Abstract
Neurotransmitter- or neurotrophin-regulated intracellular signaling in the hippocampus is hypothesized to contribute to depression and antidepressant (ADT) efficacy. Extracellular signal-regulated kinase 1/2 (ERK1/2) is downstream of several receptor types and regulates transcriptional activity of many targets; ERK1/2 may thereby influence mood and affect. Using a novel, ADT-sensitive depression model in mice, we show that prior corticosterone exposure decreases motivated behavior, sucrose consumption, and pERK1/2 in the dentate gyrus, but not in CA1/CA3. Notably, prefrontal cortical targets were also regulated. Our data suggest ADTs restore hippocampal pERK1/2 after stress-related insult, and potentially reveal a novel role for prefrontal neurotrophins in depressive-like symptomology.
Keywords: stress, antidepressant, anhedonia, prefrontal cortex, BDNF, dentate gyrus, amitriptyline, fluoxetine
Introduction
Neurotrophic hypotheses of depression and antidepressant (ADT) efficacy argue stress and ADTs chronically regulate mood via opposing action on neurotrophins, such as brain-derived neurotrophic factor (BDNF), and downstream transcription factors associated with cellular growth, motility, and survival.1 Enhancing the activity of cortico-limbic neurotrophin-related signaling pathways has been proposed as a common ADT mechanism, based largely on studies of ADT action in the naïve rodent.2 Notably, the intracellular events associated with ADT efficacy have largely not been verified in animal models of depression, nor have the major proteins linking receptor binding and transcription factor activation been identified, though a likely candidate is extracellular signal-regulated kinase 1/2 (ERK1/2).
The ERK MAP kinase pathway is an intracellular signaling cascade implicated in several forms of learning, memory, and neuroplasticity.3 ERK1/2 is activated by BDNF binding of the tyrosine kinase receptor B (trkB) via the Ras-Raf-MEK(MAP kinase kinase)-ERK cascade, inducing nuclear translocation and phosphorylation of target transcription factors.3 While ERK1/2 phosphorylation has been hypothesized to be an intracellular signaling mechanism mediating ADT efficacy in depressed humans and animal models of depression, supporting evidence is indirect and largely limited to studies in otherwise naïve rodents or in vitro models.cf.4 Nonetheless, we demonstrate amitriptyline (AMI), a tricyclic ADT, restores ERK1/2 activity in the dentate gyrus, otherwise suppressed by glucocorticoid exposure. AMI also restored hedonic response to sucrose and progressive ratio (PR) responding in a classic task of motivation. Finally, phosphorylated ERK1/2 (pERK1/2) and BDNF in the pre-frontal cortex (PFC) covaried with responding, implicating this region in depressive-like loss of motivation.
Materials and Methods
Subjects
Naïve C57BL/6J mice (8–10 wks, Charles River Laboratories, Kingston, VA) were used. Fifty seven were initially food restricted (allowed 75 min food access daily) to prevent weight gain during conditioning experiments. During corticosterone (CORT) exposure and ADT treatment, all mice had free access to food and water. Mice were maintained on a 12-h light cycle. Procedures were approved by the Yale University Animal Care and Use Committee.
Corticosterone (CORT) Exposure
CORT (4-pregnen-11β 21-DIOL-3 20-DIONE 21-hemisuccinate; Steraloids, Inc., Newport, RI) was dissolved in tap water (25 μg/mL free base unless otherwise noted, resulting in ~6.9 mg/kg/day, p.o) and neutralized to a pH of 7.0–7.4 with HCl. Mice were presented with CORT in place of drinking water for 14 days, then weaned with 3 days of 1/2 dose, then 3 days of 1/4 dose to allow for recovery of endogenous CORT secretion.
Sucrose Intake
The effects of CORT (25–100 μg/mL) on sucrose intake in a test of anhedonia were evaluated. Then, in a separate group, one of the following replaced drinking water after CORT (25 μg/mL) in an attempt to reverse the anhedonic response to sucrose with chronic ADT administration: 1) 2% saccharin (sacc.) for 3 weeks; 2) sacc.+200 μg/mL AMI for 2 weeks followed by 1 week of sacc. only; 3) sacc.+160 μg/mL fluoxetine (FLX; kindly donated by Dr. Ronald Duman for comparison to AMI) for 3 weeks, as 2 weeks was insufficient; or 4) sacc. for 3 weeks with AMI or FLX for 4 days (“sub-chronic” ADT control group). In both experiments, mice were habituated to a palatable sucrose solution (1% w/v) for 2 days beginning 3 weeks after CORT. Mice were then habituated to modest water deprivation over 3 days. Finally, each individual mouse was allowed 1 h access to sucrose in the home cage while its cagemates were temporarily housed in a clean cage. The average deprivation period per cage was 19 h; ANOVA using order as the independent variable confirmed no effects of testing order. CORT's effects were evaluated by ANOVA and Student's post-hocs; findings were unchanged by normalizing to body weight.
PR Experiments
Using operant chambers for mice with grid floors controlled by MedPC software (Med Associates Inc., George, VT), mice were initially trained to nose poke for grain-based food pel-lets (20 mg; Bio-Serv, Frenchtown, NJ) as previously described,5 but in the absence of a discrete cue signaling reinforcement. All mice acquired the task within 10–15 days and were then exposed to CORT (25 μg/mL). Three days after CORT, mice were administered AMI for 1 week. Instrumental responding was then restored. Finally, PR responding was assessed. Here, the response requirement progressively increased (1, 5, 9, 13, etc.), such that mice had to respond progressively more for subsequent reinforcement. Tests ended when no responses were emitted for 5 min or the mouse had not “timed out” within 4 h. The highest ratio of responses:reinforcements earned is the “break point ratio” and is the dependent variable. Other mice were trained to nose poke, then tested in extinction (with food hopper and magazine disconnected) to confirm CORT did not increase sensitivity to nonreinforcement. Data were analyzed by one- or two-factor (CORT × ADT) ANOVA with repeated measures (RM) as needed.
Biochemistry
Mice from PR experiments were sacrificed (see Fig. 2A for experimental design), and frozen brains were sliced into 1-mm sections and dissected with a 1.2-mm tissue core. Hippocampal samples were bilateral; the PFC sample was collected with a midline punch. Western blotting was conducted using standard methods; data were quantified by LiCor (Lincoln, NE). pERK1/2 mouse ((Ms); 1:1000; Cell Signaling, Beverly, MA) was normalized to ERK1/2 rabbit ((Rb); 1:2000; Cell Signaling). For timecourse experiments, samples were collected 1 day and 2 weeks after CORT. Both phospho-trkB (ptrkB) (Tyr490) (1:500; Cell Signaling) and trkB (Ms; 1:1000; BD Biosciences, San Jose CA) were normalized to the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Ms; 1:80,000; Advanced Immunochemical Inc., Long Beach, CA) loading control values, as both were regulated by CORT, unlike total ERK. BDNF was analyzed by ELISA (Promega, Madison, WI) in accordance with manufacturer's instructions. To control for variance, data were converted to percent of control samples on the same gel/plate and analyzed by one- or two-factor ANOVA or Pearson's r.
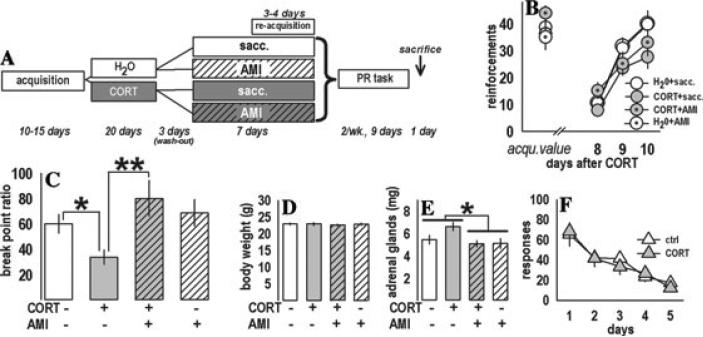
Figure 2.
Prior CORT exposure selectively decreased motivated responding in the PR task. (A) Experimental design. The contents of the drinking water (CORT and/or AMI) are displayed. “Sacc.” refers to saccharin. (B) After chronic CORT exposure and wash-out, previously trained mice reacquired the instrumental response to the same levels as during predrug acquisition sessions (“acqu.”). (C) During the PR task, levels of responding were decreased by CORT, while AMI subsequent to CORT restored responding. AMI alone had no effect. (D) Body weights were unchanged at test. (E) Adrenal gland weights—an approximate reflection of recent HPA axis activity—were decreased by AMI, but no main effects of CORT were detected. (F) Finally, nonreinforced responding was unchanged in a separate cohort of CORT-exposed mice, suggesting decreased PR responding reflects motivational deficit, and not heightened sensitivity to extinction conditions. Bars and symbols represent group means (±SEM) (*P ≤ 0.05, **P ≤ 0.001). Reprinted with permission from Reference 4.
Lesions
To evaluate the consequences of PFC neuronal insult, NMDA (20 μg/μL, 0.2 μL per hemisphere; Sigma, St. Louis, MO) was infused into anesthetized, trained mice with needles aimed at +2.0 AP, –2.5 DV, ±0.1 ML.6 Mice had 1-week recovery before PR testing. Lesions were verified using standard immunofluorescence techniques. Data were calculated as percent of each individual's day 1 value to adjust for variability introduced by surgery and analyzed by one-factor RM-ANOVA.
Results
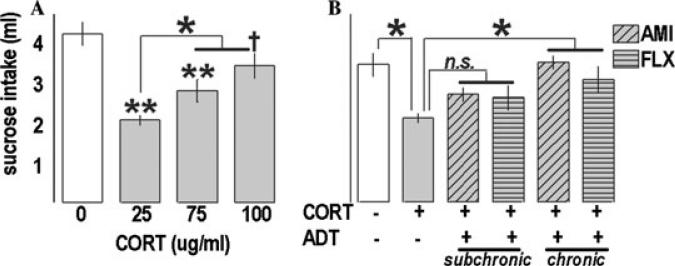
CORT (25 and 75 μg/mL) suppressed sucrose intake [F(3,25) = 15.2, P < 0.001; post-hoc Ps < 0.001] (Fig. 1A) in a test of anhedonia, with a trend detected at the 100 μg/mL dose (P = 0.07). In a separate test, CORT (25 μg/mL) again decreased intake [F(5,46) = 5.49, P < 0.001; post-hoc P = 0.002], restored by chronic ADTs (Ps ≤ 0.03 versus CORT) (Fig. 1B). Mice administered chronic ADTs did not differ from controls (Ps ≥ 0.3), while “subchronic” and CORT-only groups did not differ from each other (Ps ≥ 0.14).
Figure 1.
Prior CORT exposure persistently decreased sucrose consumption in a model of anhedonia. (A) CORT suppressed intake of a palatable sucrose solution 1 month after exposure. Interestingly, the highest dose was least effective; further experiments will investigate this finding. (B) In a subsequent experiment, chronic, but not subchronic, AMI and FLX administered subsequent to CORT (25 μg/mL) restored intake. Bars represent group means (±SEM) (†P = 0.07, *P ≤ 0.05, **P < 0.001 versus control unless noted).
During re-acquisition of the instrumental task after CORT and AMI, mice earned an increasing number of reinforcements [F(2,64) = 61.0, P < 0.001, post-hoc Ps < 0.05] with no main drug effects, and no CORT × AMI interaction (all Ps ≥ 0.18) (Fig. 2B). Each animal's three PR break point ratios were averaged and analyzed, revealing a CORT × AMI interaction (F(1,32) = 4.42, P = 0.04). CORT decreased break points (P = 0.03), and performance was restored by subsequent AMI (P = 0.001 versus CORT only) with no effects of AMI alone (P = 0.5 versus water controls) (Fig. 2C). Body weights did not differ [F(1,32) < 1] (Fig. 2D). AMI decreased adrenal gland weights [F(1,34) = 6.0, P = 0.02] (Fig. 2E), suggesting decreased endogenous CORT as previously reported,7 while prior CORT had no effects at test. CORT did not affect responding in extinction [F(1,16) < 1] (Fig. 2F).
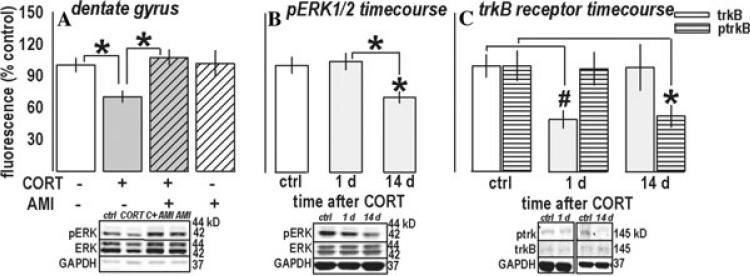
A CORT × AMI interaction effect was detected when analyzing pERK1/2 in the dentate gyrus [F(1,31) = 4.6, P = 0.04] (Fig. 3A). CORT decreased pERK1/2, a deficit that was reversed by AMI (Ps ≤ 0.01), while AMI-only and controls did not differ (P = 0.9). Only a main effect of AMI was observed in CA1/CA3 samples [F(1,32) = 5.1, P = 0.03] (not shown), suggesting CORT selectively decreased pERK1/2 in the dentate, while AMI increased pERK1/2 in both regions. A time-course analysis of the dentate gyrus revealed pERK1/2 was decreased 14 days after CORT [F(2,16) = 4.6, P = 0.03, post-hoc Ps ≤ 0.02] (Fig. 3B). Because decreased ERK1/2 phosphorylation may result from decreased BDNF binding, we also analyzed its receptor, trkB. Immunoreactivity showed a trend for an effect [F(2,14) = 3.2, P = 0.07] (Fig. 3C); however, t-test comparisons of 1-day CORT-exposed against 1-day control mice or all control mice revealed a significant down-regulation in that group (Ps = 0.01). By contrast, ptrkB was decreased on the same timecourse as pERK1/2 [F(2,16) = 4, P = 0.04; post-hoc P = 0.04] (Fig. 3C).
Figure 3.
CORT chronically regulates pERK1/2 in the dentate gyrus: Reversal by AMI. (A) Prior CORT and subsequent ADT regulated pERK1/2 in a manner consistent with behavioral responding after these compounds: CORT decreased pERK1/2 in this region (but not in the surrounding CA1/CA3 region, not shown), which has been previously implicated in ADT efficacy in otherwise naïve rodents. The decrease was restored to control levels by AMI, which had no effects alone. (B) A timecourse analysis of pERK1/2 after CORT revealed significantly decreased pERK1/2 14 days after exposure. (C) While trkB receptor expression was regulated on a different timecourse, receptor phosphorylation was regulated on the same timecourse, suggesting decreased trkB receptor activity contributed, at least in part, to decreased pERK1/2. Bars represent means (±SEM) per treatment group (*P ≤ 0.05, compared to control samples unless noted; #P < 0.05 in a t-test against same-day and all control samples). “C + AMI” refers to the CORT + AMI group. Reprinted with permission Reference 4.
ANOVA revealed no effects of CORT/AMI in medial PFC (mPFC) samples (Ps > 0.1); however, pERK1/2 significantly covaried with break point ratios (r = 0.4, P = 0.03), such that the lowest responders exhibited the lowest levels of pERK1/2 (not shown). Because low responders predominated in the CORT-only group, we analyzed expression of a target neurotrophin, BDNF, in this group. Within the CORT-exposed, but not control mice, prefrontal BDNF predicted break point ratios (CORT: r = 0.7, P = 0.03; control: r = 0.2, P = 0.59) (Fig. 4A,B). Neural damage in this region may thus contribute to decreased responding. As predicted, lesions of the mPFC mimicked CORT by decreasing break point ratios [F(1,48) = 11.1, P = 0.01] (Fig. 4C,D).
Figure 4.
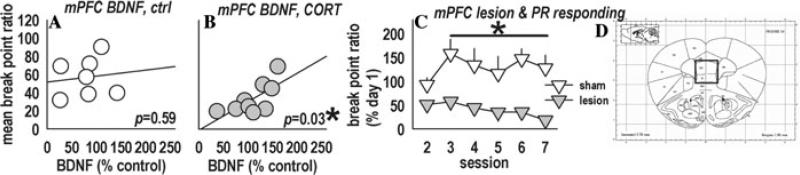
Because prefrontal pERK1/2 covaried with behavioral outcomes (P = 0.03, not shown), we analyzed BDNF, both a target and effector of ERK1/2 implicated in depression, in this region. (A) BDNF in the medial prefrontal cortex (referred to as “mPFC”) did not covary with break point ratios in control mice; (B) however, BDNF significantly covaried with break point ratios in CORT-exposed mice, potentially revealing a major role for the mPFC in motivation and/or sensitivity to outcome value, and suggesting decreased prefrontal BDNF may confer vulnerability for motivational loss in depression. (C) Consistent with the former hypothesis, lesions aimed at the prelimbic mPFC mimicked the persistent effects of CORT on break point ratios. Further experiments aimed at locally manipulating BDNF and ERK1/2 are currently ongoing. (D) Representative region of interest.6 Symbols represent individual animals in (A) and (B) and group means (±SEM) in (C) (*P < 0.05).
Discussion
We demonstrate that prior chronic oral CORT exposure persistently decreases motivated and hedonic responding in an ADT-sensitive manner. Using this depression model that carries face, predictive, and construct validity, we also reveal selectively disrupted pERK1/2 in the dentate gyrus (but not in CA1/CA3), implicating ERK1/2 phosphorylation events in this region in ADT efficacy, as previously hypothesized, and the depressive-like state. Decreased pERK1/2 temporally coincided with depressive-like behavioral responding, as well as decreased ptrkB, suggesting decreased receptor activation contributes to these deficits.
Interestingly, both pERK1/2 and BDNF in the mPFC covaried with break point ratios after CORT exposure, where selective excitotoxic lesions mimicked the chronic effects of CORT. Decreased neural integrity in this region may thus serve as a vulnerability factor for depressive symptomology,cf.8 consistent with recent work with unpredictable stress in rodents9 and novel therapeutic approaches to depression in humans.10 Ongoing experiments are aimed at locally manipulating BDNF after CORT to blunt its suppressive effects on PR responding. Elucidating how stress substrates and ADTs act on pERK1/2 and BDNF throughout cortico-limbic circuits should aid in understanding neurotrophic- and neuroplasticity-related contributions to ADT efficacy, and in the development of more rapidly acting therapies.
Acknowledgments
We thank D. Kiraly and A. Kedves for aid with the timecourse work, J. Howell for surgical expertise, and Dr. J. Quinn for comments on the manuscript. Supported by PHS MH025642. The authors have no competing financial interests.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch. Gen. Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- 2.Thome J, et al. cAMP response element-mediated gene transcription is upregulated by chronic antidepressant treatment. J. Neurosci. 2000;20:4030–4036. doi: 10.1523/JNEUROSCI.20-11-04030.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mazzucchelli C, Brambilla R. Ras-related and MAPK signaling in neuronal plasticity and memory formation. Cell. Mol. Life Sci. 2000;57:604–611. doi: 10.1007/PL00000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gourley SL, et al. Regionally-specific regulation of pERK1/2 MAP kinase in a model of antidepressant-sensitive chronic depression. Biol. Psychiatry. 2008;63:353–359. doi: 10.1016/j.biopsych.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baldwin AE, et al. Appetitive instrumental learning is impaired by inhibition of cAMP-dependent protein kinase within the nucleus accumbens. Neurobiol. Learn. Mem. 2002;77:44–62. doi: 10.1006/nlme.2000.4002. [DOI] [PubMed] [Google Scholar]
- 6.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Academic Press; San Diego, CA: 2002. [Google Scholar]
- 7.Rousse I, et al. Test-specific effects of amitriptyline administration on behavioral deficits in transgenic mice with impaired glucocorticoid receptor function. Dev. Brain Dysfunction. 1997;10:418–431. [Google Scholar]
- 8.Drevets WC. Neuroimaging studies of mood disorders. Biol. Psychiatry. 2000;48:813–829. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- 9.Liston C, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J. Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayberg HS, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]