Abstract
Background
Mesenchymal Stromal Cells (MSC) are gaining in popularity as an experimental therapy for a number of conditions that often require expansion ex vivo prior to use. Data comparing clinical-grade MSC from various ages of donors is scant. We hypothesized that MSC from older donors may display differences in cellular fitness when expanded for clinical use.
Methods
We evaluated the expression of several markers of aging, oxidative stress, and growth kinetics, and telomere length in MSCs obtained from a wide age range (8 months to 58 years).
Results
To evaluate cellular fitness we compared MSC expanded from younger (8 months - 6 years) versus older (38 - 58 years) donors in terms of selected cell surface markers, lipofuscin, migration ability, telomere length, and expression of iNOS, PGE2, p16INK, and SOD. Results did not differ between these groups. Neither SOD activity (0.025 vs 0.028 U/ml) nor death after oxidative challenge were significantly different (1% vs 1.5%, p=0.14). We did find that although MSC from older individuals produced slightly fewer cells over a 28-day culture period and have slightly longer doubling time (54 hrs vs 42 hrs), a satisfactory clinical product can still be ob tained regardless of age cohort.
Discussion
Collectively, these data show that MSC can be expanded without significant alterations in expansile properties or obvious changes in parameters associated with senescence. Because cellular fitness was equivalent in these cohorts, MSC from donors up to age 58 years can be used as a source of cells for cellular therapy.
Keywords: Mesenchymal stromal cell, aging, oxidative stress, bone marrow stroma, telomere
Introduction
Mesenchymal stromal cells (MSC), the supporting cells in the bone marrow microenvironment, were definitively described in the mid-1970's by Friedenstein et al. as a fibroblastic shaped cell with plastic adherent properties (1, 2). Their popularity has grown in the recent years due to their multiple biologic effects and reported multilineage differentiation abilities. Classically, they differentiate into osteoblasts, adipocytes, and cartilage, but others have also cultured neurons and smooth muscle from MSC (3, 4). They also have a potent immunosuppressive property that may be both contact and non-contact mediated (5, 6). MSC are in clinical studies for a wide range of diseases including graft-versus-host disease following bone marrow transplant, myocardial infarction, amyotrophic lateral sclerosis, and Crohn's disease (7, 8). MSC have considerable regenerative capacity with expansion potential of 4-5 logs, but at the end of this expansive lifespan, they do undergo cellular senescence (9, 10). Some work has been done to characterize the “aging” process of MSC in vitro. As measured by shortening of telomeres, increased DNA damage, and increased oxidation (11, 12), there does occur a loss of cellular “fitness”. With prolonged culture, MSC can become karyotypically abnormal which may pose a risk of tumorigenesis (13, 14). Although, tumorigenesis of human MSC in mice has not yet been shown, this is not true with murine-derived MSC, which have been shown to be karyotypically unstable and form tumors in mice (15).
While cellular aging occurs during in vitro culture expansion, the effect of donor age on the “cellular health” of MSC has not been intensely investigated, although studies have shown that MSC from older individuals undergo earlier cellular senescence after extended time in culture as evidenced by a recent report that fetal MSC can be expanded to larger numbers in culture and senesce later than MSC from post-natal sources (16). One study has shown that there is some decrease in colony forming unit-fibroblast (CFU-F) numbers as well as cell surface maker changes in MSC from “aged” donors compared to that of younger donors (11). The same study also showed that MSC from older donors undergo increased oxidative damage and have higher reactive oxygen species (ROS) though the number of individuals was small (3 per age group) and some of the critical incubation conditions (e.g. oxygen tension) were not provided.
We have isolated a wide range of MSC “lines” from donors of various ages (8 months – 58 years) that were expanded under conditions identical to those for a clinical product. Specifically, MSC were expanded in a 5% oxygen environment, thereby mimicking the bone marrow microenvironment and permitting cells to grow more robustly and with less oxidative stress than the traditional 21% oxygen concentration (17-19). MSC were also cultured no longer than 21-28 days (about 3 passages), since more prolonged culture times may increase karyotypic abnormalities. While all MSC isolated were robust appearing and differentiated equally irrespective of donor age, we sought to test the hypothesis that MSC isolated from donors of different ages display differences in “cellular fitness”. After measuring multiple markers of cellular fitness and quantifying total culture yield, we find that clinical-grade MSC have similar fitness regardless of donor age. Growth curve data showed the total cell yield tended to be higher from younger donors (2.7×1010 vs 3.8×109, p=0.08), although sufficient MSC (≥ 106/kg recipient body weight) were readily obtained from any aged donor. Notably, our poorest expansion yielded sufficient MSC to provide 14 doses at 106 cells/kg for a 70 kg person. Taken together, we conclude that MSC expanded for clinical use have similar cellular “fitness” at donor ages up to the highest age tested, age 58 years.
Materials and Methods
Human MSC Isolation
The filters from bone marrow harvest kits (Baxter, Deerfield, IL) were obtained after healthy donors underwent a bone marrow harvest. The filters were each cut into two pieces and washed with 40 milliliters MSC medium consisting of minimal essential medium-alpha (Life Technologies, Carlsbad, CA) with 10% FBS (Hyclone, Omaha, NE), 1X Glutamax, and 1X Penicillin-Streptomycin. This solution was layered over an equal volume of Isolymph (CTL Scientific Supply Corp., Deer Park, NY) and centrifuged at 400 × g for 30 minutes (without brake). The middle layer containing the bone marrow mononuclear cells (BM MCs) was removed, washed with phosphate buffered saline (PBS), and centrifuged at 400 × g for 5 minutes. The BMMCs were re-suspended in MSC medium, plated, and incubated at 37 °C with 5% O2. After 24 hours, non-adhered cells were removed. Adherent cells were washed with PBS and fresh media was added every 3-4 days. Cells were harvested at 80% confluency using 0.25% trypsin/0.53 mM EDTA (Life Technologies, Carlsbad, CA) for 10 minutes at room temperature. Cells were re-plated at a density of 500 cells/cm2 and expanded. The use of all tissue was approved by the Committee on the Use of Human Subjects in Research at the University of Minnesota.
Flow Cytometry
Single-cell suspensions of MSC were prepared in buffer (PBS + 2% fetal bovine serum). Flow Cytometry was performed using directly conjugated mAb (FITC or PE) to assess cell surface antigen expression. MSC were and incubated for 30 minutes at 4°C with the antibody of interest, washed once with PBS, and analyzed by FACS. The following mAbs were obtained from BD Biosciences (San Jose, CA): anti-CD31, anti-CD34, anti-CD43, anti-CD45, anti-CD29, anti-CD166, anti-CD73, anti-CD90, Annexin V. Anti-CD105 mAb was obtained from eBioscience (San Diego, CA). Samples were analyzed on a FACSCanto (Becton Dickinson, San Jose, CA) using FACSDiva software. Forward and 90 degree side-scatter were used to identity and gate the live cell population.
Positively gated cells were determined using an isotype control antibody. A minimum of 10,000 events was examined.
Sudan Black Staining
25,000 MSCs were plated in each well of a 12-well plate and incubated at 37°C for 2 days. Cells were then rinsed with 70% ethanol to fix and stained with saturated Sudan Black (Sigma) for 2 hours at room temperature. Cells were photographed at 10X magnification and 8-bit gray-scale. The entire image was processed with ImageJ; setting a gray threshold level of 137 then using the particle analysis function to determine number of stained vesicles which was then normalized to the number of cells in the field determined by hand count.
Western Blot
Human MSC were lysed using 50uL of reporter lysis buffer (Invitrogen, Carlsbad, CA). Samples were placed on ice for 20 minutes, and then centrifuged at 10000 × g at 4°C for 2 minutes. The protein concentrations were normalized using the Coomassie Plus Assay Kit (Thermo Scientific, Rockford, IL) per the manufacturer's instructions. 50 micrograms of protein was separated on a 10% Bis/Tris gel using XCell SureLock Mini-Cell system (Invitrogen) following the manufacturer's instructions. Protein was transferred to a polyvinylidene difluoride membrane and blocked in PBS with 5% non-fat milk. Antibodies used for western blot included anti-SOD-2 (Santa Cruz, Santa Cruz, CA) and anti-p16INK (Santa Cruz), anti-iNOS (Abcam, Cambridge, MA), anti-progerin (Santa Cruz), and anti-β-actin (Cell Signaling Technology, Danvers, MA). Primary antibodies were applied for 1 hour at room temperature followed by the addition of goat anti-primary species IgG linked to horse radish peroxidase (Santa Cruz) at 1:5000 for 1 hour. Blots were washed and developed using ECL Western Blotting Analysis System (GE Healthcare, Piscataway, NJ).
Hydrogen Peroxide Assay
100,000 MSCs were plated in each well of a 12-well plate and incubated overnight at 37°C. Hydrogen Peroxide (Sigma, St. Louis, MO) was added to the culture to 150 μM and incubated at 37°C for 1 hour. Cells were harvested using 0.05% trypsin/0.53mM EDTA for 10 minutes at room temperature, washed, and stained using Annexin V-FITC Apoptosis Detection Kit per the manufacturer's instructions (BD Pharmingen, San Jose, CA). Ratios of annexin V staining post- to pre- hydrogen peroxide treatment were calculated.
Superoxide Dismutase Assay
Superoxide Dismutase activity was assessed using the Superoxide Dismutase Assay Kit II (Calbiochem, San Diego, CA). Briefly, 100,000 MSC were plated in each well of a 6-well plate and incubated at 37°C for 3 days. Cells were removed by scraping, pelleted at 400 × g for 5 minutes, and resuspended in 150 microliters of cold 2 0 mM HEPES buffer, pH 7.2, containing 1mM EGTA, 210 mM mannitol, and 70 mM sucrose. Cells were pulse-sonicated 3 times for 10 seconds each on ice, centrifuged at 1500 × g for 5 minutes at 4°C, and the supernatant was collected. The assay was continued per manufacturers instructions using 5 microliters of sample. SOD results were normalized to protein using the Coomassie Plus Assay Kit (Thermo Scientific) per the manufacturer's instructions.
Migration assay
Migration was assessed using the Cultrex 24-Well Cell Migration Assay (Trevigen Gaithersburg, MD). After overnight starvation in medium with 0.2% fetal bovine serum, 50,000 MSCs were combined with 5 mg/ml Cultrex Beta-mercaptoethanol (BME) Growth Factor Reduced Pathclear. This BME/cell mixture was added to the top chamber of each well and plates incubated at 37°C for 1 hour. After incubation, 0.5 milliliters of MSC medium was added to the bottom chamber and the combined apparatus was incubated at 37°C for 24 hours. Migrated cells in the lower chamber were then washed, dissociated, and the number of cells migrated was determined following manufacturers instructions using Calcein-AM.
Prostaglandin Production
Cells were grown to 80% confluence in a 12-well plate and culture media changed 1 day prior to the assay. The supernatant was centrifuged to clarify and PGE2 was assayed by Parameter™ PGE2 assay kit according to the manufacturer's instructions (R&D Systems, Minneapolis, MN).
Telomere Length Analysis
MSC were isolated from donors of various ages. Prior to passage 4 (28 days in culture), DNA was harvested from one million cells by Gentra Puregene Cell Kit (Qiagen, Valencia, CA). One microgram of DNA was digested with Hinf III and Rsa I, separated by gel electrophoresis, transferred to positively charged nylon by vacuum, and dried. Telomeric DNA was determined by hybridization to a digoxigenin-labeled telomere probe: (TTAGGG)3. Detection was by a horseradish peroxidase labeled anti-Dig antibody (Roche, Indianapolis, IN) and chemiluminescence. Average telomere lengths were determined by scanning the resulting Southern blot and using the Telometric™ software program for the analysis (20).
Results
We have isolated MSC from bone marrow donors across a wide range of ages. MSC were derived from the bone marrow harvest filters following the harvest. These filters have been previously shown to be a rich source of MSC (21, 22). In our observations of culturing cells across a wide range of donor ages (8 months to 58 years), MSC showed no phenotypic differences in cell shape or size with respect to age of the donor under light microscopy (Figure 1). Typically MSC age through repeated passage and their morphology becoming flatter and broader in shape and eventually entering into senescence (11). Though there is no known marker to quantify the differentiation of MSC, our evaluation of over 40 differentiated MSC lines showed no qualitative age-dependent differences in the ability to become osteocytes, adipocytes, and chondrocytes (shown by histologic staining) prior to passage four (data not shown).
Figure 1.
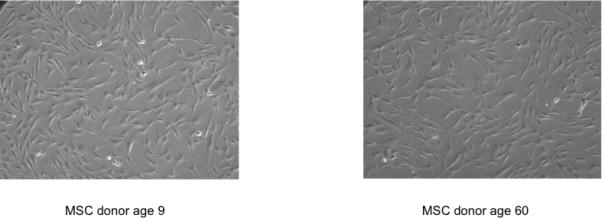
The microscopic phenotype of MSC is similar from donors 49 years apart. MSC were isolated from marrow as described and photographed under phase-contrast microscopy at 10× magnification after 17 days in culture prior to the third passage.
To evaluate for initial age-related differences, the initial growth kinetics and cell surface expression of commonly profiled MSC markers (both negative and positive) were analyzed in 41 MSC cultures. We found no significant difference in any cell surface markers with respect to age shown in Table 1. MSC from older don ors had a slightly longer doubling time than from younger donors, which varied depending on the timepoint in culture that was assessed. As shown in Figure 2, doubling time was significantly greater for the older (18 - 58 yrs) donor MSC at passages 3 and 4 but not at passage 2.
Table 1.
Cell surface analysis of MSC shows no difference between age groups. Approximately 250,000 MSC were harvest 15 days after isolation and subject to FACS analysis with antibodies as stated in the table. Each antibody was conjugated with phycoerythrin and the percentage of positive gating cells were determined based on a phycoerythrin labeled isotype control antibody. A Student's t-test was used to compare each age group and p > 0.05 was found in each comparison indicating no significant differences were found between the groups.
| Age (yrs) | CD31 | CD34 | CD43 | CD45 | CD29 | CD105 | CD166 | CD73 | CD90 | Annexin V |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.8 – 17 (n=25) | 0.77 | 0.44 | 1.27 | 1.35 | 97 | 98 | 98 | 99 | 96 | 2.63 |
| 18 - 58 (n=16) | 1.01 | 0.76 | 0.38 | 1.37 | 97 | 97 | 96 | 99 | 95 | 2.21 |
| p-value | 0.51 | 0.53 | 0.17 | 0.97 | 0.58 | 0.10 | 0.10 | 0.94 | 0.63 | 0.45 |
Figure 2.
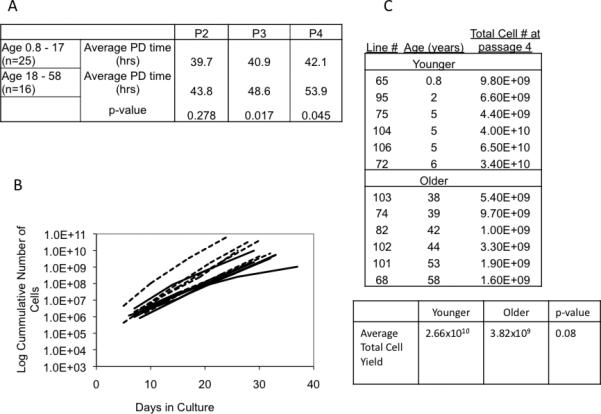
Initial growth properties of MSC are similar regardless of donor age. Panel A. Population doubling time up to the fourth passage (P2, P3, P4) of MSC from various aged donors. The p-value was calculated from a Student's t-test. Panel B shows the growth curves of MSC of the six youngest (8 mo - 6 years dashed line) and six oldest (ages 38 - 58 years solid line). Panel C. MSC were derived as previously described and the cumulative cell number was determined within the 28 days in culture.
We next assessed for age-related differences in a subgroup of our initial dataset, focused on the 6 youngest don ors (8 months – 6 years) and the 6 oldest donors (38 – 58 years) thereby maximizing the possibility of locating phenotypic differences that might be age-related. The population doubling time differences at the second to fourth passages are reflected in the slope of the growth curves of these subsets of MSC shown in Figure 2B.
For a clinical product a cell dose of 106, cells per kg is a common choice; therefore a 70 kg person would require 7 × 10 cells. In every MSC expansion we performed, the total MSC number produced within 28 days ranged between 109 and 1010 cells. As shown in Figure 2C, even our lowest expansion gave 109 cells (age 42 years) and our highest yield 6.5 × 1010 gave cells (age 5 years). Although the average yield was greater from the younger donors versus the older donors, this did not reach statistical significance (p=0.08). Whereas it is possible that there may be fewer actual mesenchymal stem cells (referring to the actual progenitors of the MSC clinical product) in the initial isolation from older donors than from younger donors, this is difficult to determine as there are no reliable a priori MSC markers though we estimated the original starting population of MSC as a fraction of the bone marrow mononuclear cells in our experiments to be 0.00018 % for donors 0.8 - 17 years old (n=25) and 0.00010 % for donors 18 - 58 years (n=15 and p=0.01) (starting MSC estimate = # cells P1/BMMC in aspirate/time in culture (hours)/doubling time at P2 (hours)). Thus, despite these differences in growth kinetics, an abundant amount of MSC could be obtained from a donor of any age suitable for in vivo infusion.
As cells age they accumulate vesicles and pigment known as lipofuscin which can be stained using Sudan black (23). We stained 9 MSC lines from various ages of donors with Sudan Black and found no difference in staining between younger MSC donors (ages 8 months to 5 years) versus older donors (ages 51 to 58 years) (Figure 3).
Figure 3.
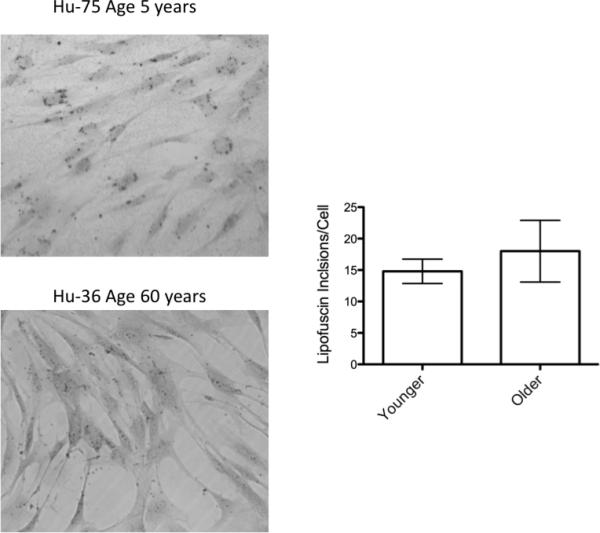
No difference in lipofuscin staining in MSC of various age donors. MSC were isolated as described in the Methods section. Prior to 28 days in culture (between passage three and four), cells were washed and stained with Sudan Black to detect lipofuscin deposits (n = 5 younger and 4 older donors). Photos taken by bright field microscopy at 10X magnification. A representative photo is shown on the left. Lipofuscin stained vesicles were quantified by a particle counting algorithm in ImageJ and normalized to number of cells. A Student's t-test was performed on the data and indicated no significance was found with a p-value of 0.53.
Finding there were no substantial histological or MSC surface marker differences in MSC with regard to age, we next investigated several biological functions known to change with age. All assays were performed on MSC less than 28 days in culture. The first assays tested markers related to oxidative stress which are generally found to increase in aging experimental models; this includes the ability of cells to directly handle oxidative stress (24, 25). Our first evaluation was of superoxide dismutase (SOD) which is involved in the catabolism of reactive oxygen species and regarded as an antioxidant (26). Therefore, the expression level of SOD was evaluated in the subset of the 6 youngest and 6 oldest MSC lines we had derived. SOD activity, previously shown to decline with increased donor age, also was measured (11). No difference in either MSC SOD expression or functional activity in MSC was seen with respect to age (Figure 4). An oxidative challenge can be used to evaluate how cells respond to high levels of reactive oxygen species, and this response is often decreasing with increased time in culture. No difference in the ability of MSC to respond to an oxidative challenge was observed using a hydrogen peroxide assay (Figure 4). These data support the notion that MSC have similar antioxidant abilities regardless of donor age.
Figure 4.
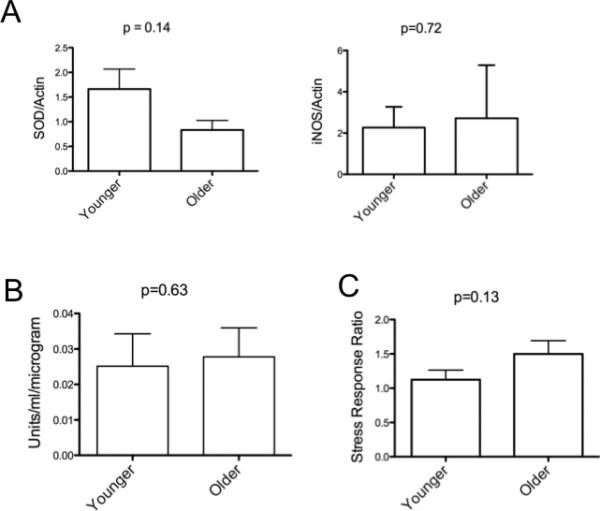
Markers of oxidative stress are not different between young and older MSC donors. MSC from same 2 donors groups described in Figure 2 were harvested 26 days after isolation (this equates to 3 passages). Panel A. Protein from whole cell lysates was immunoblotted with antibody to SOD or iNOS (or actin for no rmalization) and signal quantified by scanning densitometry. Results displayed as minimum-maximum boxed values as well as the mean for the group. Error bars represent standard deviation, and the p-value was calculated from a Student's t-test. Panel B. 100,000 MSCs (after 20 days in culture) were plated in each well of a 6-well plate at incubated at 37°C for 3 days. The supernatant from clarified cell lysates was collected and the SOD assay was performed using the Superoxide Dismutase Assay Kit II (Calbiochem) per the manufacturer's instructions and results were normalized to protein using by Bradford assay. Data represent 3 independent experiments. Panel C. Oxidative challenge by exposure to hydrogen peroxide in young versus old donor derived MSC. 100,000 MSC at 20 days post isolation were plated in each well of a 12-well plate and incubated overnight at 37°C. Hydrogen Peroxide was added to the culture to 150 μM and cells incubated at 37°C for 1 hour. Cells were stained for apoptosis using an Annexin V-FITC antibody. Data is displayed as ratio of cells staining for Annexin V post vs pre treatment with mean and standard deviation indicated. The p-value was calculated from a Student's t-test.
Inducible Nitric Oxide Synthase (iNOS) is involved in inflammatory processes and can be regulated by downstream effects of SOD (27). Chronic inflammation involving iNOS activation may contribute to the aging process (28). Therefore, we next quantified the expression levels of iNOS. Similar to SOD, iNOS levels in MSC were unaffected by age as shown in Figure 4.
Additional inflammatory markers of aging include cyclooxygenase-2 (COX2) which regulates the production prostaglandin (PGE2 in cells) production in cells. COX2 and PGE2 production has variably been found to be increased or decreased in association with aging, dependent upon the tissue or model being studied (29-33). In early passage MSC, we found no difference in PGE2 production with regard to donor age as shown in Figure 5. Another phenomenon related to prostaglandin synthesis is cytoskeleton organization changes (34). It has been shown that as cells age they become less migratory in the case of rat MSC (25). Our evaluation of MSC migration in Figure 5 shows that there is no difference in the migratory ability of MSC with respect to the age of the donor.
Figure 5.
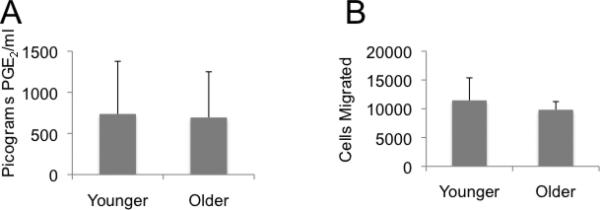
PGE2 production and cell migration from younger and older donor derived MSC are similar. Panel A. Cells were cultured to 80% confluence after 20 days in culture in a 12-well plate. PGE2 in the supernatant was assayed by Parameter™ PGE2 assay kit according to the manufacturer's instructions (R&D Systems). A Student's t-test of the results shows p=0.73. Panel B. Migration of the same groups of MSC was assessed using Cultrex 24-Well Cell Migration Assay. After overnight serum starvation, 50,000 MSC were then evaluated for their ability to migrate toward a serum source using a chamber assay for 24 hours. Afterwards, the number of migrated cells was determined. A Student's t-test of the results shows p=0.40.
Recently, a novel finding has shown the structural nuclear protein progerin to be associated with the aging process (35, 36). Progerin is a mutated form of the nuclear protein lamin A causing one of the classic aging syndromes, Huthinson-Gilford Syndrome (37). Wild-type cells also can accumulate progerin over time, which may play a role in the normal aging process (38). Interestingly, there was a statistical trend toward a higher progerin level of expression in MSC derived from older donors (Figure 6). Whether progerin will be a robust and reliable marker of aging for MSC has yet to be determined.
Figure 6.
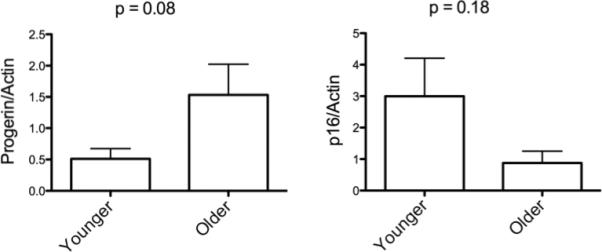
Expression of other Progerin or P16INK are not different between younger and older donor derived MSC. MSC from the younger and older donors groups were harvested 20 days after isolation. Whole cell lysates were immunoblotted with antibody as denoted (anti-actin for normalization) and signal quantified by scanning densitometry. Y-axis indicates relative expression to actin. Results displayed as minimum-maximum boxed values and the mean for the group. Error bars represent standard deviation.
Another important nuclear protein that is a marker of the aging process is p16INK which is an inhibitor for CDK4 and CDK16 and negatively regulates cell-cycle progression leading to a decrease in cell proliferation (correlating to an increase in senescence) (39). It has been previously shown that P16INK accumulates as MSC age in vitro (35, 40). Our evaluation of p16INK measured early in culture showed some variability in expression in younger donor, but no significant difference in expression le vels according to age (Figure 6).
A widely recognized phenomenon that occurs as MSC age in culture is telomere attrition due to the fact that MSC typically lack telomerase (9). Telomeres shorten linearly and consistently during extended culture periods. Although one group determined there was no correlation between telomere length and MSC donor age at the time of second passage (n=28), telomere length as related to age, though most of the donors were over 20 years old (40). We examined the telomeres from 38 of our MSC lines (11 were age 12 or less) prior to 28 days in culture and also found there was no statistically significant correlation between age and telomere length agreeing with the prior observation but increasing the younger age range of evaluation (Figure 7).
Figure 7.
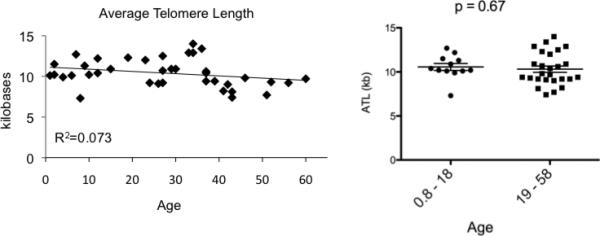
The average telomere length (ATL) of early passage MSC does not correlate to donor age. MSC were isolated from donors of various ages. At 28 days in culture, DNA was harvested from one million cells, digested by restriction enzymes and subject to Southern blot using a dig-labeled telomere probe: (TTAGGG)3. Average telomere lengths were determined by scanning the resulting Southern blot and using the Telometric™ software program for the analysis (20). Pearson correlation for ATL was -0.270, p-value = 0.101. Results in Panel B are displayed comparing the age groups 0.8 - 18 years and 19 - 58 years where a Student's t-test showed p = 0.67.
Discussion
We have shown here that MSC isolated from a wide age-range of donors, incubated in hypoxic conditions and propagated for 21-28 days are very similar in terms of cellular fitness. There is little doubt that MSC do experience age-related changes while in prolonged culture in vitro (41-46). MSC from older donors likely do not have the life expectancy as those from younger donors, but importantly MSC expanded to produce a clinical product would not be cultured for extended periods of time primarily due to the risk of karyotypic abnormalities and hence transformation events that may be acquired with increased passage number (13, 14, 47). As compared to adult marrow, fetal MSC seem to be more robust source for these cells. Fetal MSC are known to express pluripotency antigens, have long telomeres, and express more telomerase than adult MSC (16), though fetal tissue is an unlikely source for a clinical product.
The long-term culture issues of karyotopic abnormality development and increased oxidative damage are most likely due to an improper environment (i.e. not a native in vivo environment). Also, is the fact that human MSC cultured in vitro undergo several log-fold more cell divisions than they would in vivo which increases the likelihood of karyotypic abnormality. Because our clinical grade MSC products are cultured for a maximum of 28 days (are not more than 3 passages), we restricted our analysis to that time period which is more than enough time to generate multiple doses of a MSC clinical product.
The growth curves from our data did show a significant difference between younger and older donor MSC. This is the result of longer doubling time and possibly a smaller starting MSC population in older donors. The number of starting MSC cannot be known as there are no known markers to absolutely identify MSC. Interestingly, we found population doubling times to be significantly different in MSC at passages 3 and 4. This may be due to heterogeneous cellular division ongoing in the MSC cultures. It is accepted the MSC cultures in vitro are composed of a heterogeneous group of cells; these cells may have different diving times reflecting, ultimately, in differences in the whole population doubling time.
The culture period of our MSC expansion was less than 28 days, which is currently acceptable for generating a clinical product. Our attempts to estimate the starting number of potential MSC showed an increase in MSC in younger donors (0.000 18% vs 0.00010 %, p = 0.01). Despite this small differnce, we were able to generate more than enough cells needed from any age of donor taking into account that clinical trials use 0.5 × 106 – 9 × 106 cells per kg recipient body weight (48-50) or for a 70 kg person, up to 70 × 107 MSC for injection. We achieved these cell numbers in any MSC culture expanded, with 14 doses for our lowest expanded MSC line.
Our data on cell surface analysis is contrary to a prior report by Stolzing et al. who showed that several of the “classical” MSC antigens declined with increasing donor age when measured in early in passage; particularly CD105 and CD90 (11). We found that all surface antigens evaluated were very similar in expression regardless of age assayed early in passage. This may be due to the fact that we use a low-oxygen incubation of our cells to more closely mimic the native marrow environment rather than the 21% O2 often used in other studies. Additionally, that study stated at least 3 donors were represented in each age group, while we had over 15 per group. Several studies have shown that hypoxic (3-5% O2) conditions promote MSC growth, extend lifespan, reduced oxidative stress, and promoted engraftment in transplant models (17-19, 51-53). Our hypoxic conditions also may explain why we have seen little difference in the markers of oxidative stress measured by SOD activity, response to oxidative challenge, or iNOS expression in MSC from various ages of donors. This is also supported by data from other cell types in which hypoxic conditions decreased SOD production and prolonged cell life span of fibroblasts and vascular smooth muscle cells (54, 55).
We found no difference in p16INK or progerin expression. P16INK regulates cellular senescence by inhibition of CDK4 and CDK6 and accumulates as cells age in vitro (35, 40). Since we evaluated the cells early in culture, we speculate that the mechanisms that lead to p16INK accumulation do not predominate at that time. Progerin has been shown to activate members of the Notch signaling pathway when overexpressed in human MSC, and it has been also shown to accumulated in with age, though how robust an aging marker progerin will be and for what tissues remains to be seen (36, 38). Furthermore, finding no difference in iNOS or SOD expression allows us to speculate our short time in culture is not sufficient to cause an age-related effect and is perhaps inconsequential to the cell. It is also possible that there are perhaps yet undiscovered more sensitive markers of aging that may be expressed at lower levels earlier in culture and upstream of the proteins we evaluated that ultimately dictate a genetic “aging program” leading to the accumulation of the downstream known age-associated proteins.
Our evaluation of telomeres of MSC showed no significant difference when assayed from cells less than 28 days in culture. This is in agreement with the data from Shibata et al. who also showed the mean telomere length of early passage MSC did not correlate with donor age, although mean telomere length did predict ultimate expansion potential of the cells (40). While MSC telomeres shorten very consistently in the in vitro culture setting, this does not seem to be the case in vivo. Being that MSC typically lack telomerase, the only way they retain the ends of their chromosomes is by not dividing, and in situ human MSC probably undergo very few founds of division consistent with the main function of MSC in maintaining homeostasis of the bone marrow niche microenvironment. This slow turnover of MSC in situ is supported by evidence that MSC have some resistance to chemotherapy and radiation (56). Both are mechanisms that inflict the most injury on rapidly dividing cells.
Therefore, as we go forward in finding new clinical applications for MSC, our data suggest donors up to age 58 years are, in general, of equal “cellular fitness” and can be robustly expanded. MSC from any age donor should be acceptable as long as they are in good health and the MSC grown under proper conditions during their expansion.
Acknowledgments
Supported by the Children's Cancer Research Fund, Minneapolis, MN
Abbreviations
- MSC
mesenchymal stromal cell
- SOD
superoxide dismutase
Footnotes
Author Contributions
TCL designed the experiments, performed the western and Southern blots, and wrote the manuscript. AK performed in vitro assays and maintained the cell lines, BRB edited the manuscript, JT designed experiments and edited the manuscript.
References
- 1.Friedenstein AJ, Piatetzky S, II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966 Dec;16(3):381–90. [PubMed] [Google Scholar]
- 2.Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976 Sep;4(5):267–74. [PubMed] [Google Scholar]
- 3.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997 Apr 4;276(5309):71–4. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 4.Caplan AI, Bruder SP. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med. 2001 Jun;7(6):259–64. doi: 10.1016/s1471-4914(01)02016-0. [DOI] [PubMed] [Google Scholar]
- 5.Jones BJ, McTaggart SJ. Immunosuppression by mesenchymal stromal cells: from culture to clinic. Exp Hematol. 2008 Jun;36(6):733–41. doi: 10.1016/j.exphem.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Lazennec G, Jorgensen C. Concise review: adult multipotent stromal cells and cancer: risk or benefit? Stem Cells. 2008 Jun;26(6):1387–94. doi: 10.1634/stemcells.2007-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giordano A, Galderisi U, Marino IR. From the laboratory bench to the patient's bedside: an update on clinical trials with mesenchymal stem cells. J Cell Physiol. 2007 Apr;211(1):27–35. doi: 10.1002/jcp.20959. [DOI] [PubMed] [Google Scholar]
- 8.Dryden GW. Overview of stem cell therapy for Crohn's disease. Expert Opin Biol Ther. 2009 Jul;9(7):841–7. doi: 10.1517/14712590902956615. [DOI] [PubMed] [Google Scholar]
- 9.Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells. 2004;22(5):675–82. doi: 10.1634/stemcells.22-5-675. [DOI] [PubMed] [Google Scholar]
- 10.Banfi A, Muraglia A, Dozin B, Mastrogiacomo M, Cancedda R, Quarto R. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: Implications for their use in cell therapy. Exp Hematol. 2000 Jun;28(6):707–15. doi: 10.1016/s0301-472x(00)00160-0. [DOI] [PubMed] [Google Scholar]
- 11.Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008 Mar;129(3):163–73. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Garinis GA, van der Horst GT, Vijg J, Hoeijmakers JH. DNA damage and ageing: new-age ideas for an age-old problem. Nat Cell Biol. 2008 Nov;10(11):1241–7. doi: 10.1038/ncb1108-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang ZX, Guan LX, Zhang K, Wang S, Cao PC, Wang YH, et al. Cytogenetic analysis of human bone marrow-derived mesenchymal stem cells passaged in vitro. Cell Biol Int. 2007 Jun;31(6):645–8. doi: 10.1016/j.cellbi.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 14.Rubio D, Garcia-Castro J, Martin MC, de la Fuente R, Cigudosa JC, Lloyd AC, et al. Spontaneous human adult stem cell transformation. Cancer Res. 2005 Apr 15;65(8):3035–9. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]
- 15.Tolar J, Nauta AJ, Osborn MJ, Panoskaltsis Mortari A, McElmurry RT, Bell S, et al. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells. 2007 Feb;25(2):371–9. doi: 10.1634/stemcells.2005-0620. [DOI] [PubMed] [Google Scholar]
- 16.Guillot PV, Gotherstrom C, Chan J, Kurata H, Fisk NM. Human first-trimester fetal MSC express pluripotency markers and grow faster and have longer telomeres than adult MSC. Stem Cells. 2007 Mar;25(3):646–54. doi: 10.1634/stemcells.2006-0208. [DOI] [PubMed] [Google Scholar]
- 17.Rosova I, Dao M, Capoccia B, Link D, Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008 Aug;26(8):2173–82. doi: 10.1634/stemcells.2007-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krinner A, Zscharnack M, Bader A, Drasdo D, Galle J. Impact of oxygen environment on mesenchymal stem cell expansion and chondrogenic differentiation. Cell Prolif. 2009 Aug;42(4):471–84. doi: 10.1111/j.1365-2184.2009.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fehrer C, Brunauer R, Laschober G, Unterluggauer H, Reitinger S, Kloss F, et al. Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell. 2007 Dec;6(6):745–57. doi: 10.1111/j.1474-9726.2007.00336.x. [DOI] [PubMed] [Google Scholar]
- 20.Grant JD, Broccoli D, Muquit M, Manion FJ, Tisdall J, Ochs MF. Telometric: a tool providing simplified, reproducible measurements of telomeric DNA from constant field agarose gels. Biotechniques. 2001 Dec;31(6):1314–6. 8. doi: 10.2144/01316bc02. [DOI] [PubMed] [Google Scholar]
- 21.Mageed AS, Pietryga DW, DeHeer DH, West RA. Isolation of large numbers of mesenchymal stem cells from the washings of bone marrow collection bags: characterization of fresh mesenchymal stem cells. Transplantation. 2007 Apr 27;83(8):1019–26. doi: 10.1097/01.tp.0000259752.13304.0b. [DOI] [PubMed] [Google Scholar]
- 22.Capelli C, Salvade A, Pedrini O, Barbui V, Gotti E, Borleri G, et al. The washouts of discarded bone marrow collection bags and filters are a very abundant source of hMSCs. Cytotherapy. 2009;11(4):403–13. doi: 10.1080/14653240902960437. [DOI] [PubMed] [Google Scholar]
- 23.Glees P, Hasan M. Lipofuscin in neuronal aging and diseases. Norm Pathol Anat (Stuttg) 1976;32:1–68. [PubMed] [Google Scholar]
- 24.Ksiazek K. A comprehensive review on mesenchymal stem cell growth and senescence. Rejuvenation Res. 2009 Apr;12(2):105–16. doi: 10.1089/rej.2009.0830. [DOI] [PubMed] [Google Scholar]
- 25.Kasper G, Mao L, Geissler S, Draycheva A, Trippens J, Kuhnisch J, et al. Insights into mesenchymal stem cell aging: involvement of antioxidant defense and actin cytoskeleton. Stem Cells. 2009 Jun;27(6):1288–97. doi: 10.1002/stem.49. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995 Dec;11(4):376–81. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 27.Lee JA, Song HY, Ju SM, Lee SJ, Kwon HJ, Eum WS, et al. Differential regulation of inducible nitric oxide synthase and cyclooxygenase-2 expression by superoxide dismutase in lipopolysaccharide stimulated RAW 264.7 cells. Exp Mol Med. 2009 Sep 30;41(9):629–37. doi: 10.3858/emm.2009.41.9.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, et al. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009 Jan;8(1):18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saini A, Sei Y. Age-related impairment of early and late events of signal transduction in mouse immune cells. Life Sci. 1993;52(22):1759–65. doi: 10.1016/0024-3205(93)90464-e. [DOI] [PubMed] [Google Scholar]
- 30.Badawi AF, Liu Y, Eldeen MB, Morrow W, Razak ZR, Maradeo M, et al. Age-associated changes in the expression pattern of cyclooxygenase-2 and related apoptotic markers in the cancer susceptible region of rat prostate. Carcinogenesis. 2004 Sep;25(9):1681–8. doi: 10.1093/carcin/bgh176. [DOI] [PubMed] [Google Scholar]
- 31.Naik AA, Xie C, Zuscik MJ, Kingsley P, Schwarz EM, Awad H, et al. Reduced COX-2 expression in aged mice is associated with impaired fracture healing. J Bone Miner Res. 2009 Feb;24(2):251–64. doi: 10.1359/jbmr.081002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han JH, Roh MS, Park CH, Park KC, Cho KH, Kim KH, et al. Selective COX-2 inhibitor, NS-398, inhibits the replicative senescence of cultured dermal fibroblasts. Mech Ageing Dev. 2004 May;125(5):359–66. doi: 10.1016/j.mad.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Lee CH, Yoo KY, Choi JH, Park OK, Hwang IK, Kang IJ, et al. Cyclooxygenase-2 immunoreactivity and protein level in the gerbil hippocampus during normal aging. Neurochem Res. 2010 Jan;35(1):99–106. doi: 10.1007/s11064-009-0034-5. [DOI] [PubMed] [Google Scholar]
- 34.Tajima T, Murata T, Aritake K, Urade Y, Hirai H, Nakamura M, et al. Lipopolysaccharide induces macrophage migration via prostaglandin D(2) and prostaglandin E(2). J Pharmacol Exp Ther. 2008 Aug;326(2):493–501. doi: 10.1124/jpet.108.137992. [DOI] [PubMed] [Google Scholar]
- 35.Ressler S, Bartkova J, Niederegger H, Bartek J, Scharffetter-Kochanek K, Jansen-Durr P, et al. p16INK4A is a robust in vivo biomarker of cellular aging in human skin. Aging Cell. 2006 Oct;5(5):379–89. doi: 10.1111/j.1474-9726.2006.00231.x. [DOI] [PubMed] [Google Scholar]
- 36.Scaffidi P, Misteli T. Lamin A-dependent misregulation of adult stem cells associated with accelerated ageing. Nat Cell Biol. 2008 Apr;10(4):452–9. doi: 10.1038/ncb1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Worman HJ, Fong LG, Muchir A, Young SG. Laminopathies and the long strange trip from basic cell biology to therapy. J Clin Invest. 2009 Jul;119(7):1825–36. doi: 10.1172/JCI37679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McClintock D, Ratner D, Lokuge M, Owens DM, Gordon LB, Collins FS, et al. The mutant form of lamin A that causes Hutchinson-Gilford progeria is a biomarker of cellular aging in human skin. PLoS One. 2007;2(12):e1269. doi: 10.1371/journal.pone.0001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Serrano M, Hannon GJ, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993 Dec 16;366(6456):704–7. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- 40.Shibata KR, Aoyama T, Shima Y, Fukiage K, Otsuka S, Furu M, et al. Expression of the p16INK4A gene is associated closely with senescence of human mesenchymal stem cells and is potentially silenced by DNA methylation during in vitro expansion. Stem Cells. 2007 Sep;25(9):2371–82. doi: 10.1634/stemcells.2007-0225. [DOI] [PubMed] [Google Scholar]
- 41.Stolzing A, Scutt A. Age-related impairment of mesenchymal progenitor cell function. Aging Cell. 2006 Jun;5(3):213–24. doi: 10.1111/j.1474-9726.2006.00213.x. [DOI] [PubMed] [Google Scholar]
- 42.Sethe S, Scutt A, Stolzing A. Aging of mesenchymal stem cells. Ageing Res Rev. 2006 Feb;5(1):91–116. doi: 10.1016/j.arr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Roura S, Farre J, Soler-Botija C, Llach A, Hove-Madsen L, Cairo JJ, et al. Effect of aging on the pluripotential capacity of human CD105(+) mesenchymal stem cells. Eur J Heart Fail. 2006 Oct;8(6):555–63. doi: 10.1016/j.ejheart.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 44.Mohyeddin Bonab M, Alimoghaddam K, Talebian F, Ghaffari SH, Ghavamzadeh A, Nikbin B. Mesenchymal stem cell and in vitro Aging. BMC Cell Biol. 2006 Mar 10;7(1):14. doi: 10.1186/1471-2121-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fehrer C, Laschober G, Lepperdinger G. Aging of murine mesenchymal stem cells. Ann N Y Acad Sci. 2006 May;1067:235–42. doi: 10.1196/annals.1354.030. [DOI] [PubMed] [Google Scholar]
- 46.Bonab MM, Alimoghaddam K, Talebian F, Ghaffari SH, Ghavamzadeh A, Nikbin B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006;7:14. doi: 10.1186/1471-2121-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim J, Kang JW, Park JH, Choi Y, Choi KS, Park KD, et al. Biological characterization of long-term cultured human mesenchymal stem cells. Arch Pharm Res. 2009 Jan;32(1):117–26. doi: 10.1007/s12272-009-1125-1. [DOI] [PubMed] [Google Scholar]
- 48.Sun L, Akiyama K, Zhang H, Yamaza T, Hou Y, Zhao S, et al. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells. 2009 Jun;27(6):1421–32. doi: 10.1002/stem.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kebriaei P, Isola L, Bahceci E, Holland K, Rowley S, McGuirk J, et al. Adult human mesenchymal stem cells added to corticosteroid therapy for the treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant. 2009 Jul;15(7):804–11. doi: 10.1016/j.bbmt.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 50.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008 May 10;371(9624):1579–86. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 51.Hung SC, Pochampally RR, Hsu SC, Sanchez C, Chen SC, Spees J, et al. Short-term exposure of multipotent stromal cells to low oxygen increases their expression of CX3CR1 and CXCR4 and their engraftment in vivo. PLoS One. 2007;2(5):e416. doi: 10.1371/journal.pone.0000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stolzing A, Scutt A. Effect of reduced culture temperature on antioxidant defences of mesenchymal stem cells. Free Radic Biol Med. 2006 Jul 15;41(2):326–38. doi: 10.1016/j.freeradbiomed.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 53.Annabi B, Lee YT, Turcotte S, Naud E, Desrosiers RR, Champagne M, et al. Hypoxia promotes murine bone-marrow-derived stromal cell migration and tube formation. Stem Cells. 2003;21(3):337–47. doi: 10.1634/stemcells.21-3-337. [DOI] [PubMed] [Google Scholar]
- 54.Minamino T, Mitsialis SA, Kourembanas S. Hypoxia extends the life span of vascular smooth muscle cells through telomerase activation. Mol Cell Biol. 2001 May;21(10):3336–42. doi: 10.1128/MCB.21.10.3336-3342.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Poulios E, Trougakos IP, Chondrogianni N, Gonos ES. Exposure of human diploid fibroblasts to hypoxia extends proliferative life span. Ann N Y Acad Sci. 2007 Nov;1119:9–19. doi: 10.1196/annals.1404.025. [DOI] [PubMed] [Google Scholar]
- 56.Mueller LP, Luetzkendorf J, Mueller T, Reichelt K, Simon H, Schmoll HJ. Presence of mesenchymal stem cells in human bone marrow after exposure to chemotherapy: evidence of resistance to apoptosis induction. Stem Cells. 2006 Dec;24(12):2753–65. doi: 10.1634/stemcells.2006-0108. [DOI] [PubMed] [Google Scholar]


