Abstract
Background
The World Health Organization Antiretroviral Treatment Guidelines recommend phasing-out stavudine because of its risk of long-term toxicity. There are two mutational pathways of stavudine resistance with different implications for zidovudine and tenofovir cross-resistance, the primary candidates for replacing stavudine. However, because resistance testing is rarely available in resource-limited settings, it is critical to identify the cross-resistance patterns associated with first-line stavudine failure.
Methods
We analyzed HIV-1 resistance mutations following first-line stavudine failure from 35 publications comprising 1,825 individuals. We also assessed the influence of concomitant nevirapine vs. efavirenz, therapy duration, and HIV-1 subtype on the proportions of mutations associated with zidovudine vs. tenofovir cross-resistance.
Results
Mutations with preferential zidovudine activity, K65R or K70E, occurred in 5.3% of individuals. Mutations with preferential tenofovir activity, ≥two thymidine analog mutations (TAMs) or Q151M, occurred in 22% of individuals. Nevirapine increased the risk of TAMs, K65R, and Q151M. Longer therapy increased the risk of TAMs and Q151M but not K65R. Subtype C and CRF01_AE increased the risk of K65R, but only CRF01_AE increased the risk of K65R without Q151M.
Conclusions
Regardless of concomitant nevirapine vs. efavirenz, therapy duration, or subtype, tenofovir was more likely than zidovudine to retain antiviral activity following first-line d4T therapy.
Keywords: HIV-1, drug resistance, mutations, nucleoside reverse transcriptase inhibitor, NRTI, stavudine, d4T, zidovudine, AZT, tenofovir, TDF, subtypes
The global scale-up of antiretroviral (ARV) therapy has dramatically reduced human immunodeficiency virus (HIV) morbidity and mortality. Stavudine (d4T) has been among the most commonly used ARVs in resource-limited settings because of its efficacy, short-term tolerability, low cost, and availability in coformulated form. However, many countries are transitioning away from d4T, mainly because of mitochondrial toxicities associated with long-term d4T use. In 2010, the World Health Organization (WHO) recommended phasing out d4T even in patients without documented virological failure [1].
There are two distinct mutational pathways of d4T resistance with different implications for zidovudine (AZT) and tenofovir (TDF) cross-resistance, the primary candidates for replacing d4T [2–4]. However, genotypic resistance testing is usually not available in the resource-limited settings where d4T-containing regimens are most commonly used. It is therefore important to determine the extent of nucleoside reverse-transcriptase inhibitor (NRTI) cross-resistance in viruses from individuals with virological failure on d4T-containing first-line therapy undergoing genotypic resistance testing. In this study, we combined genetic sequence data from 35 different studies to characterize the patterns of NRTI resistance mutations in viruses from individuals with virological failure on the 2 widely used d4T-containing ARV regimens: d4T/lamivudine(3TC)/nevirapine(NVP) and d4T/3TC/efavirenz(EFV). We also examine the effects of HIV-1 subtype, duration of therapy, and concomitant nonnucleoside reverse-transcriptase inhibitor (NNRTI) on the selection of specific mutations. We focused our analysis on NRTI resistance mutations with differential effects on the residual activity of AZT compared with TDF. The implications of these data for optimal replacement of d4T and subsequent NRTI options are discussed.
METHODS
First-Line ARV Therapy and HIV-1 Reverse Transcriptase (RT) Sequences
We analyzed the RT sequences of HIV-1 isolates from individuals in the Stanford HIV Drug Resistance Database (HIVDB) who received first-line therapy with either d4T/3TC/NVP or d4T/3TC/EFV [5]. RT sequences were eligible for analysis if they were reported in a peer-reviewed publication that contained ≥5 RT sequences from previously ARV-naive individuals receiving 1 of the 2 first-line d4T-containing regimens. For individuals from whom multiple RT sequences were available, the last sequence on therapy was used in our analyses.
NRTI Resistance Mutations
Mutations were defined as differences from the subtype B consensus sequence (http://hivdb.stanford.edu/DR/asi/releaseNotes/index.html#consensusbsequences). NRTI resistance mutations included the 34 surveillance drug-resistance mutations at 16 RT positions (M41L, K65R, D67NGE, T69D, T69insertions, K70RE, L74VI, V75MTAS, F77L, Y115F, F116Y, Q151M, M184VI, L210W, T215YFISCDVE, K219QERN) [6] and several less common NRTI resistance mutations, including K65N, T69deletions, and K70QG. Mutations at the same position with similar effects on NRTI susceptibility were pooled, including K65RN, D67NG, K70EGQ, L74VI, M184VI, T215YF, and K219QE. Thymidine analog–associated mutations (TAMs) were defined as M41L, D67NG, K70R, L210W, T215YF, and K219QE. Type 1 TAMs, which are associated with higher levels of reduced susceptibility to both AZT and TDF, include M41L, L210W, and T215Y.
We focused on NRTI resistance mutations with different implications for AZT and TDF cross-resistance. The most common of these mutations include (1) K65R, which reduces TDF susceptibility but increases AZT susceptibility; (2) TAMs, which reduce AZT susceptibility more than they reduce TDF susceptibility; (3) Q151M, which confers high-level AZT resistance and intermediate TDF resistance; and (4) K70EGQ, which is a rare mutation that reduces susceptibility to TDF but not AZT. K65R often occurs in combination with Q151M and has different implications for cross-resistance depending on whether it occurs alone or with Q151M [7]. Indeed, the combination of K65R plus Q151M is associated with high-level resistance to both AZT and TDF. In contrast, K65R and the TAMs are usually mutually exclusive [3, 7]. The most commonly occurring NRTI resistance mutation, M184VI, which increases susceptibility to both AZT and TDF, was also included in our analysis [4].
Based on the phenotypic correlates of these mutations, viruses with K65RN or K70EGQ in the absence of Q151M were considered to be preferentially inhibited by AZT. K65R is the most important of these because it is the primary mutation selected in vivo by TDF and because it increases AZT activity in vivo as well as in vitro [8, 9]. Viruses with ≥2 TAMs were considered to be preferentially inhibited by TDF. Although viruses with a single TAM may also be preferentially inhibited by TDF, the relative benefit of TDF compared with AZT against viruses with a single TAM is unlikely to be as great as the relative benefit of AZT compared with TDF in the treatment of viruses with K65R.
The T215 revertant mutations, T215SCDEIV, were excluded from analysis because they occurred much less frequently than the TAMs T215YF and because they have little if any effect on NRTI susceptibility [10]. The Q151M accessory mutations A62V, V75I, F77L, and F116Y were excluded because they occurred rarely in the absence of Q151M. The multi–NRTI resistance insertions and deletions at position 69 were excluded because T69 insertions occurred in just 2 individuals, and, although T69 deletions occurred in 23 individuals, they occurred solely in combination with K65R and/or Q151M. Although Y115F is associated with reduced TDF susceptibility, it was not included because it occurred rarely and because its effect on AZT susceptibility is not well characterized [11].
An additional analysis compared the estimated reductions in susceptibility to AZT and TDF using the HIVDB drug resistance interpretation system. Each submitted sequence was characterized as susceptible, potentially low-level resistant, low-level resistant, intermediately resistant, or highly resistant to AZT and TDF. In contrast with the analysis of mutation frequencies, this analysis takes into account the varying effects of specific TAMs on AZT and TDF susceptibility. For this analysis, the potentially low-level resistant category was pooled with the susceptible category.
Concomitant NNRTI, Duration of Therapy, and HIV-1 Subtype
We sought to determine whether the proportions of different mutations were influenced by the following variables: (1) the concomitantly administered NNRTI (NVP compared with EFV); (2) the duration of first-line d4T-containing therapy; and (3) the HIV-1 subtype. The number of weeks of therapy was known for about one-third of the individuals in the study. For the remaining individuals, the median number of weeks of therapy for all individuals in a study was used. Duration of therapy was classified as 1 year for individuals with up to 52 weeks of therapy, 2 years for those with 53–104 weeks of therapy, 3 years for those with 105–156 weeks of therapy, and 4 years for those with >156 weeks of therapy. HIV-1 subtype was determined primarily by using the automated Rega Institute subtyping tool [12]. For indeterminate results, subtype was determined using Los Alamos National Laboratories HIV Database jumping profile hidden Markov model algorithm [13].
We used stepwise forward logistic regression to assess the influence of ARV duration, NNRTI, and subtype on the following 10 mutation categories using the R step function [14]: M184VI (henceforth, M184V), ≥1 TAM (TAMsGE1), ≥2 TAMs (TAMsGE2), ≥3 TAMs (TAMsGE3), ≥1 type 1 TAM (Type1TAMsGE1), ≥2 type 1 TAMs (Type1TAMsGE2), three type 1 TAMs (Type1TAMs3), K65RN without Q151M (K65R alone), Q151M without K65RN (Q151M alone), and K70EGQ (henceforth, K70E). Because previous published studies of K65R have not distinguished between those with and without Q151M, we also performed separate logistic regression models to examine the effects of ARV duration, NNRTI, and subtype on the proportion of viruses with this mutation.
RESULTS
Summary of Analyzed Studies
Thirty-five studies comprising 1825 individuals receiving first-line ART with d4T/3TC/NVP or d4T/3TC/EFV were analyzed. Sixty-five percent (1182 individuals) were from 13 countries in sub–Saharan Africa. Twenty-three percent (417 individuals) were from the Southeast Asian countries of Thailand and Cambodia. The remaining 12% of individuals were from the United States (117 individuals), India (86 individuals), China (14 individuals), and Europe (7 individuals). Adults and children comprised at least 75% and 7% of the study population, respectively; the age group was not available for the remaining 18% of the study population. A prior history of single-dose nevirapine was noted for <80 individuals from 5 studies.
The median duration of therapy per study was 72 weeks (interquartile range, 52–104 weeks). Sixty-seven percent of individuals received d4T/3TC/NVP, and 33% received d4T/3TC/EFV. The most common subtypes were C (42.3% of individuals), CRF01_AE (AE; 23.6%), CRF02_AG (AG; 9.0%), B (7.2%), G (6.5%), and A (5.5%). The majority of isolates from subtype C, AE, and B were from individuals living in sub–Saharan Africa (89%), Southeast Asia (97%), and the United States (86%) respectively. More than 95% of AG, subtype A, and subtype G isolates were from individuals in sub–Saharan Africa. Supplementary Table 1 summarizes the contribution of each reference to the dataset.
The 10 categories of NRTI resistance mutations occurred in the following proportions of the 1825 individuals: 72% for M184V, 31% for TAMsGE1, 19% for TAMsGE2, 12% for TAMsGE3, 12% for Type1TAMsGE1, 5.8% for Type1TAMsGE2, 2.1% for Type1TAMs3E3, 4.5% for K65R alone, 2.9% for Q151M alone, and 0.8% for K70E. Overall, K65R occurred in 6.2% of individuals: 4.5% alone and 1.7% with Q151M. Supplementary Table 2 contains the proportion of each NRTI resistance mutation and mutation category for the complete dataset and for subsets determined by the NNRTI used, duration of therapy in years, and HIV-1 subtype.
NRTI Resistance Mutation Prevalence: Influence of Concomitant NNRTI, Duration of ART, and HIV-1 Subtype
Figure 1 illustrates the proportion of individuals with 2 categories of mutations according to the covariables of HIV-1 subtype, concomitant NNRTI, and duration of therapy. It also shows the number of individuals sharing the same covariable pattern at the base of each plot. In the figure, K65R includes the the presence of K65R or K70E, the mutations associated with increased AZT susceptibility and decreased TDF susceptibility. The 2 categories of TAMs in the figure are associated with greater reductions in susceptibility to AZT than to TDF. Q151M alone, which occurred in 2.9% of individuals and is also associated with a greater reduction in susceptibility to AZT than to TDF, is not shown. K65R occurred in 5%–10% of NVP-treated individuals with subtype C, CRF01_AE, and subtype A viruses; however, a much higher proportion of NVP-treated individuals with these subtypes had viruses with ≥2 TAMs.
Figure 1.
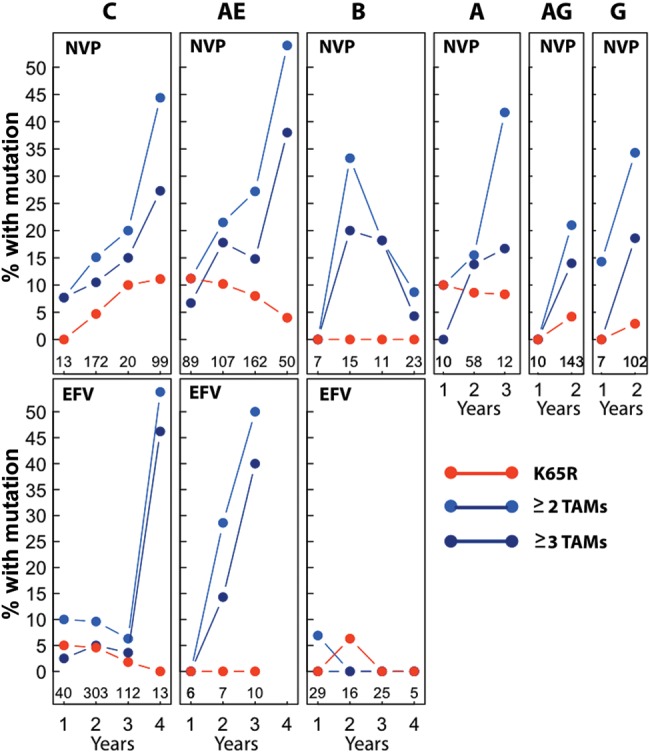
Proportion of viruses with 3 categories of nucleoside reverse-transcriptase inhibitor mutations according to subtype, concomitant nonnucleoside reverse-transcriptase inhibitor (NNRTI), and years of antiretroviral therapy. The K65R category includes viruses with K65R but not Q151M. It also includes a small number of viruses with K65N or K70EGQ. Thymidine analog mutations include M41L, D67NG, K70R, L210W, T215YF, and K219QE. The numbers of viruses with each covariable pattern (subtype, NNRTI, and year) are shown at the base of each plot. Sequences were available from 31 subtype A, 7 CRF02_AG, and 2 subtype G viruses from individuals who received stavudine/lamivudine + efavirenz. Plots of data from these 40 individuals are not shown. Abbreviations: NVP, individuals receiving stavudine/lamivudine/nevirapine; EFV, individuals receiving stavudine/lamivudine/efavirenz.
Table 1 summarizes the results of stepwise logistic regression modeling to assess the influence of the concomitant NNRTI, duration of therapy, and subtype on the 10 categories of NRTI resistance mutations. M184V, the TAMs, and Q151M were positively associated with a longer duration of ART and the use of NVP (as opposed to EFV). M184V and the TAMs were negatively associated with subtype B. K65R alone was associated only with CRF01_AE. In contrast, K65R with and without Q151M was positively associated with NVP, subtype C, and CRF01_AE and negatively associated with subtype B.
Table 1.
Logistic Regression Models For Nucleoside Reverse-Transcriptase Inhibitor Resistance Mutations: Influence of Treatment Duration, Nonnucleoside Reverse-Transcriptase Inhibitor (nevirapine vs efavirenz), and Subtype (odds ratios and 95% confidence intervals)
| Mutation | Model | Years | NVP | C | AE | B |
|---|---|---|---|---|---|---|
| M184V | Years + NVP + B | 1.3 (1.2–1.5) | 1.7 (1.4–2.1) | … | … | 0.4 (0.3–0.6) |
| P = 3E−5 | P < 1E−6 | … | … | P < 1E−6 | ||
| TAMsGE1 | Years + NVP + B | 1.7 (1.5–1.9) | 1.7 (1.4–2.2) | … | … | 0.2 (0.1–0.4) |
| P < 1E−6 | P = 5E−6 | … | … | P = 1E−6 | ||
| TAMsGE2 | Years + NVP + B | 1.8 (1.5–2.0) | 2.3 (1.7–3.2) | … | … | 0.3 (0.2–0.7) |
| P < 1E−6 | P < 1E−6 | … | … | P = .002 | ||
| TAMsGE3 | Years + NVP + B | 1.8 (1.5–2.1) | 2.4 (1.6–3.5) | … | … | 0.3 (0.1–0.8) |
| P < 1E−6 | P = 7E−6 | … | … | P = .01 | ||
| Type1TAMsGE1 | Years + NVP + B | 1.7 (1.4–2.0) | 2.0 (1.4–2.8) | … | … | 0.4 (0.2–0.8) |
| P < 1E−6 | P = 1E−4 | … | … | P = .01 | ||
| Type1TAMsGE2 | Years + NVP + B | 1.9 (1.5–2.4) | 2.1 (1.2–3.5) | … | … | 0.2 (0.1–0.9) |
| P < 1E−6 | P = .006 | … | … | P = .03 | ||
| Type1TAMsE3 | Years | 1.8 (1.3–2.6) | … | … | … | |
| P = 9E−4 | … | … | … | … | ||
| K65R alone | AE | … | … | … | 1.7 (1.1–2.8) | |
| … | … | … | P = .03 | |||
| Q151M alone | Years + NVP | 1.7 (1.2–2.3) | 3.6 (1.5–8.6) | … | … | |
| P = 7E − 4 | P = .005 | … | … |
K65R often occurred in combination with Q151M (Supplemental Table 2). Because K65R and Q151M have different implications for treatment with zidovudine vs tenofovir, we examined only those cases in which K65R occurred without Q151M (K65R alone) and those cases in which Q151M occurred without K65R (Q151M alone). K65R included a small number of patients with K65N. There were no significant predictors of the rare mutation K70E (K70E ± K70G ± K70Q).
Abbreviations: M184V, (M184V ± M184I); NVP, nevirapine; TAMs, thymidine analog mutations (M41L, D67NG, K70R, L210W, T215YF, K219QE); TAMsGE1 (≥1 TAM); Type1TAMsGE1 (≥1 type 1 TAMs [type 1 TAMs: M41L, L210W, T215Y]).
Estimated Levels of AZT and TDF Cross-resistance According to the HIVDB Interpretation System
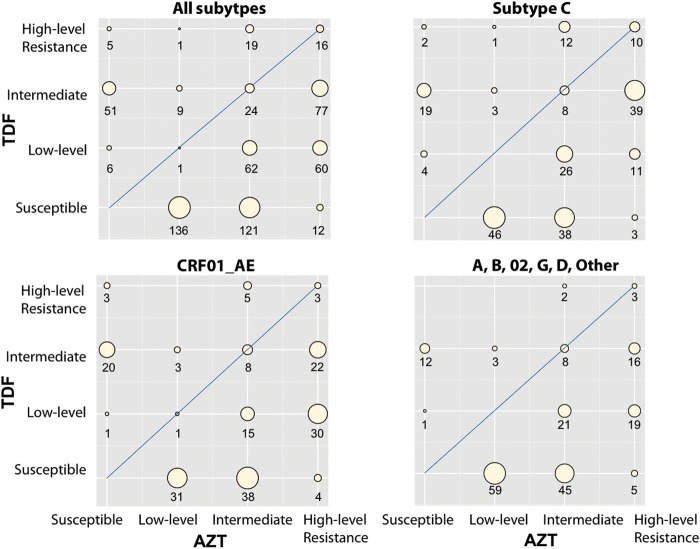
To estimate the levels of AZT and TDF cross-resistance, we submitted the 1825 RT sequences to the HIVDB resistance interpretation program. Sixty-seven percent (1225) of the sequences were categorized as susceptible to both AZT and TDF; 33% (600) were categorized as having decreased susceptibility to either AZT and/or TDF. Figure 2 summarizes the HIVDB interpretations for the entire set of these 600 sequences (upper left plot), the 212 subtype C isolates (upper right plot), the 184 CRF01_AE isolates (lower left plot), and the 194 isolates belonging to the remaining subtypes (lower right plot).
Figure 2.
Summary of the zidovudine (AZT) and tenofovir (TDF) drug resistance estimates reported by the Stanford HIV Drug Resistance Database (HIVDB) drug resistance interpretation system (http://hivdb.stanford.edu; accessed 10 December 2012). Each of the 4 subplots indicates the numbers of individuals having viruses with 16 different resistance profiles according to a 4-tiered susceptibility estimate: susceptible, low-level resistance (low-level), intermediate resistance (intermediate), and high-level resistance. The HIVDB drug resistance interpretation system also contains a fifth tier, potential low-level resistance, but for this analysis these viruses were reclassified as susceptible. The number of viruses estimated to be susceptible to both AZT and TDF is not shown but can be calculated from the total number of viruses in each category minus the sum of the remaining 15 categories. Abbreviations: AZT, zidovudine; TDF, tenofovir.
In each plot, a higher proportion of viruses were estimated to be more susceptible to TDF than to AZT (the circles to the right of the diagonal). Among the entire set of 600 isolates with decreased susceptibility to AZT and/or TDF, 41 (6.8%) were equally susceptible to AZT and TDF, 91 (15.2%) were more susceptible to AZT than TDF, and 468 (78%) were more susceptible to TDF than AZT. Fifty of the 51 isolates categorized as intermediately resistant to TDF but susceptible to AZT had K65R in the absence of Q151M.
DISCUSSION
During the global ARV scale-up, d4T-containing regimens have been the most common initial ARV regimens used by many national HIV treatment programs. Although these regimens are efficacious in a high proportion of HIV-1–infected patients, the numbers of patients developing virological failure while receiving a d4T-containing regimen is steadily increasing and necessitates the use of second-line treatment regimens [15–18]. In addition, the potential for serious toxicity associated with the long-term use of d4T has led the WHO to recommend phasing out d4T for first-line therapy [1].
The genetic mechanisms of d4T resistance are relevant to the selection of the NRTIs used as part of a second-line ART regimen and are also considerations for the choice of NRTIs to be substituted for d4T in patients without known virological failure. The most commonly occurring mutations with different implications for AZT and TDF cross-resistance include the TAMs, K65R, and Q151M. The TAMs reduce susceptibility to both AZT and TDF; however, TDF retains significant antiretroviral activity unless three type 1 TAMs are present [19]. In contrast, K65R reduces susceptibility to TDF while increasing susceptibility to AZT [4]. Q151M confers high-level AZT and intermediate TDF resistance. K65R and Q151M often occur in combination and in this context cause high-level resistance to both AZT and TDF [11]. As a result of the dichotomy between the TAM and K65R pathways, the frequency with which K65R occurs in the absence of Q151M largely determines the proportions of individuals likely to respond to subsequent therapy with AZT or TDF.
After combining data from 35 publications comprising 1825 individuals from whom RT sequences were available, we estimated the relative proportions of NRTI resistance mutations occurring in patients receiving d4T and assessed the influence of concomitant NNRTI, duration of therapy, and subtype on these estimates. Our analysis showed that the mutation patterns associated with increased susceptibility to AZT relative to TDF—K65R alone and less commonly K70E—occurred in 5.3% of individuals, whereas those associated with increased susceptibility to TDF relative to AZT—≥2 TAMs or Q151M alone—occurred in 22% of individuals. Q151M occurred nearly as commonly as K65R in patients receiving prolonged d4T therapy.
Several factors influenced the likelihood of specific NRTI resistance mutations. The use of NVP (vs EFV) was associated with increased proportions of M184V, TAMs, K65R, and Q151M. The number of years of therapy was associated with increased proportions of M184V, TAMs, and Q151M but not K65R. Subtype B was associated with a decreased proportion of all mutations, but this is almost certainly a result of frequent virological monitoring of these individuals who were each from the United States [20, 21]. Subtype C was associated with an increased proportion of K65R, but only CRF01_AE was associated with K65R in the absence of Q151M. However, regardless of the combination of concomitant NNRTI, duration of therapy, or subtype, TDF was more likely than AZT to retain antiviral activity against viruses emerging during a first-line d4T-containing regimen.
Subtype C viruses have a unique pattern of 5 consecutive adenosines preceding the position of the mutation responsible for K65R. This nucleic acid template predisposes subtype C viruses to an increased frequency of K65R in biochemical and in vitro passage experiments [22–24]. However, K65R did not occur at a higher rate in subtype C viruses compared with CRF01_AE or subtype A viruses. In contrast, the NNRTI resistance mutation V106M, which is preferentially selected in subtype C viruses as a result of a codon bias at position 106, occurred in 21% of subtype C viruses but in <1% of viruses from other subtypes, indicating that this study was powered to detect meaningful intersubtype differences in mutation frequency.
Our study has 2 main limitations that might influence the estimated relative benefit of AZT compared with TDF in different clinical scenarios. Because the duration of therapy for most individuals was the median duration for the study rather than the precise duration for the individual, we were not able to identify individuals with rapid virological failure. Such individuals may be more likely to have viruses with K65R because this mutation results from a single nucleotide change. Moreover, K65R was the only mutation in our analysis that did not increase in frequency with the duration of therapy. A second limitation is the possibility that undocumented substitutions of d4T for AZT (eg, for toxicity or intolerance) may have biased our findings because any prior therapy with AZT would increase the likelihood of TAMs on a d4T-containing regimen.
In regions where routine virus load testing is available, d4T-treated patients with stable virological suppression can be switched either to AZT or TDF. However, TDF is likely to be preferable because it has fewer toxicities and can be administered once daily. In these same regions, d4T-treated patients with virological failure can be identified and changed to a second-line boosted protease inhibitor–containing treatment regimen. In settings where genotypic resistance testing is available, the subset of patients for whom AZT is likely to be more effective than TDF can be identified. However, in regions where genotypic resistance testing is not available, our study suggests that TDF is more likely to be effective than AZT as part of a second-line treatment regimen.
In regions where virus load testing is not available, it will not be known whether virus levels are suppressed at the time d4T is switched to AZT or TDF. Based on the reported high virological responses to d4T-containing first-line ARV regimens, it is expected that the majority of adherent adult patients receiving a first-line d4T-containing regimen will be virologically suppressed at the time d4T is switched [18, 25]. Because of its favorable toxicity profile, TDF will usually be preferable to AZT in these patients. In patients without virological suppression, a single NRTI substitution is unlikely to achieve virological resuppression. However, TDF will be more likely than AZT to retain residual antiviral activity against emerging drug-resistant variants.
In conclusion, this study strongly suggests that whether patients are switched off of d4T as a result of virological failure or to avoid long-term toxicities, TDF will be more advantageous than AZT for the majority of patients in regions where genotypic resistance testing is not available.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Disclaimer. Some authors are staff members of WHO. The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions or stated policies of WHO.
Financial support. M. W. T., S.-Y. R., and R. W. S. are supported by a grant from the National Institutes of Health (1R01AI68581). M. W. T. is supported by a National Research Service Award (1F32 A1098594) and has received funding from the Bristol Meyers Squibb Virology Fellows program.
Potential conflicts of interest. M. W. T. has received funding from the Bristol-Meyers Squibb Virology Fellows program. R. W. S. has received research funding from Gilead Sciences, Bristol-Meyers Squibb, and Hoffman La Roche Pharmaceuticals. He also has unrestricted funding from Celera, Roche Molecular Diagnostics, and Siemens Healthcare System. H. J. F. has served as a consultant for Beckman Coulter and Bio-Rad Laboratories and has received grants from Merck and Viiv Healthcare. He has also had paid speaking engagements at Roche and Beckman Coulter and meeting expenses from Merck and Roche. P. J. K. has received royalties from Encyclopedia Sustainability. K. R. has served as a consultant for Merck and Tibotec. He has had paid speaking engagements with Bristol-Meyers Squibb, Merck, Roche, Jensen-Cilag, GlaxoSmithKline, and GPO. C. L. W. has served as a consultant for Celera and received honoraria from Abbott Laboratories. G. v. Z. has served as a consultant for two Abbott Laboratories sponsored workshops. P. C. served on the advisory board of Viiv Healthcare and holds stock at GlaxoSmithKline. C. C. has had paid speaking engagements at Bristol-Meyers Squibb and Janssen Cilag, developed educational presentations for Janssen Cilag and Viiv Healthcare, and received meeting expenses from Gilead Sciences and MSD. S. H. E. has collaborated on research projects but received no funding from Abbott Diagnostics and Monogram Biosciences. V. C. M. has received grant and travel support from Gilead Biosciences and Bayer. N. A. M. is employed by Gilead Sciences, Inc. J. M. S. is a consultant for Abbott Laboratories, Merck, Teva Pharmaceuticals, Gilead Sciences, GlaxoSmithKline, Pfizer, Tibotec-Janssen, Bristol-Meyers Squibb, and Viiv Healthcare. D. S. has served as a consultant for GenProbe. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents—recommendations for a public health approach: 2010 revision. http://www.who.int/hiv/pub/arv/adult2010/en/index.html . Accessed 20 March 2013. [PubMed] [Google Scholar]
- 2.Garcia-Lerma JG, MacInnes H, Bennett D, et al. A novel genetic pathway of human immunodeficiency virus type 1 resistance to stavudine mediated by the K65R mutation. J Virol. 2003;77:5685–93. doi: 10.1128/JVI.77.10.5685-5693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parikh UM, Bacheler L, Koontz D, Mellors JW. The K65R mutation in human immunodeficiency virus type 1 reverse transcriptase exhibits bidirectional phenotypic antagonism with thymidine analog mutations. J Virol. 2006;80:4971–7. doi: 10.1128/JVI.80.10.4971-4977.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitcomb JM, Parkin NT, Chappey C, Hellmann NS, Petropoulos CJ. Broad nucleoside reverse-transcriptase inhibitor cross-resistance in human immunodeficiency virus type 1 clinical isolates. J Infect Dis. 2003;188:992–1000. doi: 10.1086/378281. [DOI] [PubMed] [Google Scholar]
- 5.Rhee SY, Gonzales MJ, Kantor R, Betts BJ, Ravela J, Shafer RW. Human immunodeficiency virus reverse transcriptase and protease sequence database. Nucleic Acids Res. 2003;31:298–303. doi: 10.1093/nar/gkg100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett DE, Camacho RJ, Otelea D, et al. Drug resistance mutations for surveillance of transmitted HIV-1 drug-resistance: 2009 update. PloS One. 2009;4:e4724. doi: 10.1371/journal.pone.0004724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhee SY, Liu TF, Holmes SP, Shafer RW. HIV-1 subtype B protease and reverse transcriptase amino acid covariation. PLoS Comput Biol. 2007;3:e87. doi: 10.1371/journal.pcbi.0030087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ross L, Elion R, Lanier R, et al. Modulation of K65R selection by zidovudine inclusion: analysis of HIV resistance selection in subjects with virologic failure receiving once-daily abacavir/lamivudine/zidovudine and tenofovir DF (study COL40263) AIDS Res Hum Retroviruses. 2009;25:665–72. doi: 10.1089/aid.2008.0302. [DOI] [PubMed] [Google Scholar]
- 9.Stephan C, Dauer B, Bickel M, et al. Intensification of a failing regimen with zidovudine may cause sustained virologic suppression in the presence of resensitising mutations including K65R. J Infect. 2010;61:346–50. doi: 10.1016/j.jinf.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Lerma JG, Nidtha S, Blumoff K, Weinstock H, Heneine W. Increased ability for selection of zidovudine resistance in a distinct class of wild-type HIV-1 from drug-naive persons. Proc Natl Acad Sci U S A. 2001;98:13907–12. doi: 10.1073/pnas.241300698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melikian GL, Rhee SY, Taylor J, et al. Standardized comparison of the relative impacts of HIV-1 reverse transcriptase (RT) mutations on nucleoside RT inhibitor susceptibility. Antimicrob Agents Chemother. 2012;56:2305–13. doi: 10.1128/AAC.05487-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Oliveira T, Deforche K, Cassol S, et al. An automated genotyping system for analysis of HIV-1 and other microbial sequences. Bioinformatics. 2005;21:3797–800. doi: 10.1093/bioinformatics/bti607. [DOI] [PubMed] [Google Scholar]
- 13.Schultz AK, Zhang M, Bulla I, et al. jpHMM: improving the reliability of recombination prediction in HIV-1. Nucleic Acids Res. 2009;37:W647–51. doi: 10.1093/nar/gkp371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vienna, Austria: R Development Core Team; 2007. R Foundation for Statistical Computing. R: A language and environment for statistical computing. [Google Scholar]
- 15.Gupta RK, Hill A, Sawyer AW, et al. Virological monitoring and resistance to first-line highly active antiretroviral therapy in adults infected with HIV-1 treated under WHO guidelines: a systematic review and meta-analysis. Lancet Infect Dis. 2009;9:409–17. doi: 10.1016/S1473-3099(09)70136-7. [DOI] [PubMed] [Google Scholar]
- 16.Stadeli KM, Richman DD. Rates of emergence of HIV drug resistance in resource-limited settings: a systematic review. Antivir Ther. 2013;18:115–23. doi: 10.3851/IMP2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamers RL, Sigaloff KC, Wensing AM, et al. Patterns of HIV-1 drug resistance after first-line antiretroviral therapy (ART) failure in 6 sub-Saharan African countries: implications for second-line ART strategies. Clin Infect Dis. 2012;54:1660–9. doi: 10.1093/cid/cis254. [DOI] [PubMed] [Google Scholar]
- 18.Barth RE, van der Loeff MF, Schuurman R, Hoepelman AI, Wensing AM. Virological follow-up of adult patients in antiretroviral treatment programmes in sub-Saharan Africa: a systematic review. Lancet Infect Dis. 2010;10:155–66. doi: 10.1016/S1473-3099(09)70328-7. [DOI] [PubMed] [Google Scholar]
- 19.Miller MD, Margot N, Lu B, et al. Genotypic and phenotypic predictors of the magnitude of response to tenofovir disoproxil fumarate treatment in antiretroviral-experienced patients. J Infect Dis. 2004;189:837–46. doi: 10.1086/381784. [DOI] [PubMed] [Google Scholar]
- 20.Margot NA, Lu B, Cheng A, Miller MD. Resistance development over 144 weeks in treatment-naive patients receiving tenofovir disoproxil fumarate or stavudine with lamivudine and efavirenz in Study 903. HIV Med. 2006;7:442–50. doi: 10.1111/j.1468-1293.2006.00404.x. [DOI] [PubMed] [Google Scholar]
- 21.Riddler SA, Haubrich R, DiRienzo AG, et al. Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med. 2008;358:2095–106. doi: 10.1056/NEJMoa074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coutsinos D, Invernizzi CF, Xu H, et al. Template usage is responsible for the preferential acquisition of the K65R reverse transcriptase mutation in subtype C variants of human immunodeficiency virus type 1. J Virol. 2009;83:2029–33. doi: 10.1128/JVI.01349-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brenner BG, Oliveira M, Doualla-Bell F, et al. HIV-1 subtype C viruses rapidly develop K65R resistance to tenofovir in cell culture. AIDS. 2006;20:F9–13. doi: 10.1097/01.aids.0000232228.88511.0b. [DOI] [PubMed] [Google Scholar]
- 24.Varghese V, Wang E, Babrzadeh F, et al. Nucleic acid template and the risk of a PCR-induced HIV-1 drug resistance mutation. PloS One. 2010;5:e10992. doi: 10.1371/journal.pone.0010992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamers RL, Schuurman R, Sigaloff KC, et al. Effect of pretreatment HIV-1 drug resistance on immunological, virological, and drug-resistance outcomes of first-line antiretroviral treatment in sub-Saharan Africa: a multicentre cohort study. Lancet Infect Dis. 2012;12:307–17. doi: 10.1016/S1473-3099(11)70255-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.