Abstract
The MDR1 gene encodes P-glycoprotein, a transmembrane drug efflux transporter that confers multidrug resistance in cancer cells and affects drug pharmacokinetics by virtue of its expression in the liver, kidney, and colon. Nuclear receptors human steroid and xenobiotic receptor (SXR) and constitutive androstane receptor (CAR) are possible master regulators of xenobiotic-inducible MDR1 expression in drug processing organs, but the mechanism of MDR1 regulation has yet to be directly demonstrated in vivo. Moreover, it has previously been impossible to determine the sustained or cumulative effect of repeated doses of xenobiotics on in vivo MDR1 expression. We previously reported a mouse model containing firefly luciferase (fLUC) knocked into the mdr1a genomic locus, allowing noninvasive bioimaging of intestinal mdr1a gene expression in live animals. In the current study, we crossed mdr1a.fLUC mice into the pxr knockout (pxr−/−) genetic background and injected mice with pregnenolone-16α-carbonitrile (PCN), a strong mouse pregnane X receptor (PXR) ligand, and two therapeutically relevant taxanes, paclitaxel and docetaxel. All three agents induced mdr1a.fLUC expression (bioluminescence), but only PCN and docetaxel appeared to act primarily via PXR. Luminescence returned to baseline by 24–48 hours after drug injection and was reinducible over two additional rounds of drug dosing in pxr+/+ mice. TCPOBOP, a CAR ligand, modestly induced mdr1a.fLUC in pxr+/+ and pxr−/− strains, consistent with CAR’s minor role in mdr1a regulation. Collectively, these results demonstrate that the mdr1a.fLUC bioimaging model can capture changes in mdr1 gene expression under conditions of repeated xenobiotic treatment in vivo and that it can be used to probe the mechanism of gene regulation in response to different xenobiotic agents.
Introduction
P-glycoprotein (Pgp), encoded by the human MDR1 gene or the murine mdr1a and mdr1b genes, is a transmembrane drug transporter that mediates the efflux from cells of a broad range of structurally unrelated drugs (Gottesman and Pastan, 1993; Kane, 1996). Pgp was initially discovered by its ability to confer multidrug resistance on cell lines and cancers that overexpress it (Debenham et al., 1982; Kartner et al., 1983), presumably by reducing the intracellular accumulation of its substrate drugs. Although its normal physiologic function has yet to be proven, several studies suggest that it and other structurally related transporter proteins play a protective role against environmental toxins and other xenobiotics (Fojo et al., 1987; Thiebaut et al., 1987; Cordon-Cardo et al., 1989). Pgp is expressed in normal tissues, such as placenta, blood brain barrier, liver, kidney, and intestine (Croop et al., 1989), that serve as access barriers and/or mediate excretion of xenobiotics. It plays an important role in limiting the absorption and accumulation of foreign toxic agents. A change in Pgp expression levels or activity also affects the pharmacokinetics of uptake, distribution, and disposition of its substrate drugs and is one of the underlying mechanisms by which drug-drug interactions may occur (Schinkel et al., 1995; Chen et al., 1997; Luker et al., 1997; Sikic et al., 1997).
The mechanism by which Pgp expression is regulated in normal tissues by physiologic and xenobiotic stimuli is not fully understood. The nuclear receptors human steroid and xenobiotic receptor (SXR)/mouse pregnane X receptor (PXR) and constitutive androstane receptor (CAR) have been suggested as possible master regulators of the inducible expression of MDR1 by foreign substances (Synold et al., 2001; Urquhart et al., 2007). These nuclear receptors are proposed to be major sensors of xenobiotics and in response upregulate the expression of genes, including MDR1, that are involved in detoxification. The properties of SXR/PXR- and CAR-mediated regulation of MDR1/Pgp expression in vivo have not been well characterized. Other transcription factors, such as p53 (Chin et al., 1992; Zastawny et al., 1993; Thottassery et al., 1997) and forkhead box 03 (Hui et al., 2008), have also been implicated as MDR1 regulators in cancer cells, while their role in normal tissues has not been determined.
To facilitate the study of MDR1 gene regulation in vivo, we previously reported a mouse model that allows noninvasive bioimaging of mdr1a gene expression in live animals and in real time (Gu et al., 2009). Mouse mdr1a is the nearest homolog to human MDR1 (Devault and Gros, 1990; Hsu et al., 1990; Tang-Wai et al., 1995), expressed predominantly in the intestine and at lower levels in liver, kidney, and spleen (Croop et al., 1989). Our reported bioimaging model has a firefly luciferase (fLUC) cDNA inserted into the murine mdr1a genetic locus by homologous recombination and we have demonstrated that abdominal luminescence intensity is an accurate reporter of intestinal mdr1a and fLUC mRNA expression in mdr1a.fLUC mice (Gu et al., 2009). The purpose of the current study was to examine the dynamic effect of repeated drug dosing on mdr1a expression and to determine the role of PXR in drug-mediated mdr1a induction in vivo.
Materials and Methods
Mice.
The mdr1a+/fLUC (Gu et al., 2009) and pxr−/− mice (Xie et al., 2000) were described previously and were bred to create pxr−/−/mdr1a+/fLUC and pxr+/+/mdr1a+/fLUC mice. Briefly, we first created four littermate homozygous strains (strain A: pxr−/−/mdr1afLUC/fLUC; strain B: pxr−/−/mdr1a+/+ mice; strain C: pxr+/+/mdr1afLUC/fLUC; and strain D: pxr+/+/mdr1a+/+) for easy strain maintenance by standard breeding. The experimental strain pxr−/−/mdr1a+/fLUC was produced by crossing strain A with strain B and the control strain pxr+/+/mdr1a+/fLUC was produced by crossing strain C with strain D. Mice were analyzed by PCR to ensure correct genotypes. To identify the mdr1a.fLUC reporter gene allele, DNA extracted from tail snips was amplified using primers 5′-ACAGTGGAACAGCGGTTT and 5′-CTTCCAGCGGATAGAATG, which recognize sequences in mdr1a and in the fLUC cDNA inserted into the mdr1a locus, respectively. The wild-type mdr1a allele was identified with primers 5′-TTGAAGAGGACCTTAAGGGAAG and 5′-CCCACCATTAAAGTTCTAATCACTT, which recognize mdr1a sequences located on each side of the insertion site of the fLUC gene in the mdr1a.fLUC allele. The disrupted pxr allele was identified by PCR using the primers 5′-CTTGACGAGTTCTTCTGAGGGGATC and 5′-AGAAACACATAGAAACCCATCCATG, which recognize sequences in the inserted neo gene and the pxr gene, respectively. The wild-type pxr allele was identified with primers 5′-GTCCACCAAGCCTGAGCCTCCTAC and 5′-AGAAACACATAGAAACCCATCCATG, which recognize pxr sequences located on each side of the insertion site of the neo gene in the knockout allele. Mice were kept on Purina PicoLab Rodent Diet 20 #5053 (Newco Distributors, Rancho Cucamonga, CA) in a specific-pathogen-free facility at City of Hope. All experiments involving live animals were reviewed and approved by the City of Hope Institutional Animal Care and Use Committee.
Xenobiotic Treatment and Imaging.
Pregnenolone-16α-carbonitrile (PCN) and 1,4-bis[2-(3,5-dichloropyridyloxy)]-benzene (TCPOBOP) were purchased from Sigma-Aldrich (St. Louis, MO). Paclitaxel and docetaxel were purchased from Bristol-Myers Squibb (New York, NY) and Sanofi (Bridgewater, NJ), respectively. PCN and TCPOBOP were dissolved in corn oil and administered to mice by i.p. injection (200 and 3 mg/kg, respectively). Paclitaxel was dissolved in 50.3% Cremophor EL and 49.7% dehydrated alcohol (vol/vol) as a concentrate (6 mg/ml) and then diluted in saline to a final concentration of 1.2 mg/ml. Docetaxel (20 mg in 0.5 ml of polysorbate 80) was initially diluted to 10 mg/ml by 13% ethanol and was further diluted in saline to a final concentration of 1.2 mg/ml. The diluted paclitaxel and docetaxel were administrated to mice by i.p. injection (10 mg/kg each). Matched vehicles were used as controls. In vivo imaging of abdominal luminescence was performed in mice heterozygous for the mdr1a.fLUC allele (mdr1a+/fLUC) as described previously (Gu et al., 2009). Briefly, for each imaging time point before (t = 0) and after drug injection (time points indicated in the text for each experiment), mice were given 0.2 ml of luciferin at 15 mg/ml by i.p. injection and then anesthetized by inhalation of isoflurane (4% isoflurane carried by a flow of 1.5 l/min oxygen). Bioimaging was carried out on a Xenogen IVIS imager (Caliper Life Sciences/PerkinElmer, Hopkinton, MA) 8 minutes after luciferin injection. Consistent regions of interest were drawn for quantifying luminescence intensities in each animal.
Imaging Data Analysis.
To eliminate the effect of different baseline (t = 0) luminescence intensities among the cohort of mice used in a given experiment, we normalized each luminescence reading to the average of the t = 0 luminescence readings for the entire set of genotype-matched mice receiving drug and vehicle injections in a given experiment. Very low luminescence values (<107 luminescence units, where the average of all baseline values was 4.4 × 109 ± 2.7 × 109 luminescence units) were removed from the analysis and from the figure representations. Statistical analysis was performed on combined datasets from 2–3 independent experiments using normalized 8-hour luminescence intensities after each drug injection. When data from multiple experiments were analyzed, all mice and data points above the luminescence threshold were included in the analysis. Statistical analysis used unpaired two-sided t tests to compare the 8-hour normalized time points between genotypes (for a given treatment type) and between treatment types (for a given genotype).
Cell-Based Reporter Assays.
CV-1 cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% resin-charcoal stripped fetal bovine serum, 50 IU/ml penicillin G, and 50 μg/ml streptomycin sulfate (DMEM-FBS) at 37°C in 5% CO2. One day prior to transfection, cells were plated to 50–80% confluence using phenol-red–free DMEM-FBS. Cells were transiently transfected by lipofection with N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium methyl-sulfate (DOTAP) (Boehringer Mannheim, Ridgefield, CT). Cytomegalovirus expression vectors were used to express full-length human SXR or mouse PXR. Reporter constructs contained three copies of the human CYP3A4 SXR response element or three copies of the rat cyp3a2 PXR response element upstream of TK-Luc (Blumberg et al., 1998). Reporter constructs (300 ng/105 cells) and SXR/PXR expression vectors (20–50 ng/105 cells) were added to cells along with pCMX-βgal (500 ng/105 cells) as an internal control. After 2 hours the liposomes were removed and cells were incubated for approximately 45 hours with phenol-red–free DMEM-FBS containing the indicated compounds. After 40 hours, the cells were harvested and assayed for luciferase and β-galactosidase activity. All transfections were performed in triplicate and normalized reporter activity was calculated as luciferase (reporter) units divided by βgal (internal control) units. Statistical significance was determined by one-way analysis of variance with a Dunnett’s multiple comparisons post-test to compare each drug treatment with the no-drug control. Each experiment was repeated at least three times with similar results.
Results
PXR Is Required for Drug-Inducible But Not Basal mdr1a.fLUC Expression.
We previously reported that intestinal mdr1a.fLUC expression, measured as abdominal luminescence intensity, in the mdr1a+/fLUC imaging model is inducible by PCN (Gu et al., 2009). To determine if this induction is mediated by PXR, we crossed mdr1a.fLUC mice with pxr−/− mice and created a strain that is knocked out for pxr and heterozygous for the mdr1a.fLUC allele (pxr−/−/mdr1a+/fLUC). A control strain with the pxr+/+/mdr1a+/fLUC genotype was used as comparison. To determine if pxr knockout affected basal expression from the mdr1a.fLUC locus, we analyzed baseline luminescence intensities of all mice used in the experiments reported herein. This included 152 pxr+/+/mdr1a+/fLUC mice and 202 pxr−/−/mdr1a+/fLUC mice. The average luminescence intensity in the pxr+/+ mice was 4.2 × 109 ± 2.5 × 109 luminescence units and the average in the pxr−/− mice was 4.6 × 109 ± 2.8 × 109 luminescence units. The difference between the two groups was not statistically significant (P = 0.26), indicating that PXR does not play a significant role in regulating basal intestinal mdr1a.fLUC expression.
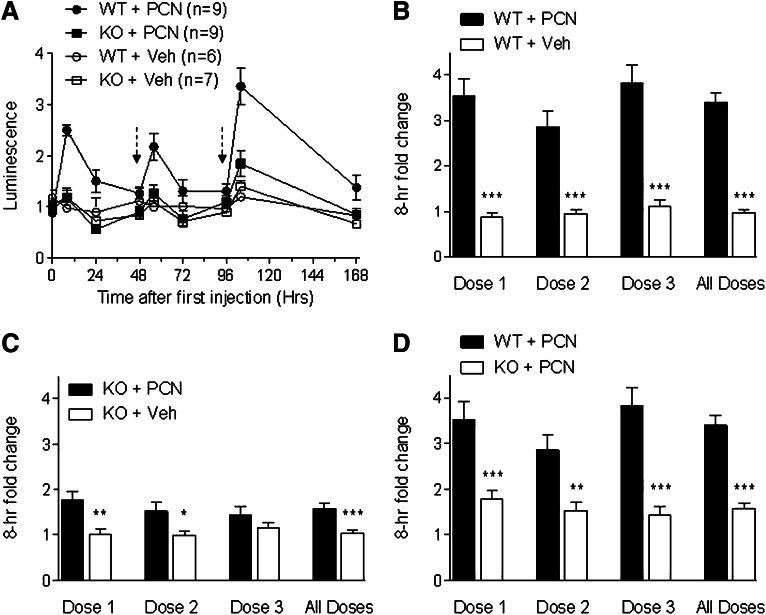
To determine if pxr knockout affected mdr1a.fLUC induction by PCN, we first took an initial baseline image of pxr+/+/mdr1a+/fLUC and pxr−/−/mdr1a+/fLUC mice, then injected PCN (200 mg/kg) or vehicle (corn oil) into the two strains and measured their intestinal mdr1a.fLUC expression by luminescence imaging at 8, 24, and (in some experiments) 48 hours. Pooling results from three independent experiments, PCN induced mdr1a.fLUC expression by 3.5 ± 0.4-fold over the average of all baselines (see Materials and Methods) in pxr+/+/mdr1a+/fLUC mice, but only by 1.8 ± 0.2-fold in pxr−/−/mdr1a+/fLUC mice. The difference in induction magnitude was statistically significant (see Fig. 1). Notably, the pxr−/−/mdr1a+/fLUC mice still exhibited a statistically significant increase in luminescence over the controls of the same genotype injected with vehicle alone, suggesting that there might be residual PXR-independent induction of mdr1a.fLUC expression by PCN.
Fig. 1.
Dynamic induction of mdr1a.fLUC by multiple doses of PCN. (A) pxr+/+ (WT) and pxr−/− (KO) mice that were heterozygous for mdr1a.fLuc allele were given PCN (200 mg/kg) or the same volume of vehicle control (Veh) by i.p. injection at time 0 and at 48 and 96 hours after the initial PCN treatment as indicated by the dashed arrows. Results are from a single representative experiment. Mdr1a.fLUC expression was quantified by measuring luminescence intensity in the abdominal area at various time points before and after PCN or vehicle injection. The luminescence intensity for each animal at each time point was normalized to the average baseline (t = 0) luminescence intensity for the entire group of mice of the same genotype within a given experiment. Each data point represents the average (± S.E.M.) normalized intensities for the respective genotypes and treatment groups at each time point.. (B–D) Normalized luminescence intensities at the 8-hour time points after each dosing cycle. Data from all mice from three multi-dosing experiments are pooled and averaged (± S.E.M.). (B) shows the comparison between WT mice treated with PCN and WT mice treated with Veh. (C) shows the comparison between KO mice treated with PCN and KO mice treated with Veh. (D) shows the comparison between WT mice treated with PCN and KO mice treated with PCN. In the All Doses columns, data from all mice from all experiments and all dosings are averaged. WT + PCN (n = 27), KO + PCN (n = 27), WT + Veh (n = 15), KO + Veh (n = 16). *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
mdr1a.fLUC Is Inducible over Multiple Rounds of Drug Dosing.
We treated the same mice with two additional doses of PCN or vehicle after luminescence induced by the previous dose had fallen back to near-basal levels (48–72 hours between drug injections). Results from a representative experiment for all three rounds of PCN injection are shown in Fig. 1A. Results comparing the 8-hour time point after each PCN injection (combined data from three independent experiments) are shown in Fig. 1, B–D. Over the course of three experiments in pxr+/+/mdr1a+/fLUC mice, luminescence was reinducible in rounds 2 and 3 of PCN dosing (Fig. 1B). The fold-induction after each round of PCN dosing was statistically significantly different from the changes seen with vehicle alone. Luminescence returned to near baseline levels after each drug dose, suggesting that there was no significant “fixing” or selection of high mdr1a.fLUC expression as a result of multiple exposures to xenobiotics.
In contrast to the significant reinduction seen after each PCN injection in the pxr+/+ background, we again observed only a small increase in luminescence after the second and third rounds of PCN injection in the pxr−/− background (Fig. 1, A and C). The comparison with vehicle control was statistically significant for dose 2 but not for dose 3. There was a statistically significant difference between the 8-hour postinjection luminescence intensities in pxr+/+/mdr1a+/fLUC mice treated with PCN versus the pxr−/−/mdr1a+/fLUC mice treated with PCN after each drug dosing (Fig. 1D). Thus, the effect of PCN on mdr1a.fLUC expression appears to be mediated primarily through PXR, and the PXR-dependent effect of drug is experienced over multiple rounds of drug dosing.
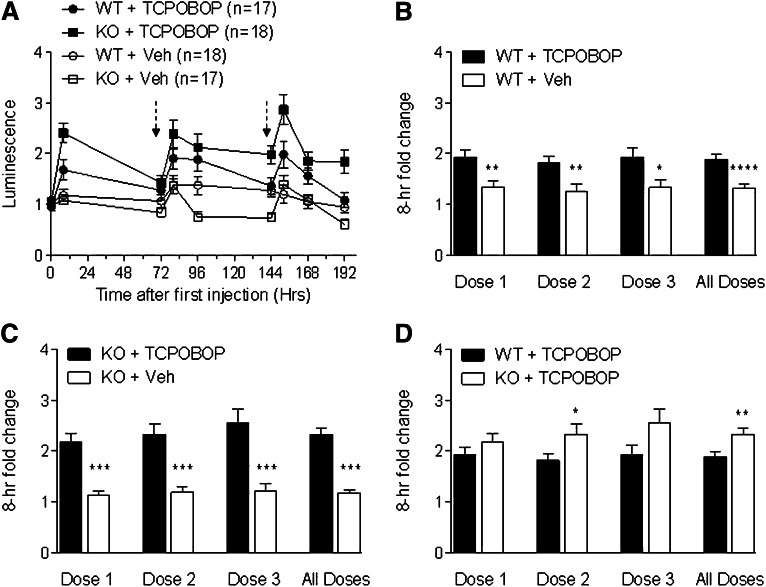
As a further control, we injected separate cohorts of pxr−/−/mdr1a+/fLUC and pxr+/+/mdr1a+/fLUC mice with TCPOBOP, a known agonist for the CAR nuclear receptor (Moore et al., 2000). CAR is believed to be a minor regulator of mdr1 expression in response to its ligands (Burk et al., 2005a,b). The experiment was performed three times, first with a single round of drug dosing and subsequently with three rounds of dosing. In the multidosing experiments, TCPOBOP was injected at times 0, 72, and 144 hours. The longer interval between each round of dosing was based on the reported long half-life of TCPOBOP in rodents (Poland et al., 1981), thus attempting to maximize the time for any induced levels of mdr1a.fLUC expression and luminescence to return to baseline between dosings. As seen in Fig. 2, TCPOBOP induced luminescence to a small extent after all three dosings in both pxr+/+ and pxr−/− backgrounds, indicating that its effect on mdr1a.fLUC expression was not dependent on PXR. In the experiment shown in Fig. 2A, TCPOBOP seemed to result in a persistent induction of luminescence over a prolonged period of time, possibly reflecting the long half-life of this compound in vivo. Nevertheless, the overall inductions by TCPOBOP in both strains were statistically or nearly statistically significant compared with their own vehicle controls for pxr+/+ mice and pxr−/− mice (Fig. 2, B and C). Interestingly, TCPOBOP seemed to cause a greater induction in pxr−/− mice than in pxr+/+ mice after each drug dosing and overall (Fig. 2D), although the difference between the two strains reached statistical significance only for dose 2 and when all dosings were combined.
Fig. 2.
Mdr1a.fLUC is inducible by TCPOBOP regardless of PXR status. (A) pxr+/+ (WT) and pxr−/− (KO) mice that were heterozygous for the mdr1a.fLuc allele were given TCPOBOP (3 mg/kg) or corn oil (Veh) by i.p. injection at times 0, 72, and 144 hours after the initial TCPOBOP injection, as indicated by the dashed arrows. Results are from a single representative experiment. Luminescence was quantified as in Fig. 1. (B–D) Normalized luminescence intensities at the 8-hour time points after each dosing cycle. Data for all mice from one single-dosing experiment (included in dose 1 only) and two multidosing experiments (doses 1–3) are pooled and averaged (± S.E.M.). Panels show comparisons as in Fig. 1. WT + TCPOBOP (n = 38 for dose 1, n = 26 for doses 2 and 3), KO + TCPOBOP (n = 39 for dose 1, n = 27 for dose 2, and n = 25 for dose 3), WT + Veh (n = 39 for dose 1, n = 27 for dose 2, and n = 26 for dose 3), KO + Veh (n = 38 for dose 1, n = 26 for dose 2, and n = 25 for dose 3). *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001.
PXR Preferentially Activated by Docetaxel In Vitro.
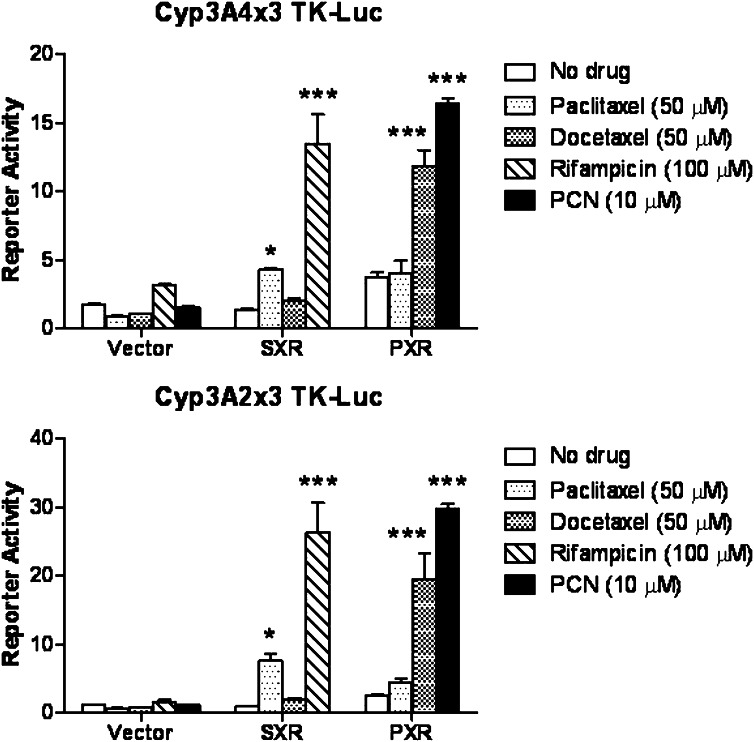
We previously reported that paclitaxel is a human SXR agonist that promotes SXR-mediated transcriptional activity, whereas docetaxel does not effectively activate SXR (Synold et al., 2001). To determine the ligand specificity of the murine PXR toward these two chemotherapeutic drugs, we first conducted a cell-based reporter assay as described in Materials and Methods. Briefly, CV-1 cells were transfected with human SXR or murine PXR expression plasmid plus a SXR or PXR response element linked to fLUC. Transfected cells were treated with various agents and assayed for luminescence (Fig. 3). As expected, PCN and the antibiotic drug rifampicin preferentially activated mouse PXR and human SXR, respectively. Consistent with our previous report, paclitaxel caused greater activation of SXR than did docetaxel. In contrast, mouse PXR-mediated transcription responded more strongly to docetaxel than paclitaxel, suggesting that murine PXR has different ligand specificity from human SXR.
Fig. 3.
Ligand specificity of the murine PXR for docetaxel vs. paclitaxel. A plasmid expressing human SXR or murine PXR was cotransfected into CV-1 cells along with a SXR or PXR reporter construct (CYP3A4x3-TK-Luc or Cyp3A2x3-TK-Luc, respectively). After transfection, CV-1 cells were treated with the indicated compounds and fold activation was determined relative to untreated cells. Shown are results from a single experiment performed in triplicate (averages ± S.D.). Statistical analysis is described in Materials and Methods; *P ≤ 0.05; ***P ≤ 0.001.
Docetaxel and Paclitaxel Have Different Effects on mdr1a.fLUC Expression In Vivo.
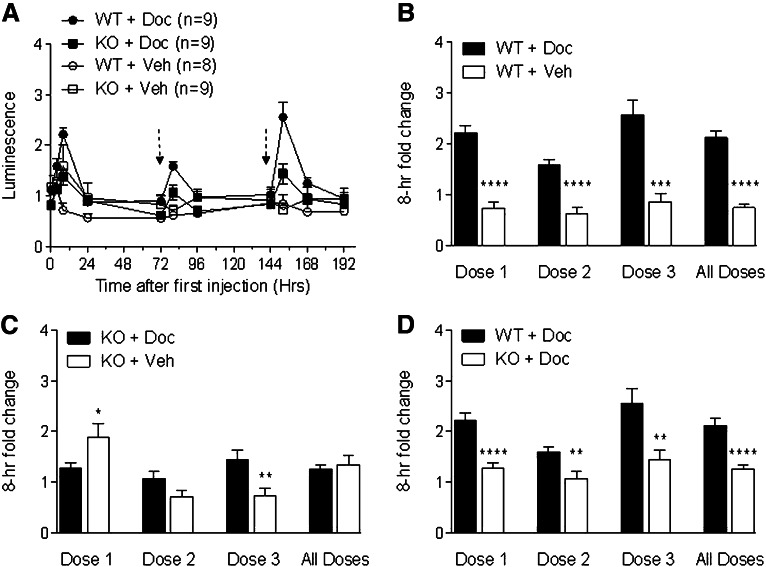
To determine if the same PXR ligand specificity observed in vitro (Fig. 3) was also seen in vivo, we injected three separate dosings of docetaxel or paclitaxel (10 mg/kg each, chosen to be consistent with our published study) into pxr+/+/mdr1a+/fLUC and pxr−/−/mdr1a+/fLUC mice and measured abdominal luminescence intensities at 4, 8, 24, and 48 or 72 hours after each drug injection. Results from a single multidosing experiment with each drug are shown in Fig. 4A (docetaxel) and Fig. 5A (paclitaxel), and the 8-hour fold-induction results from these experiments are shown in Figs. 4, B–D, and 5, B–D). The 8-hour fold-induction results for the first dosings also include pxr−/− mice from a separate single-dosing experiment with each drug. Docetaxel induced luminescence in pxr+/+/mdr1a+/fLUC mice after each drug injection (Fig. 4, A and B). The induction was reduced in the pxr−/− background (Fig. 4C) such that the difference between the pxr+/+ background and the pxr−/− background was statistically significant for each drug dosing (Fig. 4D). These data suggest that induction by docetaxel was at least partially dependent on PXR.
Fig. 4.
Docetaxel induces mdr1a.fLUC expression via PXR. (A) pxr+/+ (WT) and pxr−/− (KO) mice that were heterozygous for the mdr1a.fLUC allele were given 10 mg/kg docetaxel (Doc) or polysorbate 80 (Veh) by i.p. injection at times 0, 72, and 144 hours after the initial injection as indicated by dashed arrows. Results are from a single experiment, with expression quantified as in previous figures. (B–D) Normalized luminescence intensities at the 8-hour time points after each dosing cycle. Data from all mice from one single-dosing experiment with the KO mice (included in dose 1 only) and one multidosing experiment with WT and KO mice (included in doses 1–3) are pooled and averaged (± S.E.M.). Panels show comparisons as in Fig. 1. WT + Doc (n = 9 for all doses), KO + Doc (n = 21 for dose 1, n = 9 for doses 2 and 3), WT + Veh (n = 8 for all doses), KO + Veh (n = 21 for dose 1, n = 9 for doses 2 and 3). *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001.
Fig. 5.
Paclitaxel induces mdr1a.fLUC expression via PXR-independent mechanism. (A) pxr+/+ (WT) and pxr−/− (KO) mice that were heterozygous for the mdr1a.fLUC allele were given 10 mg/kg paclitaxel (Pac) or Cremophor EL (Veh) by i.p. injection at times 0, 48, and 120 hours after the initial injection as indicated by dashed arrows. Results are from a single experiment, with expression quantified as in previous figures. (B–D) Normalized luminescence intensities at the 8-hour time points after each dosing cycle. Data from all mice from one single-dosing experiment with the KO mice (included in dose 1 only) and one multidosing experiment with WT and KO mice (included in doses 1–3) are pooled and averaged (± S.E.M.). Panels show comparisons as in Fig. 1. WT + Pac (n = 9 for all doses), KO + Pac (n = 20 for dose 1, n = 8 for doses 2 and 3), WT + Veh (n = 9 for doses and 2, n = 8 for dose 3), KO + Veh (n = 19 for dose 1, n = 8 for doses 2 and 3). *P ≤ 0.05; **P ≤ 0.01.
With paclitaxel, we observed a small induction of luminescence in both pxr+/+ and pxr−/− backgrounds, although a statistically significant difference from the vehicle control was seen only in the pxr+/+ background after the first drug dosing and to a lesser extent when all drug dosings were combined (Fig. 5, A and B). A similar magnitude of luminescence induction was observed for the first dosing of paclitaxel in pxr−/− mice but there was also an effect of the vehicle control in these mice (Fig. 5C). Notably, the effect of paclitaxel in pxr−/− mice was not statistically different from its effect in pxr+/+ mice, even when all 8-hour data points from a given genotype were combined for analysis (Fig. 5D). The results with docetaxel and paclitaxel suggest that these two taxane analogs exert different effects on mdr1a expression, with docetaxel acting predominantly through PXR and paclitaxel having very small effects (if any), possibly via a PXR-independent mechanism.
Discussion
Results in this report demonstrate that the mdr1a gene is regulated by nuclear receptors PXR and CAR in vivo. Knocking out pxr in mdr1a.fLUC mice had no effect on basal mdr1a.fLUC expression, as measured by luminescence intensity, but nearly abolished induction of mdr1a.fLUC by PCN, a strong PXR ligand. The slight residual induction of luminescence by PCN could be due to its lower activity toward CAR, another nuclear receptor thought to be a weak inducer of mdr1 expression. Indeed, TCPOBOP, a strong CAR agonist, was able to induce luminescence in both pxr+/+ and pxr−/− backgrounds, consistent with CAR acting as an in vivo transactivator of mdr1a. Notably, PXR-mediated gene induction by PCN- and CAR-mediated gene induction by TCPOBOP have been shown to be active in the intestine (Xu et al., 2009), the overwhelming source of luminescence signal in our model (Gu et al., 2009), and in the liver, whereas another CAR-specific ligand activates target genes preferentially in the liver (Xu et al., 2009). We are not able to distinguish intestinal and liver luminescence in this model due to the physical proximity of those two organs, but future studies using a tissue-specific knock-in of the mdr1a.fLUC reporter could potentially confirm the intestine- or liver-specific effects of pxr knockout and individual drug induction.
Interestingly, the CAR ligand caused a larger luminescence induction in the pxr knockout background than in pxr wild-type mice, suggesting that CAR activity is perhaps enhanced in the absence of PXR. This is consistent with at least one earlier report in which both basal and CAR-induced expression of the mouse mrp2 gene is greater in hepatocytes derived from pxr−/− mice than in wild-type hepatocytes (Kast et al., 2002). Although the mechanism by which CAR-mediated mdr1a induction was enhanced in the absence of PXR is not known, we did not observe an increased level of CAR in pxr−/− mice by Western analysis (unpublished data). Several other possible mechanisms can be envisioned, including lack of competition for overlapping DNA binding sites or common coregulators when PXR is absent. Alternatively, although pxr knockout did not appear to reduce basal mdr1a expression in our system, PXR deficiency could reduce the expression of other PXR-target genes that are involved in drug transport, thus allowing higher TCPOBOP accumulation in CAR-expressing cells and greater luminescence induction by the CAR ligand.
Consistent with our previous report (Gu et al., 2009), we observed similar magnitudes of mdr1a.fLUC induction with both of the chemotherapeutic drugs docetaxel and paclitaxel, at least in the first dosings with these drugs, but the drugs appeared to affect mdr1a.fLUC expression through different mechanisms. Induction by docetaxel was mainly mediated by PXR, although there also appeared to be some residual induction even in the absence of PXR. Paclitaxel caused about a 2-fold induction of luminescence in pxr+/+ mice after dose 1, similar to that seen with docetaxel, but we did not see the same pattern of reinduction in doses 2 and 3. Moreover, although data shown in Fig. 5A suggests that paclitaxel induction might be PXR-dependent, this conclusion is not borne out when data from all mice in two independent experiments were combined for the analysis shown in Fig. 5, B–D. There was not a significant difference between pxr−/− and pxr+/+ mice in their response to paclitaxel in any of the three drug dosings (Fig. 5D). Further studies in car−/− and pxr−/−/car−/− backgrounds will determine if the residual effects of docetaxel and the induction by paclitaxel are perhaps mediated through CAR.
It should be noted that the vehicles used for paclitaxel and docetaxel (Cremophor EL and polysorbate 80, respectively) appeared to cause some inducing effects of their own. We had not observed such effects in single-dosing experiments previously reported (Gu et al., 2009), in which both drugs were dissolved in Cremophor EL, and the magnitude of induction by docetaxel and paclitaxel were similar to each other in that study as well. We used the different formulation (polysorbate) for docetaxel in the current study because we were following current packaging instructions from the pharmacy where the drug was obtained. However, we did not see significant differences in head-to-head comparisons of drug in Cremophor EL versus polysorbate, either in terms of the magnitude of luminescence induction or the effect of vehicle on luminescence (unpublished observations).
Studies of PXR’s ligand specificity in cell-based reporter assays revealed that docetaxel caused greater PXR activation than paclitaxel. This is consistent with their respective activities on PXR-mediated luminescence in vivo. We previously reported that paclitaxel is a strong agonist of the human ortholog SXR, whereas docetaxel is unable to activate SXR-mediated transcription in a similar cell-based reporter gene assay (Synold et al., 2001). Mechanistically, the lack of agonistic activity by docetaxel was primarily due to its inability to displace corepressors that interact with SXR. It remains to be determined whether the different activities of docetaxel and paclitaxel on PXR also involve corepressor displacement. The structural determinants that underlie the differential responses of SXR and PXR to the taxane analogs may involve the receptors, their coregulators, or a combination of factors. An intriguing question is whether the differential activities of docetaxel and paclitaxel on PXR and SXR are tissue-specific, possibly associated with the expression of specific corepressors. Indeed, this might account for the somewhat ambiguous nature of the paclitaxel effects in mice in both the pxr+/+ and pxr−/− background, given that the predominance of luminescence signal (mdr1a expression) comes from the intestine in our model. Understanding these questions will allow us to develop more refined mouse models that more closely reflect pharmacological interactions found in humans. The adaptability of our imaging model to genetic manipulation and the potential to measure tissue-specific mdr1a.fLUC expression (by virtue of spatially-controlled Cre recombinase-mediated recombination of the mdr1a.fLUCflox/flox allele) provide a unique opportunity to address important questions of pharmacological gene regulation in vivo.
Longitudinal tracking of mdr1a.fLUC expression, another unique capability of a real-time bioimaging system, demonstrated the reinducibility of the mdr1a.fLUC gene by multiple rounds of drug treatment in vivo. Each round of PCN treatment caused a similar baseline-to-peak induction that fell back to near-basal levels by 48 hours after each drug dosing. A similar induction and reinduction pattern was observed in animals treated with docetaxel. This result perhaps reflects the short half-life of these two agents in vivo. TCPOBOP has a longer half-life in rodents and also produced a more sustained effect on luminescence over the course of a 72-hour dosing cycle. The persistent effect of TCPOBOP on mdr1a.fLUC induction was especially evident by the third dosing in the pxr−/− mice. The sustained effect could be due to the relatively long pharmacokinetic half-life of TCPOBOP in mice and/or enhanced pharmacodynamics due to effects on intracellular drug accumulation in the absence of PXR. The lack of a cumulative induction effect at the later drug dosings could be due to physiologic restraints, such as receptor or coactivator saturation or certain feedback controls, but this remains to be determined. Regardless of the underlying mechanisms that cause the different induction patterns with different xenobiotics, the dynamics of mdr1a.fLUC induction and reinduction captured by real-time imaging demonstrates that our mouse model is suitable for studying mdr1a expression under conditions of chronic treatment with drugs and environmental toxins. The noninvasive longitudinal measurement capability coupled with other genetic manipulations will enable studies of a broad range of drugs in various genetic backgrounds at a practical throughput to establish the variety of agents capable of inducing mdr1a at the transcriptional level and to understand the magnitude and mediators of these effects under physiologic conditions. The clinical implications of such information about mdr1 gene regulation are significant. Both paclitaxel and docetaxel are widely used chemotherapeutic agents for treating a broad spectrum of cancers and both are Pgp substrates. Knockout studies in mice and inhibitor trials in humans have demonstrated that Pgp plays an important role in the oral uptake and elimination through excretion of taxanes and other substrate chemotherapeutics. Thus, changes in intestinal Pgp expression can have profound effects on drug pharmacology and finding new agents that do not induce such changes is a goal of contemporary drug development efforts. The ability to study the dynamics of induction in an in vivo setting adds considerable power to the search for more effective anticancer agents.
Acknowledgments
The authors thank Donna Isbell, Min Lin, and Kathleen Higgins for assistance with animal breeding and care. The authors thank Isabelle Dussault for contribution to the reporter assays and Robert Lingeman for technical assistance. The authors are also grateful to M. Suzette Blanchard for advice on biostatistical analysis.
Abbreviations
- CAR
constitutive androstane receptor
- DMEM-FBS
Dulbecco’s modified Eagle’s medium supplemented with 10% resin-charcoal stripped fetal bovine serum
- docetaxel
1,7β,10β-trihydroxy-9-oxo-5β,20-epoxytax-11-ene-2α,4,13α-triyl 4-acetate 2-benzoate 13-{(2R,3S)-3-[(tert-butoxycarbonyl)amino]-2-hydroxy-3-phenylpropanoate}
- DOTAP
N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium methyl-sulfate
- fLUC
firefly luciferase
- MDR1
human multidrug resistance-1 gene
- mdr1a
mouse homolog of MDR1 gene
- paclitaxel
(2α,4α,5β,7β,10β,13α)-4,10-bis(acetyloxy)-13-{[(2R,3S)-3-(benzoylamino)-2-hydroxy-3-phenylpropanoyl]oxy}-1,7-dihydroxy-9-oxo-5,20-epoxytax-11-en-2-yl benzoate
- PCN
pregnenolone-16α-carbonitrile
- Pgp
P-glycoprotein
- PXR
mouse pregnane X receptor
- SXR
human steroid and xenobiotic receptor
- TCPOBOP
1,4-bis[2-(3,5-dichloropyridyloxy)]-benzene
Authorship Contributions
Participated in research design: Gu, Synold, Forman, Kane.
Conducted experiments: Gu, Chen.
Performed data analysis: Gu, Synold, Forman, Kane.
Wrote or contributed to the writing of the manuscript: Gu, Synold, Forman, Kane.
Footnotes
This work was supported in part by the National Institutes of Health National Cancer Institute [Grants CA137914 and CA33572].
References
- Blumberg B, Sabbagh W, Jr, Juguilon H, Bolado J, Jr, van Meter CM, Ong ES, Evans RM. (1998) SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev 12:3195–3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk O, Arnold KA, Geick A, Tegude H, Eichelbaum M. (2005a) A role for constitutive androstane receptor in the regulation of human intestinal MDR1 expression. Biol Chem 386:503–513 [DOI] [PubMed] [Google Scholar]
- Burk O, Arnold KA, Nussler AK, Schaeffeler E, Efimova E, Avery BA, Avery MA, Fromm MF, Eichelbaum M. (2005b) Antimalarial artemisinin drugs induce cytochrome P450 and MDR1 expression by activation of xenosensors pregnane X receptor and constitutive androstane receptor. Mol Pharmacol 67:1954–1965 [DOI] [PubMed] [Google Scholar]
- Chen CC, Meadows B, Regis J, Kalafsky G, Fojo T, Carrasquillo JA, Bates SE. (1997) Detection of in vivo P-glycoprotein inhibition by PSC 833 using Tc-99m sestamibi. Clin Cancer Res 3:545–552 [PubMed] [Google Scholar]
- Chin KV, Ueda K, Pastan I, Gottesman MM. (1992) Modulation of activity of the promoter of the human MDR1 gene by Ras and p53. Science 255:459–462 [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C, O’Brien JP, Casals D, Rittman-Grauer L, Biedler JL, Melamed MR, Bertino JR. (1989) Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci USA 86:695–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croop JM, Raymond M, Haber D, Devault A, Arceci RJ, Gros P, Housman DE. (1989) The three mouse multidrug resistance (mdr) genes are expressed in a tissue-specific manner in normal mouse tissues. Mol Cell Biol 9:1346–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debenham PG, Kartner N, Siminovitch L, Riordan JR, Ling V. (1982) DNA-mediated transfer of multiple drug resistance and plasma membrane glycoprotein expression. Mol Cell Biol 2:881–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devault A, Gros P. (1990) Two members of the mouse mdr gene family confer multidrug resistance with overlapping but distinct drug specificities. Mol Cell Biol 10:1652–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fojo AT, Ueda K, Slamon DJ, Poplack DG, Gottesman MM, Pastan I. (1987) Expression of a multidrug-resistance gene in human tumors and tissues. Proc Natl Acad Sci USA 84:265–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman MM, Pastan I. (1993) Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem 62:385–427 [DOI] [PubMed] [Google Scholar]
- Gu L, Tsark WM, Brown DA, Blanchard S, Synold TW, Kane SE. (2009) A new model for studying tissue-specific mdr1a gene expression in vivo by live imaging. Proc Natl Acad Sci USA 106:5394–5399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SI-H, Cohen D, Kirschner LS, Lothstein L, Hartstein M, Horwitz SB. (1990) Structural analysis of the mouse mdr1a (P-glycoprotein) promoter reveals the basis for differential transcript heterogeneity in multidrug-resistant J774.2 cells. Mol Cell Biol 10:3596–3606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui RC-Y, Francis RE, Guest SK, Costa JR, Gomes AR, Myatt SS, Brosens JJ, Lam EW-F. (2008) Doxorubicin activates FOXO3a to induce the expression of multidrug resistance gene ABCB1 (MDR1) in K562 leukemic cells. Mol Cancer Ther 7:670–678 [DOI] [PubMed] [Google Scholar]
- Kane SE. (1996) Multidrug resistance of cancer cells, in Advances in Drug Research (Testa B, Meyer UA. eds) pp 181–252, Academic Press, San Diego [Google Scholar]
- Kartner N, Riordan JR, Ling V. (1983) Cell surface P-glycoprotein associated with multidrug resistance in mammalian cell lines. Science 221:1285–1288 [DOI] [PubMed] [Google Scholar]
- Kast HR, Goodwin B, Tarr PT, Jones SA, Anisfeld AM, Stoltz CM, Tontonoz P, Kliewer S, Willson TM, Edwards PA. (2002) Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J Biol Chem 277:2908–2915 [DOI] [PubMed] [Google Scholar]
- Luker GD, Fracasso PM, Dobkin J, Piwnica-Worms D. (1997) Modulation of the multidrug resistance P-glycoprotein: detection with technetium-99m-sestamibi in vivo. J Nucl Med 38:369–372 [PubMed] [Google Scholar]
- Moore LB, Parks DJ, Jones SA, Bledsoe RK, Consler TG, Stimmel JB, Goodwin B, Liddle C, Blanchard SG, Willson TM, et al. (2000) Orphan nuclear receptors constitutive androstane receptor and pregnane X receptor share xenobiotic and steroid ligands. J Biol Chem 275:15122–15127 [DOI] [PubMed] [Google Scholar]
- Poland A, Mak I, Glover E. (1981) Species differences in responsiveness to 1,4-bis[2-(3,5-dichloropyridyloxy)]-benzene, a potent phenobarbital-like inducer of microsomal monooxygenase activity. Mol Pharmacol 20:442–450 [PubMed] [Google Scholar]
- Schinkel AH, Wagenaar E, van Deemter L, Mol CA, Borst P. (1995) Absence of the mdr1a P-Glycoprotein in mice affects tissue distribution and pharmacokinetics of dexamethasone, digoxin, and cyclosporin A. J Clin Invest 96:1698–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikic BI, Fisher GA, Lum BL, Halsey J, Beketic-Oreskovic L, Chen G. (1997) Modulation and prevention of multidrug resistance by inhibitors of P-glycoprotein. Cancer Chemother Pharmacol 40 (Suppl):S13–S19 [DOI] [PubMed] [Google Scholar]
- Synold TW, Dussault I, Forman BM. (2001) The orphan nuclear receptor SXR coordinately regulates drug metabolism and efflux. Nat Med 7:584–590 [DOI] [PubMed] [Google Scholar]
- Tang-Wai DF, Kajiji S, DiCapua F, de Graaf D, Roninson IB, Gros P. (1995) Human (MDR1) and mouse (mdr1, mdr3) P-glycoproteins can be distinguished by their respective drug resistance profiles and sensitivity to modulators. Biochemistry 34:32–39 [DOI] [PubMed] [Google Scholar]
- Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. (1987) Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci USA 84:7735–7738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thottassery JV, Zambetti GP, Arimori K, Schuetz EG, Schuetz JD. (1997) p53-dependent regulation of MDR1 gene expression causes selective resistance to chemotherapeutic agents. Proc Natl Acad Sci USA 94:11037–11042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urquhart BL, Tirona RG, Kim RB. (2007) Nuclear receptors and the regulation of drug-metabolizing enzymes and drug transporters: implications for interindividual variability in response to drugs. J Clin Pharmacol 47:566–578 [DOI] [PubMed] [Google Scholar]
- Xie W, Barwick JL, Downes M, Blumberg B, Simon CM, Nelson MC, Neuschwander-Tetri BA, Brunt EM, Guzelian PS, Evans RM. (2000) Humanized xenobiotic response in mice expressing nuclear receptor SXR. Nature 406:435–439 [DOI] [PubMed] [Google Scholar]
- Xu C, Wang X, Staudinger JL. (2009) Regulation of tissue-specific carboxylesterase expression by pregnane x receptor and constitutive androstane receptor. Drug Metab Dispos 37:1539–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zastawny RL, Salvino R, Chen J, Benchimol S, Ling V. (1993) The core promoter region of the P-glycoprotein gene is sufficient to confer differential responsiveness to wild-type and mutant p53. Oncogene 8:1529–1535 [PubMed] [Google Scholar]