cannabinoid receptors and cannabinoid receptor-activated G proteins in rat brain. J Neurochem 73:2447–2459 [DOI] [PubMed] [Google Scholar]
- Busquets-Garcia A, Puighermanal E, Pastor A, de la Torre R, Maldonado R, Ozaita A. (2011) Differential role of anandamide and 2-arachidonoylglycerol in memory and anxiety-like responses. Biol Psychiatry 70:479–486 [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53:55–63 [DOI] [PubMed] [Google Scholar]
- Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. (1994) Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain 59:369–376 [DOI] [PubMed] [Google Scholar]
- Clapper JR, Moreno-Sanz G, Russo R, Guijarro A, Vacondio F, Duranti A, Tontini A, Sanchini S, Sciolino NR, Spradley JM, et al. (2010) Anandamide suppresses pain initiation through a peripheral endocannabinoid mechanism. Nat Neurosci 13:1265–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decosterd I, Woolf CJ. (2000) Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 87:149–158 [DOI] [PubMed] [Google Scholar]
- Dewey WL, Jenkins J, O’Rourke T, Harris LS. (1972) The effects of chronic administration of trans- 9 -tetrahydrocannabinol on behavior and the cardiovascular system of dogs. Arch Int Pharmacodyn Ther 198:118–131 [PubMed] [Google Scholar]
- Falenski KW, Thorpe AJ, Schlosburg JE, Cravatt BF, Abdullah RA, Smith TH, Selley DE, Lichtman AH, Sim-Selley LJ. (2010) FAAH-/- mice display differential tolerance, dependence, and cannabinoid receptor adaptation after delta 9-tetrahydrocannabinol and anandamide administration. Neuropsychopharmacology 35:1775–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F, Compton DR, Ward S, Melvin L, Martin BR. (1994) Development of cross-tolerance between delta 9-tetrahydrocannabinol, CP 55,940 and WIN 55,212. J Pharmacol Exp Ther 271:1383–1390 [PubMed] [Google Scholar]
- Ghosh S, Wise LE, Chen Y, Gujjar R, Mahadevan A, Cravatt BF, Lichtman AH. (2012) The monoacylglycerol lipase inhibitor JZL184 suppresses inflammatory pain in the mouse carrageenan model. Life Sci [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhaveri MD, Richardson D, Kendall DA, Barrett DA, Chapman V. (2006) Analgesic effects of fatty acid amide hydrolase inhibition in a rat model of neuropathic pain. J Neurosci 26:13318–13327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr DM, Harhan B, Okine BN, Egan LJ, Finn DP, and Roche M (2012) The monoacylglycerol lipase inhibitor JZL184 attenuates LPS-induced increases in cytokine expression in the rat frontal cortex and plasma: differential mechanisms of action. Br J Pharmacol DOI: 10.1111/j.1476-5381.2012.02237.x [published ahead of print]. [DOI] [PMC free article] [PubMed]
- Kinsey SG, Long JZ, O’Neal ST, Abdullah RA, Poklis JL, Boger DL, Cravatt BF, Lichtman AH. (2009) Blockade of endocannabinoid-degrading enzymes attenuates neuropathic pain. J Pharmacol Exp Ther 330:902–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, Naidu PS, Cravatt BF, Dudley DT, Lichtman AH. (2011a) Fatty acid amide hydrolase blockade attenuates the development of collagen-induced arthritis and related thermal hyperalgesia in mice. Pharmacol Biochem Behav 99:718–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, Nomura DK, O’Neal ST, Long JZ, Mahadevan A, Cravatt BF, Grider JR, Lichtman AH. (2011b) Inhibition of monoacylglycerol lipase attenuates nonsteroidal anti-inflammatory drug-induced gastric hemorrhages in mice. J Pharmacol Exp Ther 338:795–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, O’Neal ST, Long JZ, Cravatt BF, Lichtman AH. (2011c) Inhibition of endocannabinoid catabolic enzymes elicits anxiolytic-like effects in the marble burying assay. Pharmacol Biochem Behav 98:21–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazenka MF, Selley DE, Sim-Selley LJ. (2012) Brain regional differences in CB1 receptor adaptation and regulation of transcription. Life Sci [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Fisher J, Martin BR. (2001) Precipitated cannabinoid withdrawal is reversed by Delta(9)-tetrahydrocannabinol or clonidine. Pharmacol Biochem Behav 69:181–188 [DOI] [PubMed] [Google Scholar]
- Liu W, Okajima K, Murakami K, Harada N, Isobe H, Irie T. (1998) Role of neutrophil elastase in stress-induced gastric mucosal injury in rats. J Lab Clin Med 132:432–439 [DOI] [PubMed] [Google Scholar]
- Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, Piomelli D. (2005) The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol 67:15–19 [DOI] [PubMed] [Google Scholar]
- Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, Pavón FJ, Serrano AM, Selley DE, Parsons LH, et al. (2009a) Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol 5:37–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Nomura DK, Cravatt BF. (2009c) Characterization of monoacylglycerol lipase inhibition reveals differences in central and peripheral endocannabinoid metabolism. Chem Biol 16:744–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Nomura DK, Vann RE, Walentiny DM, Booker L, Jin X, Burston JJ, Sim-Selley LJ, Lichtman AH, Wiley JL, et al. (2009b) Dual blockade of FAAH and MAGL identifies behavioral processes regulated by endocannabinoid crosstalk in vivo. Proc Natl Acad Sci USA 106:20270–20275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BR, Dewey WL, Harris LS, Beckner JS. (1976) 3H-delta9-tetrahydrocannabinol tissue and subcellular distribution in the central nervous system and tissue distribution in peripheral organs of tolerant and nontolerant dogs. J Pharmacol Exp Ther 196:128–144 [PubMed] [Google Scholar]
- McKinney DL, Cassidy MP, Collier LM, Martin BR, Wiley JL, Selley DE, Sim-Selley LJ. (2008) Dose-related differences in the regional pattern of cannabinoid receptor adaptation and in vivo tolerance development to delta9-tetrahydrocannabinol. J Pharmacol Exp Ther 324:664–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu PS, Booker L, Cravatt BF, Lichtman AH. (2009) Synergy between enzyme inhibitors of fatty acid amide hydrolase and cyclooxygenase in visceral nociception. J Pharmacol Exp Ther 329:48–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu PS, Varvel SA, Ahn K, Cravatt BF, Martin BR, Lichtman AH. (2007) Evaluation of fatty acid amide hydrolase inhibition in murine models of emotionality. Psychopharmacology (Berl) 192:61–70 [DOI] [PubMed] [Google Scholar]
- Nomura DK, Morrison BE, Blankman JL, Long JZ, Kinsey SG, Marcondes MC, Ward AM, Hahn YK, Lichtman AH, Conti B, et al. (2011) Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science 334:809–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Hillard CJ. (2006) Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. J Pharmacol Exp Ther 318:304–311 [DOI] [PubMed] [Google Scholar]
- Rahn EJ, Makriyannis A, Hohmann AG. (2007) Activation of cannabinoid CB1 and CB2 receptors suppresses neuropathic nociception evoked by the chemotherapeutic agent vincristine in rats. Br J Pharmacol 152:765–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo R, Loverme J, La Rana G, Compton TR, Parrott J, Duranti A, Tontini A, Mor M, Tarzia G, Calignano A, et al. (2007) The fatty acid amide hydrolase inhibitor URB597 (cyclohexylcarbamic acid 3′-carbamoylbiphenyl-3-yl ester) reduces neuropathic pain after oral administration in mice. J Pharmacol Exp Ther 322:236–242 [DOI] [PubMed] [Google Scholar]
- Sánchez S, Alarcón de la Lastra C, Ortiz P, Motilva V, Martín MJ. (2002) Gastrointestinal tolerability of metamizol, acetaminophen, and diclofenac in subchronic treatment in rats. Dig Dis Sci 47:2791–2798 [DOI] [PubMed] [Google Scholar]
- Sasso O, Bertorelli R, Bandiera T, Scarpelli R, Colombano G, Armirotti A, Moreno-Sanz G, Reggiani A, Piomelli D. (2012) Peripheral FAAH inhibition causes profound antinociception and protects against indomethacin-induced gastric lesions. Pharmacol Res 65:553–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE, Blankman JL, Long JZ, Nomura DK, Pan B, Kinsey SG, Nguyen PT, Ramesh D, Booker L, Burston JJ, et al. (2010) Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat Neurosci 13:1113–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE, Boger DL, Cravatt BF, Lichtman AH. (2009a) Endocannabinoid modulation of scratching response in an acute allergenic model: a new prospective neural therapeutic target for pruritus. J Pharmacol Exp Ther 329:314–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE, Carlson BL, Ramesh D, Abdullah RA, Long JZ, Cravatt BF, Lichtman AH. (2009b) Inhibitors of endocannabinoid-metabolizing enzymes reduce precipitated withdrawal responses in THC-dependent mice. AAPS J 11:342–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciolino NR, Zhou W, Hohmann AG. (2011) Enhancement of endocannabinoid signaling with JZL184, an inhibitor of the 2-arachidonoylglycerol hydrolyzing enzyme monoacylglycerol lipase, produces anxiolytic effects under conditions of high environmental aversiveness in rats. Pharmacol Res 64:226–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoyama M, Tanaka K, Hasue F, Shimoyama N. (2002) A mouse model of neuropathic cancer pain. Pain 99:167–174 [DOI] [PubMed] [Google Scholar]
- Sim-Selley LJ. (2003) Regulation of cannabinoid CB1 receptors in the central nervous system by chronic cannabinoids. Crit Rev Neurobiol 15:91–119 [DOI] [PubMed] [Google Scholar]
- Sim-Selley LJ, Schechter NS, Rorrer WK, Dalton GD, Hernandez J, Martin BR, Selley DE. (2006) Prolonged recovery rate of CB1 receptor adaptation after cessation of long-term cannabinoid administration. Mol Pharmacol 70:986–996 [DOI] [PubMed] [Google Scholar]
- Wiley JL, Martin BR. (2003) Cannabinoid pharmacological properties common to other centrally acting drugs. Eur J Pharmacol 471:185–193 [DOI] [PubMed] [Google Scholar]