Abstract
Isl1 is a LIM homeobox transcription factor showing conserved expression in the developing and mature vertebrate pancreas. So far, functions of pancreatic Isl1 have mainly been studied in the mouse, where Isl1 has independent functions during formation of exocrine and endocrine tissues. Here, we take advantage of a recently described isl1 mutation in zebrafish to address pancreatic isl1 functions in a non-mammalian system. Isl1 in zebrafish, as in mouse, shows transient expression in mesenchyme flanking the pancreatic endoderm, and continuous expression in all endocrine cells. In isl1 mutants, endocrine cells are specified in normal numbers but more than half of these cells fail to establish expression of endocrine hormones. By using a lineage tracking approach that highlights cells leaving cell cycle early in development, we show that isl1 functions are different in first and second wave endocrine cells. In isl1 mutants, early forming first wave cells show virtually no glucagon expression and a reduced number of cells expressing insulin and somatostatin, while in the later born second wave cells somatostatin expressing cells are strongly reduced and insulin and glucagon positive cells form in normal numbers. Isl1 mutant zebrafish also display a smaller exocrine pancreas. We find that isl1 expression in the pancreatic mesenchyme overlaps with that of the related genes isl2a and isl2b and that pancreatic expression of isl-genes is independent of each other. As a combined block of two or three isl1/2 genes results in a dose-dependent reduction of exocrine tissue, our data suggest that all three genes cooperatively contribute to non-cell autonomous exocrine pancreas extension. The normal expression of the pancreas mesenchyme markers meis3, fgf10 and fgf24 in isl1/2 depleted embryos suggests that this activity is independent of isl-gene function in pancreatic mesenchyme formation as was found in mouse. This indicates species-specific differences in the requirement for isl-genes in pancreatic mesenchyme formation. Overall, our data reveal a novel interaction of isl1 and isl2 genes in exocrine pancreas expansion and cell type specific requirements during endocrine cell maturation.
Keywords: Islet1, Islet2, Lim homeodomain, Pancreas, Exocrine, Endocrine, Insulin, Glucagon, Zebrafish
Highlights
• Overlapping functions of islet1, islet2a and islet2b in exocrine pancreas formation.
• Islet1/2a/2b are not required for pancreatic mesenchyme formation.
• Islet1 but not islet2a/b is required for endocrine cell maturation.
• Endocrine cell types are differently affected by the loss of islet1.
Introduction
The pancreas is a vertebrate-specific endodermal organ with essential functions in food digestion and glucose homeostasis. The mature organ is composed of an exocrine compartment with acinar and duct cells that produce and transport digestive enzymes into the gut, and an endocrine compartment from which metabolism-regulating peptide hormones are secreted into the blood stream. In higher vertebrates, endocrine cells are arranged in small clusters termed islets (islet of Langerhans in mammals) that contain up to five cells types each expressing a specific hormone: α-, β-, δ-, ε- and PP cells expressing glucagon (gcg), insulin (ins), somatostatin (sst), ghrelin and pancreatic polypeptide (pp), respectively. Formation of the pancreas can be formally divided into two phases termed primary and secondary transition. In amniotes, primary transition refers to the outgrowth of epithelial buds of dorsal and ventral foregut endoderm into the surrounding pancreatic mesenchyme demarcating the onset of pancreas morphogenesis. During this process, a population of postmitotic endocrine cells of unknown function, also termed ‘first wave’ cells, is established. Secondary transition defines the subsequent massive proliferation and expansion of the pancreatic buds and the accompanying differentiation of exocrine and ‘second wave’ endocrine cells from which the organ is finally formed (Pan and Wright, 2011).
Genetic studies have been extremely successful in defining the importance of specific signaling pathways and transcription factors in regulating pancreas development. While the mouse has been the major model for such studies, more recently the zebrafish had gained attention as a model that is readily amenable to genetic and in vivo imaging approaches (Field et al., 2003; Kimmel and Meyer, 2010; Kinkel and Prince, 2009; Tiso et al., 2009). Importantly, pancreas in mouse and fish has a conserved physiological function, a very similar cellular architecture, and conserved expression and function of most developmental genes (Eames et al., 2010; Jurczyk et al., 2011; Yee et al., 2005). Despite these similarities, studies in fish also revealed some evolutionary differences between mammalian and fish pancreas development.
Pancreas development in zebrafish is morphologically visible at 24 h post fertilization (24 hpf) by the formation of an endocrine cluster of 50–60 cells that are located dorsal to the gut endoderm (Biemar et al., 2001; Kimmel and Meyer, 2010). This early islet separates from the endoderm and by 48 hpf becomes encircled by a second endodermal protrusion. Based on their position and by analogy to the buds formed during primary transition in mouse, the early islet and the later forming protrusion in zebrafish have been termed dorsal and ventral bud, respectively (Field et al., 2003). However, the fates of these buds are different in mouse and fish. In mouse, the dorsal and ventral bud both contributes to first and second wave endocrine, as well as to exocrine cells (Pan and Wright, 2011). By contrast, zebrafish dorsal bud consists exclusively of first wave endocrine cells (dorsal bud derived cells, DBCs), while the ventral bud gives rise to second wave endocrine (hereafter termed ventral bud derived cells, VBCs), exocrine and duct cells, and thus to all cells forming the mature pancreas (Field et al., 2003; Hesselson et al., 2009; Wang et al., 2011). Further, first wave cells in mouse mainly express Gcg, while in fish, at least 4 early endocrine cell types, expressing Gcg (α-cells), Ins (β-cells), Sst (δ-cells) and Ghrelin (ε-cells) can be distinguished (Hesselson et al., 2009; Pan and Wright, 2011; Tiso et al., 2009).
Correlating with these differences, the molecular control underlying formation of the two pancreatic buds appears slightly different in mouse and fish. Differences were found for example for the role of the homeobox transcription factors Pdx1 and Mnx1/Hb9, that both show a conserved expression in pre-pancreatic endoderm and later in differentiating β-cells. In mouse knock-out studies the two phases of expression were shown to be required for early pancreas morphogenesis and for β-cell maturation (Harrison et al., 1999; Larsson et al., 1996; Li and Edlund, 2001; Offield et al., 1996; Pan and Wright, 2011). By contrast, morpholino-based (MO) knockdown studies of these genes in fish revealed no major defects in early pancreas morphology but did provide evidence for a conserved function in specifying second wave endocrine cells (pdx1) and β-cell maturation (hb9) (Kimmel et al., 2011). These data revealed differences mainly in the regulation of early pancreas morphogenesis and in first wave endocrine specification in fish and mouse. Formation of second wave endocrine cells appears to be more similar in fish and mouse, even though not that much is known about these processes in zebrafish.
In this study, we focus on the LIM-Homeodomain (LIM-HD) transcription factor isl1 (Insulin gene enhancer protein 1) which in mouse has been shown to be required for early pancreas morphogenesis and endocrine differentiation (Ahlgren et al., 1997; Du et al., 2009; Liu et al., 2011, 2012; May, 2010). While initially identified as a direct regulator of insulin expression in RIN 14B endocrine cells (Karlsson et al., 1990), isl1 in zebrafish shows evolutionary conserved expression in pancreatic mesenchyme during embryogenesis and in endocrine cells of the embryonic and adult pancreas, including first wave endocrine cells (Manfroid et al., 2007). Mesenchymal isl1 expression is limited to the initial stages of pancreas development. In mouse, this expression is restricted to the cells flanking the dorsal pancreatic endoderm (Ahlgren et al., 1997), while in fish expression is found in the vicinity of the late forming ventral bud. Knock-out analyses in the mouse revealed that the distinct expression domains in mesenchyme and endoderm are independently required to induce formation of the dorsal pancreatic bud and pancreatic mesenchyme and to initiate endocrine hormone expression, respectively. Due to the lethality of the Isl1 mutant shortly after onset of pancreas morphogenesis, analyses of later pancreatic fates could only be done in organ culture. It was found that wild type but not Isl1 mutant pancreatic mesenchyme is able to induce differentiation of exocrine tissue in co-cultured mutant dorsal endoderm, demonstrating a non-cell autonomous function of mesenchymal Isl1 in inducing exocrine pancreas (Ahlgren et al., 1997).
More recently, a conditional knock-out approach has been used to address Isl1 functions in second wave endocrine cells. Based on a Pdx1-Cre driven knock-out strategy, it was possible to efficiently remove endodermal Isl1 expression shortly after onset of secondary transition (Du et al., 2009; Liu et al., 2011). In these mutants, expression of the endocrine progenitor marker Pax6 was initiated in normal numbers but unlike in the control embryos, only a few of these Pax6 positive cells established expression of the hormones Gcg, Ins, Sst and PP. During later development, the mutants displayed a reduced number of Pax6 expressing endocrine cells and this reduction was accompanied by a decreased rate of β-cell proliferation and an increased rate of apoptosis within the pancreatic islet (Du et al., 2009). Furthermore, a transgenic increase of Isl1 expression was shown to enhance glucose-induced insulin secretion (Liu et al., 2012). The mouse data reveal requirements for Isl1 during induction of exocrine tissue and for the expansion, maturation and physiological responses in endocrine cells. Moreover, molecular approaches provided first hints for the molecular targets and interaction partners of Isl1. MafA and Arx, two genes playing fundamental roles in the process of β- and α-cell differentiation, have been found as direct transcriptional targets of mouse Isl1 (Du et al., 2009; Liu et al., 2011). In vitro studies also identified Isl1 as a direct regulator of c-Myc and Cyclin D1, providing a possible link between Isl1 function and regulation of islet-cell proliferation (Guo et al., 2011). Most recently, the Lim-domain-binding coregulator Ldb1 was shown to be a critical cofactor for Isl1-dependent expression of MafA, Arx, ins and Glp1r, which encodes an important regulator of Ins synthesis and release (Hunter et al., 2013).
Though pancreatic expression of Isl1 is highly conserved, its functions have not been analyzed in any model system besides the mouse. In order to study pancreatic isl1 functions in a non-mammalian system, we make use of a recently described isl1 TILLING mutant in zebrafish. In these fish, an A to T transition creates a premature stop codon at amino acid 88 leading to a complete loss of the homeodomain (de Pater et al., 2009). Mutants are viable until late embryogenesis and therefore enable studies on isl1 function during both early and later stages of pancreas development. In contrast to mouse, we find that isl1 in zebrafish is not essential for pancreatic mesenchyme formation or for the formation of hormone-expressing first wave endocrine cells. We show that isl1 has overlapping function with isl2a and isl2b in pancreatic mesenchyme in promoting expansion of the exocrine pancreas. We also show that isl1 alone has cell type specific functions in endocrine cell maturation, and that these functions are different in first and second wave endocrine cells. The data reveal isl1 functions that are unexpected when considering the mouse data, and show a major difference in the molecular regulation of first and second wave cells in zebrafish.
Materials and methods
Zebrafish maintenance and fish lines
Zebrafish (danio rerio) were maintained according to standard protocols. Developing embryos were staged by hours post fertilization (hpf) when incubated at 28 °C (Kimmel et al., 1995). Embryos older than 24 hpf were treated with 0.2 mM PTU to prevent pigmentation. isl1sa0029+/− fish (de Pater et al., 2009) were obtained from the Sanger Institute (Cambridge/UK). Homozygous isl1 mutant embryos were identified either based on the absence of a touch response (at 24 hpf) or by the lack of GFP labeled motoaxons in tg[hb9:GFP]ml2 transgenic background (Arkhipova et al., 2012; Flanagan-Steet et al., 2005). tgBAC[NeuroD:EGFP]nl1 fish were obtained from Teresa Nicolson (Obholzer et al., 2008). Fixed homozygous cloche mutants (Stainier et al., 1995) and control littermates were obtained from Arndt Siekmann.
Whole-mount in situ hybridization
In situ hydridization was performed with digoxigenin labeled antisense RNA probes (DIG RNA Labeling Mix, Roche) and α-Digoxigenin-AP antibody (1:4000, Roche) using previously published protocols (Hauptmann and Gerster, 2000). Antisense RNA probes were generated after linearizing the corresponding plasmids and using T7 or Sp6 RNA Polymerase. The following probes were used: isl1 (Gong et al., 1995), isl2a (Gong et al., 1995), isl2b (gift from Hitoshi Okamoto) (Kikuchi et al., 1997; Tokumoto et al., 1995), insulin, glucagon, somatostatin, ghrelin (all kindly provided by Francesco Argenton) (Argenton et al., 1999; Biemar et al., 2001), pax6b, meis3, fgf10, fgf24, arx (gifts from Bernard Peers) (Fischer et al., 2003; Manfroid et al., 2007; Miura et al., 1997; Puschel et al., 1992; Sagerstrom et al., 2001).
Immunofluorescence
Embryos were fixed for 90 min at room temperature in 4% PFA, 1% DMSO. After fixation, the embryos were washed 3×5 min in 1x PBS, 0.1% Tween 20, the yolk was removed and embryos were incubated in blocking buffer containing 1% DMSO, 1% sheep serum, 1% BSA and 1% Triton X-100 in 1x PBS for at least 60 min at room temperature. The embryos were then incubated overnight at +4 °C with primary and secondary antibodies, at 1:200 and 1:1000 dilutions, respectively. Primary antibodies: polyclonal guinea pig anti-insulin (Dako; A0564), polyclonal rabbit anti-human glucagon (Dako; A0565), polyclonal rabbit anti-somatostatin (Dako; A0566), anti-Islet-1 & 2 homeobox (mouse, 39.4D5; DSHB) and monoclonal mouse IgG anti-GFP (Roche, Cat. no. 11814460001). Secondary antibodies: Alexa Fluor 488 goat anti-mouse IgG (Invitrogen; A-11029), Alexa Fluor 555 goat anti-guinea pig IgG (Invitrogen; A-21435), Alexa Fluor 546 goat anti-rabbit IgG (Invitrogen, A-11035), Alexa Fluor 633 goat anti-rabbit IgG (Invitrogen; A-21070), Alexa Fluor 633 goat anti-guinea pig IgG (Invitrogen; A-21105), and mouse Goat IgG, Cy3-conjugated (Chemicon; AP124C).
Morpholino and mRNA Injections
For knockdown of isl2a and isl2b, embryos were injected with a total of 4–8 pg morpholino (GenTools, Philomath, OR, USA).
MOisl2a: 5′-GAATATCCACCATACAGGAGGGTTA-3′ (Hutchinson and Eisen, 2006)
MOisl2b: 5′-TATTATATCCACCATACAGGTTGCC-3′
H2B-mRFP1 encoding mRNA was produced from NotI linearized p13-pCS-H2B-mRFP1 (kindly provided by S.G. Megason) using Sp6 RNA Polymerase and mMessageMachine (Ambion). For injection, the H2B-mRFP1 mRNA was diluted to 60 ng/µl. Eggs were collected shortly after fertilization, transferred to injection ramps and injected with 1–3 nl of mRNA solution before they reached the 2-cell stage. 6 h after injection, RFP+ eggs were sorted out and kept at 28 °C in the dark until the embryos were fixed for further analyses.
TUNEL staining
Embryos were fixed for 1 h at room temperature in 4% PFA, 1% DMSO. The yolk was removed and embryos were permeabilized by incubation in 1x PBS, 0.3% Triton X-100 for 20 min at room temperature. After Proteinase K digestion (10 µg/ml) and re-fixation in 4% PFA for 20 min at room temperature, the staining was performed using the In situ Cell Death Detection Kit, TMR red (Roche, Cat. no. 12156792910) according to manufactors instructions.
EdU staining
For EdU based detection of proliferating cells, the Click-IT 647 Kit (Invitrogen C10085) was used. At 48 hpf, 5 nl EdU solution (50 μM EdU/2% DMSO) was injected into the yolk and common cardinal vein (Kramer-Zucker et al., 2005) of PTU-treated embryos anaesthesyed with 0.6 mM tricaine. Injected fish were successively incubated in 50 µM EdU/0.4% DMSO and kept in the dark up to 96 hpf. Samples were fixed in 4% PFA/1% DMSO for 2 h at room temperature (RT), washed in 1x PBST and deyolked. EdU staining was performed as previously described (Hesselson et al., 2009).
qPCR
Total RNA samples were prepared from pools of 15–20 embryos at the stages indicated and from pools of 25–30 manually dissected embryonic pancreata of 4 dpf tg[hb9:GFP]ml2 embryos using Trizol Reagent (Ambion). cDNA was prepared using the First Strand cDNA Synthesis Kit #K1641 (Thermo Scientific). HOT FirePol EvaGreen qPCR Mix Plus (Solis BioDyne, Cat.No. 08-24-00020) was used for qPCR reactions in a CFX Connect Real-Time System (Bio Rad). Reactions were performed on two biological samples with two technical replicates each. Primers were designed using the Software “QuantPrime” (Arvidsson et al., 2008) and are listed in the supplementary information (Table. S7).
Microscopy
Confocal images were generated as previously described (Arkhipova et al., 2012) with a Zeiss LSM Exciter5 microscope equipped with the following lasers: 25 mW Argon laser (468, 488 and 514 nm), 1 mW 543 nm HeNe-laser, 5 mW 633 nm HeNe-laser and a 25 mW 405 nm diode-laser. Fluorescence detection was performed with the following filters: BP 505–530 (GFP and Alexa 488), LP 560 (Cy3, Alexa 555) and LP 650 (Alexa 633, Click-IT 647). A Leica DM 6000 microscope and Leica DFC300 FX camera was used for DIC images of whole mount in situ staining. Phenotypes of isl1 mutant embryos were captured on a Leica MZ16A stereomicroscope using a Leica DFC320 camera. Adobe Photoshop CS3 was used for image arrangement and adjustment.
Statistics
Statistical analysis was performed by GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA) using 1-way ANOVA and Tukey–Kramer Test (Figs. 1, 5I, 7G, 8 and S3) or unpaired t-test (Figs. 4, 7H & S3). For qPCR data, unpaired t-test (Fig. 1) and 1-way ANOVA with Bonferroni's Multiple Comparison Test (Figs. 2 and 5) was used. P-values for statistical significance are shown in each figure. All graphs show Mean+standard error (SEM).
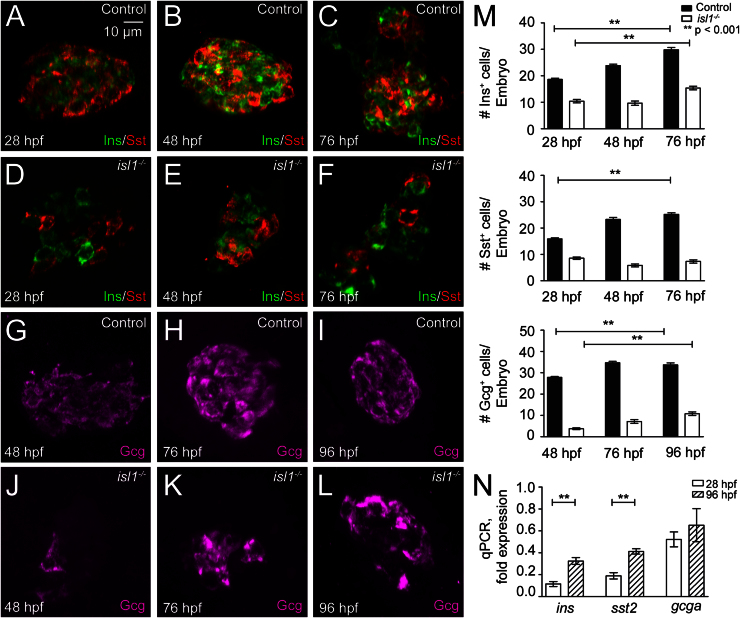
Fig. 1.
Reduced expression of endocrine hormones in isl1 mutants. (A–F) Confocal image projections of the pancreatic islet in control (A–C) and isl1−/− embryos (D–F) that were co-immunostained for Ins (green) and Sst (red) at 28 hpf (A, D), 48 hpf (B, E) and 76 hpf (C, F). (G–L) Immunostainings for Gcg (purple) in control (G–I) and in isl1−/− embryos (J–L) at 48 hpf (G, J), 76 hpf (H, K) and 96 hpf (I, L). All embryos are shown from ventral with the anterior to the left. (M) Quantitative analysis of hormone expressing cells in control and isl1−/− embryos. Bars show mean+SEM. (N) Relative expression levels of ins, gcga and sst2 mRNA in isl1−/− mutant fish in relation to control littermates as revealed by qPCR analyses of whole embryo RNA preparations, normalized to EF1α.
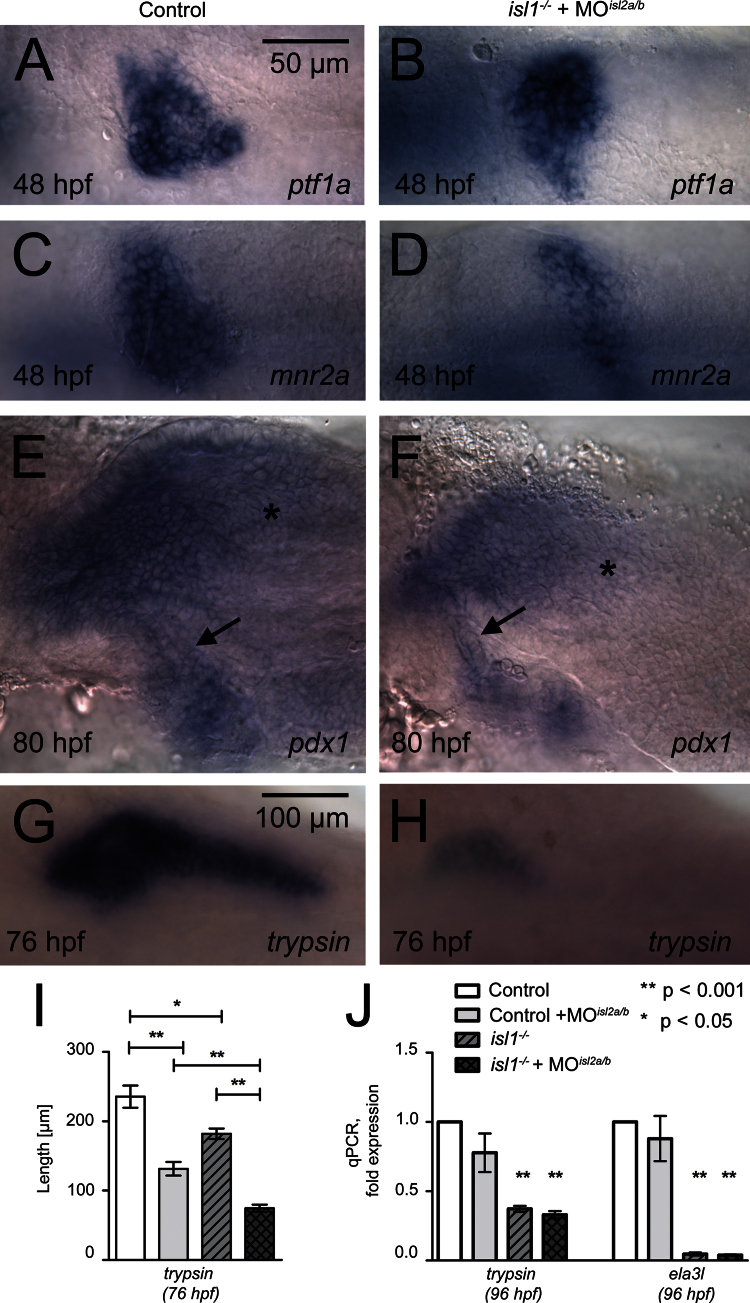
Fig. 5.
Overlapping activities of three isl-genes in exocrine tissue formation. (A, B) Pancreatic ptf1/p48 mRNA expression is similar in control (A) and isl1−/−+MOisl2a/2b embryos (B) at 48 hpf. (C, D) Differently, pancreatic mnr2a mRNA expression is reduced in isl1−/−+MOisl2a/2b embryos as compared to control embryos. (E, G) pdx1 mRNA expression at 80 hpf reveals a similar morphology of the extrapancreatic duct (arrow) in control and isl1−/−+MOisl2a/2b embryos. Note the reduced gut specific pdx1 signals (asterisk) in the isl-depleted embryos. (G, H) trypsin mRNA expressing in exocrine tissue of a control embryo (G) and in an isl1−/−+MOisl2a/2b embryos (G) at 76 hpf. (I) Quantitative analyses on the length of trypsin mRNA expression domain in control and triple isl gene deficient embryos at 76 hpf. Embryos are shown from ventral with anterior to the left (A–F) or dorsal with anterior to the left (G, H). P-values for significant changes are indicated. (J) qPCR analysis for mRNA expression levels of trypsin and ela3l in 96 hpf old whole embryos, normalized to EF1α. Coloring of the bars corresponds to the same genotypes presented in (I). Bars show mean+SEM.
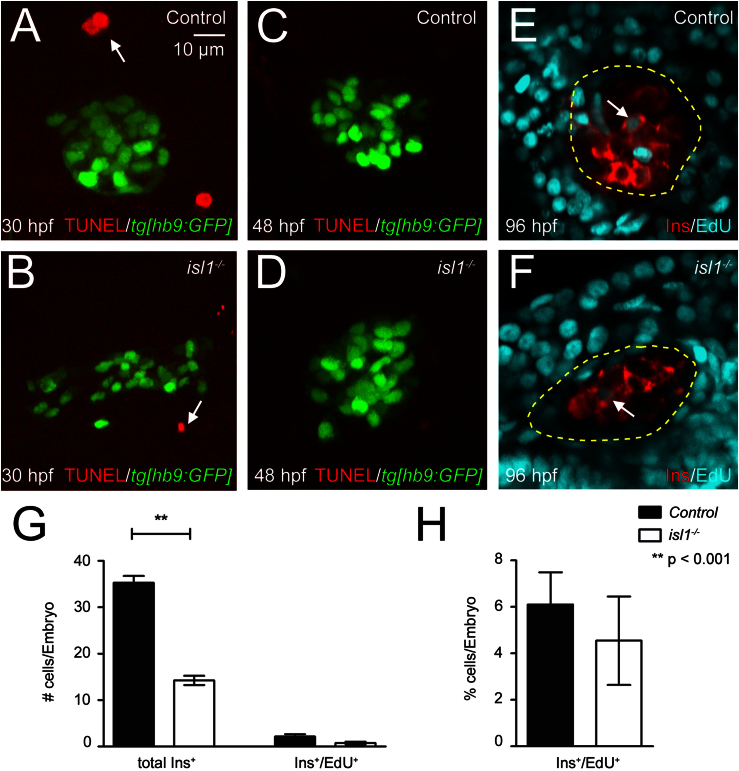
Fig. 7.
TUNEL and EdU assays on control and mutant embryos. (A–D) Confocal image projections of 30 hpf (A+B) and 48 hpf (C+D) old control and isl1 mutant embryos in the tg[hb9:GFP] background after TUNEL labeling for apoptotic cells. The arrow highlights individual TUNEL+ cells in the vicinity of the GFP labeled islet. No TUNEL+ cells were found within the pancreatic islet of control or mutant fish at all analyzed stages. (E+F) Confocal sections of 96 hpf embryos labeled for Ins (in red) and EdU (in blue). Embryos were treated with EdU from 48 hpf to 96 hpf. Note that very few EdU+ cell are present within the islet (highlighted by yellow circle), while most of the nuclei in the surrounding exocrine tissue are EdU-positive. All images show ventral view, anterior to the left. (G) Numbers of Ins+ and Ins+/EdU+ cells in control and isl1 mutants. (H) Percentage of EdU+/Ins+ cells in relation to the number of Ins+ cells. Bars show mean+SEM.
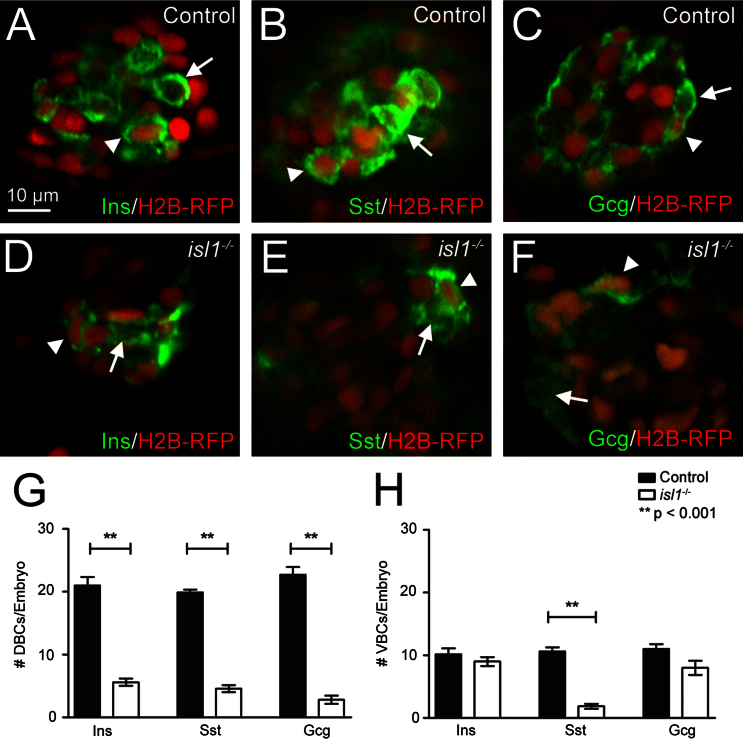
Fig. 8.
Endocrine hormone expression in first and second wave endocrine cells in control and isl1 mutant embryos. Single plane views from confocal images of the primary islets from control (A–C) and isl1 mutants (D–E). Embyros were injected with H2B-RFP (in red) encoding mRNA at the one cell stage and were immunostained (in green) for Ins (A, D), Sst (B, E) and Gcg (C, F) at 96 hpf. Cells that left cell-cycle early in development show a red nuclear label due to the injecetion of H2B-RFP encoding mRNA at the one cell stage. Arrowheads indicate examples of hormone+/RFP+ cells (refered to as DBCs), while arrows indicates examples of hormone+/RFP− cells (referred to as VBCs). (G, H) Quantification of hormone+ DBCs (G) and VBCs (H) in control (black bars) and isl1 mutant embryos (white bars). Note that isl1 mutants show significantly reduced numbers for all DBCs, while only the number of Sst expressing VBCs cells is significantly reduced. Bars show mean+SEM.
Fig. 2.
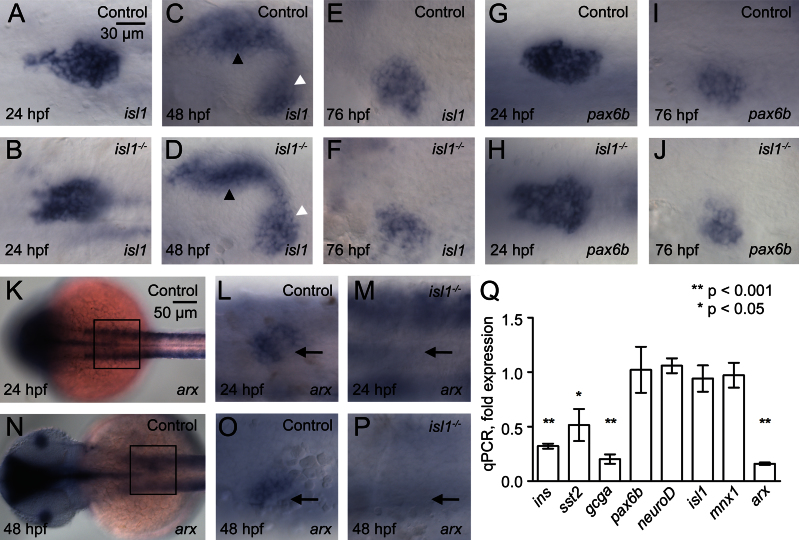
Unchanged expression of early endocrine markers in isl1 mutants. Whole mount in situ hybridizations for isl1 (A–F) and pax6b (G–J) mRNA in control (A, C, E, G, I) and in isl1−/− embryos (B, D, F, H, J) at 24 hpf (A, B, G, H), 48 hpf (C, D) and 76 hpf (E, F, I, J). Note that the pattern and intensity of isl1 signals in the pancreatic mesenchyme (black arrow head) and in the endocrine islet (white arrow head) is indistinguishable in control and mutant embryos. Embryos are shown from ventral with anterior to the left. (K–P) Whole mount in situ hybridizations for arx. (K, N) Dorsal view of control embryos at 24 hpf (K) and 48 hpf (N). Insets mark the position of the pancreatic area shown in higher magnification panels (L M, O, P). Images show a ventral view of control (L, O) and isl1−/− mutant (M, P) embryos. (Q) qPCR analysis for hormone and transcription factor encoding mRNA of isolated pancreata from 96 hpf old embryos. Shown are relative expression levels in pancreata of mutant as compared to control embryos, normalized to the relative expression levels of ins obtained for 96 hpf whole embryonic mRNA.
Measuring of exocrine pancreas
The length of the exocrine pancreas was measured from head to tail of trypsin stained embryos. Images were taken using a 10x Ph1 Objective and a Leica DM6000 microscope. The longest possible distance from the head to the tail of the exocrine pancreas was measured using the Fiji plugin “Scale Bar tools for microscope” by Gilles Carpentier (Carpentier G. Contribution: Scale Bar Tools for Microscop ImageJ News, 4 June 2009).
Results
isl1 is required for proper formation of α-, β- and δ-cells
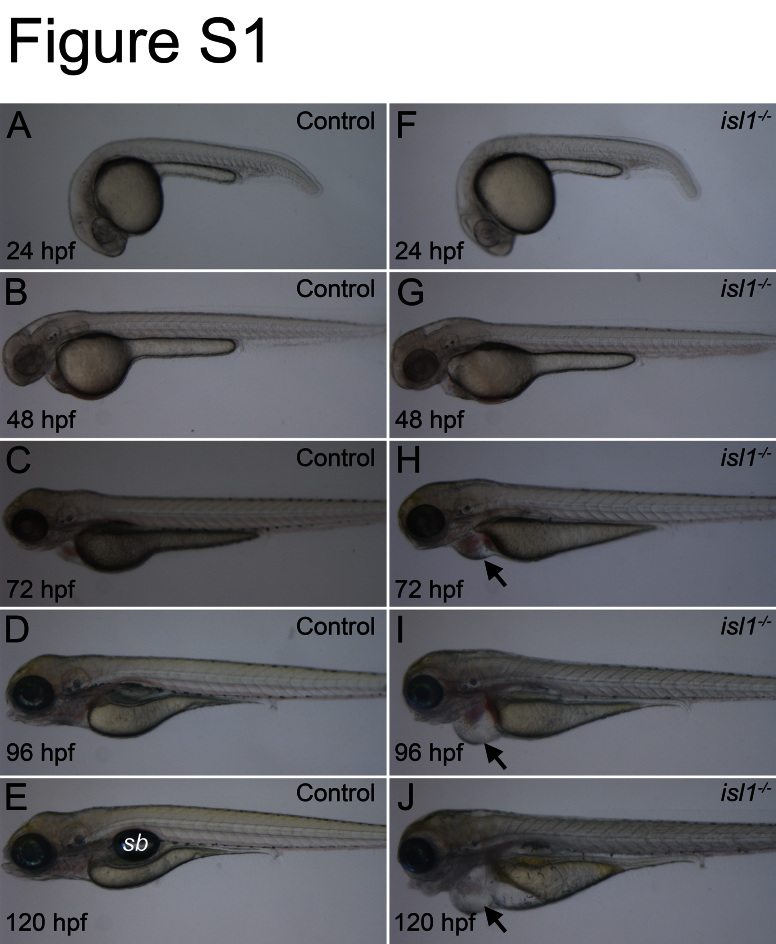
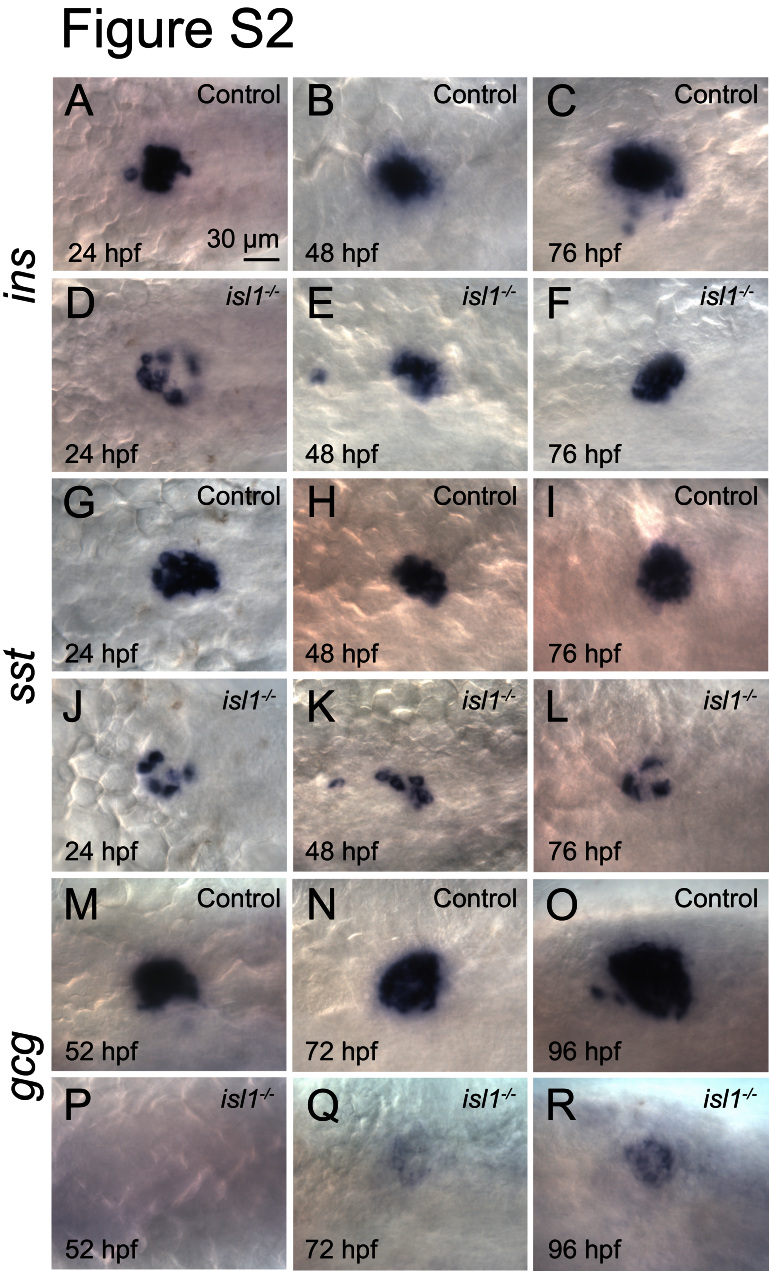
Previous analyses of the zebrafish TILLING mutation isl1sa0029 (de Pater et al., 2009) showed that homozygous mutant fish, unlike the Isl1 mouse mutants, are viable until late embryogenesis (see also Fig. S1). Taking advantage of the long survival we asked if isl1 has an evolutionary conserved requirement during pancreas formation. Immunostainings with antibodies against endocrine hormones at 28 hpf, 48 hpf and 76 hpf revealed a reduction of more than 50% in the number of Ins and Sst expressing cells in the mutants compared to phenotypic control littermates (Fig. 1A, F and M). An even more dramatic reduction was found for the number of Gcg+ cells, which were virtually absent in mutant embryos at 48 hpf and only very few Gcg+ cells could be detected at 72 hpf and 96 hpf (Fig. 1G, M, and Table. S1). The reduced number of endocrine hormone expressing cells in isl1 mutants was confirmed by whole-mount in situ hybridization analyses for ins and sst2 at 24 hpf, 48 hpf and 72 hpf (Fig. S2A–L) and for gcga at 52 hpf, 72 hpf and 96 hpf (Fig. S2M–R). Notably, control and isl1 mutant embryos both showed 1–2 ghre mRNA positive cells per embryo suggesting that ε-cell formation is not affected in isl1 mutants (data not shown). Quantitative RT-PCR (qPCR) analyses on mRNA from 28 hpf and 96 hpf embryos further confirmed reduced expression of ins (11%±2% at 28 hpf, 32%±3% at 96 hpf), sst2 (19%±2% at 28 hpf, 41%±2% at 96 hpf) and gcga mRNA (52%±6% at 28 hpf, 62%±15% at 96 hpf). The robust decrease of ins and sst2 at 28 hpf suggests that in mutants, hormone expressing cells are not only reduced in number but in addition express lower concentrations of ins and sst2 mRNA. In summary, these data reveal a conserved requirement of isl1 for α-, β- and δ-cell formation.
Conserved requirement for isl1 in endocrine cell maturation
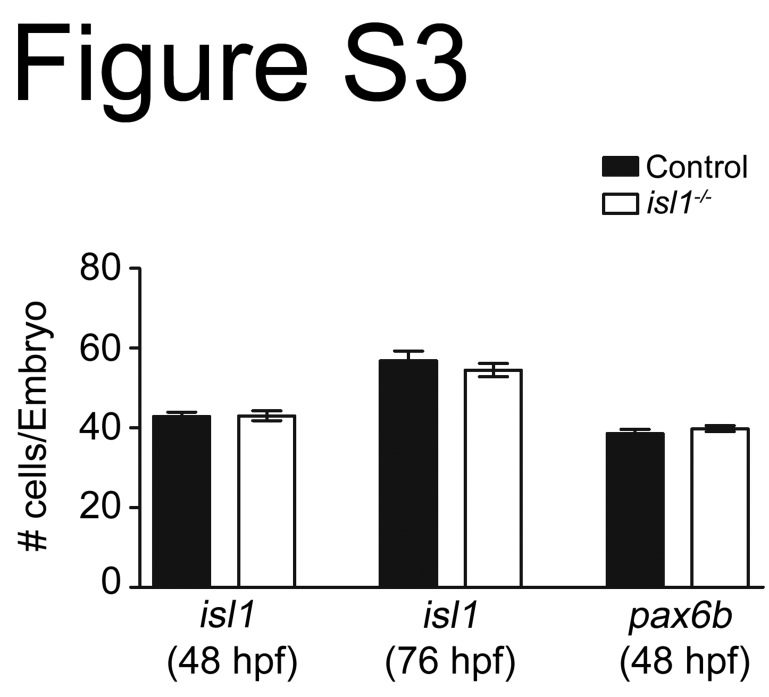
To investigate if the reduction of endocrine hormones reflects a loss of endocrine cells, we studied expression of isl1 mRNA, which is stable in isl1sa0029 mutants. The clustering of isl1 expressing cells into an islet and the size of these islets appeared indistinguishable between mutants and control embryos at 48 hpf and 76 hpf (Fig. 2A–F). Further, the numbers of isl1+ cells are similar in control embryos (48 hpf: 42.8±1,1 (n=8); 76 hpf: 56.8±2,4 (n=9)) and isl1 mutants (48 hpf: 43.0±1,2 (n=6); 76 hpf: 54.5±1.67 (n=7)), suggesting that in isl1 mutants endocrine cells are specified to adopt an endocrine fate, but fail to differentiate or mature into hormone producing cells. Consistent with this notion, we also find that the endocrine progenitor marker pax6b is expressed in similar numbers in control (38.6±1.1, n=10) and in isl1 mutants (39.8±0.7, n=10) at 24 hpf (Fig. 2G, H, and Table. S2) and in a comparable domain at 76 hpf (Fig. 2I and J).
Next, we asked if the conserved requirement of isl1 in endocrine differentiation is also associated with a conserved molecular function. Whole mount expression analyses of 24 hpf and 48 hpf embryos show that one of the few known pancreatic isl1 targets in mouse, the transcription factor arx, is selectively lost in the pancreas of isl1 mutant zebrafish embyros (Fig. 2K–P). For quantitative analyses of islet specific expression levels in 96 hpf embryos, we performed qPCR analyses on pools of 25–30 isolated pancreata. Using the relative expression levels of ins obtainted for 96 hpf whole embryonic RNA (Fig. 1N) for islet-specific normalization of these data, we found a 7-fold reduction of pancreatic arx expression in mutants. Using the same normalization approach we also determined expression level of additional transcription factors with an islet-restricted expression in the pancreas. In agreement with our whole mount expression studies, no differences were found for the expression of endocrine progenitor markers including pax6b, isl1 neuroD and mnx1, while expression of ins, gcga and sst2 were strongly reduced (Fig. 2Q). These data suggest a conserved molecular function of isl1 in endocrine cell maturation.
Overlapping expression of isl1, isl2a and isl2b in pancreas mesenchyme
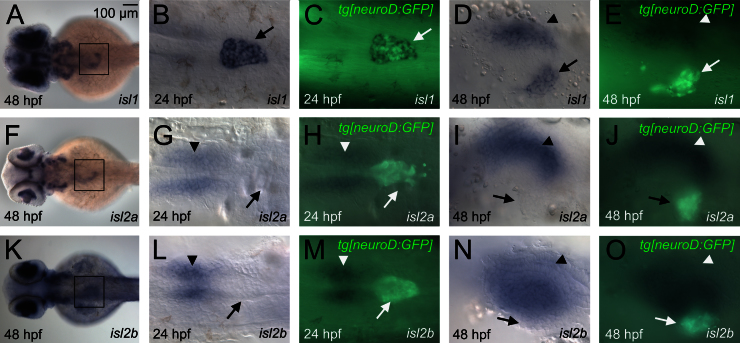
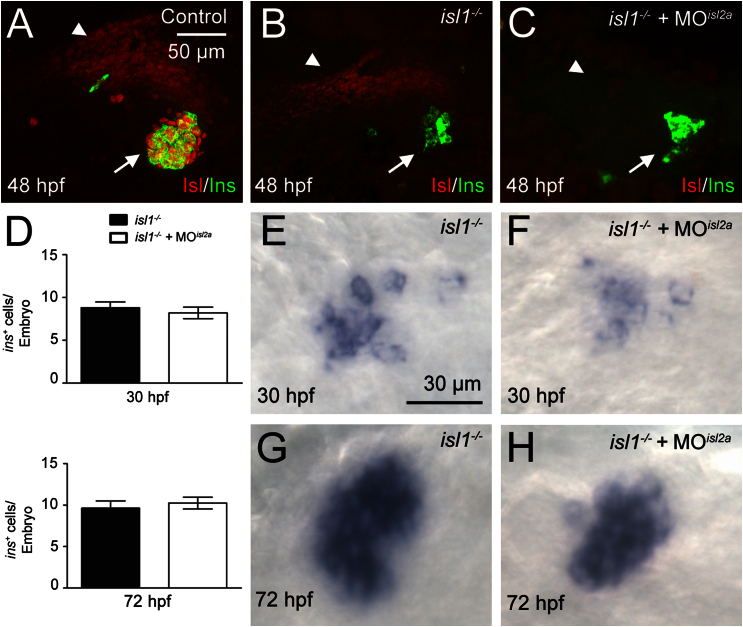
Due to the incomplete loss of endocrine hormones in isl1 mutant fish as compared to the complete loss of these hormones in early Isl1 mouse mutants, we reasoned that other factors might compensate for the loss of isl1 in fish. Promising candidates are Isl2 proteins and in particular Isl2a which was shown earlier to be able to compensate for Isl1 function during motoneuron differentiation in zebrafish (Hutchinson and Eisen, 2006). To gain insight into possible interactions between isl factors in endocrine differentiation, we analyzed pancreatic expression of isl2a and isl2b mRNA and protein and we performed functional studies using antisense morpholinos. We found that both isl2 genes are expressed in the pancreatic region between 24 and 72 hpf. Double stains for islet mRNAs and for endocrine-specific GFP in neuroD:GFP transgenics revealed that only isl1 is expressed in endocrine cells while all three isl-genes are expressed in overlapping, but slightly different patterns in the pancreatic mesenchyme (Fig. 3). To further confirm this regulation, we performed immunostainings with a pan-Isl antibody along with an anti-Ins antibody to mark β-cells. Control embryos showed robust anti-Isl signal in the pancreatic islet and a slightly weaker signal in the pancreatic mesenchyme. Consistent with an Isl1 independent regulation of isl2 genes only in pancreatic mesenchyme, loss of isl1 in the mutants was sufficient to abolish the endocrine anti-islet antibody signal but not the signal in pancreatic mesenchyme (Fig. 4B). The lack of islet antibody staining in Ins+ cells of isl1 mutants argues against a cell autonomous role of Isl2 proteins in early endocrine cell differentiation. To exclude that low levels of Isl2 might have a compensatory function in endocrine cell formation, we analyzed ins expression in isl1 mutant embryos injected with isl2a translation-blocking MO. Interestingly, immunostainings showed that injection of isl2a-MO was sufficient to entirely eliminate pancreatic anti-islet signals in isl1 mutants. While this suggests efficient knockdown of all remaining Islet proteins by the isl2a morpholino injection, it could also indicate that the levels of Isl2b expression in these embryos is below the detection limit or that Isl2b proteins are not recognized by the antibody (Fig. 4C). In the case of redundant activities, we expected to see a further reduction or even complete loss of endocrine cells in isl1 mutants/isl2a morphants. In contrast, no differences in the number of ins expressing cells at 30 hpf and 72 hpf (Fig. 4D–H and Table. S3) could be detected between uninjected isl1 mutants and isl2a MO injected mutants (Fig. 4D). In conclusion, this suggests that among the three isl-genes in zebrafish only isl1 has activity in endocrine cells, while all three genes are expressed in the pancreatic mesenchyme.
Fig. 3.
\Pancreatic expression of isl1, isl2a and isl2b. Comparison of isl1 (A–E), isl2a (F–J) and isl2b mRNA (K–O) expression at 24 hpf (B, C, G, H, L, M) and 48 hpf (A, D–F, I–K, N, O). Embryos are shown from the dorsal (A, F, K) or ventral view (rest). Insets shown in the overview images (A, F, K) indicate the relative position of the higher resolution images shown on the right side. The mRNA stains were performed in tg[neuroD:GFP] embryos in which immunofluorescence detection of GFP (green signals in C, E, H, J, M, O) was used to highlight endocrine cells (arrows mark the position of the pancreatic islet). Note that only isl1 signals overlap with GFP. Expression of isl2a is found in the mesenchyme anterior the endocrine cells at 24 hpf and left of the GFP labeled endocrine cells at 48 hpf in a pattern overlapping with that of the mesenchymal isl1 expression (arrowheads mark similar position in all embryos). Signals for isl2b are also found anterior to the endocrine cells at 24 hpf (arrowhead) and in a broader mesenchymal domain directly adjacent to the GFP signals at 48 hpf.
Fig. 4.
isl2a does not contribute to endocrine cell formation. (A–C) Immunostainings for Ins (green) and Isl-proteins (red) in the pancreatic region of control (A), isl1 mutant (B), and isl1 mutant/MOisl2a injected embryos (C) at 48 hpf. (D) Quantification of ins mRNA positive cells and examples of corresponding in situ stains for ins mRNA in isl1 mutants (E, G), and in isl1 mutant, MOisl2a injected embryos (F, H) at 30 hpf (E, F) and 72 hpf (G, H). Bars show mean+SEM.
Interaction of isl1 and isl2 genes during exocrine pancreas development
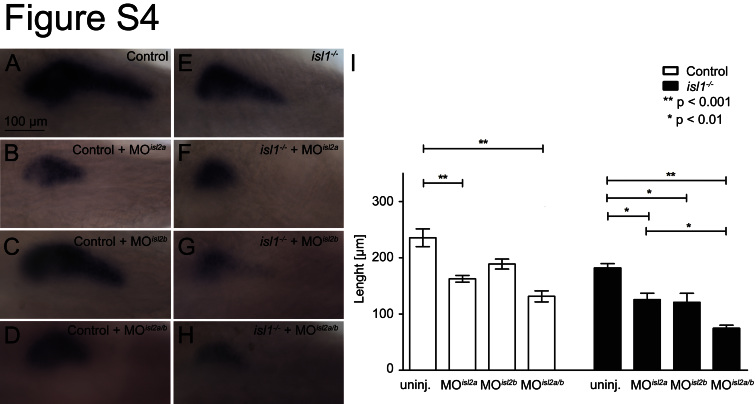
A previous study in zebrafish reported that injection of an isl1 translation-blocking morpholino caused a concentration dependent reduction of the exocrine pancreas (Wan et al., 2006). As we have shown that there is overlapping expression of three isl-genes in pancreatic mesenchyme, we hypothesized that isl1 and isl2 genes could have overlapping functions in ventral pancreatic bud formation. To test this, we analyzed pancreas formation in isl1 mutant embryos that were further injected with isl2a/isl2b-MO to generate isl1/2a/2b triple negative embryos. Analysis of early ventral bud markers at 48 hpf revealed a similar expression pattern for ptf1/p48 in control and isl1/2 depleted embryos and a reduced mnr2a expression domain in the isl1/2 depleted embryos. The data suggest that Isl proteins are dispensable for initiation ventral bud formation (Fig. 5A and B), but might be involved in ventral bud expansion (Fig. 5C and D). Analyses of isl1/2 depleted embryos at 76–80 hpf revealed normal formation of the pdx1+ extra-pancreatic duct (Fig. 5E and F), but a strong reduction of the exocrine cell marker trypsin (Fig. 5G and H, Fig. S4).
To address the question of whether isl-genes interact during exocrine tissue formation, trypsin expression was also further analyzed in embryos lacking individual isl-genes or a combination of two isl-genes (Fig. S4). The stainings displayed a variable reduction of trypsin expression, very similar to the phenotypes described after injection of isl1 translation blocking MO (Wan et al., 2006). For quantification, we measured the maximum distance from head to the tail of the trypsin expression domain (Fig. S4, Table. S4). We found that loss of either isl1 or isl2a alone results in a significant shortening of the trypsin expression domains by about 30% and that the shortening is further enhanced to 47% in isl1/2a double-deficient embryos (Fig. S4A–F and I). Isl2b appears to have only minor functions, as the knockdown did not significantly reduce the trypsin domain in control or isl2a morphants (Fig. S4C, D and I). However, isl2b morpholino injection did cause a significantly shorter trypsin expression when combined with the loss of isl1 or isl1/2a function (Fig. S4G, H and I).
For further analysis, expression of trypsin and ela3l, a second exocrine marker with a slightly later onset of expression (Wan et al., 2006), were analyzed by qPCR in 96 hpf embryos. The data confirmed the strong reduction of trypsin in isl1 mutants and revealed a loss of ela3l expression in these mutants (Fig. 5J). While whole mount analyses of 96 hpf embryos confirmed the reduction of trypsin expression seen also in 76 hpf embryos (Fig. S5), the qPCR analyses show that knockdown of isl2-genes had only mild and not statistically significant effects on the expression levels of trypsin and ela3l (Fig. 5J). In summary the data reveal interactions between mesenchymal isl1 and isl2a/b in non-cell autonomous regulation of exocrine pancreas growth and morphogenesis. The strong reduction of trypsin and loss of ela3l in isl1 mutants but not in isl2 morphants indicates a potential isl1-specific function in exocrine tissue specification or maturation.
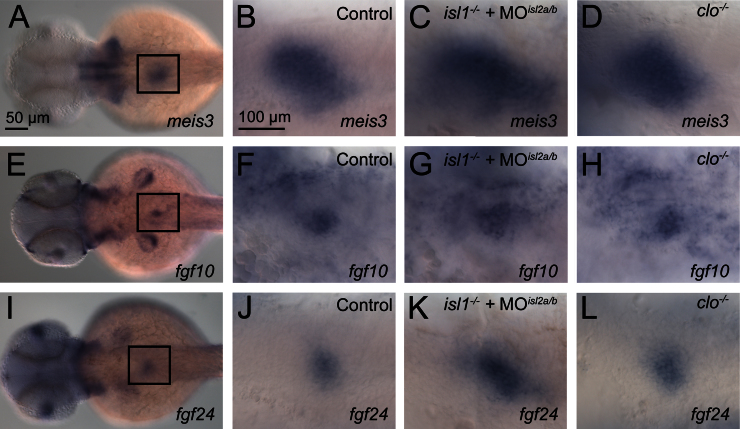
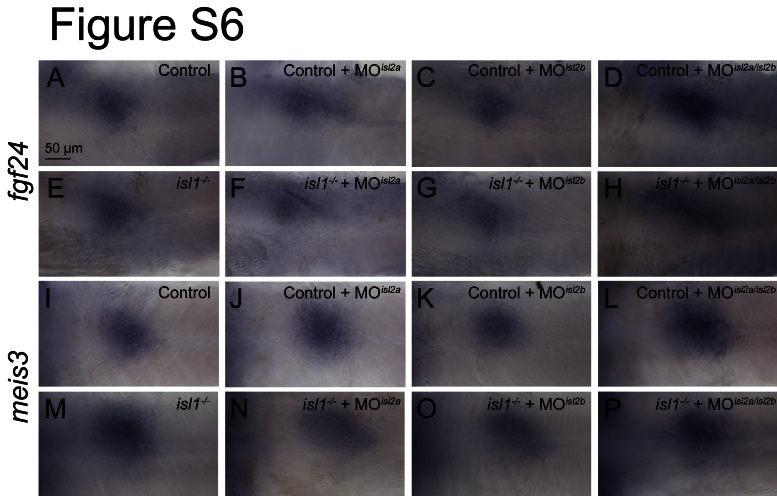
Isl1 mutant mice lack two tissues with important non-cell autonomous function during pancreas formation, the dorsal aorta endothelium and the pancreatic mesenchyme (Ahlgren et al., 1997; Lammert et al., 2003). While the dorsal aorta has been shown to form normally in the isl1 mutant zebrafish (de Pater et al., 2009), it is still possible that defects in pancreatic mesenchyme formation may account for the defects in exocrine tissue extension. Notably, the mesenchymal expression of isl1 mRNA itself is unchanged in the isl1 embryos (Fig. 2C and D), indicating that pancreatic mesenchyme is established in the mutants. Analyses of the pancreatic mesenchyme markers meis3, fgf10 and fgf24 (Manfroid et al., 2007) also revealed a similar expression in control embryos and in embryos in which the activity of individual or combinations of two or three islet genes has been disrupted (Figs. 6 and S6). Finally we investigated the contribution of the dorsal aortic endothelium to establishing pancreatic mesenchyme. Analyses of cloche mutant embryos (clo−/−), which lack endothelial cells (Stainier et al., 1995), revealed normal patterns of meis3, fgf10 and fgf24 (Fig. 6 D, H and L). Thus, neither isl-genes nor the aortic endothelium appear to be required for the formation of pancreatic mesenchyme or for the transcriptional regulation of the identified mediators of exocrine expansion, fgf10 and fgf24 within this tissue (Manfroid et al., 2007). This suggests that the requirement for Isl proteins in pancreatic mesenchyme formation differs between mouse and zebrafish, even though the non-cell autonomous function of isl-genes in exocrine pancreas extension appears to be conserved between these organisms.
Fig. 6.
Formation of pancreatic mesenchyme is independent of isl gene function. Neither the loss of all three isl-genes nor absence of vascularization in cloche mutants results in a loss of fgf10, fgf24 or meis3. Expression patterns of meis3 (A–D), fgf10 (E–H) and fgf24 (I–L) in pancreatic mesenchyme of control embryos (B, F, J), isl1/2a/2b depleted embryos (C, G, K) and cloche mutants (D, H, L). All images are ventral with the exception of A, E, I which are dorsal views. Anterior is to the left. Insets in A, E, I mark the position of the higher resolution images shown on the right side.
Distinct function of isl1 in first and second wave endocrine cells
In normal pancreas development, the endocrine cell mass increases after 36 hpf as the result of differentiation and proliferation of late forming ventral bud derived endocrine cells (Field et al., 2003; Hesselson et al., 2009; Moro et al., 2009). Consistent with published data, we find that the control embryos showed increasing numbers of hormone expressing cells types between 24–96 hpf (Figs. 1, S2 and Table S1). In isl1 mutants, we find that the number of Sst expressing cells remains unaltered, while Ins and Gcg expressing cells show an increase in numbers similar to that of the control embryos (Fig. 1M). In order to determine possible contribution of isl1 to cell death or proliferation, mutants were analyzed for the presence of apoptotic cell nuclei by TUNEL stains and for mitosis by DNA-incorporation assays using the thymidine analog EdU. TUNEL stains of hb9:GFP transgenic embryos at 30 hpf and 48 hpf revealed similar results for control and isl1 mutant embryos, with some rare TUNEL signals in the environment of the GFP labeled islets but no signals within the islets (Fig.7A–D). For the EdU incorporation assays, control animals and isl1 mutants were injected with EdU at 48 hpf and analyzed at 96 hpf. In agreement with previous reports (Hesselson et al., 2009; Kimmel et al., 2011; Moro et al., 2009), embryos showed EdU incorporation into most of the exocrine cells but in only very few endocrine cells. While isl1 mutants have less Ins+/EdU+ cells than control embryos (Fig. 7G), the proportion of Ins+/EdU+ cells is similar for both genotypes (Fig. 7H). This suggests that loss of isl1 has no major effect on endocrine cell proliferation or survival.
The age-dependent increase of α- and β-cell number in mutants could result from a delayed differentiation of early forming α- and β-cells, or alternatively from the normal onset of hormone expression in late forming second wave cells. In order to distinguish between these options, we used a recently described cell tracking approach that allows differential labeling of early born first- and later born second-wave cells (Brennand et al., 2007; Hesselson et al., 2009). In these experiments, embryos are injected with mRNA encoding the long-living fluorescent histone variant H2B-mRFP1. As the H2B-mRFP1 protein is equally distributed to daughter cells during mitosis, fluorescent intensities per nucleus exponentially decrease with every cell division. DBCs leave the cell-cycle early in development and therefore remain high levels of H2B-mRFP1, even later in development. The VBCs undergo more rounds of cell division and remain proliferative even after onset of hormone expression, which leads to the dilution of RFP label below level of detection (Hesselson et al., 2009). In the case of normal forming second-wave α- and β-cells, we expected to find RFP+ as well as RFP− α- and β-cells. As the early forming Gcg-cells are virtually absent in isl1 mutants, most ‘second wave-derived’ Gcg-expressing cells at later stages should be RFP−. By contrast, as the number of Sst-expressing cells remains constant between 28–76 hpf we expect most of the Sst-expressing cells to be RFP+ in older embryos. To address this question, we injected H2B-mRFP1 mRNA into one-cell stage eggs and analyzed RFP expression in endocrine cells at 96 hpf. As expected, Ins+/RFP+ and Ins+/RFP− cells were found in mutant fish, while most Sst expressing cells were RFP+. In contrast to our expectations, we also found a few Gcg+/RFP+ cells, possibly indicating a delayed onset of Gcg expression in isl1 mutant first wave α-cells (Fig. 8 and Table S6). To determine if hormone+ second wave cells are formed in normal numbers, we counted hormone+/RFP− endocrine cells in control and isl1 mutant embryos. While the total number of hormone+/RFP− second wave cells is reduced from 32 RFP− cells in control down to 18 RFP− cells in isl1 mutants, these embryos show no significant difference in the amount of ventral bud derived Ins+/RFP− and Gcg+/RFP− cells. This suggests that differentiation of Ins- and Gcg-expressing cells from the later-arising VBCs is not affected by the loss of isl1. However, there is a reduction of late forming Sst-expressing cells. These data show a different requirement for isl1 in early versus late forming endocrine cells.
Discussion
Here, we present a genetic analysis of pancreatic isl-gene function in zebrafish. We show that isl1 is co-expressed with isl2a and isl2b in pancreatic mesenchyme, and that all three isl-genes have additive functions in exocrine pancreas expansion. Further, we show that isl1 alone is active in endocrine cells, we reveal cell type specific requirements for isl1 in endocrine cell maturation and we show that these requirements are different in first and second wave endocrine cells.
Interactions of isl1 and isl2 genes in exocrine pancreas formation
Various studies in mouse and chicken have demonstrated that signal exchange between pancreatic mesenchyme and pre-pancreatic endoderm is a major regulator of early pancreas morphogenesis and exocrine tissue induction (Gittes, 2009). While the molecular nature of the involved signals is still not completely understood, explant studies performed as part of the Isl1 mouse knock out analyses placed Isl1 upstream of a mesenchymal signal that regulates exocrine pancreas formation (Ahlgren et al., 1997). Similarly, the strong reduction of trypsin in isl-depleted zebrafish embryos argues for a role for zebrafish isl-genes in regulating an exocrine pancreas inducing or expanding factor. Interestingly, in mouse, loss of Isl1 alone is sufficient to prevent activation of exocrine markers in dorsal pancreatic bud derived tissues. By contrast, we find that in zebrafish pancreatic mesenchyme, three isl-genes contribute in an additive manner to exocrine expansion. One possibility is that Isl proteins have redundant activities, which is also supported by the observation that isl2a can functionally compensate for loss of isl1 in motoneuron specification (Hutchinson and Eisen, 2006). While pancreatic isl2 functions have not been addressed in mammals, it is interesting to note that isl2 expression has been reported for the human pancreas (Li et al., 2007).
In this work, we show that in Isl protein-depleted embryos, mRNA expression of meis3, fgf10 and fgf24 is not significantly reduced in the pancreatic mesenchyme. This suggests that isl-genes in fish are not required for the formation of pancreatic mesoderm, as is the case in mouse. Notably, in the Isl1 mutant mouse, the loss of dorsal pancreatic mesenchyme is associated with a loss of the dorsal aorta by apoptosis (Ahlgren et al., 1997). The dorsal aorta in mouse has direct contact with the pancreatic mesenchyme and the lack of endothelial cells prevents the formation of the pancreatic mesenchyme (Jacquemin et al., 2006). While the presence of the dorsal aorta in isl1 mutant zebrafish (de Pater et al., 2009) might explain the weaker phenotype in the fish, we also show that a loss of dorsal aorta in cloche mutants has no effect on the expression of pancreatic mesenchyme markers. In agreement with a previous report showing normal expression of trypsin in cloche mutants (Field et al., 2003), our results suggest that tissues other than the dorsal aorta regulate pancreatic mesenchyme induction in fish. Consistent with this notion, it was shown that between 26 and 29 hpf, fgf10 and fgf24 expressed in the endoderm induce pancreatic mesenchyme identity within the lateral plate mesoderm and controls isl1 and meis3 expression in this mesenchyme (Manfroid et al., 2007). After 29 hpf, Fgfs expressed in the pancreatic mesenchyme are required for exocrine tissue expansion and correct specification of the hepatopancreatic duct system (Dong et al., 2007; Manfroid et al., 2007). The normal presence of fgf10 and fgf24 mRNA in the mesenchyme of isl-depleted embryos in combination with the reduced size of the exocrine pancreas in the isl1 mutants suggests that Isl proteins must regulate other paracrine factors and that Isl proteins either alone or in parallel to Fgf10/24 promote exocrine tissue expansion.
Isl functions in endocrine cell differentiation
In amniotes, first wave endocrine cells can be distinguished from second wave cells by several features including a reduced spectrum of cell types and a lack of cell renewal in first wave cells. Studies in zebrafish only recently showed that production of first and second wave cells, and the lack of self-renewal capacity, is evolutionary conserved between mammals and fish (Hesselson et al., 2009). In zebrafish, the first wave cells display the same spectrum of hormone expressing cell types as are found in second wave cells. The role of first wave cells and the reason why zebrafish embryos establish the different types of first wave cells is currently not known. One possible explanation is a requirement for a mother-independent glucose regulation that is happening already in the fish embryo (Eames et al., 2010; Jurczyk et al., 2011). Such a function implies a similar physiological role of first and second wave cells and possibly a similar molecular regulation of differentiation. However, we find that loss of isl1 has different effects on first and second wave cell maturation. This underscores the dissimilarity between first and second wave cells, and suggests that studies on first wave cells may provide only limited information relevant to the behavior of the cells of the mature organ, namely the second wave cells.
Most surprisingly, we found that loss of isl1 had no statistically significant effect on the number of Ins- and Gcg-expressing second wave cells. As these hormones are strongly reduced in the pancreas-specific Isl1 mouse knock-out (Du et al., 2009), this could indicate that isl1 functions are not only different in first and second wave zebrafish cells, but also with respect to their function in second wave cell maturation, proliferation and survival in fish versus mouse. Mutant zebrafish start to develop obvious defects after 4 dpf, e.g. in blood circulation, that affect the general fitness of the embryo. Therefore our studies were restricted to the first 4–5 days of development, during which time only few second wave cells in the vicinity of the primary islet are established. Additional experiments that overcome late embryonic lethality of isl1 fish mutants will be required to address possible later functions. Our studies revealed no obvious differences in embryonic β-cell survival and proliferation between control and isl1 mutants. However, only second wave cells are supposed to be proliferatively active (Hesselson et al., 2009). As our data suggest normal formation of second wave α- and β-cells, the reduced number of Ins+/EdU+ cells in isl1 mutants could indicate reduced β-cell proliferation. Furthermore, expression of endocrine hormones is initiated early during endocrine cell maturation and the observed presence of hormones does not exclude defects during later steps of maturation. Molecular markers that are used to distinguish later maturation stages in mammalian β-cells are currently not established in fish. To further dissect the role of isl1, it will be important to learn more about the targets of Isl proteins.
Results from transcriptome analyses of purified neuronal cells, and tissues isolated from control and Isl1 mutant animals, provided hints for potential Isl1 targets in sensory cells (Sun et al., 2008). Similar to the expression in endocrine cells, Isl1 is also active in differentiating and mature neuronal cells. It is therefore interesting to note that the transcriptome comparison links Isl1 function to the molecular machineries driving cell differentiation as well as to mature cell function. More recently, major progress has also been made in identifying additional pancreatic Isl1 targets, including transcription factors such as MafA and arx (Du et al., 2009; Liu et al., 2011) as well as known cell-cycle regulators such as Cyclin D1 or c-Myc (Guo et al., 2011). The loss of pancreas specific arx expression not only in mouse but also in zebrafish isl1 mutants provides promising evidence for a conserved molecular function for isl1 in endocrine cell maturation.
Conclusion
Pancreatic expression of vertebrate Isl1 genes is highly conserved in pancreatic mesenchyme and in post-mitotic endocrine cells. Our genetic analysis in zebrafish is the first that addresses pancreatic isl1 functions outside the mouse. Our analyses reveal a novel interaction between isl1 and isl2 genes in exocrine pancreas expansion and they implicate cell type specific requirements for isl1 in endocrine cell maturation. While our data suggest that the conserved expression correlates with conserved requirements for islet genes in non-cell autonomous exocrine pancreas expansion and cell-autonomous endocrine cell maturation, they also highlight species-specific differences in pancreatic isl1 functions. In particular we find differences with respect to the requirement for isl-genes in pancreatic mesenchyme formation, the induction of hormone expression in first wave cells, and the cell type specific requirements in endocrine cells.
Acknowledgments
We thank Marianne Voz, Justine Pirson and Bernard Peers for critical discussions during all stages of this project. Specific thanks go to Robin Kimmel and Elisabeth Ott for reading of the manuscript, suggestions and fruitful discussions. We also thank Hitoshi Okamoto and S.G. Megason for providing material and Arndt Siekmann for providing cloche mutant embryos. This project was supported by the University Innsbruck and FWF grant NFN-S105-09 (D.M.).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.ydbio.2013.03.009.
Contributor Information
Armin Wilfinger, Email: armin.wilfinger@uibk.ac.at.
Valeriya Arkhipova, Email: A.Valeriya@gmx.de.
Dirk Meyer, Email: dirk.meyer@uibk.ac.at.
Appendix A. Supplementary materials
Fig. S1.
Phenotype of isl1 mutant zebrafish compared to wildtype. Images of live control embryos (A–E) and isl1 mutant embryos (F–J) taken between 24–120 hpf. (F–J) Mutant embryos appear normal during the first two days after fertilization but show heart edema at 72 hpf (black arrow head in H, I and J). At later stages, the isl1 mutant fish show various defects including smaller eyes, malformed somites and a non-inflated swim bladder (sb).
Fig. S2.
Expression of ins, sst and gcg mRNA in control and isl1 mutants mRNA expression of ins (A–F), sst (G–L) and gcg (M–R) in the pancreatic islets of control embryos and isl1 mutants at the indicated stages. Note that in mutants the expression of ins and sst is strongly reduced at all stages analyzed, while glu is virtually absent at 52 hpf and strongly reduced at older stages. Images show a dorsal view with anterior to the left.
Fig. S3.
Control and isl1 mutants show similar numbers of isl1 and pax6b mRNA expressing cell in the pancreatic islets.
Fig. S4.
Dosedependent reduction of trypsin expression following genetic and morpholino-based loss of isl gene function at 76 hpf. Examples of trypsin mRNA stainings that were summarized in Fig. 5C. Shown are control (A–D) and isl1 mutant embryos (E–H) that were injected with indicated combinations of isl2a and isl2b morpholinos. Images show a dorsal view with anterior to the left. (I) Quantification of the length of trypsin mRNA expression in control and isl1 mutant embryos injected with the indicated morpholinos. Bars show mean+SEM.
Fig. S5.
Trypsin mRNA expression following genetic and morpholino-based loss of isl gene function at 96 hpf. Note that isl1 mutants and isl2a/2b double morphants display similar trypsin pattern.
Fig. S6.
Pancreatic mesenchyme in control and isl1 mutant/ isl2a & isl2b morphant embryos (48 hpf). Expression pattern of mesenchymal fgf24 (A–H) and meis3 (I–P). Single or multiple loss of isl genes has no impact on the mRNA expression pattern. Images show a ventral view with anterior to the left.
Supplementary material
References
- Ahlgren U., Pfaff S.L., Jessell T.M., Edlund T., Edlund H. Independent requirement for ISL1 in formation of pancreatic mesenchyme and islet cells. Nature. 1997;385:257–260. doi: 10.1038/385257a0. [DOI] [PubMed] [Google Scholar]
- Argenton F., Zecchin E., Bortolussi M. Early appearance of pancreatic hormone-expressing cells in the zebrafish embryo. Mech. Dev. 1999;87:217–221. doi: 10.1016/s0925-4773(99)00151-3. [DOI] [PubMed] [Google Scholar]
- Arkhipova V., Wendik B., Devos N., Ek O., Peers B., Meyer D. Characterization and regulation of the hb9/mnx1 beta-cell progenitor specific enhancer in zebrafish. Dev. Biol. 2012;365:290–302. doi: 10.1016/j.ydbio.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson S., Kwasniewski M., Riano-Pachon D.M., Mueller-Roeber B. QuantPrime--a flexible tool for reliable high-throughput primer design for quantitative PCR. BMC Bioinform. 2008;9:465. doi: 10.1186/1471-2105-9-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biemar F., Argenton F., Schmidtke R., Epperlein S., Peers B., Driever W. Pancreas development in zebrafish: early dispersed appearance of endocrine hormone expressing cells and their convergence to form the definitive islet. Dev. Biol. 2001;230:189–203. doi: 10.1006/dbio.2000.0103. [DOI] [PubMed] [Google Scholar]
- Brennand K., Huangfu D., Melton D. All beta cells contribute equally to islet growth and maintenance. PLoS Biol. 2007;5:e163. doi: 10.1371/journal.pbio.0050163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pater E., Clijsters L., Marques S.R., Lin Y.F., Garavito-Aguilar Z.V., Yelon D., Bakkers J. Distinct phases of cardiomyocyte differentiation regulate growth of the zebrafish heart. Development. 2009;136:1633–1641. doi: 10.1242/dev.030924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong P., Munson C., Norton W., Crosnier C., Pan X., Gong Z., Neumann C., Stainier D. Fgf10 regulates hepatopancreatic ductal system patterning and differentiation. Nat. Genet. 2007;39:397–402. doi: 10.1038/ng1961. [DOI] [PubMed] [Google Scholar]
- Du A., Hunter C., Murray J., Noble D., Cai C.-L., Evans S., Stein R., May C. Islet-1 is required for the maturation, proliferation, and survival of the endocrine pancreas. Diabetes. 2009;58:2059–2069. doi: 10.2337/db08-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eames S., Philipson L., Prince V., Kinkel M. Blood sugar measurement in zebrafish reveals dynamics of glucose homeostasis. Zebrafish. 2010;7:205–213. doi: 10.1089/zeb.2009.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H., Dong P., Beis D., Stainier D. Formation of the digestive system in zebrafish. II. Pancreas morphogenesis. Dev. Biol. 2003;261:197–208. doi: 10.1016/s0012-1606(03)00308-7. [DOI] [PubMed] [Google Scholar]
- Fischer S., Draper B., Neumann C. The zebrafish fgf24 mutant identifies an additional level of Fgf signaling involved in vertebrate forelimb initiation. Development (Cambridge, England) 2003;130:3515–3524. doi: 10.1242/dev.00537. [DOI] [PubMed] [Google Scholar]
- Flanagan-Steet H., Fox M.A., Meyer D., Sanes J.R. Neuromuscular synapses can form in vivo by incorporation of initially aneural postsynaptic specializations. Development (Cambridge, England) 2005;132:4471–4481. doi: 10.1242/dev.02044. [DOI] [PubMed] [Google Scholar]
- Gittes G. Developmental biology of the pancreas: a comprehensive review. Dev. Biol. 2009;326:4–35. doi: 10.1016/j.ydbio.2008.10.024. [DOI] [PubMed] [Google Scholar]
- Gong Z., Hui C.C., Hew C.L. Presence of isl-1-related LIM domain homeobox genes in teleost and their similar patterns of expression in brain and spinal cord. J. Biol. Chem. 1995;270:3335–3345. doi: 10.1074/jbc.270.7.3335. [DOI] [PubMed] [Google Scholar]
- Guo T., Wang W., Zhang H., Liu Y., Chen P., Ma K., Zhou C. ISL1 promotes pancreatic islet cell proliferation. PloS One. 2011;6:e22387. doi: 10.1371/journal.pone.0022387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison K.A., Thaler J., Pfaff S.L., Gu H., Kehrl J.H. Pancreas dorsal lobe agenesis and abnormal islets of Langerhans in Hlxb9-deficient mice. Nat. Genet. 1999;23:71–75. doi: 10.1038/12674. [DOI] [PubMed] [Google Scholar]
- Hauptmann G., Gerster T. Multicolor whole-mount in situ hybridization. Methods Mol. Biol. (Clifton, N.J.) 2000;137:139–148. doi: 10.1385/1-59259-066-7:139. [DOI] [PubMed] [Google Scholar]
- Hesselson D., Anderson R., Beinat M., Stainier D. Distinct populations of quiescent and proliferative pancreatic beta-cells identified by HOTcre mediated labeling. Proc. Natl. Acad. Sci. USA. 2009;106:14896–14901. doi: 10.1073/pnas.0906348106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter C.S., Dixit S., Cohen T., Ediger B., Wilcox C., Ferreira M., Westphal H., Stein R., May C.L. Islet alpha, beta, and delta cell development is controlled by the Ldb1 coregulator, acting primarily with the islet-1 transcription factor. Diabetes. 2013;62:875–886. doi: 10.2337/db12-0952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson S., Eisen J. Islet1 and Islet2 have equivalent abilities to promote motoneuron formation and to specify motoneuron subtype identity. Development (Cambridge, England) 2006;133:2137–2147. doi: 10.1242/dev.02355. [DOI] [PubMed] [Google Scholar]
- Jacquemin P., Yoshitomi H., Kashima Y., Rousseau G.G., Lemaigre F.P., Zaret K.S. An endothelial-mesenchymal relay pathway regulates early phases of pancreas development. Dev. Biol. 2006;290:189–199. doi: 10.1016/j.ydbio.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Jurczyk A., Roy N., Bajwa R., Gut P., Lipson K., Yang C., Covassin L., Racki W.J., Rossini A.A., Phillips N., Stainier D.Y., Greiner D.L., Brehm M.A., Bortell R., diIorio P. Dynamic glucoregulation and mammalian-like responses to metabolic and developmental disruption in zebrafish. Gen. Comp. Endocrinol. 2011;170:334–345. doi: 10.1016/j.ygcen.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson O., Thor S., Norberg T., Ohlsson H., Edlund T. Insulin gene enhancer binding protein Isl-1 is a member of a novel class of proteins containing both a homeo- and a cys-his domain. Nature. 1990;344:879–882. doi: 10.1038/344879a0. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y., Segawa H., Tokumoto M., Tsubokawa T., Hotta Y., Uyemura K., Okamoto H. Ocular and cerebellar defects in zebrafish induced by overexpression of the LIM domains of the islet-3 LIM/homeodomain protein. Neuron. 1997;18:369–382. doi: 10.1016/s0896-6273(00)81239-8. [DOI] [PubMed] [Google Scholar]
- Kimmel C.B., Ballard W.W., Kimmel S.R., Ullmann B., Schilling T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. (official publication of the American Association of 203) 1995:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kimmel R., Meyer D. Molecular regulation of pancreas development in zebrafish. Methods Cell Biol. 2010;100:261–280. doi: 10.1016/B978-0-12-384892-5.00010-4. [DOI] [PubMed] [Google Scholar]
- Kimmel R., Onder L., Wilfinger A., Ellertsdottir E., Meyer D. Requirement for Pdx1 in specification of latent endocrine progenitors in zebrafish. BMC Biol. 2011;9:75. doi: 10.1186/1741-7007-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkel M., Prince V. On the diabetic menu: zebrafish as a model for pancreas development and function. BioEssays. 2009;31:139–152. doi: 10.1002/bies.200800123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer-Zucker A.G., Olale F., Haycraft C.J., Yoder B.K., Schier A.F., Drummond I.A. Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer’s vesicle is required for normal organogenesis. Development. 2005;132:1907–1921. doi: 10.1242/dev.01772. [DOI] [PubMed] [Google Scholar]
- Lammert E., Cleaver O., Melton D. Role of endothelial cells in early pancreas and liver development. Mech. Dev. 2003;120:59–64. doi: 10.1016/s0925-4773(02)00332-5. [DOI] [PubMed] [Google Scholar]
- Larsson L.I., Madsen O.D., Serup P., Jonsson J., Edlund H. Pancreatic-duodenal homeobox 1 -role in gastric endocrine patterning. Mech. Dev. 1996;60:175–184. doi: 10.1016/s0925-4773(96)00609-0. [DOI] [PubMed] [Google Scholar]
- Li H., Edlund H. Persistent expression of Hlxb9 in the pancreatic epithelium impairs pancreatic development. Dev. Biol. 2001;240:247–253. doi: 10.1006/dbio.2001.0440. [DOI] [PubMed] [Google Scholar]
- Li Y., Zhang Y., He B., Wang Y., Yuan Z., Yuan W., Liao P., Deng Y., Xiao J., Zhu C., Wang Y., Wu X., Liu M. Cloning and expression of a novel human gene, Isl-2, encoded a LIM-homeodomain protein. Mol. Biol. Rep. 2007;34:19–26. doi: 10.1007/s11033-006-9003-0. [DOI] [PubMed] [Google Scholar]
- Liu J., Hunter C., Du A., Ediger B., Walp E., Murray J., Stein R., May C. Islet-1 regulates Arx transcription during pancreatic islet alpha-cell development. J. Biol. Chem. 2011;286:15352–15360. doi: 10.1074/jbc.M111.231670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Walp E., May C.L. Elevation of transcription factor Islet-1 levels in vivo increases beta-cell function but not beta-cell mass. Islets. 2012;4 doi: 10.4161/isl.19982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfroid I., Delporte F., Baudhuin A., Motte P., Neumann C., Voz M., Martial J., Peers B. Reciprocal endoderm-mesoderm interactions mediated by fgf24 and fgf10 govern pancreas development. Development (Cambridge, England) 2007;134:4011–4021. doi: 10.1242/dev.007823. [DOI] [PubMed] [Google Scholar]
- May C.L. The role of Islet-1 in the endocrine pancreas: Lessons from pancreas specific Islet-1 deficient mice. Islets. 2010;2:121–123. doi: 10.4161/isl.2.2.10908. [DOI] [PubMed] [Google Scholar]
- Miura H., Yanazawa M., Kato K., Kitamura K. Expression of a novel aristaless related homeobox gene ‘Arx’ in the vertebrate telencephalon, diencephalon and floor plate. Mech. Dev. 1997;65:99–109. doi: 10.1016/s0925-4773(97)00062-2. [DOI] [PubMed] [Google Scholar]
- Moro E., Gnugge L., Braghetta P., Bortolussi M., Argenton F. Analysis of beta cell proliferation dynamics in zebrafish. Dev. Biol. 2009;332:299–308. doi: 10.1016/j.ydbio.2009.05.576. [DOI] [PubMed] [Google Scholar]
- Obholzer N., Wolfson S., Trapani J., Mo W., Nechiporuk A., Busch-Nentwich E., Seiler C., Sidi S., Sollner C., Duncan R., Boehland A., Nicolson T. Vesicular glutamate transporter 3 is required for synaptic transmission in zebrafish hair cells. J. Neurosci. 2008;28:2110–2118. doi: 10.1523/JNEUROSCI.5230-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offield M.F., Jetton T.L., Labosky P.A., Ray M., Stein R.W., Magnuson M.A., Hogan B.L., Wright C.V. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122:983–995. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- Pan F., Wright C. Pancreas organogenesis: from bud to plexus to gland. Dev. Dyn. 2011;240:530–565. doi: 10.1002/dvdy.22584. [DOI] [PubMed] [Google Scholar]
- Puschel A.W., Gruss P., Westerfield M. Sequence and expression pattern of pax-6 are highly conserved between zebrafish and mice. Development (Cambridge, England) 1992;114:643–651. doi: 10.1242/dev.114.3.643. [DOI] [PubMed] [Google Scholar]
- Sagerstrom C.G., Kao B.A., Lane M.E., Sive H. Isolation and characterization of posteriorly restricted genes in the zebrafish gastrula. Dev. Dyn. 2001;220:402–408. doi: 10.1002/dvdy.1119. [DOI] [PubMed] [Google Scholar]
- Stainier D.Y., Weinstein B.M., Detrich H.W., 3rd, Zon L.I., Fishman M.C. Cloche, an early acting zebrafish gene, is required by both the endothelial and hematopoietic lineages. Development. 1995;121:3141–3150. doi: 10.1242/dev.121.10.3141. [DOI] [PubMed] [Google Scholar]
- Sun Y., Dykes I.M., Liang X., Eng S.R., Evans S.M., Turner E.E. A central role for Islet1 in sensory neuron development linking sensory and spinal gene regulatory programs. Nat. Neurosci. 2008;11:1283–1293. doi: 10.1038/nn.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiso N., Moro E., Argenton F. Zebrafish pancreas development. Mol. Cell Endocrinol. 2009;312:24–30. doi: 10.1016/j.mce.2009.04.018. [DOI] [PubMed] [Google Scholar]
- Tokumoto M., Gong Z., Tsubokawa T., Hew C.L., Uyemura K., Hotta Y., Okamoto H. Molecular heterogeneity among primary motoneurons and within myotomes revealed by the differential mRNA expression of novel islet-1 homologs in embryonic zebrafish. Dev. Biol. 1995;171:578–589. doi: 10.1006/dbio.1995.1306. [DOI] [PubMed] [Google Scholar]
- Wan H., Korzh S., Li Z., Mudumana S., Korzh V., Jiang Y.-J., Lin S., Gong Z. Analyses of pancreas development by generation of gfp transgenic zebrafish using an exocrine pancreas-specific elastaseA gene promoter. Exp. Cell Res. 2006;312:1526–1539. doi: 10.1016/j.yexcr.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Wang Y., Rovira M., Yusuff S., Parsons M. Genetic inducible fate mapping in larval zebrafish reveals origins of adult insulin-producing beta-cells. Development (Cambridge, England) 2011;138:609–617. doi: 10.1242/dev.059097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee N., Lorent K., Pack M. Exocrine pancreas development in zebrafish. Dev. Biol. 2005;284:84–101. doi: 10.1016/j.ydbio.2005.04.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material