Abstract
(±)-3,4-Methylenedioxymethamphetamine (MDMA, “Ecstasy”) is a popular drug of abuse. We aimed to characterize the behavioral effects of intragastric MDMA in a species closely related to humans and to relate behavioral effects to plasma MDMA and metabolite concentrations. Single doses of MDMA (0.32–7.8 mg/kg) were administered via an intragastric catheter to adult male baboons (N = 4). Effects of MDMA on food-maintained responding were assessed over a 20-hour period, whereas untrained behaviors and fine-motor coordination were characterized every 30 minutes until 3 hours postadministration. Levels of MDMA and metabolites in plasma were measured in the same animals (n = 3) after dosing on a separate occasion. MDMA decreased food-maintained responding over the 20-hour period, and systematic behavioral observations revealed increased frequency of bruxism as the dose of MDMA was increased. Drug blood level determinations showed no MDMA after the lower doses of MDMA tested (0.32–1.0 mg/kg) and modest levels after higher MDMA doses (3.2–7.8 mg/kg). High levels of 3,4-dihydroxymethamphetamine (HHMA) were detected after all doses of MDMA, suggesting extensive first-pass metabolism of MDMA in the baboon. The present results demonstrate that MDMA administered via an intragastric catheter produced behavioral effects that have also been reported in humans. Similar to humans, blood levels of MDMA after oral administration may not be predictive of the behavioral effects of MDMA. Metabolites, particularly HHMA, may play a significant role in the behavioral effects of MDMA.
Introduction
(±)-3,4-Methylenedioxymethamphetamine HCl (MDMA; Ecstasy) is a popular drug of abuse. Structurally, MDMA is related to both amphetamine (Gold et al., 1989; Verrico et al., 2008) and the hallucinogen mescaline (Kovar, 1998). According to the National Survey on Drug Use and Health, lifetime MDMA use in the United States has increased significantly from 4.3% in 2002 to 6.3% in 2010 among people aged ≥12 years.
Behavioral effects after MDMA administration are undoubtedly functional consequences of MDMA-induced changes in brain neurochemistry. Thus, it is important to note that MDMA is a potent indirect monoaminergic agonist and reuptake inhibitor that also inhibits tryptophan hydroxylase, an essential enzyme in the synthesis of serotonin (Malberg and Bonson, 2001) [for reviews, see Cole and Sumnall (2003) and Green et al. (2003)]. Biologically relevant differences in the serotonin-1 (5-HT1) and 5-HT2 receptor systems of rodent and primate species (Weerts et al., 2007), and an overall >98% gene homology between monkeys and humans in the case of monoamine transporters (Hacia et al., 1998), makes baboons an attractive species for characterizing the behavioral effects of MDMA. In addition, a characterization of the relationship between behavioral and pharmacokinetic effects of MDMA in baboons would be useful for comparison of similar effects in humans.
In addition to possible species differences in relevant physiology, there are also species differences in the metabolism of MDMA. For example, 3,4-methylenedioxyamphetamine (MDA) is a minor metabolite of MDMA in humans (3–5%), whereas the percentage of MDMA N-demethylated to form MDA was 23–34% after oral MDMA administration in rats (Baumann et al., 2009; Mueller et al., 2009c). In comparison, 3–5% of MDMA is metabolized to MDA in humans (Farré et al., 2004; Kolbrich et al., 2008) and squirrel monkeys (de la Torre et al., 2000b; Mueller et al., 2008). In baboons, as reported in humans, MDA appears to be a relatively minor metabolite of MDMA, which is only detected after higher MDMA doses. Initially, MDMA to MDA ratios in baboons seem higher compared with humans; however, ratios tend to decrease with increases in body weight (Mueller et al., 2011).
There is a surprising lack of research characterizing the behavioral effects of orally administered MDMA in nonhuman subjects given that the oral route is the one most commonly used by recreational MDMA users (Green et al., 2003). Moreover, differential behavioral and pharmacological profiles as a function of the route of administration for abused drugs are a focus of clinical research [e.g., see Kuramoto et al. (2011)], and the pharmacokinetics of MDMA do differ across routes of administration in rats (Baumann et al., 2009). In nonhuman primates, oral MDMA administration increased body temperature while suppressing locomotion in rhesus monkeys (Crean et al., 2007) and increased error rates in a cognitive task in cynomolgus monkeys (Verrico et al., 2008). In human laboratory studies, oral administration of MDMA produced increases in heart rate and blood pressure as well as increases in subjective responses such as energy level, empathy (Kolbrich et al., 2008; Peiró et al., 2013), and feelings associated with increased social behavior (Bedi et al., 2010).
The pharmacokinetics of orally administered MDMA are nonlinear across species (de la Torre et al., 2000a; Pacifici et al., 2001; Farré et al., 2004; Mechan et al., 2006; Mueller et al., 2008, 2009a, 2011). Hence, clinically relevant comparisons of the behavioral and pharmacokinetic effects of MDMA must include a consideration of the dose range and dosing schedules used. For example, human studies of the behavioral and pharmacokinetic effects of MDMA have been limited to the lower portion of the abused MDMA dose range [for review, see Parrott (2005)] and this is likely due to the neurotoxic effects of MDMA reported in rodents and nonhuman primates (Sarkar and Schmued, 2010).
We sought to follow up on our previous work with oral MDMA in the baboon (Mueller et al., 2011) to characterize the behavioral effects of MDMA across a wide dose range (0.32–7.8 mg/kg) as well as to determine the plasma concentration-over-time profiles of MDMA and its major metabolites in the same subjects. To assure reliable oral delivery of the dose throughout the assessments, we used a route of administration that mimics oral administration, that is, via chronically indwelling intragastric (i.g.) catheters.
Materials and Methods
Subjects
Four adult male baboons (Papio hamadryas anubis) designated as GD, PY, SHA, and YO served as subjects and ranged in weight from 18.4 to 33.2 kg at the start of the study (Table 1). Each of the subjects had previously served in experimental conditions that involved chronic i.g. administration of a single dose of a test drug for approximately a month, followed by evaluation of the effects of drug withdrawal. All had experience with one (PY) to five (YO) such studies of novel GABA-A allosteric modulators (Ator et al., 2010). Two baboons (GD, PY) had also served in an intravenous self-administration procedure in which they had experience with cocaine and five or six sedative/anxiolytic compounds (Ator et al., 2010), and PY had experience with oral ethanol self-administration. Otherwise, GD, SHA, and YO had experience with bolus i.g. dosing with three compounds to characterize effects on food-maintained behavior under the procedure used in this study. The baboons had not served in experimental conditions for 6 months before this investigation. They had patent i.g. catheters, which had been implanted at the time of previous studies. The catheter was protected using a tether/vest system that allowed for free movement within the cage. The procedures for catheter implantation surgery, as well as a complete description of the tether/vest system, were previously described (Lukas et al., 1982).
TABLE 1.
Mean body weight and order of study of MDMA doses for the behavioral and pharmacokinetic portions of the experiments
Vehicle sessions occurred before MDMA administration and between MDMA sessions during the behavioral assessment.
| Subject | Mean Body Weight | Order of MDMA Doses |
|||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| kg ± S.D. | mg/kg | ||||||
| Behavioral assessment | |||||||
| GD | 27.3 (0.6) | 3.20 | 1.00 | 0.56 | 5.60 | 0.32 | _ |
| PY | 27.0 (0.4) | 1.00 | 3.20 | 0.56 | 5.60 | 0.32 | 7.80 |
| SHA | 17.9 (0.5) | 1.00 | 3.20 | 0.56 | 5.60 | _ | _ |
| YO | 34.8 (0.6) | 3.20 | 1.00 | 0.56 | 5.60 | 7.80 | 0.32 |
| Plasma assessment | |||||||
| GD | 26.1 (0.7) | 0.56 | 3.20 | 0.32 | 5.60 | 1.00 | _ |
| PY | 26.0 (0.3) | 0.32 | 1.00 | 5.60 | 0.56 | 3.20 | 7.80 |
| YO | 33.9 (1.2) | 0.32 | 1.00 | 0.56 | 3.20 | 5.60 | 7.80 |
The baboons had continuous access to tap water via a drinking spout located at the front of the cage. In addition to 20-h/d access to food pellets (see Behavioral Procedures), diets were supplemented with once-daily feeding of two pieces of fresh fruit or vegetables and a multivitamin delivered late morning each day (i.e., between hours 3 and 4 in the food-maintained behavior measure). Overhead lights were on a 13/11-hour schedule, with the overhead lights being illuminated for 13 h/d and dim lighting for 11 h/d; natural light also was provided via windows in the laboratory area. Physical examinations occurred every 2–3 weeks using atropine sulfate to control secretions, and ketamine hydrochloride (HCl) to permit handling of the baboons. During these examinations, the baboon was weighed, the catheter exit site was shaved and scrubbed, nails were clipped as needed, and teeth were brushed. The program of environmental enrichment included providing two or more toys or other objects to manipulate (e.g., stainless steel mirrors), which were periodically changed.
Apparatus
Baboons were housed in individual stainless steel cages that also served as the experimental chambers. Cages were equipped with a bench along the length of one of the side walls, and a custom-made intelligence panel formed the back wall of the cage. The intelligence panel contained a plunger (Lindsley operandum) over which a cue light was mounted, a food hopper, and a speaker for the delivery of a tone. The Lindsley operandum and food hopper were within easy reach of the baboon when seated on the bench. A strain relief mount (model SR-750B; Instech-Soloman, Plymouth Meeting, PA) was attached at the top of the cage and connected to a peristaltic pump (Harvard Model 1201 or 1203; Harvard Apparatus, South Natick, MA) and to the catheter via a custom 18-gauge liquid swivel. Reverse osmosis (RO) water was continuously infused (approximately 0.3 ml/min) via the peristaltic pump to maintain catheter patency. Personal computers with MED-PC software and instrumentation (Med Associates, Inc., St. Albans, VT), located in an adjacent room, were used to collect operant data.
Drugs
MDMA was acquired through the National Institute on Drug Abuse Drug Supply Program (Research Triangle Institute International, Research Triangle, NC). Doses were chosen based on interspecies dose calculations (Dews, 1976; Mordenti and Chappell, 1989) to include the range of doses used recreationally by humans. More specifically, the US Drug Enforcement Administration reported in 2009 that seized tablets generally contained between 50 and 150 mg of MDMA, and similar ranges have been reported from regions such as the United Kingdom (range of 20–131 mg/tablet) (Wood et al., 2011) and Australia (range of 0–124 mg/tablet with an outlier at 249 mg/tablet) (Morefield et al., 2011). Self-report studies suggest that regular users of MDMA ingest 2–3 tablets at a time, whereas highly experienced users may ingest 10–25 tablets at a time [for review, see Parrott (2005)]. In addition, the majority of users escalate the number of tablets ingested over time (Parrott, 2005). The results of a retrospective self-report study conducted in the United Kingdom found that men took an average of 3.55 tablets an average of 6.33 days a month, whereas women averaged 2.26 tablets an average of 4 days a month (Verheyden et al., 2002). Thus, the dose of MDMA for recreational users may be described as generally falling between 50 and 450 mg of MDMA (0.7–6.4 mg/kg; baboon equivalent of 0.8–9.1 mg/kg using interspecies dose calculations) (Dews, 1976; Mordenti and Chappell, 1989), depending on how many tablets are ingested.
MDMA (0.32–7.8 mg/kg) was dissolved in 60 ml RO water and administered within 1 hour of mixing. MDMA or an equal volume of vehicle alone was administered via the i.g. catheter over 5–6 minutes, followed by a flush of 10 ml RO water. MDMA deliveries were separated by at least 5 days, and assessment of vehicle administration never occurred in the 3 days after administration of MDMA. Because total dose can be a relevant variable when individual subjects vary significantly in weight, Table 1 provides the average individual body weights during each phase of the study as well as the order in which doses were studied for each baboon.
Behavioral Procedures
Food-Maintained Behavior.
Baboons had daily access (20 h/d) to 1-g banana-flavored food pellets (Bio-SERV, Inc., Frenchtown, NJ) under a fixed ratio (FR) schedule of reinforcement, in which a pellet was delivered after 10 responses on the Lindsley operandum. Access generally began at approximately 9:00 AM. On test days, MDMA or vehicle was administered between 8:45 and 9:15 AM, and pellet availability began 15 minutes after the infusion was completed. A cue light illuminated above the Lindsley operandum was correlated with the availability of food pellets under the FR-10 schedule of reinforcement. After 20 hours elapsed, the cue light was extinguished and responses had no programmed consequences.
Behavioral Observations.
One of two trained observers completed a 17-item behavioral checklist noting the frequency and occurrence of the listed behaviors and postures for 2 minutes, beginning 30 minutes after MDMA or vehicle infusion and repeated 60, 90, 120, and 150 minutes after infusion. Behaviors and postures on the checklist were precisely defined and included those indicative of activity (locomotion, standing), motor coordination (ataxia), muscle tensor/convulsant effects (tremor/jerk), gastrointestinal symptoms (head-below-torso posture, gag/retch/vomit), social behaviors (lip-smacking, grunting), aggression (yawn), and other behaviors of interest (bruxism, scratching/grooming, rubbing nose, tactile-seeking, shaking cage, posture changes, responsiveness, and stereotypy). The observers had memorized the definitions of the behaviors in advance of the study. The included behaviors were based on those used to characterize the acute effects of other psychoactive drugs (Goodwin et al., 2009) and were previously described in detail (Weerts et al., 1998). Baboons had habituated to the presence of observers, and reliability between trained observers was 95% before the start of the present study. The observer was aware of whether the baboon had received vehicle or an MDMA dose, but the specific demands of the 2-minute observation task focus attention on presence or absence of defined behaviors/changes in appearance and reduce ambiguity/subjectivity in recording.
Fine-Motor Task.
The effects of MDMA on fine-motor coordination were characterized immediately after the behavioral observation described above. In this procedure, a food item (raisin, or plain M&M if the baboon did not readily perform the task for raisins) was placed in each of six small cups on a Plexiglas tray, and the tray was then presented to the baboon at the front of the cage for a maximum duration of 2 minutes. The positioning of the cups was such that the baboon had easy access to each through the bars of the cage. The number of items retrieved and the duration to retrieve all six was recorded, as well as whether there was evidence of manual incoordination (e.g., dropping retrieved items, difficulty grasping), hand tremor, ataxia in movement toward the task, and whether there was a failure to engage in the task itself (i.e., failure to approach the front of the cage).
Behavioral Data Analysis.
A single-subject design was used in which each baboon served as its own control (Sidman, 1960). Multiple observations after infusion of vehicle were completed in each baboon to characterize the range of behaviors to serve as a baseline for comparison of drug effects for each baboon. Vehicle test results were used to calculate z-scores for each behavioral measure for comparison with results after i.g. MDMA administration. Total food pellets obtained, duration to complete the fine-motor task, and some behaviors recorded in observation sessions (i.e., locomotion, standing, lip-smacking, grunting, yawn, bruxism, scratching/grooming, rubbing nose, tactile-seeking, shaking cage, posture changes, responsiveness, and stereotypy) were judged as significant if the value was outside the 95% confidence limits of the calculated z-scores that delineated the top 2.5% and bottom 2.5% of the distribution (i.e., a two-tailed test). For behaviors that were predicted to increase under drug (ataxia, tremor/jerk, head-below-torso posture, gag/retch/vomit), a change was judged as significant when the frequency of the behavior exceeded the vehicle control z-score above which only 5% of the scores would fall (i.e., a one-tailed test).
Blood Collection Procedures and Analysis
Plasma concentration-over-time profiles of MDMA and its major metabolites were determined after completion of study of the effects of MDMA on behavior because sedation of the baboon was required to be able to draw blood. Using the same administration procedures described above, MDMA or vehicle was administered in mixed order via the i.g. catheter in three (GD, PY, and YO) of the four subjects (see Table 1). The fourth subject (SHA) no longer had a functioning catheter during the blood collection phase of the study. In addition, due to the loss of the catheter in baboon GD, blood collection after the highest dose of MDMA (7.8 mg/kg) occurred in only two subjects (PY and YO).
To allow handling and blood collection, baboons were sedated with a commercially available solution of 50:50 tiletamine HCl and zolazepam HCl (Telazol; Fort Dodge Animal Health, Fort Dodge, IA). Atropine sulfate (Penn Vet, Lancaster, PA) was given to control secretions. Approximately 1.5–2.0 ml of blood was collected from a saphenous vein into a Vacutainer (BD, Franklin Lakes, NJ) containing EDTA as an anticoagulant at the following time points after administration: 30 minutes and 1, 2, 2.5, 3, 3.5, 6, and 24 hours. Blood for all time points was collected sequentially in the same 24-hour period. After collection, samples were centrifuged at 3200 rpm for 12 minutes, and serum was drawn off and transferred to polypropylene tubes that were kept frozen at −80°C until analysis.
Plasma concentrations of MDMA and its metabolites 3,4-dihydroxymethamphetamine (HHMA), 4-hydroxy-3-methoxymethamphetamine (HMMA), and MDA were determined using liquid chromatography/mass spectrometry (LC/MS) methods as described and validated previously (Mueller et al., 2009b). Briefly, aliquots (100 µl) of plasma were preserved with 20 µl sodium metabisulfite \ (250 mM) and 10 µl EDTA (250 mM). We added 100 µl of the corresponding analytical standard solution to the calibrator samples. Accordingly, 100 µl vehicle was added to each noncalibrator sample to adjust the volume. After addition of 100 µl of an aqueous solution of the racemic internal standards MDMA-d5, MDA-d5, and pholedrine (1.0 µg/ml, each) and 300 µl 0.5 M of HCl, the samples were mixed (15 seconds) on a rotary shaker and left at 100°C for 80 minutes to perform conjugate cleavage. After cooling to room temperature, 20 µl 4-methylcatechol was added to the samples and then briefly mixed. We then added 10 µl perchloric acid and the samples were mixed again on a rotary shaker for 15 seconds to perform protein precipitation. The samples were centrifuged (16,000 rpm for 5 minutes), and the supernatant transferred to autosampler vials. Aliquots (5 µl) were injected into the LC/MS system. Analysis was performed using an Agilent Technologies (Waldbronn, Germany) AT Series 1100 LC/MSD, VL version, using electrospray ionization in positive ionization mode, and including an AT 1100 Series high-performance liquid chromatography system that consisted of a degasser, a quaternary pump, a column thermostat, and an autosampler. Isocratic elution was performed on a Zorbax 300-SCX column (Narrow-Bore 2.1 × 150 mm, 5 μm) and a Zorbax SCX guard column (4.6 × 12.5 mm, 5 μm). The limit of quantification of the method for each analyte was as follows: 20 ng/ml each for MDMA, HHMA, and HMMA, and 10 ng/ml for MDA.
Results
Behavioral Data
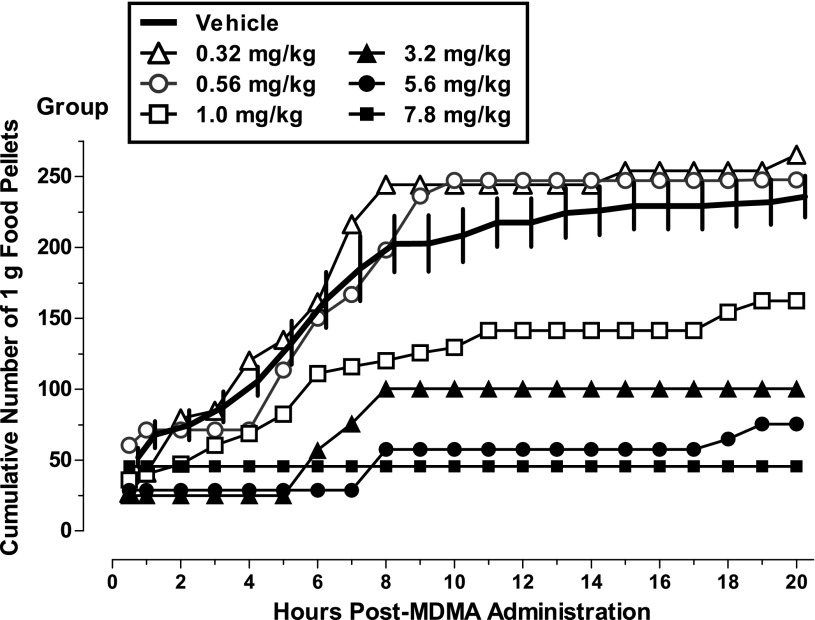
The numbers of 1-g food pellets obtained across the 20 hours of availability were recorded in 30-minute bins, and the cumulative numbers earned per hour by individual baboons are shown in Fig. 1. Three baboons (GD, PY, and YO) typically obtained 50–100 pellets within the first hour or two of pellet availability after vehicle infusion, whereas the fourth baboon (SHA) did not do so until after the second hour of pellet availability. Two baboons (PY and YO) generally did not continue earning pellets beyond hour 7, whereas the other two baboons tended to earn at least small numbers of pellets beyond that time point. MDMA changed the timing and/or patterning of feeding bouts, and did so at most doses in the largest baboon (YO) (Table 1). Although lower doses shifted the curves upward in some cases, MDMA doses of 1.0 mg/kg and above generally produced dose-related decreases in food-maintained behavior, compared with vehicle, during the 6 hours after administration (Fig. 1), and feeding for the day was severely disrupted. At doses of 3.2 mg/kg and higher, feeding was almost completely eliminated for the entire 20 hours. Because of unprogrammed restricted access to pellets (i.e., feeder malfunction) on the day preceding 3.2 mg/kg MDMA, data for this dose are not included for baboon GD. Fig. 2 shows the mean number of pellets earned after administration of MDMA for all baboons. When considered as a group, MDMA doses of 3.2 mg/kg and higher produced decreases in food-maintained behavior compared with vehicle.
Fig. 1.
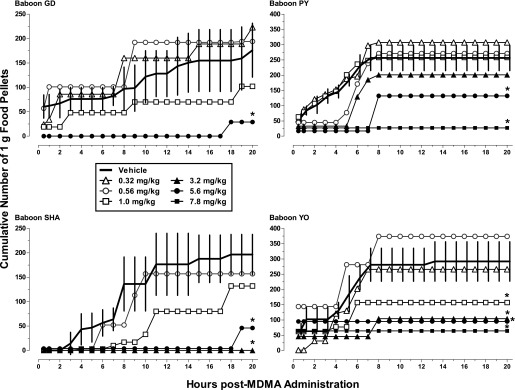
Food-maintained behavior after i.g. MDMA (0.32–7.8 mg/kg) or vehicle administration in four baboons during the daily 20-hour period of food pellet availability. The solid line presents the mean (± S.D.) cumulative total of 1 g food pellets earned from 15 minutes to 20 hours after vehicle was infused in control sessions (n = 5 for GD, n = 6 for PY and YO, and n = 4 for SHA). Open and closed symbols present the cumulative total food pellets earned from 15 minutes to 20 hours after the MDMA dose was infused. Note difference in the scale of y-axis in panels on the left compared with those on the right. MDMA doses were studied once in each baboon (see Table 1 for dose order; note that 0.32 was not studied in SHA and 7.8 mg/kg was not studied in GD and SHA). For GD, unprogrammed restricted access to pellets (i.e., feeder malfunction) on the day preceding 3.2 mg/kg MDMA preclude including data for this dose. *Indicates the total number of food pellets earned over the 20-hour period was significantly lower compared with vehicle (value fell outside the 95% confidence limits of the calculated z-scores for vehicle).
Fig. 2.
Food-maintained behavior after i.g. MDMA (0.32–7.8 mg/kg) or vehicle administration for the group (n = 4 baboons except n = 3 for 0.32 and 3.2 mg/kg and n = 2 for 7.8 mg/kg). Data for vehicle are the mean (± S.E.M.) total number of food pellets earned and data for MDMA are the mean total of food pellets earned. Other details same as for Fig. 1.
Performance of the fine-motor task is shown in Fig. 3. Three baboons typically retrieved all six raisins or M&Ms within 20 seconds or less, without dropping any, regardless of the time at which the task was offered after vehicle administration; the fourth baboon (SHA) took longer to approach completing the task when it was offered for the third, fourth, and fifth time. After MDMA infusion, there was a generally dose-related increase in time taken to complete the task such that it often was not completed when it was offered more than 30 minutes after the higher MDMA doses. Ataxia, defined as slow, uncertain movements, swaying, or falling over [Adams and Vistor (1993) and Weerts et al. (1998)], was not observed during presentations of the fine-motor task. Performance on the task was not disrupted by motor incoordination (e.g., dropping the items); rather, baboons failed to engage in the task on those occasions when it was not completed in the allotted time. Dose order (Table 1) may explain occasions when significantly longer durations were required to complete the task (or subjects failed to engage in the task) after administration of the lower MDMA doses (0.32–1.0 mg/kg). For example, baboon PY received 0.56 mg/kg MDMA after the 3.2 mg/kg dose. Observer comments repeatedly included “never approached task” and “sniffed food items but never picked any up.” Thus, MDMA (3.2–7.8 mg/kg) decreased performance of the fine-motor task in all subjects but not as a result of altering motor coordination.
Fig. 3.
Performance on the fine-motor task at multiple time points after i.g. MDMA (0.32–7.8 mg/kg) or vehicle administration in four baboons. Data for vehicle are the mean (± S.D.) durations to complete the task within 120 seconds at each time point postadministration (number of days on which vehicle was administered are the same as in Fig. 1), and data for MDMA are the durations to complete the task at each time point after administration. A value of 120 seconds indicates the task was not completed. The task was not presented to baboon YO at 30 and 60 minutes after 3.2 mg/kg MDMA administration. *Indicates the duration to complete the task was significant higher compared with vehicle at the same time point (value was outside the 95% confidence limits of the calculated z-scores for vehicle).
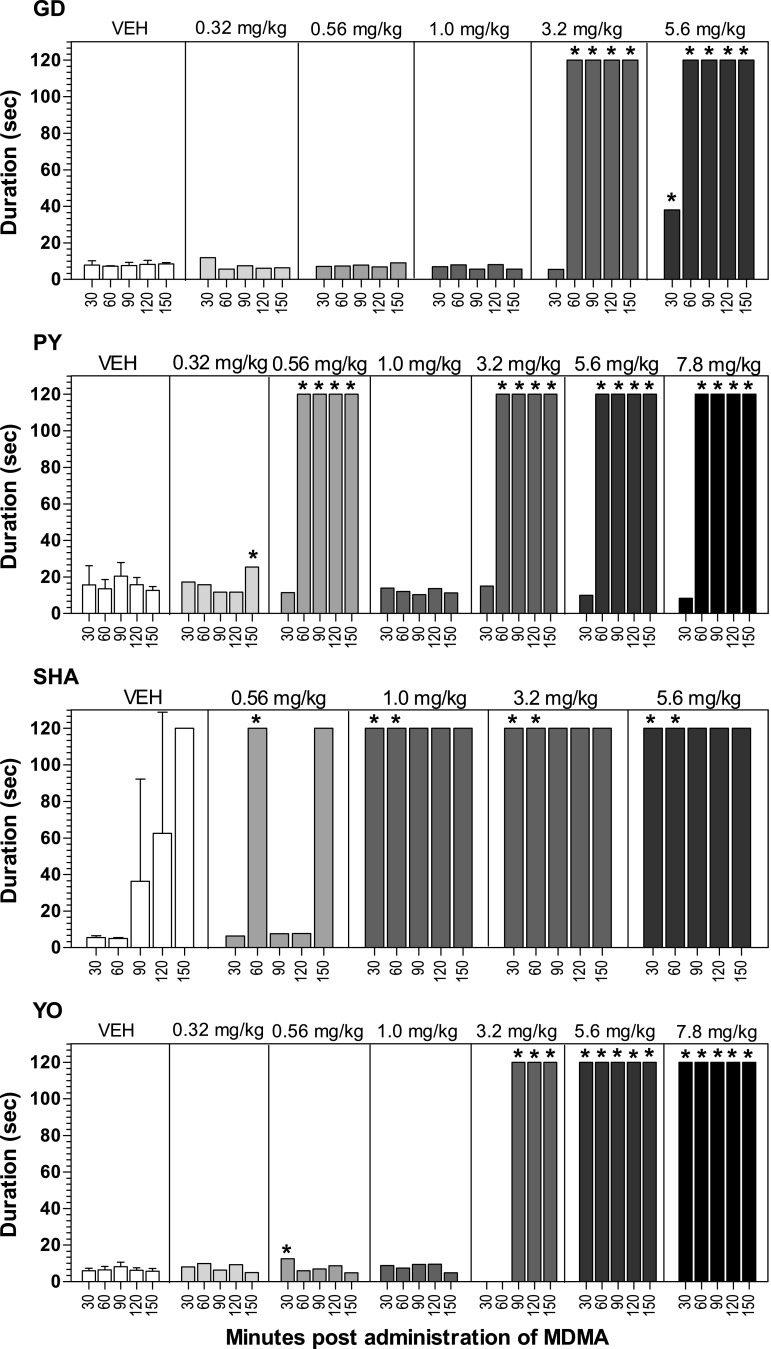
Table 2 shows the occurrences of bruxism (defined as gritting or grinding the teeth) and tremor (defined as more or less regular, rhythmic movement of the limbs, head, or trunk produced by synchronous contractions of antagonistic muscles) or jerks (defined as muscle contraction and/or relaxation leading to a quick jerking movement or twitch) during the observation periods [see Adams and Vistor (1993) and Weerts et al. (1998) for further details on the definitions of bruxism, tremor, and jerk]. After vehicle, all baboons exhibited very low rates of bruxism. After MDMA administration, dose-related increases in bruxism occurred in all four baboons, with the effect generally appearing 30–90 minutes after administration and persisting at the 150-minute observation. Although tremor and jerks were distinctively defined, they were recorded in a single category (tremor/jerk) on the behavioral checklist as indicators of muscle tensor/convulsant effects, and cannot be separated for analysis (notes permitted differentiation on some occasions). Neither tremor nor jerks were observed after vehicle administration. One baboon (SHA) exhibited only one limb tremor (30 minutes after 5.6 mg/kg MDMA) and a second baboon (GD) exhibited one limb tremor (30 minutes after 1.0 mg/kg MDMA and 30 minutes after 3.2 mg/kg MDMA). The remaining two baboons exhibited tremor/jerks after multiple doses of MDMA (see Table 2). Only one baboon exhibited signs of gastrointestinal distress (data not shown); more specifically, two episodes of vomiting were observed in baboon YO 2.5 hours after the 7.8 mg/kg MDMA dose. One baboon (PY) exhibited ataxia after 1.0 mg/kg MDMA 2.5 hours postadministration. Locomotion occurred at relatively low levels after vehicle administration, and the frequency of locomotion did not significantly change after MDMA administration (unpublished data).
TABLE 2.
Frequency after vehicle administration and frequency after MDMA (0.32–7.8 mg/kg) of bruxism and tremor/jerk during 2-minute observations that began 30 minutes after MDMA or vehicle infusion and were repeated 60, 90, 120, and 150 minutes after infusion
| Baboon | Time after Administration | Vehicle Bruxism | Vehicle Tremor/ Jerk | 0.32 Bruxism | 0.32 Tremor/ Jerk | 0.56 Bruxism | 0.56 Tremor/ Jerk | 1.0 Bruxism | 1.0 Tremor/ Jerk | 3.2 Bruxism | 3.2 Tremor/ Jerk | 5.6 Bruxism | 5.6 Tremor/ Jerk | 7.8 Bruxism | 7.8 Tremor/ Jerk |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| min | Mean (±S.D.) | ||||||||||||||
| GD | 30 | 1.3 (1.7) | 0.0 (0.0) | 1 | 0 | 4 | 0 | 6a | 1a,b | 1 | 1a,b | 11a | 0 | n/a | n/a |
| 60 | 1.9 (3.2) | 0.0 (0.0) | 1 | 0 | 4 | 0 | 5 | 0 | 3 | 0 | 31a | 0 | n/a | n/a | |
| 90 | 0.9 (1.7) | 0.0 (0.0) | 3 | 0 | 4 | 0 | 5a | 0 | 8a | 0 | 11a | 0 | n/a | n/a | |
| 120 | 0.8 (1.4) | 0.0 (0.0) | 0 | 0 | 5a | 0 | 10a | 0 | 8a | 0 | 12a | 0 | n/a | n/a | |
| 150 | 0.8 (1.1) | 0.0 (0.0) | 3 | 0 | 5a | 0 | 5a | 0 | 7a | 0 | 9a | 0 | n/a | n/a | |
| PY | 30 | 1.1 (1.1) | 0.0 (0.0) | 0 | 0 | 3 | 5a | 1 | 0 | 6a | 1a | 7a | 0 | 11a | 0 |
| 60 | 0.5 (1.1) | 0.0 (0.0) | 0 | 0 | 12a | 6a | 1 | 0 | 14a | 0 | 6a | 0 | 33a | 1a | |
| 90 | 0.0 (0.0) | 0.0 (0.0) | 0 | 0 | 18a | 0 | 3a | 0 | 7a | 1a | 8a | 0 | 5a | 1a | |
| 120 | 0.6 (1.4) | 0.0 (0.0) | 0 | 0 | 8a | 0 | 5a | 0 | 7a | 0 | 2 | 0 | 8a | 2a | |
| 150 | 0.4 (0.7) | 0.0 (0.0) | 0 | 0 | 0 | 0 | 3a | 0 | 9a | 1a | 1 | 0 | 4a | 0 | |
| SHA | 30 | 0.7 (1.0) | 0.0 (0.0) | n/a | n/a | 6a | 0 | 10a | 0 | 2 | 0 | 22a | 1a,b | n/a | n/a |
| 60 | 0.5 (0.8) | 0.0 (0.0) | n/a | n/a | 3a | 0 | 15a | 0 | 3a | 0 | 19a | 0 | n/a | n/a | |
| 90 | 0.3 (0.8) | 0.0 (0.0) | n/a | n/a | 5a | 0 | 10a | 0 | 3a | 0 | 35a | 0 | n/a | n/a | |
| 120 | 0.8 (1.0) | 0.0 (0.0) | n/a | n/a | 9a | 0 | 6a | 0 | 3 | 0 | 37a | 0 | n/a | n/a | |
| 150 | 0. (0.8) | 0.0 (0.0) | n/a | n/a | 7a | 0 | 4a | 0 | 3 | 0 | 11a | 0 | n/a | n/a | |
| YO | 30 | 1.0 (1.8) | 0.0 (0.0) | 7a | 1a | 7a | 0 | 5 | 0 | 5 | 0 | 6a | 0 | 9a | 0 |
| 60 | 0.6 (0.7) | 0.0 (0.0) | 2 | 2a | 0 | 0 | 5a | 0 | 13a | 0 | 8a | 0 | 18a | 2a | |
| 90 | 0.9 (2.1) | 0.0 (0.0) | 0 | 0 | 6a | 0 | 2 | 0 | 4 | 0 | 17a | 1a | 6a | 1 | |
| 120 | 0.8 (1.8) | 0.0 (0.0) | 2 | 0 | 9a | 0 | 3 | 0 | 4 | 0 | 5a | 0 | 12a | 0 | |
| 150 | 1.0 (2.1) | 0.0 (0.0) | 0 | 0 | 3 | 0 | 1 | 0 | 5 | 0 | 7a | 1a | 20a | 0 | |
n/a, dose was not administered.
Value was outside the 95% confidence limits of the calculated z-scores for vehicle.
Limb tremor specifically noted.
Pharmacokinetic Data
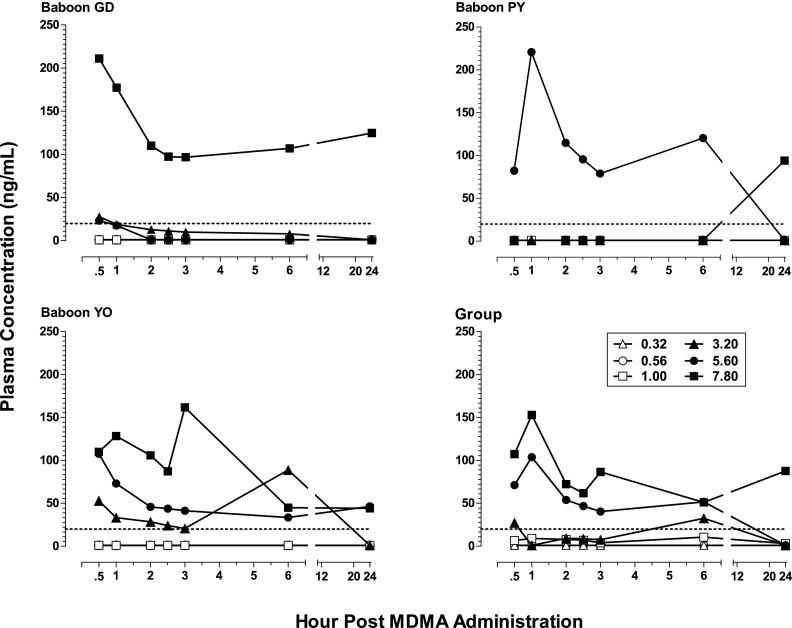
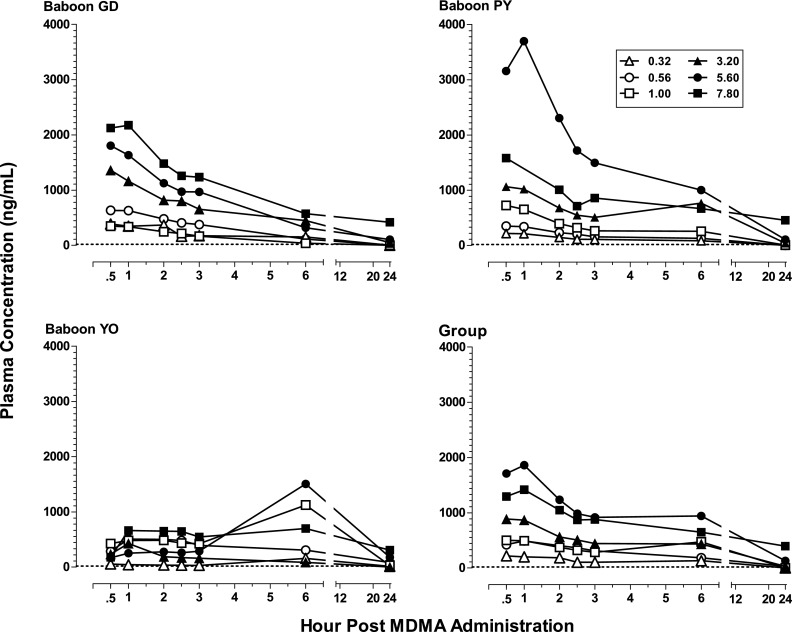
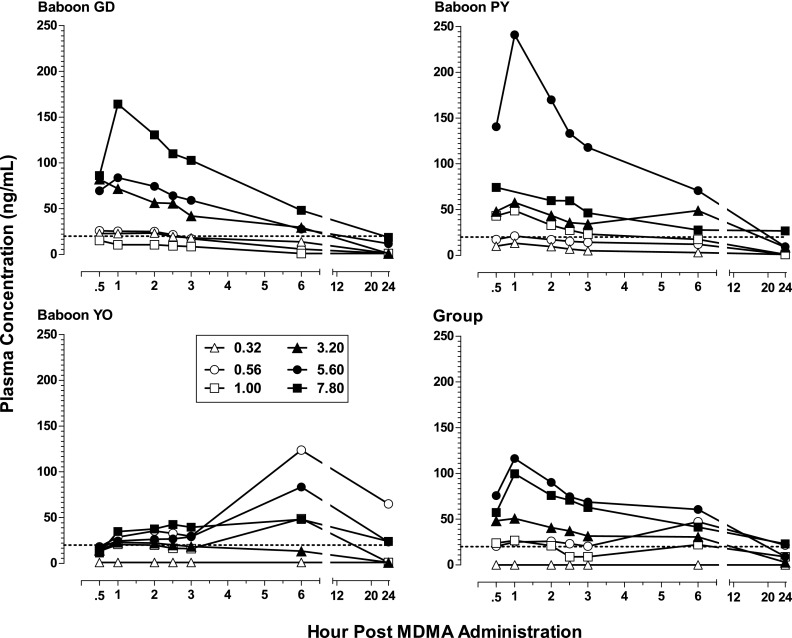
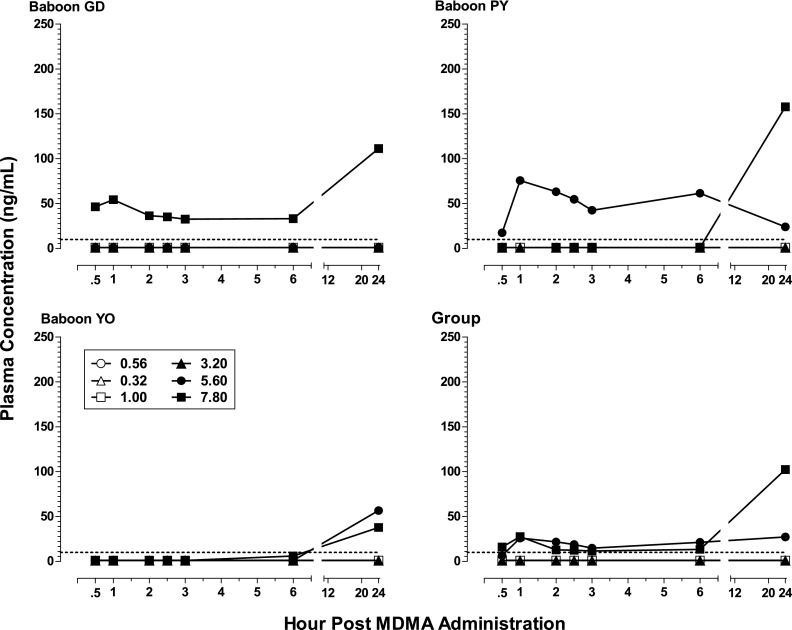
Figure 4 shows plasma concentration levels (nanograms per milliliter) of MDMA in the three subjects (GD, PY, and YO) for which the blood study could be conducted as well as for the group. Levels of the metabolites HHMA, HMMA, and MDA are shown, respectively, in Figs. 5, 6, and 7. Neither MDMA, nor the psychoactive metabolite MDA, was detected in plasma at any time point for any subject after i.g. administration of the lower MDMA doses (0.32–1.0 mg/kg). After the higher doses (3.2–7.8 mg/kg), MDMA was detected in all subjects (except one after the 3.2 mg/kg) during at least one time point postadministration. In contrast, HHMA (Fig. 5) was detected in all subjects after administration of all MDMA doses. HMMA (Fig. 6) was detected in all subjects at MDMA doses above 0.32 mg/kg. There were striking individual differences in the metabolism of MDMA across subjects. For example, much lower levels of MDMA were generally detected for GD compared with PY and YO, lower levels of HHMA were generally observed for YO compared with GD and PY, and MDA was detected only after the two highest MDMA doses.
Fig. 4.
Plasma levels (nanograms per milliliter) of MDMA at consecutive sampling intervals after administration of i.g. MDMA (0.32–7.8 mg/kg) to baboons (n = 3, except n = 2 for 7.8 mg/kg) as well as mean plasma levels of MDMA for the group. The limit of quantification was 20 ng/ml and is indicated with a dashed line.
Fig. 5.
Plasma levels (nanograms per milliliter) of HHMA at consecutive sampling intervals after administration of i.g. MDMA (0.32–7.8 mg/kg) to baboons (n = 3, except n = 2 for 7.8 mg/kg), as well as mean plasma levels of HHMA in for the group. The limit of quantification was 20 ng/ml and is indicated with a dashed line.
Fig. 6.
Plasma levels (nanograms per milliliter) of HMMA at consecutive sampling intervals after administration of i.g. MDMA (0.32–7.8 mg/kg) to baboons (n = 3, except n = 2 for 7.8 mg/kg), as well as mean plasma levels for the group. The limit of quantification was 20 ng/ml and is indicated with a dashed line.
Fig. 7.
Plasma levels (nanograms per milliliter) of MDA at consecutive sampling intervals after administration of i.g. MDMA (0.32–7.8 mg/kg) to baboons (n = 3, except n = 2 for 7.8 mg/kg), as well as mean plasma levels for the group (n = 3, except n = 2 for 7.8 mg/kg). The limit of quantification was 10 ng/ml and is indicated with a dashed line
For the group, mean levels of MDMA, HMMA, and MDA generally did not exceed 150 ng/ml, whereas mean levels of HHMA were greater than 500 ng/ml at all MDMA doses above 0.56 mg/kg. The highest mean levels (± S.E.M.) of MDMA (103.8 ± 60.6 ng/ml; n = 3), HHMA (1860.2 ± 1000.6 ng/ml; n = 3), and HMMA (116.4 ng/ml ± 64.6; n = 3) were measured 1 hour after administration of the 5.6 mg/kg MDMA dose. In contrast, the highest mean level (± S.E.M.) of MDA (26.9 ± 16.4 ng/ml) in plasma after the 5.6 mg/kg MDMA dose was measured at the 24-hour time point. After administration of 7.8 mg/kg MDMA, albeit in only two baboons, the highest mean level of MDA was also at the 24-hour time point, but was considerably higher (97.9 ± 60.0 ng/ml; n = 2).
Relation between Behavior and Pharmacokinetic Data
There are limitations on relating the behavioral effects observed to the plasma levels achieved at the same doses in the same animals because the pharmacokinetic data were collected in the context of a second dose-effect study after the behavioral data had been collected and only three of the four baboons could be studied. To the extent that some generalizations can be made, the behavioral effects observed in the present study seemed to be most clearly associated with the detection of high levels of HHMA and/or HMMA in the plasma. For example, feeding behavior measured under the FR schedule was completely suppressed within 30 minutes after a high (5.6 mg/kg) MDMA dose in baboons (GD and PY; Fig. 1) that later showed high levels of those metabolites 30 minutes after administration of that dose (Figs. 5 and 6). Feeding behavior in the third baboon (YO) did not differ from vehicle levels until 5 hours after that dose (Fig. 1), which was paralleled by the peaking of HHMA and HMMA measured 6 hours after that dose (Figs. 5 and 6). By contrast, performance on the fine-motor task, involving retrieval of food treats, was hardly affected in baboons GD and PY when tested 30 minutes after the 5.6 mg/kg dose even though feeding behavior per se had been completely suppressed. For baboon YO, for which MDMA, but not metabolite, levels were high 30 minutes after the 5.6 mg/kg dose, performance of the fine-motor task was eliminated despite feeding behavior showing little change.
Discussion
This study demonstrates, for the first time, that MDMA is behaviorally active in an Old World Monkey, the baboon, when administered via an i.g. catheter. Moreover, some of the behavioral effects reported for the baboons in this study have also been reported in humans, suggesting that the baboon is a useful animal model for study of oral MDMA. All four baboons exhibited significant amounts of bruxism and decreases in food-maintained behavior, and human MDMA users also experience bruxism and consistently report decreases in appetite (Baylen and Rosenberg, 2006). Overt behavioral changes in the baboons were observed beginning 30–60 minutes post-MDMA administration and continued to occur at the last observation 2.5 hours postadministration. Decreases in food-maintained responding after MDMA were generally observed across the 20-hour experimental sessions at the higher doses. In humans, many of the behavioral effects of MDMA began occurring by 1 hour postadministration and continued for several hours (Farré et al., 2004; Kolbrich et al., 2008; Bedi et al., 2010; Peiró et al., 2013). In addition, significant increases in movements defined as tremor and/or limb or torso jerking movements were observed in baboons, and the latter resembled myoclonic jerks. These types of movements have also been described in humans (Baylen and Rosenberg, 2006; Rodgers et al., 2006) and are considered to be one result of altered serotonin activity (Rodnitzky, 2005; Dvir and Smallwood, 2008). Indeed, myoclonus may be an early indicator of serotonin syndrome, an adverse effect of MDMA reported in humans (Sarkar and Schmued, 2010).
Consistent with studies in humans (Kolbrich et al., 2008; Mueller et al., 2009a; Peiró et al., 2013), other primate species (Mechan et al., 2006; Mueller et al., 2008, 2009a), as well as rodents (Baumann et al., 2009; Scheidweiler et al., 2011), the pharmacokinetics of MDMA were nonlinear in baboons. Similar to our previous results with oral administration (Mueller et al., 2011), MDMA was absent from baboon plasma after administration of the lower MDMA doses (0.32–1.0 mg/kg; human equivalent of approximately 0.26–1.42 mg/kg; Dhuman=Danimal (Whuman/Wanimal)0.7 where D = dose in milligrams, W = weight of the animal in kilograms, and 0.7 is a commonly used and empirically derived exponent). Higher doses of MDMA (3.2–7.8 mg/kg; human equivalent of approximately 2.26–5.5 mg/kg) resulted in a mean maximum concentration of 135 ng/ml MDMA in plasma in the present study and 249 ng/ml (after 5.0 mg/kg) in our previous study (Mueller et al., 2011). In humans, lower doses of MDMA (0.75–1.6 mg/kg) produced somewhat similar ranges of maximum MDMA concentrations in blood, ranging from 161 ng/ml to 306 ng/ml (Farré et al., 2004; Kolbrich et al., 2008; Dumont et al., 2010). Although the dose range of MDMA investigated in baboons (0.32–7.8 mg/kg) can be considered to encompass the range of abused doses in humans (0.7–6.4 mg/kg; baboon equivalent of 0.8–9.1 mg/kg using interspecies dose calculations) (Dews, 1976; Mordenti and Chappell, 1989), such a range has not been examined in humans under laboratory conditions.
Despite the absence of MDMA in baboon plasma after the lower MDMA doses, the HHMA and HMMA metabolites of MDMA were detected within the first 30 minutes after every MDMA dose (present study; Mueller et al., 2011). In addition, vastly higher levels of the metabolite HHMA were observed in baboons compared with levels reported in humans. Since MDMA is metabolized to HHMA mainly via O-demethylenation and subsequent O-methylation to HMMA in humans and other primate species, the absence of MDMA after low doses may be related to a remarkably efficient rate of O-demethylenation during first-pass metabolism of MDMA to HHMA in baboons. There is no evidence that Telazol alters MDMA metabolism, and preliminary studies in our laboratory comparing Telazol with ketamine, as well as ketamine with saline, have yielded similar findings with regard to the metabolic pattern of MDMA (unpublished observation). We cannot, however, totally rule out the possible influence of Telazol in the detection of MDMA and its metabolites in plasma. Ideally, behavioral and pharmacological effects after peripheral administration of HHMA and HMMA should be examined. In addition, to better understand the pharmacokinetics of MDMA in baboons after intragastric administration, analysis of plasma at additional time points post-MDMA administration (e.g., 15 minutes and 7, 9, 11 hours, etc.) is needed.
The lack of a strict correspondence between doses and subjects included in both the behavioral and pharmacokinetic studies, as well as the variability in metabolism found in the present study, precluded useful statistical analysis of the correlation between plasma levels of MDMA and its metabolites. However, study of the correspondences for individual subjects permits certain generalizations to be made. First, there did not appear to be a clear correlation between MDMA-induced onset of behavioral changes and baboon plasma levels of MDMA in the present study. The detection of MDMA in baboon plasma sometimes corresponded with the onset of a specific behavioral effect. For example, the appearance of bruxism and tremor/jerks, as well as disruption of the fine-motor task, generally occurred in the first 60 minutes post-MDMA administration at higher doses, as did peak MDMA levels in plasma at these doses. However, these same behavioral effects were also observed after lower MDMA doses, at which no MDMA was detected in baboon plasma. Indeed, the behavioral effects observed in the present study seem to be most clearly associated with the detection of high levels of HHMA and/or HMMA in baboon plasma.
There are few studies in humans that have examined behavioral changes and pharmacokinetics after oral MDMA administration. The earliest study reported that peak levels of MDMA in plasma occurred around the same time peak subjective effects were reported (1–2 hours postadministration of 100 mg MDMA; Farré et al., 2004). A second study found that the behavioral effects of MDMA (1.6 mg/kg; total dose of 111–150 mg) were correlated with MDMA plasma concentrations but correlation coefficients were low and clinically insignificant (Kolbrich et al., 2008). A third study reported that the correlation between oxytocin blood levels and the subjective effects of MDMA after MDMA administration (100 mg) was significantly stronger than the correlation between the subjective effects and MDMA blood levels (Dumont et al., 2010). The most recent study in humans (Peiró et al., 2013), reported that the subjective effects of MDMA (100 mg, orally administered) peaked between 1–2 hours and returned to baseline by 4 hours after administration. Plasma MDMA levels, however, peaked at 3.75 hours (Peiró et al., 2013). Thus, blood levels of MDMA after peripheral administration may not be the best predictor of the behavioral effects of MDMA.
Similar to data with human subjects (Farré et al., 2004; Kolbrich et al., 2008), we observed high intersubject variability in the maximum concentration of MDMA and its metabolites in baboon plasma, as well individual differences in some behavioral effects. Such individual differences in humans, combined with the identification of polymorphisms in the catechol-O-methyltransferase and CYP2D6 genes in humans, has led some to conclude there may be significant individual differences in genetic vulnerability to the effects of MDMA (Carmo et al., 2006; Schilt et al., 2009; Fagundo et al., 2010). Taken together, genetic vulnerability to the effects of MDMA may explain at least some of the individual differences observed in the present study. Indeed, the notion of significant individual differences in response to MDMA being mediated via genetic polymorphisms is reminiscent of current findings in drug toxicity (Spanagel et al., 2010; Johansson and Ingelman-Sundberg, 2011). The role of genetics in mediating the magnitude of individual responses after MDMA administration will be more readily addressed as further models (e.g., genetic knock-outs, etc.) become available for use in this area of study.
In conclusion, MDMA administered via an i.g. catheter produced observable behavioral effects in baboons that were similar to those reported after oral MDMA administration in humans. Behavioral effects in baboons were generally evident 30–60 minutes after MDMA administration, similar to the onset of behavioral effects in humans. Overall, the baboon appears to be a suitable model for investigations of the behavioral effects of MDMA administered via an i.g. catheter (or orally), although first-pass effects are more pronounced in baboons. Studies in humans confirm the notion that the behavioral effects of MDMA may not be readily correlated with detection of MDMA in plasma. HHMA levels, however, were vastly higher in baboons compared with humans. This species difference in metabolism may render the baboon an ideal model for examining the relative contributions of MDMA versus HHMA and other downstream metabolites after peripheral MDMA administration. Moreover, a characterization of species differences in the pharmacokinetics of orally administered MDMA will be useful for understanding relationships between behavior and pharmacokinetic effects.
Acknowledgments
Expert technical support was provided by Kelly Lane and Rebecca Rodgerson. The authors thank the National Institute on Drug Abuse Drug Supply Program for providing the MDMA used in the studies.
Abbreviations
- FR
fixed ratio
- HCl
hydrochloride
- HHMA
3,4-dihydroxymethamphetamine
- HMMA
4-hydroxy-3-methoxymethamphetamine
- i.g.
intragastric
- LC/MS
liquid chromatography/mass spectrometry
- MDA
3,4-methylenedioxyamphetamine
- MDMA
3,4-methylenedioxymethamphetamine
- RO
reverse osmosis
Authorship Contributions
Participated in research design: Goodwin, Mueller, Ricaurte, Ator.
Conducted experiments: Goodwin, Mueller, Shell.
Performed data analysis: Goodwin, Mueller.
Wrote or contributed to the writing of the manuscript: Goodwin, Mueller, Shell, Ricaurte, Ator.
Footnotes
This research was supported by the National Institutes of Health National Institute on Drug Abuse [Grants DA-05707 and DA-01796401 (to G.A.R.), and DA-021616 (to N.A.A.)].
References
- Adams RD, Vistor M. (1993) Principles of Neurology, McGraw-Hill, Inc., New York [Google Scholar]
- Ator NA, Atack JR, Hargreaves RJ, Burns HD, Dawson GR. (2010) Reducing abuse liability of GABAA/benzodiazepine ligands via selective partial agonist efficacy at alpha1 and alpha2/3 subtypes. J Pharmacol Exp Ther 332:4–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Zolkowska D, Kim I, Scheidweiler KB, Rothman RB, Huestis MA. (2009) Effects of dose and route of administration on pharmacokinetics of (+ or -)-3,4-methylenedioxymethamphetamine in the rat. Drug Metab Dispos 37:2163–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylen CA, Rosenberg H. (2006) A review of the acute subjective effects of MDMA/ecstasy. Addiction 101:933–947 [DOI] [PubMed] [Google Scholar]
- Bedi G, Van Dam NT, Redman J. (2010) Ecstasy (MDMA) and high prevalence psychiatric symptomatology: somatic anxiety symptoms are associated with polydrug, not ecstasy, use. J Psychopharmacol 24:233–240 [DOI] [PubMed] [Google Scholar]
- Carmo H, Brulport M, Hermes M, Oesch F, Silva R, Ferreira LM, Branco PS, Boer Dd, Remião F, Carvalho F, et al. (2006) Influence of CYP2D6 polymorphism on 3,4-methylenedioxymethamphetamine (‘Ecstasy’) cytotoxicity. Pharmacogenet Genomics 16:789–799 [DOI] [PubMed] [Google Scholar]
- Cole JC, Sumnall HR. (2003) The pre-clinical behavioural pharmacology of 3,4-methylenedioxymethamphetamine (MDMA). Neurosci Biobehav Rev 27:199–217 [DOI] [PubMed] [Google Scholar]
- Crean RD, Davis SA, Taffe MA. (2007) Oral administration of (+/-)3,4-methylenedioxymethamphetamine and (+)methamphetamine alters temperature and activity in rhesus macaques. Pharmacol Biochem Behav 87:11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre R, Farré M, Ortuño J, Mas M, Brenneisen R, Roset PN, Segura J, Camí J. (2000a) Non-linear pharmacokinetics of MDMA (‘ecstasy’) in humans. Br J Clin Pharmacol 49:104–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre R, Farré M, Roset PN, Lopez CH, Mas M, Ortuño J, Menoyo E, Pizarro N, Segura J, Cami J. (2000b) Pharmacology of MDMA in humans. Ann N Y Acad Sci 914:225–237 [DOI] [PubMed] [Google Scholar]
- Dews PB. (1976) Interspecies differences in drug effects: behavioral, in Psychotherapeutic Drugs, Part 1 (Usdin E, Forrest IS, eds) pp 175–214, Marcel Dekker, New York [Google Scholar]
- Dumont GJ, Schoemaker RC, Touw DJ, Sweep FC, Buitelaar JK, van Gerven JM, Verkes RJ. (2010) Acute psychomotor effects of MDMA and ethanol (co-) administration over time in healthy volunteers. J Psychopharmacol 24:155–164 [DOI] [PubMed] [Google Scholar]
- Dvir Y, Smallwood P. (2008) Serotonin syndrome: a complex but easily avoidable condition. Gen Hosp Psychiatry 30:284–287 [DOI] [PubMed] [Google Scholar]
- Fagundo AB, Cuyàs E, Verdejo-Garcia A, Khymenets O, Langohr K, Martín-Santos R, Farré M, de la Torre R. (2010) The influence of 5-HTT and COMT genotypes on verbal fluency in ecstasy users. J Psychopharmacol 24:1381–1393 [DOI] [PubMed] [Google Scholar]
- Farré M, de la Torre R, Mathúna BO, Roset PN, Peiró AM, Torrens M, Ortuño J, Pujadas M, Camí J. (2004) Repeated doses administration of MDMA in humans: pharmacological effects and pharmacokinetics. Psychopharmacology (Berl) 173:364–375 [DOI] [PubMed] [Google Scholar]
- Gold LH, Geyer MA, Koob GF. (1989) Neurochemical mechanisms involved in behavioral effects of amphetamines and related designer drugs. NIDA Res Monogr 94:101–126 [PubMed] [Google Scholar]
- Goodwin AK, Brown PR, Jansen EE, Jakobs C, Gibson KM, Weerts EM. (2009) Behavioral effects and pharmacokinetics of gamma-hydroxybutyrate (GHB) precursors gamma-butyrolactone (GBL) and 1,4-butanediol (1,4-BD) in baboons. Psychopharmacology (Berl) 204:465–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AR, Mechan AO, Elliott JM, O’Shea E, Colado MI. (2003) The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”). Pharmacol Rev 55:463–508 [DOI] [PubMed] [Google Scholar]
- Hacia JG, Makalowski W, Edgemon K, Erdos MR, Robbins CM, Fodor SP, Brody LC, Collins FS. (1998) Evolutionary sequence comparisons using high-density oligonucleotide arrays. Nat Genet 18:155–158 [DOI] [PubMed] [Google Scholar]
- Johansson I, Ingelman-Sundberg M. (2011) Genetic polymorphism and toxicology—with emphasis on cytochrome p450. Toxicol Sci 120:1–13 [DOI] [PubMed] [Google Scholar]
- Kolbrich EA, Goodwin RS, Gorelick DA, Hayes RJ, Stein EA, Huestis MA. (2008) Plasma pharmacokinetics of 3,4-methylenedioxymethamphetamine after controlled oral administration to young adults. Ther Drug Monit 30:320–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar KA. (1998) Chemistry and pharmacology of hallucinogens, entactogens and stimulants. Pharmacopsychiatry 31 (Suppl 2):69–72 [DOI] [PubMed] [Google Scholar]
- Kuramoto SJ, Bohnert AS, Latkin CA. (2011) Understanding subtypes of inner-city drug users with a latent class approach. Drug Alcohol Depend 118:237–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas SE, Griffiths RR, Bradford LD, Brady JV, Daley L. (1982) A tethering system for intravenous and intragastric drug administration in the baboon. Pharmacol Biochem Behav 17:823–829 [DOI] [PubMed] [Google Scholar]
- Malberg JE, Bonson KR. (2001) How MDMA works in the brain, in Ecstasy: The Complete Guide (Holland J, ed), Park Street Press, Rochester, Vermont [Google Scholar]
- Mechan A, Yuan J, Hatzidimitriou G, Irvine RJ, McCann UD, Ricaurte GA. (2006) Pharmacokinetic profile of single and repeated oral doses of MDMA in squirrel monkeys: relationship to lasting effects on brain serotonin neurons. Neuropsychopharmacology 31:339–350 [DOI] [PubMed] [Google Scholar]
- Mordenti J, Chappell W. (1989) The use of interspecies scaling in toxicokinetics, in Toxicokinetics and New Drug Development (Yacobi A, Kelly J, Batra V, eds) pp 42–96, Pergamon Press, New York [Google Scholar]
- Morefield KM, Keane M, Felgate P, White JM, Irvine RJ. (2011) Pill content, dose and resulting plasma concentrations of 3,4-methylendioxymethamphetamine (MDMA) in recreational ‘ecstasy’ users. Addiction 106:1293–1300 [DOI] [PubMed] [Google Scholar]
- Mueller M, Goodwin AK, Ator NA, McCann UD, Ricaurte GA. (2011) Metabolism and disposition of 3,4-methylenedioxymethamphetamine (“ecstasy”) in baboons after oral administration: comparison with humans reveals marked differences. J Pharmacol Exp Ther 338:310–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller M, Kolbrich EA, Peters FT, Maurer HH, McCann UD, Huestis MA, Ricaurte GA. (2009a) Direct comparison of (+/-) 3,4-methylenedioxymethamphetamine (“ecstasy”) disposition and metabolism in squirrel monkeys and humans. Ther Drug Monit 31:367–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller M, Peters FT, Huestis MA, Ricaurte GA, Maurer HH. (2009b) Simultaneous liquid chromatographic-electrospray ionization mass spectrometric quantification of 3,4-methylenedioxymethamphetamine (MDMA, Ecstasy) and its metabolites 3,4-dihydroxymethamphetamine, 4-hydroxy-3-methoxymethamphetamine and 3,4-methylenedioxyamphetamine in squirrel monkey and human plasma after acidic conjugate cleavage. Forensic Sci Int 184:64–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller M, Peters FT, Maurer HH, McCann UD, Ricaurte GA. (2008) Nonlinear pharmacokinetics of (+/-)3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”) and its major metabolites in squirrel monkeys at plasma concentrations of MDMA that develop after typical psychoactive doses. J Pharmacol Exp Ther 327:38–44 [DOI] [PubMed] [Google Scholar]
- Mueller M, Yuan J, Felim A, Neudörffer A, Peters FT, Maurer HH, McCann UD, Largeron M, Ricaurte GA. (2009c) Further studies on the role of metabolites in (+/-)-3,4-methylenedioxymethamphetamine-induced serotonergic neurotoxicity. Drug Metab Dispos 37:2079–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifici R, Zuccaro P, Farré M, Pichini S, Di Carlo S, Roset PN, Ortuño J, Pujadas M, Bacosi A, Menoyo E, et al. (2001) Effects of repeated doses of MDMA (“ecstasy”) on cell-mediated immune response in humans. Life Sci 69:2931–2941 [DOI] [PubMed] [Google Scholar]
- Parrott AC. (2005) Chronic tolerance to recreational MDMA (3,4-methylenedioxymethamphetamine) or Ecstasy. J Psychopharmacol 19:71–83 [DOI] [PubMed] [Google Scholar]
- Peiró AM, Farré M, Roset PN, Carbó M, Pujadas M, Torrens M, Camí J, de la Torre R. (2013) Human pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, ecstasy) after repeated doses taken 2 h apart. Psychopharmacology (Berl) 225:883–893 [DOI] [PubMed] [Google Scholar]
- Rodgers J, Buchanan T, Pearson C, Parrott AC, Ling J, Hefferman TM, Scholey AB. (2006) Differential experiences of the psychobiological sequelae of ecstasy use: quantitative and qualitative data from an internet study. J Psychopharmacol 20:437–446 [DOI] [PubMed] [Google Scholar]
- Rodnitzky RL. (2005) Drug-induced movement disorders in children and adolescents. Expert Opin Drug Saf 4:91–102 [DOI] [PubMed] [Google Scholar]
- Sarkar S, Schmued L. (2010) Neurotoxicity of ecstasy (MDMA): an overview. Curr Pharm Biotechnol 11:460–469 [DOI] [PubMed] [Google Scholar]
- Scheidweiler KB, Ladenheim B, Barnes AJ, Cadet JL, Huestis MA. (2011) (±)-3,4-methylenedioxymethamphetamine and metabolite disposition in plasma and striatum of wild-type and multidrug resistance protein 1a knock-out mice. J Anal Toxicol 35:470–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilt T, Koeter MW, de Win MM, Zinkstok JR, van Amelsvoort TA, Schmand B, den Brink Wv. (2009) The effect of Ecstasy on memory is moderated by a functional polymorphism in the cathechol-O-methyltransferase (COMT) gene. Eur Neuropsychopharmacol 19:116–124 [DOI] [PubMed] [Google Scholar]
- Sidman M. (1960) Tactics of Scientific Research, Basic Books, New York [Google Scholar]
- Spanagel R, Bartsch D, Brors B, Dahmen N, Deussing J, Eils R, Ende G, Gallinat J, Gebicke-Haerter P, Heinz A, et al. (2010) An integrated genome research network for studying the genetics of alcohol addiction. Addict Biol 15:369–379 [DOI] [PubMed] [Google Scholar]
- Verheyden SL, Hadfield J, Calin T, Curran HV. (2002) Sub-acute effects of MDMA (+/-3,4-methylenedioxymethamphetamine, “ecstasy”) on mood: evidence of gender differences. Psychopharmacology (Berl) 161:23–31 [DOI] [PubMed] [Google Scholar]
- Verrico CD, Lynch L, Fahey MA, Fryer AK, Miller GM, Madras BK. (2008) MDMA-induced impairment in primates: antagonism by a selective norepinephrine or serotonin, but not by a dopamine/norepinephrine transport inhibitor. J Psychopharmacol 22:187–202 [DOI] [PubMed] [Google Scholar]
- Weerts EM, Ator NA, Grech DM, Griffiths RR. (1998) Zolpidem physical dependence assessed across increasing doses under a once-daily dosing regimen in baboons. J Pharmacol Exp Ther 285:41–53 [PubMed] [Google Scholar]
- Weerts EM, Fantegrossi WE, Goodwin AK. (2007) The value of nonhuman primates in drug abuse research. Exp Clin Psychopharmacol 15:309–327 [DOI] [PubMed] [Google Scholar]
- Wood DM, Stribley V, Dargan PI, Davies S, Holt DW, Ramsey J. (2011) Variability in the 3,4-methylenedioxymethamphetamine content of ‘ecstasy’ tablets in the UK. Emerg Med J 28:764–765 [DOI] [PubMed] [Google Scholar]