Highlights
► Constitutive phenotype in nitrate-reductase mutants depends on nitrate transporters. ► Intracellular nitrate derives from media components. ► Internal nitrate generation from nitric oxide. ► Nitrate transporters are functional in cells lacking nitrate reductase.
Keywords: Nitrate cluster; Pseudo-constitutive; Nitrate reductase; NirA, AreA; Nitric oxide; Nitrate transporter; Gene expression
Abstract
In fungi, transcriptional activation of genes involved in assimilation requires the presence of an inducer (nitrate or nitrite) and low intracellular concentrations of the pathway products ammonium or glutamine. In Aspergillus nidulans, the two transcription factors NirA and AreA act synergistically to mediate nitrate/nitrite induction and nitrogen metabolite derepression, respectively. In all studied fungi and in plants, mutants lacking nitrate reductase (NR) activity express nitrate-metabolizing enzymes constitutively without the addition of inducer molecules. Based on their work in A. nidulans, Cove and Pateman proposed an “autoregulation control” model for the synthesis of nitrate metabolizing enzymes in which the functional nitrate reductase molecule would act as co-repressor in the absence and as co-inducer in the presence of nitrate. However, NR mutants could simply show “pseudo-constitutivity” due to induction by nitrate which accumulates over time in NR-deficient strains. Here we examined this possibility using strains which lack flavohemoglobins (fhbs), and are thus unable to generate nitrate internally, in combination with nitrate transporter mutations (nrtA, nrtB) and a GFP-labeled NirA protein. Using different combinations of genotypes we demonstrate that nitrate transporters are functional also in NR null mutants and show that the constitutive phenotype of NR mutants is not due to nitrate accumulation from intracellular sources but depends on the activity of nitrate transporters. However, these transporters are not required for nitrate signaling because addition of external nitrate (10 mM) leads to standard induction of nitrate assimilatory genes in the nitrate transporter double mutants. We finally show that NR does not regulate NirA localization and activity, and thus the autoregulation model, in which NR would act as a co-repressor of NirA in the absence of nitrate, is unlikely to be correct. Results from this study instead suggest that transporter-mediated accumulation in NR deficient mutants, originating from traces of nitrate in the media, is responsible for the constitutive expression of NirA-regulated genes, and the associated phenotype is thus termed “pseudo-constitutive”.
1. Introduction
With few exceptions, like the yeast Saccharomyces cerevisiae, most fungi are able to use nitrate as an assimilatory nitrogen source and recent work showed that fungal nitrate assimilation significantly contributes to biogeochemical nitrogen cycling in nitrate-dominated agricultural soils (Gorfer et al., 2011). Bacteria, algae and plants also assimilate nitrate and in all systems needs to be reduced to ammonium in order to serve as nitrogen source for incorporation into amino acids. These sequential reaction steps are carried out by the enzymes nitrate reductase (NR, to ) and nitrite reductase (NiR, to ) (Cove, 1979). Nitrate assimilation in Neurospora crassa and Aspergillus nidulans served as early eukaryotic model systems to study adaptive enzyme formation due to the ease with which the enzymatic activity can be assayed in cell extracts (Cove and Coddington, 1965; Kinsky and McElroy, 1958). Genetic dissection of the pathway in A. nidulans resulted in the characterization of mutants affected in structural genes (Cove and Pateman, 1963; Pateman et al., 1967). Amongst others, NirA and one of the first eukaryotic regulatory mutations were identified using this model pathway (Cove, 1969). This work revealed that de novo synthesis of NR and NiR are subject to induction by nitrate or nitrite and to repression by ammonium (Cove, 1966; Kinsky, 1961). Both inducer molecules are internalized by active transport via the two nitrate permeases nrtA (crnA) and nrtB (crnB), also capable to transport nitrite (Brownlee and Arst, 1983; Unkles et al., 1991, 2001), whereas nitA encodes a specific nitrite transporter only (Unkles et al., 2011; Wang et al., 2008).
Today, the molecular basis of nitrate-responsive gene regulation in fungi is well understood. In A. nidulans, nitrate induction is mediated by the binuclear Zn-cluster protein NirA (Burger et al., 1991a,b; Cove, 1976) which accumulates in the nucleus in the presence of intracellular nitrate. Nuclear accumulation is the consequence of nitrate-or nitrite- mediated disruption of the interaction between the NirA nuclear export signal (NES) and the nuclear exportin KapK (Bernreiter et al., 2007). Subsequently, NirA binds to specific recognition sites in target promoters (Strauss et al., 1998). Transcriptional activation of most nitrate-responsive genes additionally requires interaction of NirA with AreA (Arst and Cove, 1973; Arst, 1982; Caddick et al., 1986; Kudla et al., 1990; Starich et al., 1998), a GATA-type transcriptional co-activator regulating genes involved in nitrogen metabolism. In the nitrate pathway AreA is required for in vivo DNA binding of NirA (Berger et al., 2006; Narendja et al., 2002), for recruitment of histone acetylation activities (Berger et al., 2008) and chromatin remodeling in nitrate-responsive promoters (Muro-Pastor et al., 1999, 2004). In such a way, NirA and AreA act synergistically to activate transcription of nitrate-responsive genes including the genes coding for NR (niaD) and NiR (niiA).
Cove and Pateman noted that mutants affected in NR activity constitutively synthesize NiR without the need of inducer addition and proposed an autoregulation model in which the NR holoenzyme would act as co-repressor of its own synthesis and that of NiR in the absence of nitrate or nitrite (Cove and Pateman, 1969). This hypothesis was further supported by findings that cnx mutants, unable to synthesize the molybdenum-containing cofactor of NR, are likewise constitutive for NiR activity (Mac Donald and Cove, 1974; Pateman et al., 1964). Interestingly, also the enzymes of the pentose phosphate pathway, later shown to be up-regulated in a NirA-dependent manner (Schinko et al., 2010), were found to be constitutively produced in cnx and niaD mutants (Hankinson and Cove, 1974). A molecular study confirmed the constitutive expression of both niaD and niiA in selected niaD and cnx mutants (Hawker et al., 1992).
However, an alternative way to explain the constitutive phenotype would be the intracellular accumulation of nitrate in NR-negative mutants from either external or internal sources. Such external trace amounts of nitrate may occur as contaminants of media components which might accumulate over time inside NR-mutant cells by nitrate transporter activity, and eventually lead to activation of NirA. In the yeast Hansenula polymorpha (Navarro et al., 2003) and in the algae Chlamydomonas reinhardtii (Llamas et al., 2002) this was shown to be the underlying mechanism of constitutive gene expression. In both organisms, constitutivity of the nitrate assimilatory genes observed in NR mutants was lost when nitrate transporters were non-functional. In C. reinhardtii, the authors showed also that intracellular levels of nitrate are detectable in NR mutants grown on “nitrate-free” medium. These results weaken the hypothesis that in these organisms NR itself possesses a regulatory function. However, from these studies it cannot be formally excluded that internal sources of nitrate – such as or derived from nitric oxide (NO) – artificially induce the system in cooperation with the transporters. In this alternative model nitrate transporters would additionally act as signalers, similar to what has been shown for Arabidopsis thaliana (Guo et al., 2003).
Although so far no clear evidence for the existence of mammalian-type classical NO synthases is available for fungi, algae and plants, NO can be generated in these organisms by the nitrate reductase enzyme itself as a by-product of the main enzymatic reaction (Besson-Bard et al., 2008; de Montaigu et al., 2010; Modolo et al., 2005; Rockel et al., 2002; Schinko et al., 2010; Wendehenne et al., 2001; Yamasaki, 2000; Yamasaki and Sakihama, 2000). Additionally, a variety of (bio)chemical pathways are known to generate NO in metabolically active cells (Nagase et al., 1997; Zweier et al., 1999). Notably, many processes have been shown to be regulated by NO in plants, e.g. stomatal closure, flowering, gravitropsim, and stress response (reviewed in Besson-Bard et al., 2008). NO is harmful to cells at higher concentrations causing proteins to become nitrosylated or nitrated impairing their proper function. Levels of NO are antagonized by spontaneous oxidation to nitrite and peroxinitrite and by enzymatic detoxification involving flavohemoglobins (fhb). These evolutionary conserved di-oxygenases have the ability to convert NO directly to and thereby efficiently remove excess NO (Gardner et al., 1998; Poole and Hughes, 2000). We have recently characterized two flavohemoglobin genes (fhbA and fhbB) in A. nidulans and cells lacking both enzymes show hypersensitivity to elevated environmental NO levels. FhbA was shown to be induced by nitrate in a strictly NirA-dependent manner but interestingly, and in contrast to all other known nitrate-responsive genes, fhbA expression does not require the function of the general nitrogen regulator AreA (Schinko et al., 2010).
To clarify whether in A. nidulans NR itself has a real regulatory role and if external or internal sources might evoke a “pseudo-constitutive” phenotype in NR loss-of-function mutants, we used mutant strains affected in nitrate transport and metabolism, and combined them with flavohemoglobin mutants lacking NO to conversion. Our results show that NR mutants accumulate significant levels of intracellular nitrate leading to NirA nuclear accumulation and expression of the assimilatory genes. Flavohemoglobins apparently are not required for this activity whereas nitrate permeases are. This suggests, that also in A. nidulans external traces of nitrate in supposedly “nitrate-free” media are accumulating in NR mutants leading to a “pseudo-constitutive” phenotype. The transporters themselves, however, do not seem to be required for the induction process when standard levels of nitrate are present in the medium.
2. Materials and methods
2.1. Strains, media and growth conditions
Genotypes of A. nidulans strains used in this study are given in Table 1. Minimal (MM) and complete (CM) media for A. nidulans were as described by Cove (1966). Supplements were added when necessary at adequate concentrations (http://www.gla.ac.uk/acad/ibls/molgen/aspergillus/supplement.html). Strains were grown on 1% glucose minimal media (GMM) supplemented according to the relevant genotypes. Liquid cultures were grown for 14 h at 37° C, 180 rpm. 3 mM l-arginine or 5 mM l-proline were used as sole nitrogen sources. For nitrate or nitrite induction 10 mM NaNO3 or 10 mM NaNO2 were added 15 min prior to harvesting. Mycelia were harvested by filtration through Miracloth (Merck) including a washing step with 100 ml modified and chilled MM. The washing media lacked glucose and supplements, and MgSO4 was substituted by equal amounts of MgCl2.
Table 1.
A. nidulans strains used in this study.
| Strain name | Genotype | Reference |
|---|---|---|
| WT | veA1 biA1 yA2 | Schinko et al. (2010) |
| niaDΔ | veA1 biA1 pyrG89 niaDΔ wA3 | Schinko et al. (2010) |
| nrtA− nrtB−∗ | veA1 biA1 pabaA1 argB2 crnA747 crnB110 | Ukles et al. (2001) |
| nrtA− nrtB− | veA1 biA1 pabaA1 argB complemented crnA747 crnB110 | Schinko et al. (2010) |
| nrtA− nrtB− niaDΔ | veA1 biA1 pabaA1 niaDΔ::argB crnA747 crnB110 | Schinko et al. (2010) |
| fhbΔΔ | veA1 biA1 fhbAΔ::argB fhbB::argB yA2 | Schinko et al. (2010) |
| fhbΔΔ niaDΔ | veA1 biA1 fhbAΔ::argB fhbB::argB niaDΔ wA3 | Schinko et al. (2010) |
| nirA− | veA1 pabaA1 nirA637 | Schinko et al. (2010) |
| niiA− | veA1, biA1, niiA4, pyroA4, nkuAΔ::bar | Schinko et al. (2010) |
| niiAΔ:AFpyrG | veA1 nkuAΔ::argB niiAΔ::AFpyrG pyroA4 riboB2 | This study |
| niaDΔ niiAΔ | veA1 biA1 niaDΔ niiAΔ::AFpyrG wA3 | This study |
| nirAΔ:AFriboB | veA1 nkuAΔ::argB nirAΔ::AFriboB pyroA4 pyrG89 | This study |
| FLAG:nirAcDNA:GFP | veA1 nkuAΔ::argB 5′UTRnirA:AFpyrG:gpdAp Matg:FLAG:nirAcDNA:GFP:3′UTRnirA pyroA4 riboB2 | This study |
| niaDΔ FLAG:nirAcDNA:GFP | veA1 biA1 5′UTRnirA:AFpyrG:gpdAp Matg:FLAG:nirAcDNA:GFP:3′UTRnirA | This study |
| nrtA− nrtB− FLAG:nirAcDNA:GFP | veA1 crnA747 crnB110 biA1 5′UTRnirA:AFpyrG:gpdAp Matg:FLAG:nirAcDNA:GFP:3′UTRnirA | This study |
| nrtA− nrtB− niaDΔ FLAG:nirAcDNA:GFP | veA1 crnA747 crnB110 biA1 5′UTRnirA:AFpyrG:gpdAp Matg:FLAG:nirAcDNA:GFP:3′UTRnirA | This study |
| TNO2A7 | veA1 nkuAΔ::argB pyroA4 pyrG89 riboB2 | Nayak et al. (2006) |
2.2. DNA and RNA manipulations
Plasmid preparation from E. coli strains was carried out using the Qiagen Plasmid Mini kit following the instructions of supplier. Genomic DNA extraction from A. nidulans was according to Lockington et al. (1985). Southern blot analysis was carried out according to Sambrook and Russell (2001). Restriction enzymes were used according to the manufacturer’s instructions (Thermo Fisher Scientific). High fidelity PCR reactions were carried out using the Phusion® Flash High-Fidelity PCR Master Mix (Thermo Fisher Scientific). For conventional PCR reactions RED Taq® ReadyMix™ (Sigma Aldrich) was used. DNA fragments were purified from agarose gels using the MinElute Gel Extraction Kit (Qiagen). Total RNA was isolated from pulverized mycelia using TRIzol® reagent according to the manufacturer’s instructions (Invitrogen). Northern blots were performed as described by Narendja et al. (2002), using 15 μg of total RNA per lane and membrane. 32P α-dCTP labeled DNA molecules, used as gene-specific probes, were prepared using the Random Primed DNA labeling kit following the supplier’s instructions (Roche Applied Science). 32P α-dCTP labeled DNA probes for nitrite reductase (niiA), flavohemoglobin A (fhbA) and ribosomal 18S rRNA (18S) were used. Signal intensities were captured on a phosphoimager screen (Storm, Molecular Dynamics, Inc.) and quantified using the ImageJ software. Readout values were normalized to the values of the loading control (18S rRNA). Normalized signals were referred to the transcriptional level in the wild type strain (WT) under inducing conditions, which were arbitrarily set to 1.0. Experimental setup was repeated at least three times and representative results from these repeats are shown.
2.3. Generation of gene deletion cassettes
Gene replacement was performed by exchanging the open reading frame (ORF) or parts thereof by sequences from Aspergillus fumigatus encoding orotidine-5′-phosphate -decarboxylase (AFpyrG, Afu2g0836) or GTP-cyclohydrolase II (AFriboB, Afu1g13300) and the A. nidulans ornithine carbamoyltransferase (argB, ANID_04409.3), thus complementing the corresponding A. nidulans mutations pyrG89, riboB2 and argB2, respectively. Details of the primer combinations used are given in Supplementary information (Supplementary Table 2).
The nirA deletion cassette (nirAΔ::AFriboB) was assembled using the 5′- and 3′-UTRnirA sequences amplified from genomic DNA using primer 1&2 and primer 3&4 and Taq polymerase. Introduced T-overhangs allowed the insertion of the fragments into pGEM-Teasy being spaced by the AFriboB sequence which was amplified from A. fumigatus genomic DNA using oligonucleotide combinations primer 5&6. Assembly of the deletion cassette was achieved in a single ligation step generating the plasmid pGEM-Teasy_nirAΔ::AFriboB.
The construction of the niaD deletion cassette (niaDΔ::argB) followed the same principal but replacing only part of the ORF. The 5′- and 3′- regions of the ORF were amplified using primer combinations 7&8 and 9&10. Again, a single ligation step was used to join the argB encoding fragment, obtained from plasmid pMS12 (FGSC, http://www.fgsc.net), together with the 5′- and 3′-ORFniaD sequences resulting into plasmid pGEM-Teasy_5′-ORFniaD:argB:3′-ORFniaD.
Finally, for the niiA deletion cassette (niiAΔ::AFpyrG), the 5′- and 3′- UTRniiA regions amplified using oligonucleotide combinations primer 11&12 and primer 13&14 were ligated into pGEM-Teasy creating the intermediate plasmid pGEM-Teasy_5′-UTRniiA:3′-UTRniiA. After the re-opening with SphI the AFpyrG fragment (primer 15&16), obtained from plasmid p1439 (Szewczyk et al., 2006) was inserted resulting the plasmid pGEM-Teasy_5′-UTRniiA:AFpyrG:3′-UTRniiA.
All deletion cassettes were NotI released from the final plasmids and used for A. nidulans transformations.
2.4. Construction of nirA:GFP fusion cassette
The A. nidulans strain harboring a FLAG:nirA cDNA:GFP fusion protein originally was designed for another experimental setup though was used for crossings. Since its construction has not been published elsewhere the cloning is described here. A step by step strategy was applied to assemble the chimeric cassette starting with the amplification of the 5′-UTRnirA from genomic DNA (primer 17&18) and the 3′-UTRnirA using oligonucleotides primer 19&20. The two fragments were inserted into the ApaI/PstI sites of the pGEM-Teasy vector, possessing a common KpnI restriction site. The sGFP sequence used originated from pERE-nirAc1-GFP (Bernreiter et al., 2007) using primer 21&22 by which a FLAG-tag coding sequence was added at the 5′-end spaced by an NcoI site from the sGFP sequence. The KpnI/NotI construct was inserted into pGEM-Teasy_5′- UTRnirA:3′UTRnirA along with a 0.65 kb gpdAp promoter fragment region (Vogt et al., 2005) amplified using primer 23&24. The resulting plasmid was termed pGEM-Teasy_5′-UTRnirA:FLAG:sGFP:3′UTRnirA,. The nirA cDNA sequence, amplified using primer 25&26, was inserted between the FLAG and sGFP sequence and the AFpyrG encoding sequence, generated with primer 27&28, was inserted upstream of the gpdAp promoter resulting in plasmid pGEM-Teasy_5′UTRnirA:AFpyrG:gpdAp Matg:FLAG:nirA cDNA:GFP:3′UTRnirA. For A. nidulans transformation a linear fragment of the cassette was amplified using primer 17&20. Details on plasmids generated during this work and primers used for amplifications are given in Supplementary Information (Supplementary Tables 1 and 2).
2.5. Determination of intracellular nitrate () levels
The method follows the procedure described in Schinko et al. (2010), with minor modifications. Briefly, mycelia were broken by grinding in liquid nitrogen and then transferred into pre-weighed Eppendorf tubes and extracted twice using 50% methanol (v/v). The pooled supernatant (SN) was centrifuged and directly analyzed for and concentrations. Values were normalized to the dry weight (DW) of the extracted biomass and are given as μg g−1 DW. Negative values are calculatory artefacts which derive from nitrite concentrations which were higher before the to reduction step than after this step (nitrite levels should be at least equally high) and are thus expressed as “not detected” (n.d.). Mean values of three independent biological experiments, including standard deviation values, were calculated.
2.6. Fluorescence microscopy
Microscopic studies were carried out as described in Berger et al. (2006) using a Leica TCS SP2 confocal laser scanning microscope. Strains were grown on cover slips for 16 h at 25 °C in GMM with 3 mM l-arginine as a sole nitrogen source. For inducing conditions 10 mM NaNO3 was added to the media 2 min prior to image acquisition. Images were processed with ImageJ 1.41o and Photoshop CS software but without changing relevant characteristics of the image.
3. Results and discussion
3.1. Nitrate transporters are required for constitutive nitrate cluster expression in niaDΔ strains
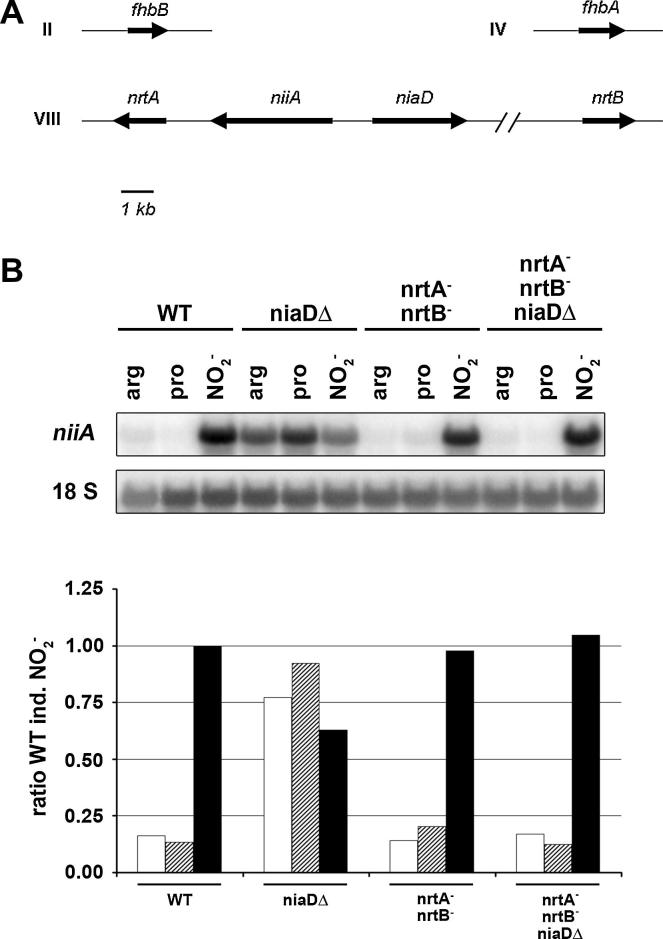
An overview of the genomic arrangement of the nitrate-induced genes tested in this work is presented in Fig. 1A. The small nitrate assimilation gene cluster comprises one of the two nitrate transporters (nrtA) and the two reductase genes niaD, coding for NR and niiA, coding for nitrite reductase (NiR). The second nitrate transporter gene (nrtB) is also located on chromosome VIII but is not linked to the cluster. The nitrate-induced flavohemoglobin gene fhbA as well as the constitutively expressed second flavohemoglobin gene fhbB (Schinko et al., 2010) are neither linked to the cluster. The promoter regions of nrtA, nrtB, fhbA and the bidirectional promoter separating the divergently transcribed niaD and niiA ORFs, all contain binding sites for the pathway-specific activator NirA. It is well established that mutant strains lacking nitrate reductase activity constitutively transcribe genes of the nitrate assimilation cluster when grown on non-inducing media supposedly free of inducer molecules (Cove and Pateman, 1969; Hawker et al., 1992). To test if in A. nidulans nitrate transporters and/or flavohemoglobins are required for the constitutive expression of nitrate-responsive genes on these non-inducing media, we tested strains carrying mutations in niaD singly or in combination with the two nitrate transporters nrtA and nrtB, and flavohemoglobins fhbA and fhbB. Fig. 1B shows that niiA is highly expressed on non-inducing (NI) media containing arginine or proline as sole nitrogen (N) sources only in the niaDΔ strain, but not in the wild type. We used as inducer to exclude indirect starvation effects that could appear when nitrate would be used in niaDΔ strains and to have a positive induction control also for the nitrate transporter double mutants, in which nitrite is still transported by the specific nitrite transporter NitA (Wang et al., 2008). The constitutive expression level in niaDΔ almost reached the nitrite-induced level in the wild type strain. As expected from a strain possessing a functional nitrite transporter, induced niiA levels in nrtA− nrtB− double mutants are identical to wild type but expression of niiA on a non-inducing N-source is lost in the triple mutant strain lacking niaD and both transporters. However, all strains normally respond to nitrite induction by up-regulation of niiA.
Fig. 1.
(A). Overview on chromosomal positions of nitrate-responsive genes. Chromosome numbers are indicated in Roman numerals and the scale bar represents 1 kb. The nitrate cluster is located on chromosome VIII and comprises nitrate reductase (niaD), nitrite reductase (niiA) and one nitrate/nitrite transporter (nrtA/crnA). The second nitrate/nitrite transporter (nrtB/crnB) is located on the same chromosome but separated from the nitrate cluster. Genes encoding the two flavohemoglobins fhbA and fhbB are located on chromosome IV and II, respectively. Arrows indicate direction of transcription. (B). Northern blot (upper panel) and densitometric analysis (lower panel) of niiA steady-state levels in A. nidulans wild type and mutant strains lacking nitrate-related functions. Strains deficient in either nitrate reductase (niaDΔ), both nitrate transporters (nrtA− and nrtB−) or all three genes were grown in liquid GMM for 14 h with either 3 mM l-arginine (white bars) or 5 mM l-proline (shaded bars) as sole nitrogen source. For induction cells (black bars) were grown on l-arginine and induced by addition of 10 mM for 15 min. 18S rRNA was used as loading control and reference for densitometric analysis. 32P signal intensities were recorded by phosphoimaging and expression levels were calculated relative to induced wild type transcript levels which were arbitrarily set to 1.
Our observations demonstrate that nitrate permeases are not required for the signal to activate NirA but they are required to confer a constitutive phenotype on NR loss-of-function strains. Similar to the situation in the methylotrophic yeast H. polymorpha (Navarro et al., 2003) and the algae C. reinhardtii (Fernandez and Galvan, 2008) it is thus likely that also in A. nidulans constitutive expression of nitrate-responsive genes in niaDΔ strains is related to the transport of trace amounts of contaminating nitrate from the medium into niaDΔ mutant cells in which nitrate subsequently accumulates. These traces of nitrate, however, apparently are too low to be detected by our methods directly in the growth media or in the concentrated stock solutions and certificates of chemical analysis of the supplier of media components (e.g. l-arginine and l-proline) do not specify nitrate as contamination. The apparent Km of NrtA and NrtB are roughly 100 μM and 10 μM (Unkles et al., 2001), respectively. Thus it is likely that in NR deficient strains nitrate levels accumulate which are sufficiently high for NirA activation.
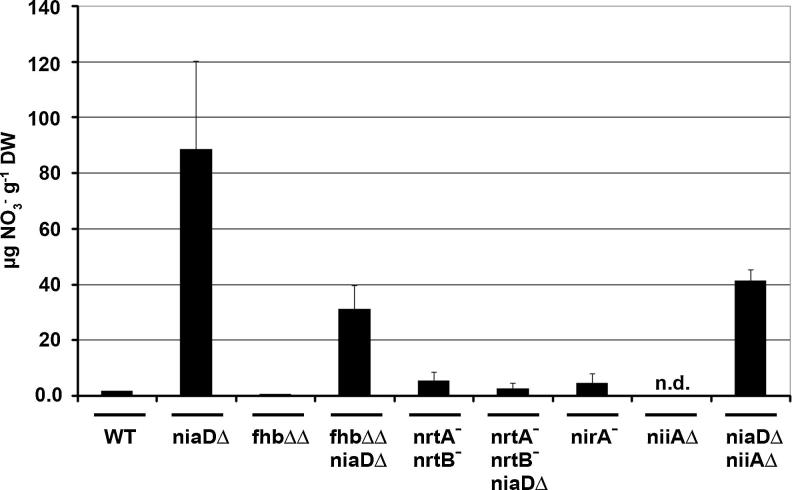
Consistent with this interpretation, we find significantly higher levels of in niaDΔ cells grown for 14 h on l-proline or l-arginine compared to wild type cells which readily metabolize the traces of entering these cells (Fig. 2). In contrast, cells lacking nitrate transporters nrtA− and nrtB− show very low nitrate levels regardless whether niaD is present or not. These measurements are in line with the loss of constitutivity in the triple nrtA− nrtB− niaDΔ mutant and thus loss of constitutivity can be best explained by the lack of sufficiently high intracellular inducer levels in transporter negative strains. Based on these observations we conclude that transporter activity is necessary for inducer accumulation in a NR negative strain and that this subsequently leads to induction of responsive genes. Thus, it is unlikely that in A. nidulans NR plays a regulatory role in its own synthesis, but the source for nitrate – external or internal – is not yet clear from these results (see below).
Fig. 2.
Determination of intracellular nitrate levels. Intracellular nitrate concentrations were determined in A. nidulans wild type and different mutants strains grown for 14 h with 3 mM l-arginine as sole nitrogen source. Nitrate levels are expressed as μg g−1 dry weight (DW). Mean values and standard deviations represent data from three biological repetitions. Strain designations are wild type (WT), nitrate reductase deletion (niaDΔ), nitrite reductase deletion (niiAΔ), nitrate transporter loss-of-function mutants (nrtA−nrtB−), flavohemoglobin A and B deletions (fhbΔΔ) and a nirA loss-of-function mutant (nirA−). n.d, not detected.
At the molecular level this phenotype has a different basis compared to “real” constitutive nirAc modification-of-function mutations (Cove and Pateman, 1969; Rand and Arst, 1978; Tollervey and Arst, 1981). NirAc1 is now well characterized and known to carry a glycine to valine (G167V) exchange in the nuclear export signal (NES) of the protein. This amino acid substitution abolishes the interaction of the NirA-nuclear export signal (NES) with the A. nidulans exportin KapK. Consequently, NirA permanently accumulates in the nucleus, binds to target promoters, interacts with AreA and eventually activates target genes also under non-inducing conditions (Bernreiter et al., 2007). Because gene expression under non-inducing conditions in NR mutants is triggered by the intracellular presence of inducer we term the expression of nitrate-responsive genes in NR null mutants on “neutral” media “pseudo-constitutive”.
It is surprising, however, that nitrate transporters seem to function in a niaDΔ background as Unkles and associates have shown that under their experimental conditions a niaD171 loss-of-function strain does not take up tracer from the medium and concluded that NR activity is required for functional transport (Unkles et al., 2004). The main difference in the experimental setup was pre-loading of cells with nitrate in the case of tracer studies whereas in the experiments reported here arginine or proline were used as sole nitrogen sources. We speculate that nitrate pre-loading of niaD loss of function strains would result in very high intracellular nitrate concentrations since intracellular nitrate is not metabolized. These high levels of in the cell might block uptake of additional nitrate from the medium. In any case, data presented here formally demonstrate that nitrate reductase activity is not required for transporter function under physiological conditions.
3.2. Flavohemoglobins are not required for constitutive expression of nitrate-responsive genes
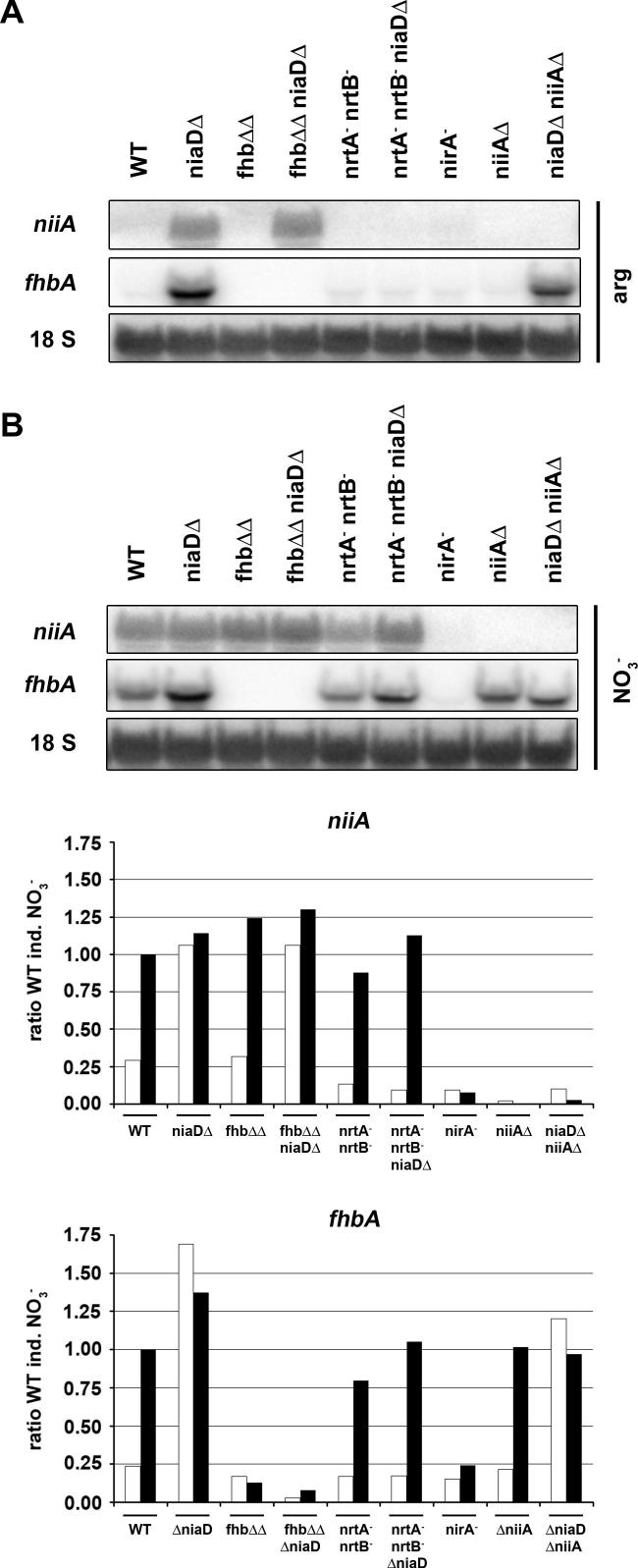
Our own experimental results as well as those obtained in H. polymorpha and C. reinhardtii NR mutants do not exclude an intracellular source of nitrate which is secreted and re-imported by transporters. We tested this hypothesis in A. nidulans by measuring nitrate-responsive gene expression in a triple mutant background lacking niaD and both flavohemoglobin genes fhbA and fhbB. We reasoned that in this strain the intracellular generation of at least from NO is impaired and thus pseudo-constitutivity in a niaDΔ background should be lost or reduced. Fig. 3A shows that this is not the case because in the niaDΔ strain lacking flavohemoglobin function (fhbΔΔ niaDΔ) expression of niiA on non-inducing arginine is equally strong as in the niaDΔ single mutant control strain. These results demonstrate that flavohemoglobins are not required for pseudo-constitutivity and reinforce the view that nitrate from external sources accumulates in NR-deficient cells and subsequently induces the assimilatory genes.
Fig. 3.
Northern blot and densitometric analysis of niiA and fhbA steady-state levels in A.nidulans wild type and mutant strains lacking nitrate-related functions (for strain designation see Legend to Fig. 2) grown on arginine (A) or grown on arginine and subsequently induced by nitrate (B). (A). Northern blot probed for niiA and fhbA expression in strains grown on 5 mM arginine as sole N source for 14 h. (B). Northern blot (upper panel) probed for niiA and fhbA expression in strains grown on 5 mM arginine as sole N source for 14 h and induced for 15 min by addition of 10 mM nitrate. Graphs (lower panels) summarize relative expression levels of niiA and fhbA in the different strains when arginine (white bars) and arginine + nitrate (black bars) conditions are compared. 18S rRNA in each case was used as loading control and as reference for densitometric analysis. Values were calculated relative to the induced wild type level which was arbitrarily set to 1.
Despite this clear evidence it is still puzzling why pseudo-constitutivity is not observed in niiAΔ strains grown on non-inducing media. In such strains inducing nitrite should accumulate due to the inability of the cells to metabolize nitrite (derived from nitrate by NR or from media contaminated by ). However, niiAΔ strains behave like wild type, as observed before (Cove, 1979; Hawker et al., 1992) and seen also here for the nitrate and nitrite-responsive fhbA gene (Fig. 3A). In fact, we have observed equal levels of intracellular nitrite in all strains including niiAΔ strains on neutral media (data not shown). We neither found higher levels of intracellular nitrate in niiAΔ cells (Fig. 2). Nitrate, theoretically, could be formed by flavohemoglobins that would convert NO derived from spontaneous decomposition under acidic conditions (e.g. if excess nitrite would be transported into the vacuole). The reason why nitrate but not nitrite accumulates in cells is not known at the moment. One possibility might be nitrite secretion from niiAΔ cells into the medium. But since the amount of secreted into the medium was too low to be detected by our experimental system, this possibility was difficult to verify. Another plausible explanation for lack of intracellular in niiAΔ cells is its conversion to another, non-inducing metabolite. Recently, functional dissimilatory copper-containing nitrite reductases have been identified in a number of fungi, including Aspergillus oryzae (Kim et al., 2009, 2010; Nakanishi et al., 2010). Although protein blast did not reveal putative orthologues of dissimilatory nitrite reductases in A. nidulans it cannot be excluded that proteins with more distant relation function in the same way. Although the genes encoding nitrite dissimilation activities have been shown to be strongly induced only under anaerobic conditions, a basal level of activity could be sufficient to remove the small amount of nitrite produced by nitrate reductase in niiAΔ strains growing on media containing trace amounts of nitrate (or nitrite). The difference between niaDΔ and niiAΔ strains in respect to pseudo-constitutivity might thus be explained by the presence of alternative nitrite reduction but the lack of alternative nitrate reduction systems.
3.3. Nitrate transporters and nitrate reductase are dispensable for nitrate signaling and NirA activation
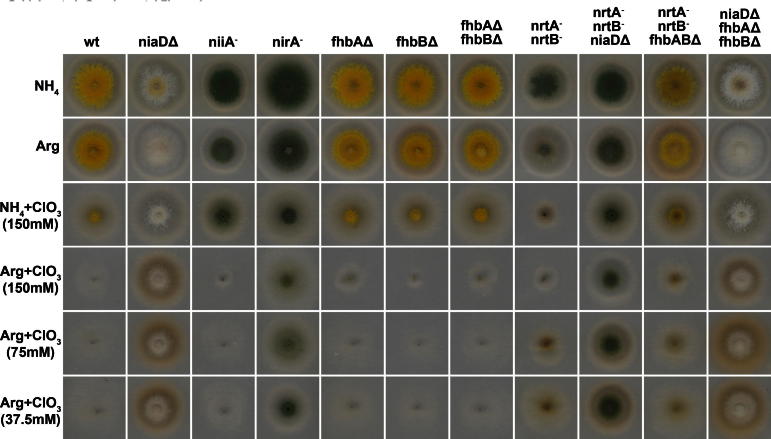
The fact that pseudo-constitutivity is lost in the nrtA− nrtB− niaDΔ triple mutant could suggest that one or both nitrate transporters are involved in the signaling process for NirA-dependent transcriptional activation. A. thaliana contains 67 predicted nitrate transporters (De Angeli et al., 2009), among which CHL1 has been shown to display phosphorylation-dependent dual nitrate affinities and additionally carries out nitrate sensing function (Ho et al., 2009). We therefore tested our strains for transcriptional response to nitrate and nitrite induction and found that niiA and fhbA are both induced in strains carrying mutations in both nitrate transporters (Figs. 1B and 3B). Unkles and colleagues have shown that no other nitrate transporters exist in A. nidulans and it is thus surprising to observe an almost full transcriptional response (i.e. 80% of wild type) after 15 min of induction with 10 mM nitrate in nrtA− nrtB− strains. These results on the one hand demonstrate that the nitrate permeases do not participate in signaling towards NirA activation. On the other hand this study and previous results from our lab (Berger et al., 2008) show that intracellular nitrate is required for the NirA-dependent activation process and thus nitrate must enter nrtA−nrtB− cells to some extent. This amount of nitrate must be sufficient to activate NirA but insufficient to serve as nitrogen source for growth because transporter double mutants do not grow on nitrate (10 mM) as sole N-source in plate assays (Schinko et al., 2010; Unkles et al., 2001). The mechanism of unspecific nitrate uptake is unknown but is further supported by growth tests which monitor sensitivity to the toxic analogue chlorate () (Suppl. Fig. 1). Chlorate is the precursor taken up and subsequently reduced to the toxic metabolite chlorite by nitrate reductase activity and thus mutants lacking NR activity (niaDΔ or nirA−) are resistant to . This also explains why ammonium in the growth medium (Suppl. Fig. 1, row ) protects against chlorate toxicity because nitrogen metabolite repression shuts down expression of nitrate transporters and niaD (Arst and Cove, 1973; Cove, 1976). In contrast to niaD or nirA mutants strains lacking nrtA and nrtB function are only moderately chlorate resistant, and high concentrations lead to strong toxicity whereas lower chlorate concentrations support residual growth of the mutant strains. This suggests that also chlorate, similar to nitrate, is taken up unspecifically when present at high concentrations. Collectively, our results suggest that the very low concentration of nitrate present in the NI medium is taken up by the highly effective specific transporters NrtA and NrtB, but unspecific alternative nitrate channels are ineffective for such low external nitrate concentrations thus leading to loss of pseudo-constitutivity of niaDΔ strains lacking transporter function.
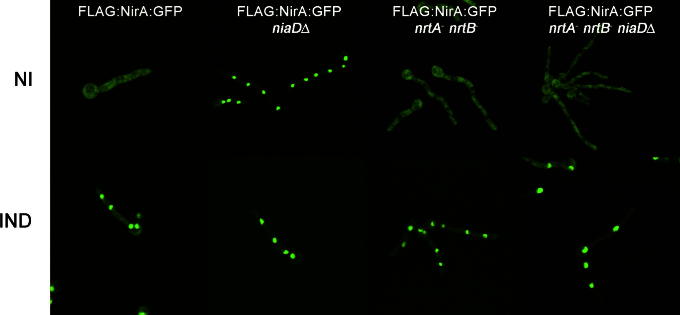
To additionally clarify the relationship between nitrate permeases and the activity status of NirA, we crossed the transporter double mutant to a strain expressing a NirA-GFP fusion which functionally complements a nirA loss-of-function mutation (Berger et al., 2006). Panel 3 in Fig. 4 shows that in the presence of 10 mM NirA-GFP accumulates in the nucleus in a nrtA−nrtB− background, identically to what is observed in wild type cells (first panel). Thus, small amounts of nitrate must be able to enter transporter deficient cells via an alternative route. This putative alternative nitrate channel(s), however, can only be effective when high external nitrate concentrations are present because at low nitrate concentrations, like those leading to pseudo-constitutivity in niaDΔ strains, they do not function (loss of pseudo-constitutivity in nrtA−nrtB− niaDΔ triple mutants, compare Fig. 1B). This is further substantiated by the finding that NirA-GFP does not accumulate in the nucleus on the neutral nitrogen source arginine (NI) in the nrtA−nrtB− niaDΔ triple mutant (Fig. 4, lane NI, last panel).
Fig. 4.
Localization of the functional NirA-GFP fusion protein. Wild type and mutant strains expressing an N-terminally FLAG-tagged NirA-GFP fusion protein from the constitutive gpdAp promoter were grown at 25 °C for 16 h on 3 mM arginine (NI) or 3 mM arginine plus 10 mM nitrate (IND). In the case of nitrate induction, pictures were captured on average 2 min after the addition of inducer.
Using this experimental approach we were also able to finally show that nitrate reductase does not act as a co-repressor of NirA in the absence of nitrate, as suggested by the autoregulation model put forward by Cove and coworkers. NR is a cytosolic protein (Takasaki et al., 2004) and if it would scavenge NirA preventing nuclear accumulation, then NirA-GFP should be nuclear in all niaDΔ strains. However, this is not the case and NirA-GFP only accumulates in the nucleus under non-inducing conditions when the nitrate transporters are active. It thus is highly unlikely that NR inactivates NirA by physical interaction in the absence of nitrate and that the inducer acts to compete for the NirA-NR interaction surface thereby releasing NirA from NR inhibition. Instead, NirA seems to require only small amounts of intracellular inducer – not sufficient to promote hyphal growth – which eventually leads to disruption of the NirA–KapK interaction and subsequent NirA nuclear accumulation, specific DNA binding, interaction with AreA and finally promoting transcriptional activity of nitrate responsive genes.
Acknowledgments
The authors thank Sheila Unkles for nitrate transporter mutant strains and David Canovas and Claudio Scazzocchio for fruitful discussions. The work was supported by Austrian Science Fund (FWF) Project P20630 to JS.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.fgb.2013.02.003.
Appendix A. Supplementary material
Supplementary Figure 1.
References
- Arst H.N., Jr., Cove D.J. Nitrogen metabolite repression in Aspergillus nidulans. Mol. Gen. Genet. 1973;126:111–141. doi: 10.1007/BF00330988. [DOI] [PubMed] [Google Scholar]
- Arst H.N., Jr. A near terminal pericentric inversion leads to nitrogen metabolite derepression in Aspergillus nidulans. Mol. Gen. Genet. 1982;188:490–493. doi: 10.1007/BF00330054. [DOI] [PubMed] [Google Scholar]
- Berger H., Pachlinger R., Morozov I., Goller S., Narendja F., Caddick M., Strauss J. The GATA factor AreA regulates localization and in vivo binding site occupancy of the nitrate activator NirA. Mol. Microbiol. 2006;59:433–446. doi: 10.1111/j.1365-2958.2005.04957.x. [DOI] [PubMed] [Google Scholar]
- Berger H., Basheer A., Bock S., Reyes-Dominguez Y., Dalik T., Altmann F., Strauss J. Dissecting individual steps of nitrogen transcription factor cooperation in the Aspergillus nidulans nitrate cluster. Mol. Microbiol. 2008;69:1385–1398. doi: 10.1111/j.1365-2958.2008.06359.x. [DOI] [PubMed] [Google Scholar]
- Bernreiter A., Ramon A., Fernandez-Martinez J., Berger H., Araujo-Bazan L., Espeso E.A., Pachlinger R., Gallmetzer A., Anderl I., Scazzocchio C., Strauss J. Nuclear export of the transcription factor NirA is a regulatory checkpoint for nitrate induction in Aspergillus nidulans. Mol. Cell. Biol. 2007;27:791–802. doi: 10.1128/MCB.00761-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson-Bard A., Pugin A., Wendehenne D. New insights into nitric oxide signaling in plants. Annu. Rev. Plant Biol. 2008;59:21–39. doi: 10.1146/annurev.arplant.59.032607.092830. [DOI] [PubMed] [Google Scholar]
- Brownlee A.G., Arst H.N., Jr. Nitrate uptake in Aspergillus nidulans and involvement of the third gene of the nitrate assimilation gene cluster. J. Bacteriol. 1983;155:1138–1146. doi: 10.1128/jb.155.3.1138-1146.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger G., Strauss J., Scazzocchio C., Lang B.F. NirA, the pathway-specific regulatory gene of nitrate assimilation in Aspergillus nidulans, encodes a putative GAL4-type zinc finger protein and contains four introns in highly conserved regions. Mol. Cell. Biol. 1991;11:5746–5755. doi: 10.1128/mcb.11.11.5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger G., Tilburn J., Scazzocchio C. Molecular cloning and functional characterization of the pathway – specific regulatory gene nirA, which controls nitrate assimilation in Aspergillus nidulans. Mol. Cell. Biol. 1991;11:795–802. doi: 10.1128/mcb.11.2.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caddick M.X., Arst H.N., Jr., Taylor L.H., Johnson R.I., Brownlee A.G. Cloning of the regulatory gene areA mediating nitrogen metabolite repression in Aspergillus nidulans. EMBO J. 1986;5:1087–1090. doi: 10.1002/j.1460-2075.1986.tb04326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cove D.J., Pateman J.A. Independently segregating genetic loci concerned with nitrate reductase activity in Aspergillus nidulans. Nature. 1963;198:262–263. doi: 10.1038/198262a0. [DOI] [PubMed] [Google Scholar]
- Cove D.J., Coddington A. Purification of nitrate reductase and cytochrome c reductase from Aspergillus nidulans. Biochim. Biophys. Acta. 1965;110:312–318. doi: 10.1016/s0926-6593(65)80038-8. [DOI] [PubMed] [Google Scholar]
- Cove D.J. The induction and repression of nitrate reductase in the fungus Aspergillus nidulans. Biochim. Biophys. Acta. 1966;113:51–56. doi: 10.1016/s0926-6593(66)80120-0. [DOI] [PubMed] [Google Scholar]
- Cove D.J. Evidence for a near limiting intracellular concentration of a regulator substance. Nature. 1969;224:272–273. doi: 10.1038/224272b0. [DOI] [PubMed] [Google Scholar]
- Cove D.J., Pateman J.A. Autoregulation of the synthesis of nitrate reductase in Aspergillus nidulans. J. Bacteriol. 1969;97:1374–1378. doi: 10.1128/jb.97.3.1374-1378.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cove D.J. Chlorate toxicity in Aspergillus nidulans. Studies of mutants altered in nitrate assimilation. Mol. Gen. Genet. 1976;146:147–159. doi: 10.1007/BF00268083. [DOI] [PubMed] [Google Scholar]
- Cove D.J. Genetic studies of nitrate assimilation in Aspergillus nidulans. Biol. Rev. Camb. Philos. Soc. 1979;54:291–327. doi: 10.1111/j.1469-185x.1979.tb01014.x. [DOI] [PubMed] [Google Scholar]
- De Angeli A., Monachello D., Ephritikhine G., Frachisse J.M., Thomine S., Gambale F., Barbier-Brygoo H. Review. CLC-mediated anion transport in plant cells. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009;364:195–201. doi: 10.1098/rstb.2008.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Montaigu A., Sanz-Luque E., Galvan A., Fernandez E. A soluble guanylate cyclase mediates negative signaling by ammonium on expression of nitrate reductase in Chlamydomonas. Plant Cell. 2010;22:1532–1548. doi: 10.1105/tpc.108.062380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez E., Galvan A. Nitrate assimilation in Chlamydomonas. Eukaryot. Cell. 2008;7:555–559. doi: 10.1128/EC.00431-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner P.R., Gardner A.M., Martin L.A., Salzman A.L. Nitric oxide dioxygenase: an enzymic function for flavohemoglobin. Proc. Natl. Acad. Sci. USA. 1998;95:10378–10383. doi: 10.1073/pnas.95.18.10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorfer M., Blumhoff M., Klaubauf S., Urban A., Inselsbacher E., Bandian D., Mitter B., Sessitsch A., Wanek W., Strauss J. Community profiling and gene expression of fungal assimilatory nitrate reductases in agricultural soil. ISME J. 2011;5:1771–1783. doi: 10.1038/ismej.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F.Q., Okamoto M., Crawford N.M. Identification of a plant nitric oxide synthase gene involved in hormonal signaling. Science. 2003;302:100–103. doi: 10.1126/science.1086770. [DOI] [PubMed] [Google Scholar]
- Hankinson O., Cove D.J. Regulation of the pentose phosphate pathway in the fungus Aspergillus nidulans. The effect of growth with nitrate. J. Biol. Chem. 1974;249:2344–2353. [PubMed] [Google Scholar]
- Hawker K.L., Montague P., Kinghorn J.R. Nitrate reductase and nitrite reductase transcript levels in various mutants of Aspergillus nidulans: confirmation of autogenous regulation. Mol. Gen. Genet. 1992;231:485–488. doi: 10.1007/BF00292720. [DOI] [PubMed] [Google Scholar]
- Ho C.H., Lin S.H., Hu H.C., Tsay Y.F. CHL1 functions as a nitrate sensor in plants. Cell. 2009;138:1184–1194. doi: 10.1016/j.cell.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Kim S.W., Fushinobu S., Zhou S., Wakagi T., Shoun H. Eukaryotic nirK genes encoding copper-containing nitrite reductase: originating from the protomitochondrion? Appl. Environ. Microbiol. 2009;75:2652–2658. doi: 10.1128/AEM.02536-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.W., Fushinobu S., Zhou S., Wakagi T., Shoun H. The possible involvement of copper-containing nitrite reductase (NirK) and flavohemoglobin in denitrification by the fungus Cylindrocarpon tonkinense. Biosci. Biotechnol. Biochem. 2010;74:1403–1407. doi: 10.1271/bbb.100071. [DOI] [PubMed] [Google Scholar]
- Kinsky S.C., McElroy W.D. Neurospora nitrate reductase: the role of phosphate flavine and cytochrome c reductase. Arch. Biochem. Biophys. 1958;73:466–483. doi: 10.1016/0003-9861(58)90290-x. [DOI] [PubMed] [Google Scholar]
- Kinsky S.C. Induction and repression of nitrate reductase in Neurospora crassa. J. Bacteriol. 1961;82:898–904. doi: 10.1128/jb.82.6.898-904.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudla B., Caddick M.X., Langdon T., Martinez-Rossi N.M., Bennett C.F., Sibley S., Davies R.W., Arst H.N., Jr. The regulatory gene areA mediating nitrogen metabolite repression in Aspergillus nidulans. Mutations affecting specificity of gene activation alter a loop residue of a putative zinc finger. EMBO J. 1990;9:1355–1364. doi: 10.1002/j.1460-2075.1990.tb08250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llamas A., Igeno M.I., Galvan A., Fernandez E. Nitrate signalling on the nitrate reductase gene promoter depends directly on the activity of the nitrate transport systems in Chlamydomonas. Plant J. 2002;30:261–271. doi: 10.1046/j.1365-313x.2002.01281.x. [DOI] [PubMed] [Google Scholar]
- Lockington R.A., Sealy-Lewis H.M., Scazzocchio C., Davies R.W. Cloning and characterization of the ethanol utilization regulon in Aspergillus nidulans. Gene. 1985;33(2):137–149. doi: 10.1016/0378-1119(85)90088-5. [DOI] [PubMed] [Google Scholar]
- MacDonald D.W., Cove D.J. Studies on temperature-sensitive mutants affecting the assimilatory nitrate reductase of Aspergillus nidulans. Eur. J. Biochem. 1974;47:107–110. doi: 10.1111/j.1432-1033.1974.tb03673.x. [DOI] [PubMed] [Google Scholar]
- Modolo L.V., Augusto O., Almeida I.M., Magalhaes J.R., Salgado I. Nitrite as the major source of nitric oxide production by Arabidopsis thaliana in response to Pseudomonas syringae. FEBS Lett. 2005;579:3814–3820. doi: 10.1016/j.febslet.2005.05.078. [DOI] [PubMed] [Google Scholar]
- Muro-Pastor M.I., Gonzalez R., Strauss J., Narendja F., Scazzocchio C. The GATA factor AreA is essential for chromatin remodelling in a eukaryotic bidirectional promoter. EMBO J. 1999;18:1584–1597. doi: 10.1093/emboj/18.6.1584. [published erratum appears in EMBO J 1999 May 4;18(9):2670] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muro-Pastor M.I., Strauss J., Ramon A., Scazzocchio C. A paradoxical mutant GATA factor. Eukaryot. Cell. 2004;3:393–405. doi: 10.1128/EC.3.2.393-405.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase S., Takemura K., Ueda A., Hirayama A., Aoyagi K., Kondoh M., Koyama A. A novel nonenzymatic pathway for the generation of nitric oxide by the reaction of hydrogen peroxide and d- or l-arginine. Biochem. Biophys. Res. Commun. 1997;233:150–153. doi: 10.1006/bbrc.1997.6428. [DOI] [PubMed] [Google Scholar]
- Nakanishi Y., Zhou S., Kim S.W., Fushinobu S., Maruyama J., Kitamoto K., Wakagi T., Shoun H. A eukaryotic copper-containing nitrite reductase derived from a NirK homolog gene of Aspergillus oryzae. Biosci. Biotechnol. Biochem. 2010;74:984–991. doi: 10.1271/bbb.90844. [DOI] [PubMed] [Google Scholar]
- Narendja F., Goller S.P., Wolschek M., Strauss J. Nitrate and the GATA factor AreA are necessary for in vivo binding of NirA, the pathway-specific transcriptional activator of Aspergillus nidulans. Mol. Microbiol. 2002;44:573–583. doi: 10.1046/j.1365-2958.2002.02911.x. [DOI] [PubMed] [Google Scholar]
- Navarro F.J., Perdomo G., Tejera P., Medina B., Machin F., Guillen R.M., Lancha A., Siverio J.M. The role of nitrate reductase in the regulation of the nitrate assimilation pathway in the yeast Hansenula polymorpha. FEMS Yeast Res. 2003;4:149–155. doi: 10.1016/S1567-1356(03)00163-6. [DOI] [PubMed] [Google Scholar]
- Pateman J.A., Cove D.J., Rever B.M., Roberts D.B. A common co-factor for nitrate reductase and xanthine dehydrogenase which also regulates the synthesis of nitrate reductase. Nature. 1964;201:58–60. doi: 10.1038/201058a0. [DOI] [PubMed] [Google Scholar]
- Pateman J.A., Rever B.M., Cove D.J. Genetic and biochemical studies of nitrate reduction in Aspergillus nidulans. Biochem J. 1967;104:103–111. doi: 10.1042/bj1040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R.K., Hughes M.N. New functions for the ancient globin family: bacterial responses to nitric oxide and nitrosative stress. Mol. Microbiol. 2000;36:775–783. doi: 10.1046/j.1365-2958.2000.01889.x. [DOI] [PubMed] [Google Scholar]
- Rand K.N., Arst H.N., Jr. Mutations in nirA gene of Aspergillus nidulans and nitrogen metabolism. Nature. 1978;272:732–734. doi: 10.1038/272732a0. [DOI] [PubMed] [Google Scholar]
- Rockel P., Strube F., Rockel A., Wildt J., Kaiser W.M. Regulation of nitric oxide (NO) production by plant nitrate reductase in vivo and in vitro. J. Exp. Bot. 2002;53:103–110. [PubMed] [Google Scholar]
- Sambrook J., Russel D. Cold Spring Harbor Laboratory Press; New York: 2001. Molecular Cloning: A Laboratory Manual third ed. [Google Scholar]
- Schinko T., Berger H., Lee W., Gallmetzer A., Pirker K., Pachlinger R., Buchner I., Reichenauer T., Guldener U., Strauss J. Transcriptome analysis of nitrate assimilation in Aspergillus nidulans reveals connections to nitric oxide metabolism. Mol. Microbiol. 2010;78:720–738. doi: 10.1111/j.1365-2958.2010.07363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starich M.R., Wikstrom M., Arst H.N., Jr., Clore G.M., Gronenborn A.M. The solution structure of a fungal AREA protein-DNA complex: an alternative binding mode for the basic carboxyl tail of GATA factors. J. Mol. Biol. 1998;277:605–620. doi: 10.1006/jmbi.1998.1625. [DOI] [PubMed] [Google Scholar]
- Strauss J., Muro-Pastor M.I., Scazzocchio C. The regulator of nitrate assimilation in ascomycetes is a dimer which binds a nonrepeated, asymmetrical sequence. Mol. Cell. Biol. 1998;18:1339–1348. doi: 10.1128/mcb.18.3.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szewczyk E., Nayak T., Oakley C.E., Edgerton H., Xiong Y., Taheri-Talesh N., Osmani S.A., Oakley B.R. Fusion PCR and gene targeting in Aspergillus nidulans. Nat. Protoc. 2006;1:3111–3120. doi: 10.1038/nprot.2006.405. [DOI] [PubMed] [Google Scholar]
- Takasaki K., Shoun H., Yamaguchi M., Takeo K., Nakamura A., Hoshino T., Takaya N. Fungal ammonia fermentation, a novel metabolic mechanism that couples the dissimilatory and assimilatory pathways of both nitrate and ethanol. Role of acetyl CoA synthetase in anaerobic ATP synthesis. J. Biol. Chem. 2004;279:12414–12420. doi: 10.1074/jbc.M313761200. [DOI] [PubMed] [Google Scholar]
- Tollervey D.W., Arst H.N.J. Mutations to constitutivity and derepression are separate and separable in a regulatory gene of Aspergillus nidulans. Curr. Genet. 1981;4:63–68. doi: 10.1007/BF00376787. [DOI] [PubMed] [Google Scholar]
- Unkles S.E., Hawker K.L., Grieve C., Campbell E.I., Montague P., Kinghorn J.R. CrnA encodes a nitrate transporter in Aspergillus nidulans. Proc Natl Acad Sci USA. 1991;88:204–208. doi: 10.1073/pnas.88.1.204. [published errata appear in Proc. Natl. Acad. Sci. USA 1991 May 15;88(10):4564 and 1995 March 28;92(7):3076] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkles S.E., Zhou D., Siddiqi M.Y., Kinghorn J.R., Glass A.D. Apparent genetic redundancy facilitates ecological plasticity for nitrate transport. EMBO J. 2001;20:6246–6255. doi: 10.1093/emboj/20.22.6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkles S.E., Wang R., Wang Y., Glass A.D., Crawford N.M., Kinghorn J.R. Nitrate reductase activity is required for nitrate uptake into fungal but not plant cells. J. Biol. Chem. 2004;279:28182–28186. doi: 10.1074/jbc.M403974200. [DOI] [PubMed] [Google Scholar]
- Unkles S.E., Symington V.F., Kotur Z., Wang Y., Siddiqi M.Y., Kinghorn J.R., Glass A.D. Physiological and biochemical characterization of AnNitA, the Aspergillus nidulans high-affinity nitrite transporter. Eukaryot. Cell. 2011;10:1724–1732. doi: 10.1128/EC.05199-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt K., Bhabhra R., Rhodes J.C., Askew D.S. Doxycycline-regulated gene expression in the opportunistic fungal pathogen Aspergillus fumigatus. BMC Microbiol. 2005;5:1. doi: 10.1186/1471-2180-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Li W., Siddiqi Y., Symington V.F., Kinghorn J.R., Unkles S.E., Glass A.D. Nitrite transport is mediated by the nitrite-specific high-affinity NitA transporter and by nitrate transporters NrtA, NrtB in Aspergillus nidulans. Fungal Genet. Biol. 2008;45:94–102. doi: 10.1016/j.fgb.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Wendehenne D., Pugin A., Klessig D.F., Durner J. Nitric oxide: comparative synthesis and signaling in animal and plant cells. Trends Plant Sci. 2001;6:177–183. doi: 10.1016/s1360-1385(01)01893-3. [DOI] [PubMed] [Google Scholar]
- Yamasaki H. Nitrite-dependent nitric oxide production pathway: implications for involvement of active nitrogen species in photoinhibition in vivo. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000;355:1477–1488. doi: 10.1098/rstb.2000.0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H., Sakihama Y. Simultaneous production of nitric oxide and peroxynitrite by plant nitrate reductase: in vitro evidence for the NR-dependent formation of active nitrogen species. FEBS Lett. 2000;468:89–92. doi: 10.1016/s0014-5793(00)01203-5. [DOI] [PubMed] [Google Scholar]
- Zweier J.L., Samouilov A., Kuppusamy P. Non-enzymatic nitric oxide synthesis in biological systems. Biochim. Biophys. Acta. 1999;1411:250–262. doi: 10.1016/s0005-2728(99)00018-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.