Abstract
Technological developments in municipal lighting are altering the spectral characteristics of artificially lit habitats. Little is yet known of the biological consequences of such changes, although a variety of animal behaviours are dependent on detecting the spectral signature of light reflected from objects. Using previously published wavelengths of peak visual pigment absorbance, we compared how four alternative street lamp technologies affect the visual abilities of 213 species of arachnid, insect, bird, reptile and mammal by producing different wavelength ranges of light to which they are visually sensitive. The proportion of the visually detectable region of the light spectrum emitted by each lamp was compared to provide an indication of how different technologies are likely to facilitate visually guided behaviours such as detecting objects in the environment. Compared to narrow spectrum lamps, broad spectrum technologies enable animals to detect objects that reflect light over more of the spectrum to which they are sensitive and, importantly, create greater disparities in this ability between major taxonomic groups. The introduction of broad spectrum street lamps could therefore alter the balance of species interactions in the artificially lit environment.
Keywords: animals, artificial light spectra, pollution, species interactions, street lighting, vision ecology
Introduction
Artificial lights have been used to illuminate the night-time environment for over a century, during which numerous alternative lighting technologies have arisen, each emitting light with unique spectral characteristics (Elvidge et al., 2010). Now widely distributed, artificial lighting is spreading at a rate of 6% per year globally (Hölker et al., 2010). Indeed, by 2001 it was estimated that the fraction of land under skies that were artificially brightened above natural background levels already exceeded 10% in 66 nations across the planet (Cinzano et al., 2001). Organisms have evolved under the intensities, timings and spectral composition of light emitted from the sun and stars, and reflected from the moon. However, artificial lighting is changing all these aspects of natural light regimes (Gaston et al., 2012) leading to a potentially diverse array of ecological impacts (Longcore & Rich, 2004; Perkin et al., 2011; Gaston et al., 2013). A recent surge in research activity has revealed that artificially lighting the nocturnal environment can have impacts ranging from changes in animal behaviour (Rydell, 1992; Bird et al., 2004; Eisenbeis, 2006; Stone et al., 2009; Titulaer et al., 2012) to the composition of whole communities (Davies et al., 2012). Yet, because artificial light pollution has only recently become widely recognized as an environmental issue, studies on its ecological effects remain relatively scarce.
Since the second half of the 20th century, narrow spectrum Low Pressure Sodium (LPS) lighting, with a characteristic orange hue, has been the most common form of street lighting in many regions. However, ongoing advances in street lighting technology have led to the increasing adoption of broader spectrum light sources such as High Pressure Sodium (HPS), Light Emitting Diode (LED) and Metal Halide (MH) lamps (Elvidge et al., 2010), which provide improved colour rendering capabilities for humans. Shifting and broadening the spectra of street lamps may lead to unforeseen environmental impacts because the spectral signature reflected from objects is an important cue that guides a number of animal behaviours, including, for example, the detection of resources (Chittka et al., 1994; Hempel de Ibarra & Vorobyev, 2009; Macedonia et al., 2009; Chiao et al., 2011; Zou et al., 2011), mate selection (Andersson et al., 1998; Hunt et al., 2001; Robertson & Monteiro, 2005; Lim et al., 2008) and navigation (Cheng et al., 1986; Möller, 2002; Mappes & Homberg, 2004; Reppert et al., 2004; Ugolini et al., 2005). Here, we ask whether the use of broad spectrum street lighting technologies is likely to improve the ability of animals to perform tasks during the night which are guided by the detection of light reflected from objects, and whether this could alter the balance of species interactions.
Materials and methods
Overview
We based our analysis on a novel collation of previously published wavelengths at which the visual pigments contained within the photoreceptors of 213 species of animal (comprising 7 arachnids, 112 insects, 16 birds, 32 reptiles and 46 mammals) maximally absorb light (λmax) (see Table S1). Using a previously derived formula which describes the absorbance properties of visual pigments based on their λmax (see Govardovskii et al., 2000 for details), we then modelled the absorbance of the visual pigments in each species from 200 to 750 nm and estimated the maximum (maxλ0.5) and minimum (minλ0.5) wavelengths of half maximum absorbance (Fig. 1) to determine the range of wavelengths detectable by each species (λ0.5 range). By comparing the region of the light spectrum over which LPS, HPS, LED and MH lamps emit light (λlight range) with the region of the light spectrum over which the visual pigments in animal eyes absorb more than half of the light passing through them (λ0.5 range), we obtained a value of the proportion of the visually detectable wavelength range at greater than half maximum absorbance which is stimulated by each type of street lamp (% λ0.5 range). By way of example, Fig. 1 illustrates how the % λ0.5 range of the visual system in humans relates to the objects we can detect in the environment under each type of street lamp. LPS lamps emit light over a narrow region of the light spectrum to which human photoreceptors are sensitive, hence objects that reflect light mainly outside this region appear dim or are not seen at all. HPS, LED and MH lamps emit light over a greater proportion of the light spectrum to which humans are sensitive (Fig. 1), hence more objects are easily detected under these lighting technologies because they are better discriminated in colour and brightness. The % λ0.5 range is a useful comparative index of the ability of animals to detect light reflected from ecologically relevant objects in their environment, because it represents the proportion of the visually detectable region of the light spectrum illuminated by a light source.
Fig. 1.
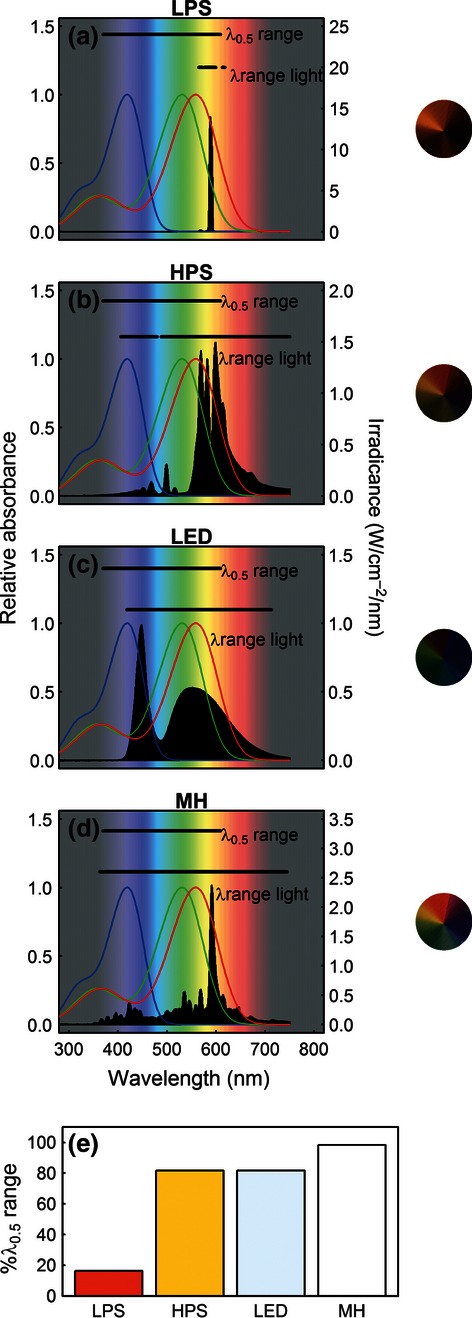
The colour vision performance of human beings under light emitted from four contrasting street lighting technologies. (a). LPS lamps which emit light over a narrow region of the light spectrum (λrange light) stimulate a smaller proportion of the region of the light spectrum to which human visual pigments are half maximally sensitive (λ0.5 range) (dashed line), hence objects which reflect light outside of this range appear less bright (colour wheel insert). (b,c,d). Broad spectrum street lighting technologies (HPS, LED, MH) emit light across a broader region of the light spectrum to which humans are sensitive, allowing us to identify objects reflecting light across a broader range of wavelengths. (e). The visual performance of humans under each of the street lighting types can be compared using an index (% λ0.5 range stimulated) calculated as the percentage of λ0.5 range overlapped by λrange light. A–D. Solid black lines represent the α and β band absorbance curves for the three visual pigments used to detect light in the human visual system. The emission spectrum of each street light is represented by the filled curve. The plot background approximates the colour of the light detected at each wavelength by the human visual system. UV light is emitted below 400 nm and infrared light above 700 nm. Colour wheel inserts are photographic images taken of the same colour wheel under each of the street lighting types using a standard digital SLR camera which detects red, green and blue light at approximately the same wavelengths as human visual pigments are maximally sensitive.
Values of % λ0.5 range were compared both between lighting technologies within each animal class, and between animal classes within each lighting technology using Markov Chain Monte Carlo regression (see below). To aid the interpretation of any patterns observed in the data, we also estimated the average maximum and minimum wavelengths at which the visual pigments of each animal group absorb more than half of the light entering the photoreceptor (max λ0.5 and min λ0.5) (Fig. 2a).
Fig. 2.
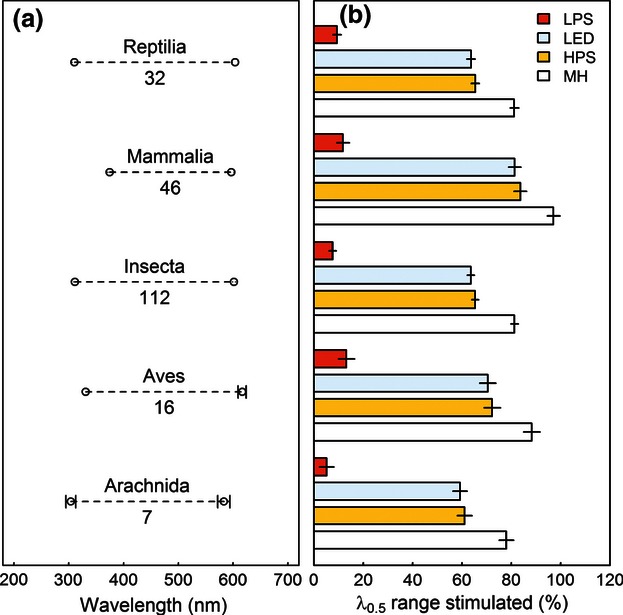
The percentage of the visual range stimulated by four contrasting street lighting technologies in five classes of animal. (a) The λ0.5 range of animals estimated for five classes. The average minimum and maximum wavelengths of half maximum visual pigment absorbance are denoted by points with error bars representing 95% credibility intervals estimated using MCMC regression. Values quoted under dashed lines represent the number of species on which derived values are based. (b) The percentage of the visual range at more than half maximum absorbance stimulated by each street light in each of five classes of animal. Means and 95% credibility intervals (error bars) were estimated using MCMC regression.
Data collection (a) visual pigment λmax
Values of photoreceptor visual pigment λmax were collected for 248 species of animal through an extensive literature search (see Table S1), and used to model the α and the β absorbance curves of the corresponding visual pigments using a standard formula (Govardovskii et al., 2000). Values of min λ0.5, max λ0.5 and λ0.5 range were then calculated for each of the 213 species. The remaining 35 species were omitted from the analysis due to missing values of λmax for the visual pigments of known photoreceptors. For example, while the majority of insects possess UV photoreceptor cells, they have not been characterized in every studied species due to technological limitations (Bernard & Stavenga, 1979; Peitsch et al., 1992; Briscoe & Chittka, 2001). In species of insect for which the spectral sensitivity functions were available separately for females and males, either the sex for which fewer visual pigment λmax wavelengths were quantified was omitted from the analysis, or if the number of visual pigments quantified was identical between sexes the male was omitted from the analysis. New World primates of the same species can be either dichromatic or trichromatic (Jacobs & Deegan, 2003). To prevent duplicating results for any one species, it was assumed that all individuals of each polymorphic species were trichromatic. The short, medium and long wave sensitive photoreceptors of birds and some diurnal reptiles are associated with oil droplets which alter the transmittance of light to the visual pigment and change the maximum wavelengths at which these pigments half maximally absorb light (UV sensitive photoreceptors and all photoreceptors in nocturnal reptiles possess clear oil droplets which do not affect the transmittance of light to the visual pigment; e.g., Hart & Vorobyev, 2005). For the birds and diurnal reptiles, changes in the absorbance curves of short, medium and long wavelength photoreceptors due to oil droplet transmittance were modelled prior to the estimation of min λ0.5 and max λ0.5 using the method outlined by Hart & Vorobyev (2005) and published values of oil droplet cut-off wavelengths (λcut) or wavelengths at half maximum absorbance (λmid) (see Table S1). In a few species, lens absorption produces a cut-off effect slightly limiting the visual range, but it has not been measured widely across species, therefore it was not included.
Data collection (b) light spectra
The spectral compositions of four glass housed street lamps, one representative of each of the LPS (35W Thorn Beta 5 installed 12/2009), HPS (250W ZX3, Urbis installed 07/2008), LED (120W Ledway, Ruud installed 11/2010) and MH (45W Evolo lantern, Urbis installed 12/2009) technologies, were collected from municipal lighting installations in Cornwall, UK. While some variation exists in the exact spectra emitted by different makes and models of each technology, our selection was representative of the common differences between these technologies (narrow vs. broad spectrum, and UV vs. non-UV emissive). Light spectra were quantified using a MAYA2000-Pro spectrometer collecting light from a CC-3-UV-S cosine corrector connected via a 1000 μm fibre optic cable (Ocean Optics). The cosine corrector was held at ground level during measurements to capture the light spectra that most animals are likely to be exposed to. The resulting light spectra were used to quantify the region over which each lamp technology emits light (λlight range).
Data analysis
Photoreceptor signals are mainly determined by the maximum absorption of the photopigment at the wavelength λmax, and a photoreceptor's sensitivity decreases steeply with increasing distance from this peak sensitivity wavelength (Fig. 1). Visual systems have mostly evolved sets of receptors where sensitivities are well separated to avoid overlapping within the receptor's most sensitive range, usually between the half maximally sensitive (λ0.5) and λmax. Such spacing of photoreceptor sensitivities enables the effective coding of colours and increases an animal's ability to discriminate and recognize colours. Visual performance is influenced to a much lesser extent by the absorption of light in the low-sensitivity wavelength range. Therefore, the region of the light spectrum to which each species is more than half maximally sensitive (λ0.5 range) was determined as the visual range. The percentage of λ0.5 range stimulated by each street lamp technology (% λ0.5 range) was then estimated as the fraction of the λ0.5 range overlapped by the λlight range (Fig. 1).
Means and 95% credibility interval values of min λ0.5, max λ0.5 and % λ0.5 range were estimated for each animal class perceiving light emitted from each type of street lamp using zero intercept Markov Chain Monte Carlo regression (MCMCregress; CRAN: MCMCpack; Martin et al., 2010) (1001 : 11000 iterations). Means and 95% credibility intervals of the difference in % λ0.5 range between street lamp types were estimated separately for each animal class, and separately for each street lamp type when comparing between animal classes, using MCMC regression with a fitted intercept (1001 : 11000 iterations). The resulting pairwise comparisons were interpreted in a manner analogous to parametric pairwise comparison tests. Where the credibility intervals of the difference between two street lamp types or animal classes did not bound 0, there is a 95% probability that they are different.
Results
The results indicate that the four street lighting technologies can be divided into three categories based on how likely they are to facilitate the detection of objects reflecting light in different regions of the spectrum (Table 1; Fig. 2b): narrow spectrum lamps which do not emit UV light (LPS), broad spectrum lamps which do not emit UV light (HPS and LED) and broad spectrum lamps which do emit UV light (MH). There was a greater than 95% probability that the narrow spectral range of light emitted by LPS lamps stimulated the smallest proportion of the light spectrum to which animals are sensitive (Table 1) spanning from 5 ± 3.66% λ0.5 range in the arachnids to 13.1 ± 2.4% λ0.5 range in the birds (Fig. 2b). Metal Halide (MH) lamps stimulated the largest proportion of the light spectrum to which animals are sensitive spanning from 77.9 ± 5.4% λ0.5 range in the arachnids to 97.1 ± 2.1% λ0.5 range in the mammals (Fig. 2b). There was a greater than 95% probability that MH lamps stimulated a larger percentage of the λ0.5 range than each of the remaining lighting technologies (Table 1). HPS and LED lighting technologies stimulate similar percentages of the λ0.5 range in all classes of animal studied (Table 1). The broad emission spectra of these technologies stimulate a higher percentage of the λ0.5 range in comparison to LPS lamps with greater than 95% probability (Table 1) in all five animal classes, but to a lesser extent than MH lamps (Table 1; Fig. 2b).
Table 1.
The difference in the percentage of the visual range at greater than half maximum absorbance (% λ0.5 range) stimulated by each of the four contrasting street lighting technologies compared within five classes of animal
| Street lamp type | ||||
|---|---|---|---|---|
| Class | LPS | HPS | LED | |
| Arachnida | HPS | 55.9(51.7,60.1) | ||
| LED | 54.1(50.0,58.3) | −1.8(-6.0,2.3) | ||
| MH | 72.9(68.6,77.0) | 16.9(12.7,21.1) | 18.8(14.5,22.9) | |
| Aves | HPS | 59.2(54.4,63.8) | ||
| LED | 57.4(52.7,62.1) | −1.8(−6.5,2.9) | ||
| MH | 75.1(70.4,79.9) | 16.0(11.2,20.6) | 17.8(13.0,22.5) | |
| Insecta | HPS | 57.8(55.7,59.8) | ||
| LED | 56.1(54.0,58.1) | −1.7(−3.8,0.3) | ||
| MH | 73.7(71.6,75.7) | 15.9(13.8,18.0) | 17.7(15.6,19.7) | |
| Mammalia | HPS | 71.9(68.2,75.5) | ||
| LED | 69.7(66.0,73.3) | −2.3(−5.9,1.3) | ||
| MH | 85.4(81.7,89.0) | 13.5(9.7,17.1) | 15.7(12.0,19.4) | |
| Reptiles | HPS | 56.1(53.6,58.5) | ||
| LED | 54.4(51.9,56.8) | −1.7(−4.2,0.7) | ||
| MH | 71.9(69.4,74.3) | 15.8(13.3,18.2) | 17.5(15.0,20.0) | |
Values represent the mean difference and 95% credibility intervals of the difference (values in parentheses) in % λ0.5 range stimulated by each lamp type. Values are derived from the pairwise comparison outputs from Markov Chain Monte Carlo simulations performed between factor levels going across the table subtracted from factor levels going down the table. Where values in parentheses do not bound zero there is a 95% probability that the two factor levels are different (underlined results).
In addition to changing the ability of animals to detect light reflected from objects in general, the contrasting lighting technologies also affected the comparative ability of different taxonomic groups to detect light reflected from objects. LPS lamps stimulate more of the λ0.5 range of birds and mammals compared to arachnids, insects and reptiles with greater than 95% probability (Fig. 2b; Table 2). HPS, LED and MH lights, however, increase the number and magnitude of differences in % λ0.5 range between animal classes with a greater than 95% probability (Table 2). These differences are greatest between the mammals and the remaining animal classes under LED and HPS lamp types (Table 2) because mammals detect light over a narrower region of the light spectrum (Fig. 2a). Similarly, the λ0.5 range of birds extends less into the shorter wavelengths compared to insects, arachnids and reptiles (Fig. 2a), hence there was a greater than 95% probability that a higher percentage of the light spectrum detected by birds is stimulated under HPS, LED and MH lamps (Table 2).
Table 2.
The difference in the percentage of the visual range at greater than half maximum absorbance (% λ0.5 range) stimulated by each of four contrasting street lighting technologies compared between five classes of animal
| Class | |||||
|---|---|---|---|---|---|
| Street lamp type | Arachnida | Aves | Insecta | Mammalia | |
| LPS | Aves | 8.0(3.6,12.3) | |||
| Insecta | 2.5(−1.3,6.2) | −5.5(−8.1,−3.0) | |||
| Mammalia | 6.7(2.8,10.6) | −1.3(−4.1,1.4) | 4.2(2.5,5.9) | ||
| Reptilia | 4.3(0.3,8.3) | −3.8(−6.7,−0.8) | 1.8(−0.2,3.7) | −2.4(−4.6,−0.2) | |
| HPS | Aves | 11.3(3.4,18.9) | |||
| Insecta | 4.4(−2.4,11.0) | −6.9(−11.4,−2.3) | |||
| Mammalia | 22.7(15.7,29.7) | 11.4(6.4,16.4) | 18.3(15.3,21.4) | ||
| Reptilia | 4.4(−2.8,11.7) | −6.8(−12.1,−1.6) | 0.1(−3.4,3.6) | −18.2(−22.2,−14.2) | |
| LED | Aves | 11.3(3.4,18.9) | |||
| Insecta | 4.4(−2.3,11.1) | −6.9(−11.4,−2.3) | |||
| Mammalia | 22.2(15.2,29.2) | 10.9(5.9,15.9) | 17.8(14.8,20.8) | ||
| Reptilia | 4.5(−2.7,11.8) | −6.8(−12.1, −1.5) | 0.1(−3.4,3.6) | −17.7(−21.7,−13.7) | |
| MH | Aves | 10.3(3.8,16.6) | |||
| Insecta | 3.4(−2.3,8.9) | −6.9(−10.7,−3.2) | |||
| Mammalia | 19.2(13.4,25.0) | 8.9(4.8,13.0) | 15.8(13.4,18.4) | ||
| Reptilia | 3.3(−2.7,9.3) | −7.0(−11.4,−2.6) | −0.1(−2.9,2.8) | −15.9(−19.2,−12.6) | |
Values represent the mean difference and 95% credibility intervals of the difference (values in parentheses) in % λ0.5 range stimulated by each street lamp type. Values were derived from the pairwise comparison outputs from Markov Chain Monte Carlo simulations performed between factor levels going across the table subtracted from factor levels going down the table. Where values in parentheses do not bound zero there is a 95% probability that the two factor levels are different (underlined results).
Discussion
Our results suggest that the installation of broader spectrum lighting technologies in artificially lit habitats is likely to improve the ability of animals to detect light reflected from objects in their environment at night, and has the potential to generate greater disparities in this ability between different classes of animal. These improvements in object detection under broad spectrum street lights are likely to affect the execution of visually guided behaviours in animals, altering their normal activity times and spatially extending or fragmenting habitats. All three broad spectrum lighting technologies provided significant improvements in the % λ0.5 range in comparison to narrow spectrum LPS lamps. MH lamps provided the greatest improvements in all five taxonomic classes. Hence, where these are in use, a greater variety of objects reflecting light in different regions of the light spectrum will appear brighter and more colourful to animals compared with alternative street lamp technologies. While LPS lamps illuminate objects reflecting light across the smallest region of the light spectrum, our results suggest that in areas illuminated by LPS lamps, birds and mammals are better able to detect objects that reflect light in this region compared to arachnids, insects and reptiles. The introduction of broader spectrum technologies, however, increases the number, and the magnitude of the differences between animal classes, in the proportion of the visually detectable light spectrum illuminated, with mammals and birds displaying the largest improvements. Most mammals possess dichromatic vision spanning a less extended range of the light spectrum in comparison to birds, reptiles, arachnids and insects (Fig. 2a; see Table S1) that typically can detect light at wavelengths below 400 nm (UV) (Tovée, 1995; Briscoe & Chittka, 2001; Hart & Hunt, 2007; Osorio & Vorobyev, 2008). Birds do possess UV sensitive photoreceptors, but their sensitivity extends less into the shorter wavelengths compared to insects, arachnids and reptiles (Fig. 2a). Broad spectrum lamp types therefore stimulate a larger percentage of the λ0.5 range in mammals and birds in general, compared with other classes of animal, improving their ability to perform visually guided behaviours with greater acuity and potentially upsetting the balance of interspecific interactions.
Our results provide an overview of how shifting artificial light spectra are likely to affect visually guided behaviours in broad taxonomic groups of animal. However, the λ0.5 range of individual species can be variable within each taxonomic group, and therefore caution should be exercised when applying the results of a group in general to any one specific species within that group. For example, the number of photoreceptor types in insect eyes is variable between different orders (Table S1) giving rise to variation in the proportion of λ0.5 range illuminated by each type of artificial light. In addition, the number of species for which λmax values are available in the literature varies between taxonomic groups (Table S1), and while the main results of this study are unlikely to be affected, the λ0.5 range will inevitably adjust as data become available for more species and additional photoreceptors in those groups which are not currently well investigated (for example the arachnids). These results are not therefore conclusive, rather they should be considered as a platform of predictions which incentivises further studies into the impact of broadening artificial light spectra on visually guided behaviours in animals.
The ecological impacts of artificially lighting the nocturnal environment are increasingly being recognized (Frank, 2006; Stone et al., 2012; Titulaer et al., 2012), with some studies drawing attention to the potential impact of shifting spectral signatures (Eisenbeis, 2006; Stone et al., 2012). This study has highlighted that such changes may be affecting visually guided behaviours in species across the animal kingdom. The range of potential impacts are diverse and may include extending the times of foraging and sexual competition of diurnal and crepuscular animals into the night (Robertson & Monteiro, 2005; Somanathan et al., 2009; Titulaer et al., 2012), improving both prey detection and predator avoidance (Roth & Kelber, 2004), changing the ability of organisms to navigate around their environment (Warrant et al., 2004, Somanathan et al., 2008; Stone et al., 2009; van Langevelde et al., 2011) and affecting the ability of pollinating species to detect nectar resources (Kelber et al., 2002; Hempel de Ibarra & Vorobyev, 2009). Whether broadening artificial light spectra will elicit positive or negative species responses is likely to depend on the species and the behaviour being considered. For example, the presence of LED lighting increases feeding rates in nesting Great Tits Parus major (Titulaer et al., 2012), while the bat Rhinolophus hipposideros avoids areas lit by HPS and LED lighting (Stone et al., 2009, 2012) potentially due to perceived predation risk (Rydell, 1992). Metal Halide (MH) lamps are likely to provide the largest improvements in animal vision because they emit light that is both broad and contains UV in its spectral composition. Many of the above tasks depend on the perception of UV light reflected from objects by animals that can detect light at these wavelengths. Hence, the introduction of broader spectrum lighting technologies containing UV may have more profound consequences for biological systems than non-UV broad spectrum lighting technologies. All three broad spectrum technologies, however, create larger disparities in % λ0.5 between animal groups compared with narrow spectrum LPS lamps, and so have greater potential to alter the balance of interspecific interactions in the environment. Evaluating the direct environmental impacts of each of these different lamp types is clearly essential in a world where the artificially lit night-time environment is increasingly becoming ‘white’.
Acknowledgments
The research leading to this manuscript has received funding from the European Research Council under the European Union's Seventh Framework Programme (FP7/2007–2013)/ERC grant agreement no. 268504 to K.J.G.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. The wavelengths of maximum absorbance (λmax) of the visual pigments contained within the photoreceptors of animal eyes.
References
- Andersson S, Ornborg J, Andersson M. Ultraviolet sexual dimorphism and assortative mating in blue tits. Proceedings of the Royal Society B. 1998;265:445–450. [Google Scholar]
- Bernard GD, Stavenga DG. Spectral sensitivities of retinular cells measured in intact, living flies by an optical method. Journal of Comparative Physiology A. 1979;134:95–107. [Google Scholar]
- Bird BL, Branch LC, Miller DL. Effects of coastal lighting on foraging behavior of beach mice. Conservation Biology. 2004;18:1435–1439. [Google Scholar]
- Briscoe AD, Chittka L. The evolution of color vision in insects. Annual Review of Entomology. 2001;46:471–510. doi: 10.1146/annurev.ento.46.1.471. [DOI] [PubMed] [Google Scholar]
- Cheng K, Collett TS, Wehner R. Honeybees learn the colours of landmarks. Journal of Comparative Physiology A. 1986;159:69–73. [Google Scholar]
- Chiao C-C, Wickiser JK, Allen JJ, Genter B, Hanlon RT. Hyperspectral imaging of cuttlefish camouflage indicates good color match in the eyes of fish predators. Proceedings of the National Academy of Sciences, U.S.A. 2011;108:9148–9153. doi: 10.1073/pnas.1019090108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittka L, Shmida A, Troje N, Menzel R. Ultraviolet as a component of flower reflections, and the colour perception of Hymenoptera. Vision Research. 1994;34:1489–1508. doi: 10.1016/0042-6989(94)90151-1. [DOI] [PubMed] [Google Scholar]
- Cinzano P, Falchi F, Elvidge CD. The first world atlas of the artificial night sky brightness. Monthly Notices of the Royal Astronomical Society. 2001;328:689–707. [Google Scholar]
- Davies TW, Bennie J, Gaston KJ. Street lighting changes the composition of invertebrate communities. Biology Letters. 2012;8:764–767. doi: 10.1098/rsbl.2012.0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbeis G. Artificial night lighting and insects: attraction of insects to streetlamps in a rural setting in Germany. In: Rich C, Longcore T, editors. Ecological Consequences of Artificial Night Lighting. Washington: Island Press; 2006. pp. 281–304. [Google Scholar]
- Elvidge CD, Keith DM, Tuttle BT, Baugh KE. Spectral identification of lighting type and character. Sensors. 2010;10:3961–3988. doi: 10.3390/s100403961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank KD. Effects of artificial night lighting on moths. In: Rich C, Longcore T, editors. Ecological Consequences of Artificial Night Lighting. Washington: Island Press; 2006. pp. 305–344. [Google Scholar]
- Gaston KJ, Davies TW, Bennie J, Hopkins J. Reducing the ecological consequences of night-time light pollution: options and developments. Journal of Applied Ecology. 2012;49:1256–1266. doi: 10.1111/j.1365-2664.2012.02212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston KJ, Bennie J, Davies TW, Hopkins J. The ecological impacts of nighttime light pollution: a mechanistic appraisal. Biological Reviews. 2013 doi: 10.1111/brv.12036. (in press) [DOI] [PubMed] [Google Scholar]
- Govardovskii VI, Fyhrquist N, Reuter T, Kuzmin DG, Donner K. In search of the visual pigment template. Visual Neuroscience. 2000;17:509–528. doi: 10.1017/s0952523800174036. [DOI] [PubMed] [Google Scholar]
- Hart NS, Hunt DM. Avian visual pigments: characteristics, spectral tuning, and evolution. The American Naturalist. 2007;169:S7–S26. doi: 10.1086/510141. [DOI] [PubMed] [Google Scholar]
- Hart NS, Vorobyev M. Modelling oil droplet absorption spectra and spectral sensitivities of bird cone photoreceptors. Journal of Comparative Physiology A. 2005;191:381–392. doi: 10.1007/s00359-004-0595-3. [DOI] [PubMed] [Google Scholar]
- Hempel de Ibarra N, Vorobyev M. Flower patterns are adapted for detection by bees. Journal of Comparative Physiology A. 2009;195:319–323. doi: 10.1007/s00359-009-0412-0. [DOI] [PubMed] [Google Scholar]
- Hölker F, Moss T, Griefahn B, et al. The dark side of light: a transdisciplinary research agenda for light pollution policy. Ecology and Society. 2010;15 report number 13. [Google Scholar]
- Hunt S, Cuthill IC, Bennett ATD, Church SC, Partridge JC. Is the ultraviolet waveband a special communication channel in avian mate choice? Journal of Experimental Biology. 2001;204:2499–2507. doi: 10.1242/jeb.204.14.2499. [DOI] [PubMed] [Google Scholar]
- Jacobs GH, Deegan JF. Cone pigment variations in four genera of new world monkeys. Vision Research. 2003;43:227–236. doi: 10.1016/s0042-6989(02)00565-5. [DOI] [PubMed] [Google Scholar]
- Kelber A, Balkenius A, Warrant EJ. Scotopic colour vision in nocturnal hawkmoths. Nature. 2002;419:922–925. doi: 10.1038/nature01065. [DOI] [PubMed] [Google Scholar]
- van Langevelde F, Ettema JA, Donners M, WallisDeVries MF, Groenendijk D. Effect of spectral composition of artificial light on the attraction of moths. Biological Conservation. 2011;144:2274–2281. [Google Scholar]
- Lim MLM, Li J, Li D. Effect of UV-reflecting markings on female mate-choice decisions in Cosmophasis umbratica, a jumping spider from Singapore. Behavioral Ecology. 2008;19:61–66. [Google Scholar]
- Longcore T, Rich C. Ecological light pollution. Frontiers in Ecology and the Environment. 2004;2:191–198. [Google Scholar]
- Macedonia JM, Lappin AK, Loew ER, et al. Conspicuousness of Dickerson's collared lizard (Crotaphytus dickersonae) through the eyes of conspecifics and predators. Biological Journal of the Linnean Society. 2009;97:749–765. [Google Scholar]
- Mappes M, Homberg U. Behavioral analysis of polarization vision in tethered flying locusts. Journal of Comparative Physiology A. 2004;190:61–68. doi: 10.1007/s00359-003-0473-4. [DOI] [PubMed] [Google Scholar]
- Martin AD, Quinn KM, Hee Park J. 2010. MCMCpack: Markov chain Monte Carlo (MCMC) package. R package version 1.0-7.
- Möller R. Insects could exploit UV-green contrast for landmark navigation. Journal of Theoretical Biology. 2002;214:619–631. doi: 10.1006/jtbi.2001.2484. [DOI] [PubMed] [Google Scholar]
- Osorio D, Vorobyev M. A review of the evolution of animal colour vision and visual communication signals. Vision Research. 2008;48:2042–2051. doi: 10.1016/j.visres.2008.06.018. [DOI] [PubMed] [Google Scholar]
- Peitsch D, Fietz A, Hertel H, Souza J, Ventura DF, Menzel R. The spectral input systems of hymenopteran insects and their receptor-based colour vision. Journal of Comparative Physiology A. 1992;170:23–40. doi: 10.1007/BF00190398. [DOI] [PubMed] [Google Scholar]
- Perkin EK, Holker F, Richardson JS, Sadler JP, Wolter C, Tockner K. The Influence of artificial light on stream and riparian ecosystems: questions, challenges and perspectives. Ecosphere. 2011;2:1–16. [Google Scholar]
- Reppert SM, Zhu H, White RH. Polarized light helps monarch butterflies navigate. Current Biology. 2004;14:155–158. doi: 10.1016/j.cub.2003.12.034. [DOI] [PubMed] [Google Scholar]
- Robertson KA, Monteiro AN. Female Bicyclus anynana butterflies choose males on the basis of their dorsal UV-reflective eyespot pupils. Proceedings of the Royal Society B. 2005;272:1541–1546. doi: 10.1098/rspb.2005.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth LSV, Kelber A. Nocturnal colour vision in geckos. Proceedings of the Royal Society of London. Series B. 2004;271:S485–S487. doi: 10.1098/rsbl.2004.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydell J. Exploitation of insects around streetlamps by bats in Sweden. Functional Ecology. 1992;6:744–750. [Google Scholar]
- Somanathan H, Borges RM, Warrant EJ, Kelber A. Nocturnal bees learn landmark colours in starlight. Current Biology. 2008;18:R996–R997. doi: 10.1016/j.cub.2008.08.023. [DOI] [PubMed] [Google Scholar]
- Somanathan H, Kelber A, Borges R, Wallén R, Warrant E. Visual ecology of Indian carpenter bees II: adaptations of eyes and ocelli to nocturnal and diurnal lifestyles. Journal of Comparative Physiology A. 2009;195:571–583. doi: 10.1007/s00359-009-0432-9. [DOI] [PubMed] [Google Scholar]
- Stone EL, Jones G, Harris S. Street lighting disturbs commuting bats. Current Biology. 2009;19:1123–1127. doi: 10.1016/j.cub.2009.05.058. [DOI] [PubMed] [Google Scholar]
- Stone EL, Jones G, Harris S. Conserving energy at a cost to biodiversity? Impacts of LED lighting on bats. Global Change Biology. 2012;18:2458–2465. [Google Scholar]
- Titulaer M, Spoelstra K, Lange CYMJG, Visser ME. Activity patterns during food provisioning are affected by artificial light in free living Great Tits (Parus major. PLoS ONE. 2012;7:e37377. doi: 10.1371/journal.pone.0037377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovée MJ. Ultra-violet photoreceptors in the animal kingdom: their distribution and function. Trends in Ecology & Evolution. 1995;10:455–460. doi: 10.1016/s0169-5347(00)89179-x. [DOI] [PubMed] [Google Scholar]
- Ugolini A, Boddi V, Mercatelli L, Castellini C. Moon orientation in adult and young sandhoppers under artificial light. Proceedings of the Royal Society B. 2005;272:2189–2194. doi: 10.1098/rspb.2005.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrant EJ, Kelber A, Gislen A, Greiner B, Ribi W, Wcislo WT. Nocturnal vision and landmark orientation in a tropical halictid bee. Current Biology. 2004;14:1309–1318. doi: 10.1016/j.cub.2004.07.057. [DOI] [PubMed] [Google Scholar]
- Zou Y, Araujo DP, Lim MLM, Li D. Ultraviolet is a more important cue than reflection in other wavelengths for a jumping spider to locate its spider prey. Animal Behaviour. 2011;82:1457–1463. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


