Abstract
STUDY QUESTION
Do ovulatory hormone profiles among healthy premenopausal women differ between women with and without sporadic anovulation?
SUMMARY ANSWER
Women with one anovulatory cycle tended to have lower estradiol, progesterone and LH peak levels during their ovulatory cycle.
WHAT IS KNOWN ALREADY
Anovulation occurs sporadically in healthy premenopausal women, but the influence of hormones in a preceding cycle and the impact on a subsequent cycle's hormone levels is unknown.
STUDY DESIGN, SIZE, DURATION
The BioCycle Study was a prospective cohort including 250 healthy regularly menstruating women, 18–44 years of age, from Western New York with no history of menstrual or ovulation disorders. The women were followed with up to eight study visits per cycle for two cycles, most of which were consecutive.
PARTICIPANTS/MATERIALS, SETTING AND METHODS
All study visits were timed to menstrual cycle phase using fertility monitors and located at the University at Buffalo women's health research center from 2005 to 2007. The main outcomes measured were estradiol, progesterone, LH and follicle-stimulating hormone levels in serum at up to 16 visits over two cycles. Anovulation was defined as peak serum progesterone concentrations ≤5 ng/ml and no serum LH peak detected during the mid- or late-luteal phase visit.
MAIN RESULTS AND THE ROLE OF CHANCE
Reproductive hormone concentrations were lower during anovulatory cycles, but significant reductions were also observed in estradiol (−25%, P = 0.003) and progesterone (−22%, P = 0.001) during the ovulatory cycles of women with one anovulatory cycle compared with women with two ovulatory cycles. LH peak concentrations were decreased in the ovulatory cycle of women with an anovulatory cycle (significant amplitude effect, P = 0.004; geometric mean levels 38% lower, P < 0.05).
LIMITATIONS, REASONS FOR CAUTION
Follow-up was limited to two menstrual cycles, and no ultrasound assessment of ovulation was available. Data were missing for a total of 168 of a possible 4072 cycle visits (4.1%), though all women had at least five visits per cycle (94% had seven or more per cycle).
WIDER IMPLICATIONS OF THE FINDINGS
These results suggest a possible underlying cause of anovulation, such as a longer-term subclinical follicular, ovarian or hypothalamic/pituitary dysfunction, even among healthy, regularly menstruating women.
STUDY FUNDING/COMPETING INTERESTS
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD (Contract no. HHSN275200403394C). The authors have no potential competing interests.
Keywords: anovulation, sex hormones, menstrual cycle
Introduction
Whereas chronic anovulation is considered a marker of a number of potential endocrine disturbances, it is thought that among healthy, regularly menstruating women of reproductive age, intermittent anovulatory cycles may occur but that preceding and succeeding cycles are ovulatory (Wathen et al., 1984; Chatterton et al., 2005). Though anovulatory cycles tend to occur more frequently among women close to menarche and menopause, the prevalence of anovulatory cycles among regularly menstruating women is not well established (Treloar et al., 1970; Venturoli et al., 1987; Santoro et al., 1996). It is typically understood that hormonal patterns during anovulatory cycles are lower than among ovulatory cycles, but it is not clear whether anovulation may be influenced by hormone abnormalities in preceding cycles or reflected in hormone levels during subsequent cycles (Buckler et al., 1991).
Follicle-stimulating hormone (FSH) plays a major role in initiating and maintaining the development of several antral follicles during the ovarian cycle and works in conjunction with several other hormones, including estradiol, progesterone and LH (Mihm et al., 2011). Little is known about how and when the dominant follicle is selected, but researchers hypothesize that it occurs early in the menstrual cycle and may be largely affected by or characterized by hormone levels during prior menstrual cycles (Oktem and Urman, 2010). Consequently, anovulatory cycles where no ovum is released from the ovary, may be affected by preceding cycles or affect future cycles.
To address this gap, we evaluated the hormone profiles of healthy, regularly menstruating, premenopausal women who were monitored for two menstrual cycles with the specific aim of characterizing hormonal differences between anovulatory and ovulatory cycles. Understanding whether anovulation is a sporadic event or representative of an underlying endocrine or metabolic state could have important implications for women trying to become pregnant.
Materials and Methods
Study design
The BioCycle Study was a prospective cohort of 259 regularly menstruating, healthy female volunteers between the ages of 18 and 44. Only participants with data from two, not necessarily consecutive, menstrual cycles were included in this analysis (n = 250 women, 90.8% with consecutive cycles). All women were recruited from the Western New York State region. Details of the study design are described elsewhere (Wactawski-Wende et al., 2009). In brief, exclusion criteria included the use of oral contraceptives during the previous 3 months, pregnancy or breastfeeding in the past 6 months and self-reported prior diagnosis of certain chronic conditions, including thyroid disorders and a history of menstrual and ovulation disorders and uterine abnormalities, such as uterine fibroids and polycystic ovary syndrome (PCOS). Women with a self-reported body mass index (BMI; kg/m2) of <18 or >35 at screening were also excluded. The University at Buffalo Health Sciences Institutional Review Board (IRB) approved the study and served as the IRB designated by the National Institutes of Health for this study under a reliance agreement. All participants provided written informed consent.
Participants were prospectively followed for two menstrual cycles, with up to eight clinic visits per cycle, with blood samples collected for hormonal assessment timed to the cycle phase: second day of menstruation; mid-follicular phase; three visits during periovulation and early, mid- and late-luteal phase. The actual calendar days for the peri-ovulatory visits varied between women, but based on a standardized 28-day cycle would correspond to approximately Days 12, 13 and 14. Fertility monitors (Clearblue Easy Fertility Monitor; Inverness Medical, Waltham, MA, USA) were used to supplement menstrual calendars to assist in the timing of mid-cycle visits (Mumford et al., 2011a). Fertility monitors measured oestrone-3-glucoronide and LH in urine, starting on calendar Day 6 after menses and continuing for 10–20 days, depending on whether the woman reached peak levels on the monitor. Monitor indications of low, high and peak fertility were used to time mid-cycle visits, with other visits scheduled according to an algorithm that took each woman's reported cycle length into consideration. Though the fertility monitor greatly improves cycle phase classification and identification of the LH surge, misclassification may still occur, and a realignment algorithm was applied to improve cycle phase classification (Howards et al., 2009). Data from fasting blood draws were, thus, synchronized with the planned menstrual phase visits so that all women were compared at similar cycle phases (Howards et al., 2009).
Hormone and ovulation assessment
Reproductive hormones were measured in fasting serum samples collected at each clinic visit by the Kaleida Health Center for Laboratory Medicine (Buffalo, NY, USA). Cycle visits were scheduled to occur in the morning, typically between 07:00 and 08:30 h. Estradiol, FSH, LH and progesterone concentrations were measured using solid-phase competitive chemiluminescent enzymatic immunoassays by Specialty Laboratories Inc. (Valencia, CA, USA) on the DPC Immulite 2000 analyzer (Siemens Medical Solutions Diagnostics, Deerfield, IL, USA). Across the study period, the coefficients of variation for these tests were reported by the laboratory as <10% for estradiol, <5% for LH and FSH and <14% for progesterone. Cycles were defined as anovulatory if peak progesterone concentrations were ≤5 ng/ml (Fritz and Speroff, 2011) across the cycle and no serum LH peak was observed during the later cycle visits [38 of 500 cycles (7.6%)] (Gaskins et al., 2009). This joint approach was utilized as a means of conservatively defining anovulation in this cohort, so that if progesterone levels were observed to be low, we were confident that levels were measured during the luteal phase (timing of the LH peak was only evaluated in cycles with low progesterone levels). We also performed a sensitivity analysis using a cut point of 3 ng/ml progesterone to define anovulation to evaluate the robustness of our findings.
Covariate assessment
At study enrollment, height and weight were measured using standardized protocols by trained study staff and were used to calculate BMI, while age, race, education, marital status, smoking and reproductive history were obtained by self-report via in-person questionnaires (Wactawski-Wende et al., 2009). Participants completed questionnaires regarding physical activity using the International Physical Activity Questionnaire (IPAQ) long form 2002 (Craig et al., 2003). High-, moderate- and low-physical activity categories were formed on the basis of standard IPAQ cutoffs. Cycle length was defined as the number of days between the onset of menstrual bleeding. To differentiate from spotting, Day 1 was defined as menstruating by 16:00 h on that day after confirming two consecutive days of bleeding. We had at least 95% complete data for all assessed covariates.
Statistical analysis
Women were categorized into one of three ovulatory groups based on the number of anovulatory cycles they experienced: 0 (ovulatory during both cycles), 1 (anovulatory during one study cycle and ovulatory during the other) or 2 (anovulatory during both cycles). For some analyses, cycles, rather than women, were compared and these were grouped based on ovulatory status: ovulatory cycles among women with two ovulatory cycles, ovulatory cycles among women with one anovulatory cycle and anovulatory cycles. Because no differences were observed between anovulatory cycles among women with and without observed ovulatory cycles, all anovulatory cycles were collapsed into one group to improve statistical power.
Descriptive statistics were calculated for demographics, hormone levels, reproductive characteristics and health-related behaviors for both the entire cohort and by number of anovulatory cycles. Statistical comparisons were made using analysis of variance (ANOVA) for continuous variables and Fisher's exact tests for categorical variables.
Linear mixed models with random intercepts were used to evaluate differences in log-hormone levels across the cycle phases by cycle ovulatory status. Models were run adjusted for age and BMI, as age and BMI are known to be associated with hormone levels as well as with anovulation (Metcalf, 1979; Metwally et al., 2007; Pasquali et al., 2007; Moran et al., 2011). Other potential confounders, including smoking status and past oral contraceptive use, were considered but were not included in the final model for parsimony, as there were only a small number of anovulatory cycles observed in this study. We also conducted a sensitivity analysis adjusting for race, parity and age at menarche. Significant pairwise differences were evaluated for each of the linear mixed models and P-values were adjusted for multiple comparisons using the Bonferroni method. Effect modification by BMI was also explored.
The area under the hormone concentration-time curve (AUC) was calculated for each of the four hormones, for each individual, using log-hormone concentrations and compared by cycle ovulatory status. We assumed a linear relationship between hormone values at each visit and calculated the area under each trapezoid between visits. AUCs summarize overall hormonal exposure and were used to compare hormonal exposure by cycle ovulatory status.
Nonlinear mixed models with harmonic terms were used to evaluate differences in hormone profiles during ovulatory cycles for women with two ovulatory cycles versus women with one ovulatory cycle and one anovulatory cycle (Albert and Hunsberger, 2005). These models were originally developed for analyzing circadian rhythms, but have been adapted here to study menstrual cycle hormone patterns (Albert and Hunsberger, 2005). These models are especially useful because they allow for not only an estimation of differences in mean levels, but also for estimation of the amplitude (differences between the nadir and the peak), and the phase shift (differences in the timing of events in a cycle), and were adjusted for age and BMI. Models were restricted to ovulatory cycles as the hormonal patterns for anovulatory cycles are distinctly different from ovulatory cycles, and these models assume that the underlying shape of the hormonal patterns is the same between groups. These models did not require uniform visit days, but rather standardized time was derived by taking the cycle day of the clinic visit and dividing by observed cycle length so that the start of the menstrual cycle is at time 0 and the end of the cycle is at time 1, with cycles centered on the day of ovulation at 0.5.
Most of the women in the BioCycle Study were followed for two consecutive menstrual cycles. A sensitivity analysis was conducted excluding women with non-consecutive study cycles (n = 23 women). Moreover, we evaluated whether order of the anovulatory cycle was associated with differences in the hormonal profile, as it is unknown whether anovulatory cycles are influenced by preceding cycles or affect subsequent cycles, both, or neither.
A significance level of P < 0.05 was used for all analyses. Descriptive statistics, AUC analyses, linear mixed models and generalized linear mixed models were conducted with version 9.2 of SAS (SAS Institute, Cary, NC, USA). Nonlinear mixed models analysis with harmonic terms was conducted with version 2.13.0 of R (R Foundation for Statistical Computing, 2009).
Results
Demographics
Women were highly compliant; 94% of women completed at least seven clinic visits per cycle. For the purpose of this study, a completed cycle included a minimum of five visits per cycle, with at least one visit during mid-cycle. Those with less than eight visits per cycle were generally the result of having shorter cycles (e.g. 21 days) or having unexpected events occur (travel, illness). There were 168 missing visits out of a total of 4072 (4.1%).
Overall, women in the BioCycle Study were young (mean age: 27.5 years), of healthy weight (mean BMI: 24.1 kg/m2), physically active (moderate to high physical activity: 90.8%) and non-smokers (96.0%; Table I). Age was strongly associated with the number of anovulatory cycles, with women with two anovulatory cycles being on average 8.8 years younger (P = 0.0004) than women with two ovulatory cycles. In turn, women with two anovulatory cycles also tended to be unmarried and nulliparous as these factors are strongly associated with age. Number of anovulatory cycles was not associated with BMI, race, education, smoking status, past oral contraceptive use or physical activity.
Table I.
Characteristics of women participating in the biocycle study (n = 250) by number of anovulatory cycles.
| Total cohort | Number of anovulatory cycles |
P-valuea | |||
|---|---|---|---|---|---|
| Zero | One | Two | |||
| n (number of women) | 250 | 219 | 24 | 7 | — |
| Demographics mean (SD) | |||||
| Age, years | 27.5 (8.3) | 28.2 (8.3) | 23.0 (6.4) | 19.4 (1.0) | 0.0004 |
| BMI, kg/m2 | 24.1 (3.9) | 24.2 (3.9) | 24.1 (4.0) | 21.6 (2.1) | 0.2 |
| Measured cycle length (days) | 28.9 (4.1) | 28.9 (3.9) | 29.9 (5.3) | 25.3 (2.7) | <0.0001 |
| Measured menses length (days) | 5.4 (1.5) | 5.4 (1.4) | 5.7 (2.3) | 4.8 (2.2) | 0.2 |
| Reported mean cycle length (past 12 months) | 28.4 (2.2) | 28.4 (2.1) | 29.3 (1.9) | 27.3 (5.0) | 0.07 |
| Reported mean menses length (past 12 months) | 5.1 (1.1) | 5.0 (1.1) | 5.2 (1.0) | 5.3 (0.5) | 0.7 |
| Age at menarche (years) | 12.4 (1.2) | 12.4 (1.2) | 12.4 (1.4) | 13.1 (1.5) | 0.3 |
| n (%) | |||||
| Race | |||||
| White | 148 (59.2) | 130 (59.4) | 13 (54.2) | 5 (71.4) | 0.8 |
| Black | 50 (20.0) | 42 (19.2) | 7 (29.2) | 1 (14.3) | |
| Other | 52 (20.8) | 47 (21.5) | 4 (16.7) | 1 (14.3) | |
| ≤High school education | 32 (12.8) | 28 (12.8) | 2 (8.3) | 2 (28.6) | 0.3 |
| Married | 65 (26.0) | 65 (29.7) | 0 (0.0) | 0 (0.0) | 0.0004 |
| Nulliparous | 180 (73.2) | 149 (60.6) | 24 (100.0) | 7 (100.0) | 0.0002 |
| Current smoker | 10 (4.0) | 9 (4.1) | 0 (0.0) | 1 (14.3) | 0.2 |
| Physical activity | |||||
| Low | 23 (9.2) | 21 (9.6) | 1 (4.2) | 1 (14.3) | 0.5 |
| Moderate | 90 (36.0) | 81 (37.0) | 6 (25.0) | 3 (42.9) | |
| High | 137 (54.8) | 117 (53.4) | 17 (70.8) | 3 (42.9) | |
| Past OC use | 135 (54.4) | 123 (56.7) | 10 (41.7) | 2 (28.6) | 0.1 |
BMI, body mass index; OC, oral contraceptives.
aTwo-sided P-values for continuous variables calculated using ANOVA and for categorical variables using Fisher's exact test.
There were also significant differences in menstrual cycle characteristics. Measured cycle length differed by number of anovulatory cycles, with women with two anovulatory cycles having the shortest cycle lengths (P < 0.0001). We found similar differences in mean reported cycle length over the past 12 months, though these differences only achieved marginal significance (P = 0.07). There were no observed differences in reported or measured mean menses length by number of anovulatory cycles. Though women with two anovulatory cycles experienced menarche at a later age on average, the difference was not statistically significantly different.
Linear mixed models
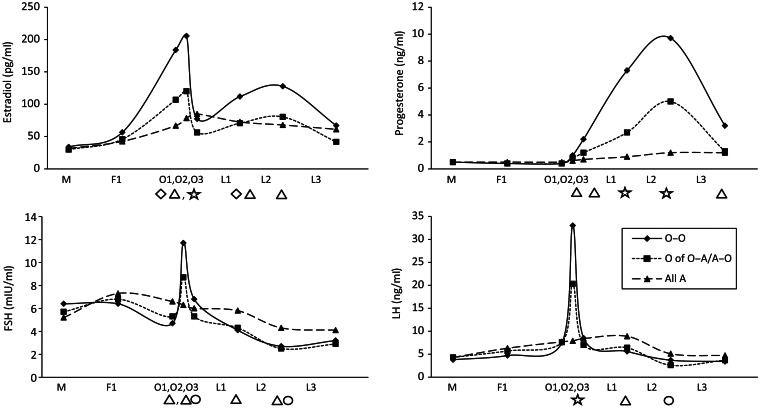
Mean hormone levels were higher during ovulatory cycles when compared with anovulatory cycles for estradiol, progesterone and LH and often lower for FSH regardless of whether a woman had one or no anovulatory cycles (Fig. 1). Lower estradiol and progesterone levels as well as a decreased LH peak were observed in the ovulatory cycles of women with one anovulatory cycle compared with those with two ovulatory cycles. These differences were particularly pronounced around the time of ovulation and during the luteal phase. No effect modification by BMI was observed (data not shown).
Figure 1.
Geometric mean hormone levels among women in the biocycle study by cycle phase and ovulatory status after adjustment for age and BMI. A, anovulatory cycle; BMI, body mass index; FSH, follicle-stimulating hormone; LH, luteinizing hormone; O, ovulatory cycle. Symbols indicate significant differences (P < 0.05) between cycle types within each hormone and each visit, adjusted for each multiple comparisons via the Bonferroni method. Diamond: O–O different from O; Triangle: O–O different from all A; Circle: O different from all A; Star: all pairwise significant. O–O includes cycles of women with two ovulatory cycles (n = 438 cycles); O of O–A/A–O includes the ovulatory cycles only of women with one ovulatory and one anovulatory cycle (n = 24 cycles), all A includes all anovulatory cycles from women with two anovulatory cycles A–A, and the anovulatory cycle of women with one ovulatory and one anovulatory cycle A of O–A/A–O (n = 38 cycles).
Area under the curve
There were significant differences in the AUC by ovulatory status for estradiol, progesterone and FSH (Table II). For estradiol, ovulatory cycles among women with two ovulatory cycles had significantly higher AUCs than the ovulatory cycles of women with one anovulatory cycle or two anovulatory cycles [3149.7 versus 2309.5 and 1962.0 (log pg ×day/ml), respectively]. Similarly, for progesterone, ovulatory cycles among women with two ovulatory cycles had significantly higher AUCs than the other two cycle types [105.6 versus 47.3 and 25.4 (log ng ×day/ml)]. For FSH, ovulatory cycles among women with two ovulatory cycles had significantly higher AUCs than all anovulatory cycles [183.9 versus 159.4 (log ng ×day/ml)]. No significant differences were observed for LH.
Table II.
Mean area under the hormone concentration-time curve (AUC) by ovulatory status.1
| Ovulatory (O–O)2 (n = 438 cycles) | Ovulatory cycles in women with one anovulatory cycle (O of O–A/A–O; n = 24 cycles) | All anovulatory (A of O–A/A–O/A–A; n = 38 cycles) | |
|---|---|---|---|
| Estradiol (log pg ×day/ml) | 3149.7a | 2309.5b | 1962.0b |
| Progesterone (log ng ×day/ml) | 105.6a | 47.3b | 25.4c |
| FSH (log mIU ×day/ml) | 183.9a | 176.0a,b | 159.4b |
| LH (log ng ×day/ml) | 247.0 | 246.0 | 243.9 |
A, anovulatory cycle; AUC, area under the curve; FSH, follicle-stimulating hormone; LH, luteinizing hormone; O, ovulatory cycle.
1AUCs were calculated with log-hormone concentrations. Superscripts indicate significant pairwise differences at the 0.05 level between cycle types within each hormone. For example, groups identified with ‘a's’ are similar to each other, but are statistically significantly different from groups identified with ‘b's’.
2Ovulatory includes cycles of women with two ovulatory cycles; ovulatory cycles in women with one anovulatory cycle includes the ovulatory cycles only of women with one ovulatory and one anovulatory cycle, all anovulatory includes all anovulatory cycles from women with two anovulatory cycles A–A and the anovulatory cycle of women with one ovulatory and one anovulatory cycle A of O–A/A–O.
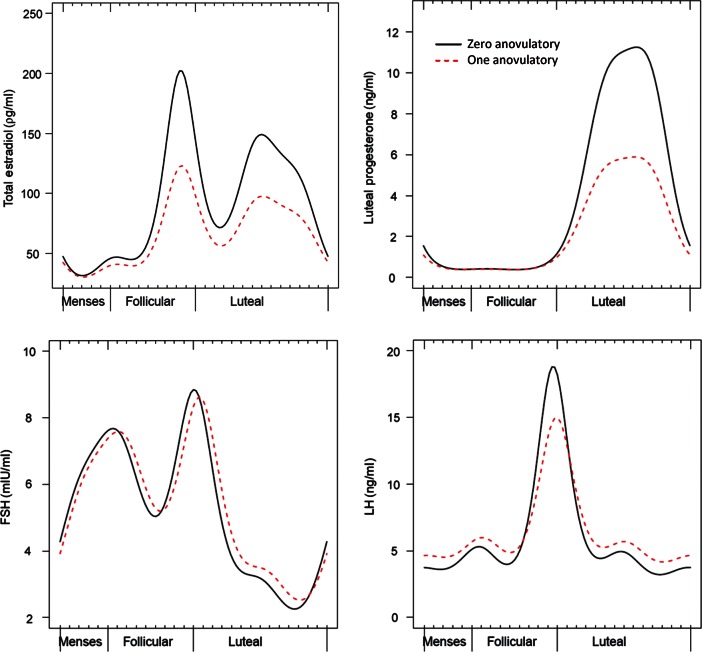
Nonlinear mixed models with harmonic terms
We observed differences in the hormonal profiles of ovulatory cycles between women with and without an anovulatory cycle (Table III). Specifically, mean estradiol levels were on average 24% lower (95% CI: −14, −32) across the cycle among women with at least one anovulatory cycle when compared with women with no anovulatory cycle. Women with at least one anovulatory cycle also had a smaller amplitude (P = 0.0004; Fig. 2).
Table III.
Results from nonlinear mixed models with harmonic terms comparing ovulatory cycles between women with and without an anovulatory cycle, adjusted for age and BMI.
| Estimate (95% CI) | ||
|---|---|---|
| Estradiol (pg/ml) | Mean (%)a | −23.80 (−14.42, −32.15)b |
| Amplitudec | −0.15 (−0.10, −0.18) | |
| Phase shift (days)d | 0.10 (−0.19, 0.38) | |
| Progesterone (ng/ml) | Mean | −25.73 (−17.78, −32.91) |
| Amplitude | −0.31 (−0.23, −0.37) | |
| Phase shift | −0.14 (−0.37, 0.07) | |
| FSH (mIU/ml) | Mean | 3.55 (−6.89, 15.15) |
| Amplitude | −0.03 (−0.07, 0.03) | |
| Phase shift | 0.57 (0.24, 0.92) | |
| LH (ng/ml) | Mean | 12.88 (−1.01, 28.72) |
| Amplitude | −0.10 (−0.06, −0.12) | |
| Phase shift | 0.33 (−0.10, 0.77) | |
BMI, body mass index; FSH, follicle-stimulating hormone; LH, luteinizing hormone.
aRatios were obtained from the model to compare the mean hormone levels across the menstrual cycle for ovulatory cycles among women with one anovulatory cycle compared with women with no anovulatory cycle. Estimates of the percentage difference in the hormone level and 95% CI were obtained by subtracting 1 from each ratio and multiplying by 100.
bBold indicates significance at the 0.05 level. All models take repeated measures and correlated cycles into account.
cAmplitude differences are interpreted as the difference in peak hormone levels when compared with women with no anovulatory cycles.
dPhase shift differences are interpreted as the difference in the number of days of a standardized 28-day cycle between the rise in hormone levels when compared with women with no anovulatory cycles (e.g. 1.12 = hormone rise is shifted 1.12 days later).
Figure 2.
Results from nonlinear mixed models with harmonic terms for hormonal profiles during ovulatory cycles comparing women with zero or one anovulatory cycle, adjusted for age and BMI. Figures display predicted hormone levels for women with a mean age of 27 years and mean BMI of 24.1 kg/m2.
Models further suggested that women with at least one anovulatory cycle had lower mean progesterone values by 26% (95% CI: −18, −33) with a smaller amplitude (P < 0.0001) when compared with women with no anovulatory cycles (Table III, Fig. 2).
Women with one anovulatory cycle had FSH peaks that occurred ∼0.6 days later during their ovulatory cycles when compared with women without an anovulatory cycle (FSH, P = 0.004). Women with one anovulatory cycle also had significantly lower amplitude of LH (Table III, Fig. 2).
Sensitivity analysis
There were 23 individuals with non-consecutive cycles in our analysis: 19 women who had two ovulatory cycles and 4 women who had 1 ovulatory cycle and 1 anovulatory cycle. The time between cycles ranged from 20 to 144 days, with a mean and median of 43.7 and 30 days, respectively. Excluding these women had little impact on the results, though some results lost statistical significance due to a loss of precision associated with the smaller sample size (data not shown). Despite this, all results were trending in the same direction as seen in previous analyses.
Further, additional analyses were conducted wherein women with one anovulatory and one ovulatory cycle were analyzed according to the order of their ovulatory and anovulatory cycles. We did not observe any differences in hormonal profiles by order of ovulatory and anovulatory cycles; hormone levels were lower during ovulatory cycles irrespective of whether the anovulatory cycle occurred prior to or following the ovulatory cycle (data not shown). Moreover, additional adjustment for race, parity and age at menarche did not appreciably alter the results (data not shown).
A sensitivity analysis comparing our definition of anovulation based on a cutpoint of ≤5 ng/ml with a stricter cut point for defining anovulatory cycles (≤3 ng/ml) showed similar results. Based on this stricter cut point, there were a total of 23 anovulatory cycles (4.6%), when compared with 38 based on the 5 ng/ml cut point (7.6%). In general, the patterns that we observed between women with and without ovulatory cycles remained, although, in some cases became non-statistically significant due to a loss in power because of the reduced number of anovulatory cycles. Specifically, the geometric mean hormone levels were observed to follow similar patterns with levels during the ovulatory cycle of those women with sporadic anovulation being lower than women with two ovulatory cycles, though these levels were closer to the levels observed during anovulatory cycles (data not shown). Moreover, the AUC results also followed similar patterns, but that the area for the ovulatory cycle of women with sporadic anovulation was even lower than when the 3 ng/ml cut point was used. Finally, the harmonic model results also follow a similar pattern, with similar point estimates and no change in inference.
Discussion
In a prospective cohort study of 250 healthy premenopausal women, we found differences in hormone profiles during ovulatory cycles between women who experienced an anovulatory cycle and women who did not. Compared with women with two ovulatory cycles, women with one anovulatory cycle had statistically significantly lower estradiol, progesterone and LH peak levels during their ovulatory cycle. We also found that women with anovulatory cycles tended to be younger, unmarried, nulliparous and have shorter cycles on average. These differences in hormone profiles during the ovulatory cycles of women with anovulatory cycles suggest that anovulation may not always be a sporadic event, even among healthy, premenopausal women like those in the BioCycle Study. Among women with presumed sporadic anovulation, we observed alterations in the hormone profiles of preceding and subsequent cycles, suggesting chronic subclinical follicular and ovarian dysfunction.
Our findings are consistent with previous research that has shown that younger women have a higher probability of anovulation (Vollman, 1977; Metcalf, 1983). In addition, previous research has also shown that unusually short and long cycles are 10–30% more likely to be anovulatory, though in our study only short cycles were associated with anovulation (Metcalf, 1979). However, longer cycles are typically more characteristic of anovulation during menopause and the perimenopausal transition, and our study population consisted of healthy premenopausal women (Treloar et al., 1970). Our finding of lower hormone levels during anovulatory cycles is expected as anovulation is typically defined based on the analysis of hormone levels, and these results are consistent with reported hormonal profiles among anovulatory cycles (Buckler et al., 1991). However, there is a lack of comparable research evaluating hormonal profiles among women who experience sporadic anovulatory cycles. Previous research in the BioCycle Study showed that the anovulatory women displayed several characteristics of endocrine and metabolic disturbances (increased LH:FSH ratios, presence of acne and decreased levels of sex hormone-binding globulin), even after adjustment for age and BMI (Mumford et al., 2011b). The current results extend these findings and demonstrate that women with anovulatory cycles tend to have reduced estradiol, progesterone and LH peak levels, and increased FSH levels even during their ovulatory cycles.
We also found using the nonlinear mixed models with harmonic terms that women with one anovulatory cycle had slightly higher FSH concentrations throughout their ovulatory cycle. Though these results were not statistically significant, they suggest that the pituitary gland may be releasing more FSH in women with anovulatory cycles in an attempt to compensate for poor follicle development and inadequate estrogen production in response to normal FSH levels. Future studies should incorporate serum anti-Mullerian hormone or antral follicle count by ultrasound to further investigate this possibility.
Research is needed to further identify the pathophysiology of incident anovulation among eumenorrheic women. Future studies should attempt to address how long it takes to transition from the healthy hormone profile typically seen during ovulatory cycles to the abnormal hormone profile found during anovulatory cycles, as well as how long it takes for the hormone profile to recover after an anovulatory cycle. In addition, investigators should consider incorporating additional markers of ovarian reserve and ovulation to more specifically evaluate the etiology of this phenomenon.
Our study includes several limitations. First, our sample size was limited, particularly for those groups involving an anovulatory cycle, as this cohort study was designed to follow healthy, regularly menstruating women. Secondly, our assessment of ovulatory status was limited in that ultrasound was not available for comparison, and some misclassification is possible as our classification of anovulation was based on multiple serum luteal progesterone measurements and LH concentrations. Misclassification of ovulatory status would tend to dilute the observed effects. However, we found similar results when using a stricter cut point for defining anovulatory cycles. In addition, some investigators question the occurrence of anovulatory cycles among regularly menstruating women and suggest that this is simply a matter of insensitivity of the biomarker approach (Malcolm and Cumming, 2003). They argue that, with ultrasound, all cycles among regularly menstruating women will be classified as ovulatory, a possibility we cannot rule out with the data available. Further, our assessment was limited to two menstrual cycles, and androgen measurements were not available for better characterization of mild PCOS-like phenotypes.
Our study also has several strengths. Study participants were highly compliant so there were relatively few missing visits. In addition, this is one of the few studies that had follow-up for more than one menstrual cycle and the majority of the women in the study had consecutive cycles, allowing for inferences to be made across the two cycles. Moreover, we were able to adjust for several important confounding factors, including age, BMI, race, parity and age at menarche, though some residual confounding may still be present.
In conclusion, we observed that among a cohort of healthy, regularly menstruating women, women with at least one anovulatory cycle had atypical hormonal and gonadotrophin profiles even during their ovulatory cycles. These results suggest that anovulation may not be a sporadic event, but rather a result of a longer-term subclinical follicular, ovarian or hypothalamic/pituitary dysfunction. Further research is needed among larger cohorts with follow-up over multiple sequential cycles to further elucidate these findings.
Authors’ roles
H.L.M., S.L.M., D.R.M., E.F.S. and J.W. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. E.F.S. and J.W. were involved in the study concept and design. E.F.S. and J.W. were responsible for acquisition of data. H.L.M., D.R.M., S.L.M., A.Y. and E.F.S. did the analysis and interpretation of data. H.L.M., D.R.M., S.L.M., E.F.S., M.S.B. and A.Z.P. drafted the manuscript. D.R.M., S.L.M., E.F.S., A.Z.P., M.S.B., M.P., K.L.L., J.W. and E.F.S. critically revised the manuscript for important intellectual content. H.L.M. and S.L.M. performed the statistical analysis. E.F.S. and J.W. supervised the study.
Funding
This study was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (contract no. HHSN275200403394C).
Conflict of interest
None declared.
Acknowledgements
The authors would like to acknowledge the staff at the University at Buffalo, the BioCycle Study Working Group members and the BioCycle participants for their assistance and participation.
References
- Albert PS, Hunsberger S. On analyzing circadian rhythms data using nonlinear mixed models with harmonic terms. Biometrics. 2005;61:1115–1120. doi: 10.1111/j.0006-341X.2005.464_1.x. doi:10.1111/j.0006-341X.2005.464_1.x. [DOI] [PubMed] [Google Scholar]
- Buckler HM, Evans CA, Mamtora H, Burger HG, Anderson DC. Gonadotropin, steroid, and inhibin levels in women with incipient ovarian failure during anovulatory and ovulatory rebound cycles. J Clin Endocrinol Metab. 1991;72:116–124. doi: 10.1210/jcem-72-1-116. doi:10.1210/jcem-72-1-116. [DOI] [PubMed] [Google Scholar]
- Chatterton RT, Jr, Mateo ET, Hou N, Rademaker AW, Acharya S, Jordan VC, Morrow M. Characteristics of salivary profiles of oestradiol and progesterone in premenopausal women. J Endocrinol. 2005;186:77–84. doi: 10.1677/joe.1.06025. doi:10.1677/joe.1.06025. [DOI] [PubMed] [Google Scholar]
- Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. doi:10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- Fritz MA, Speroff L. Female infertility. In: Speroff L, Fritz MA, editors. Clinical Gynecologic Endocrinology and Infertility. 8th edn. Philadelphia, PA: Lippincott Williams & Wilkins; 2011. Chapter 27. [Google Scholar]
- Gaskins AJ, Mumford SL, Zhang C, Wactawski-Wende J, Hovey KM, Whitcomb BW, Howards PP, Perkins NJ, Yeung E, Schisterman EF BioCycle Study Group. Effect of daily fiber intake on reproductive function: the BioCycle Study. Am J Clin Nutr. 2009;90:1061–1069. doi: 10.3945/ajcn.2009.27990. doi:10.3945/ajcn.2009.27990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howards PP, Schisterman EF, Wactawski-Wende J, Reschke JE, Frazer AA, Hovey KM. Timing clinic visits to phases of the menstrual cycle by using a fertility monitor: The BioCycle Study. Am J Epidemiol. 2009;169:105–112. doi: 10.1093/aje/kwn287. doi:10.1093/aje/kwn287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm CE, Cumming DC. Does anovulation exist in eumenorrheic women? Obstet Gynecol. 2003;102:317–318. doi: 10.1016/s0029-7844(03)00527-1. doi:10.1016/S0029-7844(03)00527-1. [DOI] [PubMed] [Google Scholar]
- Metcalf MG. Incidence of ovulatory cycles in women approaching the menopause. J Biosoc Sci. 1979;11:39–48. doi: 10.1017/s0021932000012037. [DOI] [PubMed] [Google Scholar]
- Metcalf MG. Incidence of ovulation from the menarche to the menopause: observations of 622 New Zealand women. N Z Med J. 1983;96:645–648. [PubMed] [Google Scholar]
- Metwally M, Li TC, Ledger WL. The impact of obesity on female reproductive function. Obes Rev. 2007;8:515–523. doi: 10.1111/j.1467-789X.2007.00406.x. doi:10.1111/j.1467-789X.2007.00406.x. [DOI] [PubMed] [Google Scholar]
- Mihm M, Gangooly S, Muttukrishna S. The normal menstrual cycle in women. Anim Reprod Sci. 2011;124:229–236. doi: 10.1016/j.anireprosci.2010.08.030. doi:10.1016/j.anireprosci.2010.08.030. [DOI] [PubMed] [Google Scholar]
- Moran LJ, Dodd J, Nisenblat V, Norman RJ. Obesity and reproductive dysfunction in women. Endocrinol Metab Clin North Am. 2011;40:895–906. doi: 10.1016/j.ecl.2011.08.006. doi:10.1016/j.ecl.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Mumford SL, Schisterman EF, Gaskins AJ, Pollack AZ, Perkins NJ, Whitcomb BW, Ye A, Wactawski-Wende J. Realignment and multiple imputation of longitudinal data: an application to menstrual cycle data. Paediatr Perinat Epidemiol. 2011a;25:448–459. doi: 10.1111/j.1365-3016.2011.01204.x. doi:10.1111/j.1365-3016.2011.01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford SL, Schisterman EF, Siega-Riz AM, Gaskins AJ, Steiner AZ, Daniels JL, Olshan AF, Hediger ML, Hovey K, Wactawski-Wende J, et al. Cholesterol, endocrine and metabolic disturbances in sporadic anovulatory women with regular menstruation. Hum Reprod. 2011b;26:423–430. doi: 10.1093/humrep/deq322. doi:10.1093/humrep/deq322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oktem O, Urman B. Understanding follicle growth in vivo. Hum Reprod. 2010;25:2944–2954. doi: 10.1093/humrep/deq275. doi:10.1093/humrep/deq275. [DOI] [PubMed] [Google Scholar]
- Pasquali R, Patton L, Gambineri A. Obesity and infertility. Curr Opin Endocrinol Diabetes Obes. 2007;14:482–487. doi: 10.1097/MED.0b013e3282f1d6cb. doi:10.1097/MED.0b013e3282f1d6cb. [DOI] [PubMed] [Google Scholar]
- Santoro N, Brown JR, Adel T, Skurnick JH. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81:1495–1501. doi: 10.1210/jcem.81.4.8636357. doi:10.1210/jc.81.4.1495. [DOI] [PubMed] [Google Scholar]
- Treloar AE, Boynton RE, Behn BG, Brown BW. Variation of the human menstrual cycle through reproductive life. Int J Fertil. 1970;12:77–126. [PubMed] [Google Scholar]
- Venturoli S, Porcu E, Gammi L, Magrini O, Fabbri R, Paradisi R, Flamigni C. Different gonadotropin pulsatile fashions in anovulatory cycles of young girls indicate different maturational pathways in adolescence. J Clin Endocrinol Metab. 1987;65:785–791. doi: 10.1210/jcem-65-4-785. doi:10.1210/jcem-65-4-785. [DOI] [PubMed] [Google Scholar]
- Vollman RF. The menstrual cycle. Major Probl Obstet Gynecol. 1977;7:1–193. [PubMed] [Google Scholar]
- Wactawski-Wende J, Schisterman EF, Hovey KM, Howards PP, Browne RW, Hediger M, Liu A, Trevisan M. BioCycle study: design of the longitudinal study of the oxidative stress and hormone variation during the menstrual cycle. Paediatr Perinat Epidemiol. 2009;23:171–184. doi: 10.1111/j.1365-3016.2008.00985.x. doi:10.1111/j.1365-3016.2008.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathen NC, Perry L, Lilford RJ, Chard T. Interpretation of single progesterone measurement in diagnosis of anovulation and defective luteal phase: observations on analysis of the normal range. Br Med J. 1984;288:7–9. doi: 10.1136/bmj.288.6410.7. doi:10.1136/bmj.288.6410.7. [DOI] [PMC free article] [PubMed] [Google Scholar]