Abstract
The mTORC1 protein kinase complex consists of mTOR, raptor, mLST8/GβL and PRAS40. Previously, we reported that mTOR plays an important role in regulating protein synthesis in response to alcohol (EtOH). However, the mechanisms by which EtOH regulates mTORC1 activity have not been established. Here, we investigated the effect of EtOH on the phosphorylation and interaction of components of mTORC1 in C2C12 myocytes. We also examined the specific role that PRAS40 plays in this process. Incubation of myocytes with EtOH (100 mM, 24 h) increased raptor and PRAS40 phosphorylation. Likewise, there were increased levels of the PRAS40 upstream regulators Akt and IRS-1. EtOH also caused changes in mTORC1 protein-protein interactions. EtOH enhanced the binding of raptor and PRAS40 with mTOR. These alterations occurred in concert with increased binding of 14-3-3 to raptor, while the PRAS40 and 14-3-3 interaction was not affected. The shRNA knockdown (KD) of PRAS40 decreased protein synthesis similarly to EtOH. PRAS40 KD increased raptor phosphorylation and its association with 14-3-3, while it decreased GβL-mTOR binding. The effects of EtOH and PRAS40 KD were mediated by AMPK. Both factors increased in vitro AMPK activity towards the substrate raptor. In addition, knockdown enhanced the activity of AMPK towards TSC2. Collectively, our results indicate that EtOH stabilizes the association of raptor, PRAS40 and GβL with mTOR, while likewise increasing the interaction of raptor with 14-3-3. These data suggest a possible mechanism for the inhibitory effects of EtOH on mTOR kinase activity and protein synthesis in myocytes.
Keywords: mTORC1, PRAS40, AMPK, Myocytes
INTRODUCTION
The mammalian target of rapamycin (mTOR) is a key regulator of cell growth and proliferation. The mTOR pathway integrates signals from growth factors, nutrients, and diverse environmental stresses. As such, it regulates a number of processes including mRNA translation, metabolism and cell survival [Avruch et al., 2009; Sarbassov et al., 2005; Wullschleger et al., 2006]. mTOR functions as part of two distinct signaling heteromeric complexes, the mammalian TOR complex 1 (mTORC1) and mTORC2 [Loewith et al., 2002; Sarbassov et al., 2004; Wullschleger et al., 2006]. mTOR interacts with raptor, mLST8/GβL and PRAS40 (proline-rich Akt substrate 40) to form mTORC1. This complex regulates the phosphorylation of the p70 S6 ribosomal protein (S6K1) and the eIF-4E binding protein (4EBP-1), and this, in turn, promotes protein translation and cell growth. Alternatively, mTOR can interact with GβL, rictor and mSin1 to form mTORC2. Although less well characterized, this complex plays a role in the phosphorylation of Akt/protein kinase B at Ser-473, protein kinase C (PKC), and regulation of the actin cytoskeleton [Jacinto et al., 2004; Sarbassov et al., 2004; Sarbassov et al., 2005].
The functions of most mTOR-associated proteins have not been fully defined, although some information is available. For instance, raptor has been proposed to act as a scaffold protein in the mTORC1 and, as such, it recruits mTORC1 substrates. The important function of this protein in mTOR signaling has been highlighted using deletion and knockdown procedures, both of which diminish mTORC1 activity [Guertin et al., 2006; Hara et al., 2002; Kim et al., 2002]. Another mTORC1 and mTORC2 component, GβL, appears to act as a positive regulator of mTOR kinase activity in both complexes [Kim et al., 2003; Loewith et al., 2002; Wullschleger et al., 2005]. Finally, PRAS40 is a novel substrate of Akt that is a binding partner for mTORC1 [Kovacina et al., 2003; Oshiro et al., 2007; Sato et al., 2009; Wang et al., 2007]. This protein has been postulated as a negative regulator of mTORC1. However, this inhibitory effect is suppressed when PRAS40 is phosphorylated in the presence of growth factors such as insulin [Sancak et al., 2007; Vander Haar et al., 2007].
Various factors can influence protein-protein interactions within the TORC1 and/or with other cellular proteins. For example, insulin decreases PRAS40-mTOR association [Wang et al., 2007], while others have reported that phosphorylation of PRAS40 leads to its binding to the cytosolic protein 14-3-3. This redistribution of PRAS40 appears crucial for the regulating mTOR kinase activity [Kovacina et al., 2003; Vander Haar et al., 2007; Wang et al., 2008]. The ability of altered phosphorylation to affect the interaction of PRAS40 with other proteins is evidenced by the mutations of phosphorylation sites such as Ser 183, Ser 221, or Thr 246. As a result of such mutations, the interaction of PRAS40 with the protein 14-3-3 is reduced, thereby increasing the inhibitory effect of PRAS40 on mTORC1 [Fonseca et al., 2007; Wang et al., 2008]. In contrast, recent studies reported that phosphorylation of PRAS40 and its binding to 14-3-3 was not required for the activation of mTORC1 [Fonseca et al., 2008; Sancak et al., 2007]. Thus, it is unclear whether changes in PRAS40 phosphorylation and 14-3-3 association are required to regulate mTORC1.
Energy insufficiency, amino acid, and growth factors can all regulate mTOR, albeit through different signaling pathways. For instance, mTOR directly phosphorylates Akt in response to insulin. On the other hand, Akt has been proposed to phosphorylate and activate the tumor suppressor protein TSC2, a component of the tuberous sclerosis protein complex. This, in turn, suppresses the activity of the Ras-related GTPase Rheb, which is a positive regulator of mTOR [Inoki et al., 2002; Manning et al., 2002; Memmott and Dennis, 2009]. Conversely, mTOR can be regulated via an Akt-independent pathway under conditions that deplete intracellular energy [Memmott and Dennis, 2009]. One mechanism by which this occurs is through the activation of the AMP-activated protein kinase (AMPK). AMPK downregulates energetically demanding processes like protein synthesis by negatively regulating mTORC1, and this can occur via dual mechanisms. First, AMPK can phosphorylate TSC2, thereby increasing its activity and repressing mTORC1 signaling [Inoki et al., 2003]. Second, AMPK can phosphorylate raptor [Gwinn et al., 2008], which promotes the interaction of raptor with 14-3-3. This association then inactivates the kinase activity of the target protein [Bridges and Moorhead, 2005; Yaffe, 2002]. Along these lines, the phosphorylation of raptor and its binding to 14-3-3 has been reported to inhibit mTORC1 under conditions of energy stress [Gwinn et al., 2008]. Thus, this association hinders the growth of cells under unfavorable conditions.
We have previously reported that alcohol (EtOH) inhibits protein synthesis in C2C12 myocytes [Hong-Brown et al., 2001]. This decrease was associated with a concomitant decrease in the phosphorylation of mTOR, S6K1 and 4E-BP1 [Hong-Brown et al., 2006]. At present, the mechanisms by which EtOH regulates mTORC1 signaling are unresolved, although AMPK may play a role in this process. EtOH stimulates AMPK activity in myocytes, and this activation increases phosphorylation of acetyl CoA carboxylase (ACC) and the eukaryotic elongation factor (eEF)-2 [Hong-Brown et al., 2007]. It is not known whether EtOH-induced AMPK activity regulates the mTORC1 pathway through phosphorylation of individual components such as raptor and PRAS40. If so, this could affect the protein interactions of the mTORC1.
The aim of the present study was to examine the effect of EtOH on the posttranslational modification and interaction of various components of the mTORC1 complex. We also investigated the potential role played by AMPK in regulating the interaction and function of mTORC1. EtOH increased association of mTOR with various components of mTORC1 as well as raptor and 14-3-3. Similar results were observed in shRNA knockdown (KD) cells. Therefore, changes in mTORC1 stabilization may represent a mechanism by which EtOH and PRAS40 KD inhibit mTOR kinase activity and protein synthesis.
MATERIALS AND METHODS
Ethanol was purchased from Fisher Scientific Co. (Springfield, NJ). The pLKO shRNA plasmids encoding for PRAS40 or scrambled control were from Addgene Inc. (Cambridge, MA). Antibodies against total S6K1, 14-3-3θ and TSC2 were from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against phosphorylated (p) PRAS40 at T246 and S183 were from Biosource, Invitrogen (Carlsbad, CA) and Immuno-Biological Laboratories Co. (Gunma, Japan), respectively. Antibodies against p-Akt (T308, S473), p-raptor (S792), p-mTOR (S2448), p-S6K1 (T389) p-S6 ribosomal protein (S235/S236) and p-TSC2 (T1459) were purchased from Cell Signaling Technology (Beverly, MA), as were antibodies to total Akt, IRS-1, mTOR, raptor, GβL, PRAS40 and AMPK. The AMPK inhibitor compound C was from CalBiochem (EMD Biosciences, San Diego, CA). 35S-methionine/cysteine (>1000 Ci/mol) was obtained from MP Biomedicals (Aurora, OH). Phenylmethanesulfonyl fluoride (PMSF), protease, phosphatase I and II inhibitor cocktails were from Sigma (St. Louis, MO). Cell culture media and fetal bovine serum (FBS) were from Gibco, Invitrogen Corporation (Carlsbad, CA). Protein A Sepharose CL 4B was purchased from GE Healthcare Biosciences Corp (Piscataway, NJ). The Catch and Release V2 Reversible immunoprecipitation system was from Upstate Biotechnology, Inc., (Lake Placid, NY).
Cell culture and transfection
C2C12 mouse myoblasts were purchased from American Type Culture Collection (Manassas, VA). Cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% FBS, penicillin (100 U/ml), streptomycin (100 μg/ml) and amphotericin (25 μg/ml) at 37°C in 5% CO2.
For transient expression, the pLKO shRNA vectors encoding shRNA target PRAS40 or scrambled sequences [Vander Haar et al., 2007] were transfected into C2C12 myocytes using electroporation and the cell line nucleofector kit V (Amaxa, Germany) following the manufactures’ protocol. Experiments were carried out 24-30 h post-transfection, and cells were harvested 24 h thereafter for co-immunoprecipitation and immunoblot assays.
The effects of EtOH and PRAS40 KD on protein synthesis were determined as previous described [Hong-Brown et al., 2006]. EtOH was used at 100 mM because this concentration inhibits protein synthesis without being cytotoxic to myocytes. All experiments were conducted using cells at the early passage of the myoblast stage. For metabolic labeling, control, scrambled control or PRAS40 KD cells were incubated in the absence or presence of EtOH and radioisotope for 24 h prior to harvesting. Cells were labeled with 10 μCi 35S-methionine/cysteine in 1-2% FBS media because C2C12 myocyte survival is decreased when cells are cultured for extended periods in serum free media. The rate of radiolabel incorporation into protein was linear between 1 h and 24 h (data not shown) indicating there was no significant change in the specific activity of the precursor pool. Hence, all subsequent studies were conducted using the 24 h labeling protocol. At the end of the experiment, cells were collected and precipitated in 10% trichloroacetic acid (TCA), and the incorporation of 35S-methionine/cysteine into TCA-precipitable protein was determined via liquid scintillation counting. The results were normalized with total protein and compared with the control group. Data were expressed as a percentage of the control value.
Immunoprecipitation and immunoblot analysis
C2C12 myocytes were sub-cultured in either 10 cm or 6-well plates in the presence or absence of EtOH for 24 h. Thereafter, cells were rinsed once with phosphate buffered saline and clysed in ice-cold 0.3% CHAPS buffer containing PMSF and a cocktail of protease and phosphatase inhibitors. Soluble fractions of cell extracts were isolated by centrifugation at 14,000 rpm for 5 min at 4°C. For immunoprecipitation, primary antibody was added to equal amounts of protein from cell lysates and incubated overnight at 4°C. A 50% slurry of protein A sepharose was then added and the incubation was continued for an additional 1 h with rotation. Immunoprecipitates were washed 3 times with lysis buffer and the precipitated proteins were denatured by the addition of 2X Laemmli sample buffer (LSB). Equal amounts of protein from cell lysates were electrophoresed on denaturing polyacrylamide gels and transferred to nitrocellulose. The resulting blots were blocked with 5% nonfat dry milk and incubated with the antibodies of interest. Unbound primary antibody was removed by washing with TBS containing 0.05% Tween-20 (ICI Americas, Inc, Wilmington, DE) and blots were incubated with anti-rabbit immunoglobulin conjugated with horseradish peroxidase. Blots were briefly incubated with an enhanced chemiluminescent detection system (Amersham, Bickinghamshire, England) and exposed to Kodak X-ray film (Rochester, NY). The film was scanned (ScanMaker 4, Microtek, Los Angeles, CA) and analyzed with NIH Image 1.6 software.
In vitro mTOR kinase and AMPK activity assay
For kinase activity measurements, cells were lysed in buffer 1 (0.3% CHAPS, 40 mM Hepes, 120 mM NaCl, 1 mM EDTA) or buffer 2 (1% NP-40, 20 mM Hepes, 150 mM NaCl) and a cocktail of protease and phosphatase inhibitors. AMPK and mTOR kinase activities were determined as described previously [Hong-Brown et al., 2008; Sancak et al., 2007] with minor modifications. Briefly, equal amounts of total protein from cell extracts were immunoprecipitated overnight with antibodies against total mTOR, AMPKα, S6K1, or raptor. The antibody-antigen complexes of S6K1 or raptor which are substrates of mTOR and AMPK, respectively, were precipitated using the Catch and Release reversible immunoprecipitation system following manufactures’ protocol (Cat # 17-500). The antibody-antigen complex of mTOR or AMPKα was captured by incubation for 1 h with protein A Sepharose at 4°C on a rotator. For the mTOR kinase assay, immune complexes were washed with lysis buffer and then incubated with reaction buffer A (25 mM Hepes, 50 mM KCl, 10 mM MgCl2, 0.2 mM ATP or [γ- 32P] ATP). Alternatively, immune complexes were incubated with reaction buffer B (40 mM Hepes, 0.2 mM AMP, 80 mM NaCl, 5 mM MgCl2, and 0.2 mM ATP) for measuring AMPK kinase activity. To correct for endogenous activity associated with the precipitated substrate, some reaction mixtures did not contain added kinase. Instead, substrates were combined with pre-immune precipitants and examined for activity. The reaction was allowed to proceed for 15-18 min at 30°C, and terminated by addition of LSB to a final concentration of 2X. Samples were heated for 5 min and run on SDS-PAGE gels. AMPK or mTOR kinase activities were analyzed by immunoblotting for the phosphorylation state of their substrates, raptor and S6K1, respectively. In vitro kinase results were standardized with immunoprecipitates.
Statistical analysis
For experimental protocols with more than two groups, statistical significance was determined using one-way ANOVA followed by the Dunnett’s test to compare all data to the appropriate time-matched control group. For experiments with only two groups, an unpaired Student’s t-test was performed. Data are presented as mean ± SE. A value of P < 0.05 was considered statistically significant.
RESULTS
Effect of EtOH on phosphorylation of mTORC1 proteins
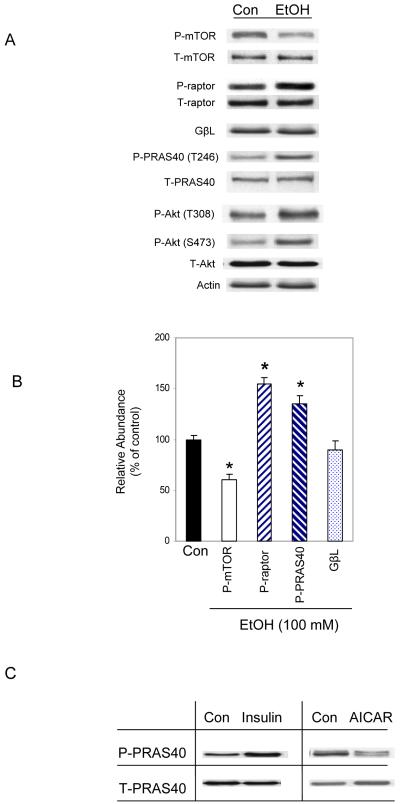
We previously reported that 100 mM EtOH suppresses the rate of protein synthesis in myocytes and concomitantly decreases the phosphorylation of S6K1 and 4E-BP1, suggesting a decrease in mTOR activity [Hong-Brown et al., 2006]. Therefore, we examined the effect of EtOH on the phosphorylation and interaction of various proteins within the mTORC1. Incubation of C2C12 myocytes with 100 mM EtOH for 18-24 h significantly altered the phosphorylation state of mTOR, raptor and PRAS40 (Fig. 1A). For example, EtOH decreased phosphorylation of mTOR by 30%, while it increased raptor and PRAS40 phosphorylation by ~55% and 35%, respectively (Fig. 1B). On the other hand, EtOH did not affect the levels of GβL. Upstream regulators of mTORC1 were also affected by EtOH, as exemplified by the increased phosphorylation of Akt (T308 and S473) (Fig. 1A).
Fig. 1. Phosphorylation of various TORC1 components in response to EtOH.
C2C12 myocytes were incubated in the presence or absence of 100 mM EtOH for 18-24 h. Equal amounts of cell extracts were collected and analyzed via Western blotting using antibodies against phosphorylated (P) mTOR (S2448), raptor (S792), PRAS40 (T246), and Akt (T308, S473) as well as the total (T) forms of the indicated proteins (panel A). Panel B, phosphorylation of mTOR, raptor, and PRAS40 was quantified from 5 independent experiments and plotted on a graph (3 replicate samples/experiment). Results for indicated phosphorylated proteins were normalized to total protein and expressed as a percentage of basal control levels. Data are mean ± SE. * P< 0.05 versus control (con) values. Panel C, myocytes were incubated with insulin (20 nM) for 15 min or with AICAR (2 mM) for 2.5 h. Cell lysates were analyzed by immunoblotting with total and phosphorylated PRAS40 (T246).
The increase in PRAS40 phosphorylation following EtOH treatment was unexpected, based on previous observations in which insulin also increased phosphorylation at this same residue [Kovacina et al., 2003; Vander Haar et al., 2007]. Since the above described insulin experiments were conducted using different cell lines, we next characterized the response of C2C12 myocytes to this anabolic stimulus. Cells were treated with insulin and phosphorylation levels were examined by Western blotting. Alternatively, cells were treated with the AMP analog AICAR, a compound that increases AMPK activity and suppresses protein synthesis. As expected, PRAS40 phosphorylation was increased by insulin and decreased by AICAR (Fig. 1C).
Role of PRAS40 in the EtOH-induced suppression of protein synthesis
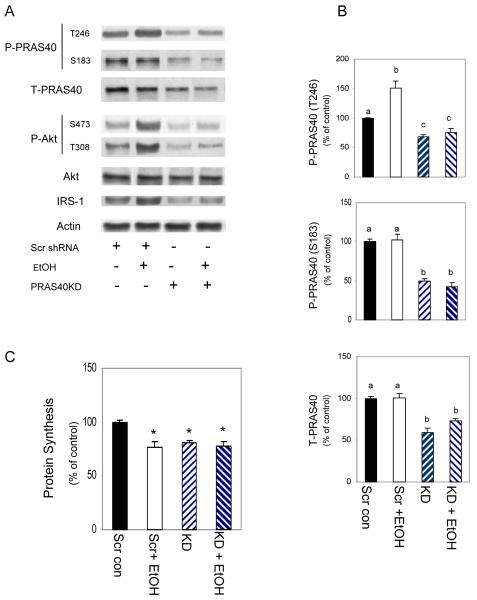
Although mTORC1 appears responsible for the suppressive effect of EtOH on myocyte protein synthesis, the role played by individual proteins within the complex remains unclear. To address this issue, we first examined the function of PRAS40. C2C12 myocytes were transfected with scrambled shRNA or a PRAS40 specific shRNA and cell extracts were collected. As shown in Figure 2 A-B, there was a 40-50% decrease in total PRAS40 in cells transfected with PRAS40 shRNA, as compared to those transfected with scrambled shRNA. Likewise, PRAS40 phosphorylation at residues T246 and S183 decreased by 35% and 50%, respectively. In addition, we observed a decreased phosphorylation of Akt (T308 and S473) and total IRS-1. PRAS40 KD also altered the manner in which cells responded to EtOH. For instance, knockdown countered the stimulatory effect of EtOH on PRAS40 phosphorylation, as this level was reduced below control values in EtOH-treated knockdown cells. It is noteworthy that myocytes transfected with scrambled shRNA and untreated control cells appeared to respond similarly to EtOH (compare 1A and 2A).
Fig. 2. Both EtOH and PRAS40 knockdown decrease basal protein synthesis.
C2C12 myocytes were transfected with scrambled shRNA or shRNA specifically targeting PRAS40. Panel A, PRAS40 knockdown decreased phosphorylation of PRAS40 at T246, S183 and levels of total PRAS40. Levels of P- Akt (T308, S473) and IRS-1 were also decreased in PRAS40 knockdown cells. Panel B, quantitated data are presented in bar graphs. Panel C, PRAS40 knockdown and scrambled control cells were labeled with [35S] methionine/cysteine in the presence or absence of 100 mM EtOH. Bar graphs represent the mean ± SE of 4 independent experiments consisting of 3 replicate samples per experiment. Groups with different letters are significantly different from one another (* P< 0.05). Group with the same letters are not significantly different.
Based on the reported inhibitory effects of PRAS40 on mTOR kinase activity [Sancak et al., 2007; Wang et al., 2007], we anticipated that decreased levels of this protein would ameliorate the adverse affect of EtOH on muscle protein synthesis. Hence, we measured protein synthesis in PRAS40 KD cells in the presence or absence of EtOH (Fig. 2C). Contrary to expectations, PRAS40 KD alone decreased protein synthesis. The protein synthetic rate decreased comparably to cells transfected with scrambled shRNA and then treated with EtOH. The combination of PRAS40 KD and EtOH did not have an additive effect on protein synthesis.
Effects of EtOH and PRAS40 knockdown on mTORC1 component phosphorylation and mTOR activity
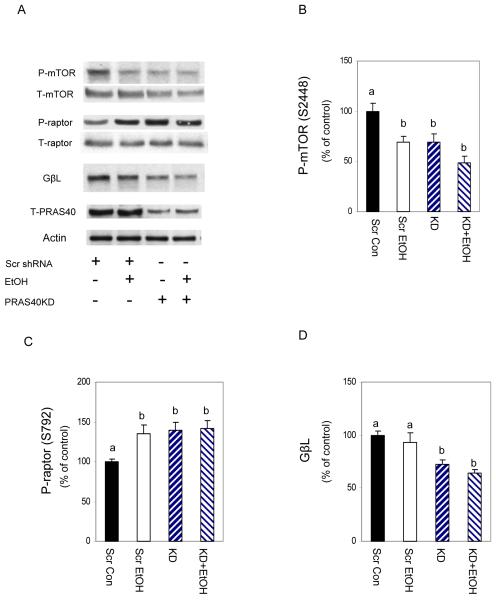
The above results indicate that PRAS40 KD and EtOH can each inhibit protein synthesis. To address possible mechanistic similarities, we first examined the effect of PRAS40 KD on the phosphorylation of members of mTORC1. Myocytes were transfected as above and immunoblotted with antibodies specific for raptor, mTOR and GβL. Knockdown of PRAS40 decreased p-mTOR, while it increased the amount of p-raptor (Fig. 3). In each case, the results were similar to those of EtOH. In contrast to EtOH, however, PRAS40 KD decreased total GβL. Again, the combination of knockdown and EtOH had no additive effect on the parameters assessed.
Fig. 3. EtOH and PRAS40 knockdown alter mTOR and raptor phosphorylation.
Scrambled control and PRAS40 knockdown cells were incubated in the presence or absence of 100 mM EtOH for 18-24 h. Cell extracts were analyzed via Western blotting using antibodies that recognize phosphorylated (P) mTOR (S2448) and raptor (S792) as well as total (T) mTOR, raptor and GβL (panel A). Panels B-D, phosphorylation levels of mTOR and raptor, as well as total GβL were quantified in bar graphs. Results were normalized to total protein and expressed as a percentage of scrambled control levels. Each bar graph represents mean ± SE of 4 independent experiments consisting of 4 replicate samples per experiment. Groups with different letters are significantly different from one another (P< 0.05). Groups with the same letters are not significantly different.
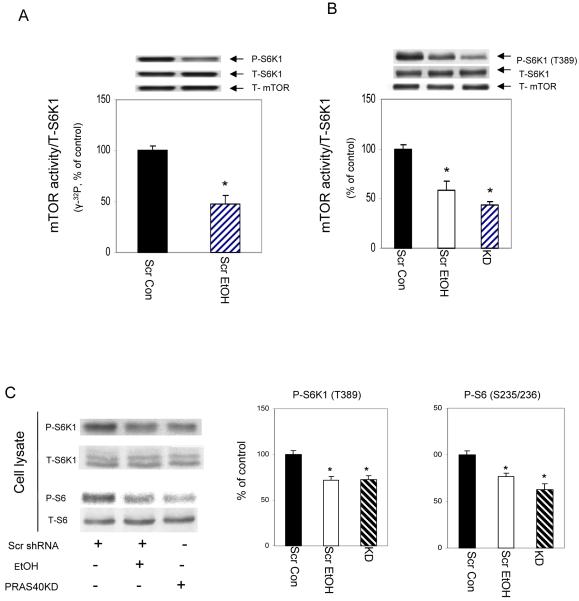
Based on the above results, we anticipated that the activity of mTOR would likely be compromised by EtOH and knockdown conditions. Therefore, we performed an in vitro kinase assay to directly determine mTOR activity per se. In this assay, total S6K1 was isolated from control myocytes and used as substrate, while mTOR was immunoprecipitated from scrambled control cells treated with or without EtOH. The activity of this protein was examined using [γ-32P] ATP labeling. As illustrated in Figure 4A, EtOH decreased the activity of mTOR. Note that this in vitro experiment measured the incorporation of [γ-32P] ATP into total S6K1 protein, and as such, we could not distinguish the specific S6K1 phosphorylation site responsible for this change. To address this issue, we performed an in vitro kinase assay as above, albeit using unlabeled ATP. Following completion of the in vitro reaction, the material was subjected to Western blot using an S6K1 antibody that recognized the phosphorylated form of the T389 site. A significant decrease in mTOR activity was observed at T389 site in cells treated with EtOH or in PRAS40 KD cells (Fig. 4B). Because no significant difference was observed between [γ-32P] ATP labeling and unlabeled ATP, subsequent in vitro kinase activity was determined using unlabelled ATP. To confirm that similar change in mTOR kinase activity occur in vivo, we performed Western blot on cell lysates. Fig. 4C shows that both p-S6K1 (T389) and p-ribosomal S6 (S235/236) levels decreased with EtOH treatment or PRAS40 knockdown.
Fig. 4. PRAS40 knockdown decreases mTOR kinase activity towards S6K1.
For in vitro kinase activity, cells were lysed in 0.3% CHAPS buffer. mTOR was immunoprecipitated from 150 μg of cell lysate, and the activity was assayed using S6K1 as the substrate. Reaction mixtures were incubated as described under “Materials and Methods Section” in the presence (panel A) or absence (panel B) of [γ- 32P] ATP. Panel B, Reaction mixtures were examined by Western blots using the anti-phosphorylated S6K1 (T389) antibody. Results were corrected with immunoprecipitated mTOR. Panel C, equal amounts of cell extracts were analyzed via Western blotting using antibodies that recognize either phosphorylated or total S6K1 and S6 ribosomal protein. Results are expressed as a percentage of scrambled control levels. Each bar graph represents mean ± SE of 4 independent experiments consisting of 4 replicate samples per experiment. * P< 0.05 versus the scrambled control values.
EtOH and PRAS40 knockdown alter TORC1 protein-protein interactions
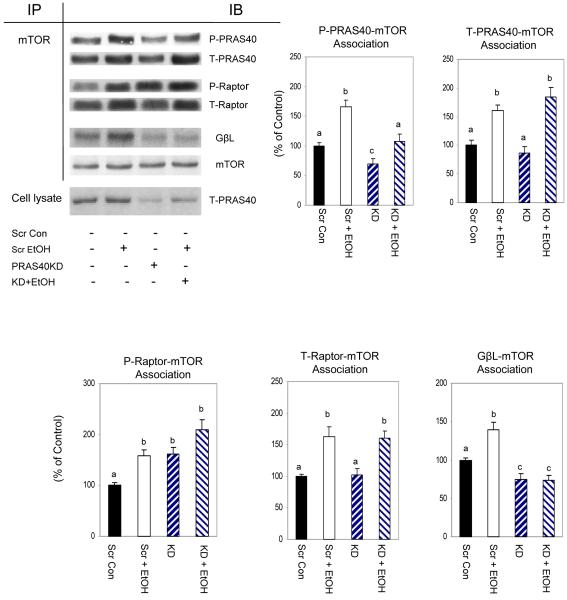
Owing to the significant changes in mTORC1 phosphorylation, we next examined whether EtOH or PRAS40 knockdown affected the extent of specific protein-protein interactions between complex members. Cell extracts from EtOH-treated or untreated myocytes were immunoprecipitated with mTOR and then immunoblotted with raptor, PRAS40 and GβL. As illustrated in Fig. 5, EtOH increased the association of various members of the mTORC1 complex, as evidenced by the increased interaction of mTOR with raptor, PRAS40 and GβL. These increased interactions were observed for both the phosphorylated and total forms of these proteins.
Fig. 5. Interaction of mTOR with PRAS40, raptor and GβL in EtOH and PRAS40 knockdown cells.
Scrambled control and PRAS40 knockdown myocytes were incubated in the presence or absence of EtOH. mTOR was immunoprecipitated from equal amounts of cell extracts and then immunoblotted with antibodies specific for phosphorylated (P) PRAS40 (T246), raptor (S792), as well as total (T) PRAS40, raptor and GβL. Results were normalized with immunoprecipitated mTOR, which was assessed by immunoblotting. Data are mean ± SE of 4-6 independent experiments consisting of 3 replicate samples per experiment. Groups with different letters are significantly different from one another (P< 0.05). Groups with the same letters are not significantly different.
The knockdown of PRAS40 also affected the associations between members of mTORC1. For example, knockdown decreased p-PRAS40 but not total PRAS40 bound to mTOR (Fig. 5). In contrast, the addition of EtOH to knockdown cells increased the amount of p-PRAS40-mTOR complex, relative to PRAS40 knockdown cells. However, this response was lower than levels observed in scrambled controls incubated with EtOH.
The raptor and mTOR interaction is believed to play an important role in regulating the process of protein synthesis and, as such, it may be one of the factors mediating the reduction in protein synthesis. PRAS40 KD increased the association of mTOR with p-raptor (Fig. 5). Although the amount of mTOR- p-raptor association tended to be further elevated in PRAS40 KD myocytes incubated with EtOH, this change did not achieve statistical significance. EtOH increased the GβL-mTOR complex in cells transfected with scrambled shRNA. However, PRAS40 KD decreased the association of these proteins.
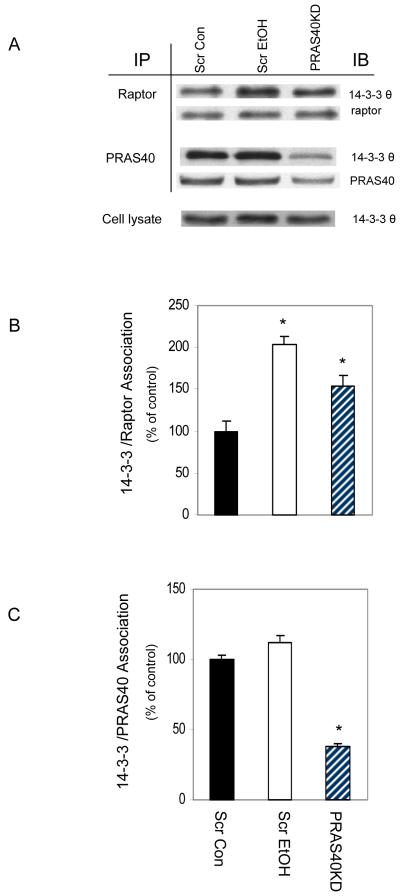
EtOH and PRAS40 knockdown alters 14-3-3/raptor association
Akt-mediated phosphorylation of PRAS40 at T246 leads to the binding of this protein to the cytosolic anchor protein 14-3-3, and this interaction causes conformational changes that can affect mTOR activity [Kovacina et al., 2003; Vander Haar et al., 2007]. Hence, we next examined whether EtOH or PRAS40 KD affected the interaction of 14-3-3 with PRAS40 or other mTORC1 component(s). As illustrated in Fig. 6, both EtOH and PRAS40 KD increased the binding of raptor with 14-3-3. In contrast, the PRAS40 and 14-3-3 association was not affected by EtOH, indicating that phosphorylation of PRAS40 at this site may not be crucial for this process. As expected, PRAS40 KD decreased the interaction of PRAS40 and 14-3-3.
Fig. 6. Association of raptor and PRAS40 with 14-3-3 in EtOH and PRAS40 knockdown cells.
Scrambled control myocytes were incubated in the presence or absence of EtOH for 18-24 h, and these were compared to PRAS40 knockdown cells. Raptor or PRAS40 were immunoprecipated from equal amounts of cell extracts and then immunoblotted with antibody against 14-3-3 θ. Levels of interaction of Raptor /14-3-3 (panel B) and PRAS40/14-3-3 (panel C) were quantified in bar graphs. Results were normalized with immunoprecipitated (IP) raptor or PRAS40. Data are mean ± SE of 3 independent experiments consisting of 3 replicate samples per experiment. * P< 0.05 versus the control values.
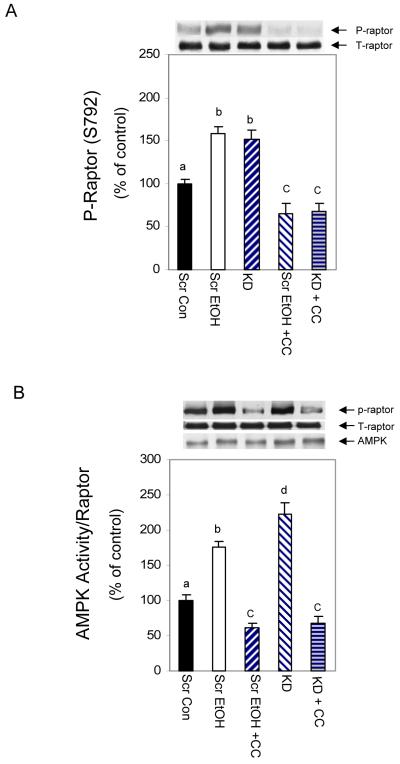
EtOH and PRAS40 knockdown increase AMPK activity towards raptor and TSC2
It was reported that raptor is regulated by AMPK under conditions of energy stress [Gwinn et al., 2008]. If AMPK mediates the effects of EtOH and PRAS40 knockdown on raptor phosphorylation, then this should be sensitive to treatment with an AMPK inhibitor compound C. As shown in Fig. 7A, EtOH and PRAS40 KD increased raptor phosphorylation, and both were suppressed by compound C. To further confirm AMPK regulation of mTORC1 activity, an in vitro AMPK activity assay was performed using raptor as a substrate. As illustrated in Fig. 7B, EtOH increased AMPK activity significantly when raptor was utilized as the substrate. However, this increase was prevented when compound C was included in the reaction mixture. In a similar manner, PRAS40 KD increased the activity of AMPK towards raptor, and this activity again was blocked by the inhibitor. Thus, these data support a model in which increased AMPK activity can negatively regulate mTORC1 by affecting the phosphorylation of raptor. This, in turn, may influence raptor’s subsequent interaction with other proteins such as 14-3-3.
Fig. 7. EtOH and PRAS40 knockdown increase AMPK activity toward raptor.
C2C12 myocytes or PRAS40 KD cells were pre-incubated for 1 h in the presence or absence of the AMPK inhibitor compound C (20 μM). Knockdown cells were harvested, while scrambled control cell were further treated with EtOH. The specificity of compound C was examined via Western blot using anti-phospho-raptor. Results were normalized to total protein and expressed as a percentage of scrambled control levels (panel A). Panel B, an in vitro AMPK activity assay was performed where raptor was utilized as the substrate in the presence of AMP. Cells were lysed in 1% NP-40 and AMPK was immunoprecipitated from 150 μg of lysate. The activity was determined in the presence or absence of 20 μM compound C as described above. Results were normalized with immunoprecipitated AMPK which was assessed by immunoblotting. Each bar graph represents mean ± SE of 3 independent experiments consisting of 4 replicate samples per experiment. Groups with different letters are significantly different from one another (* P< 0.05). Groups with the same letters are not significantly different.
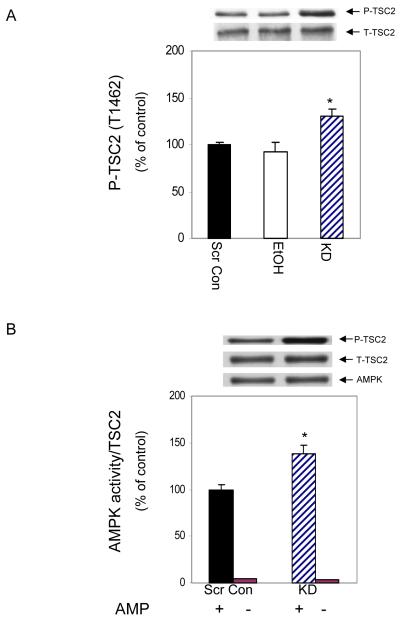
AMPK also regulates TSC2 phosphorylation and thereby inhibits mTORC1 activity [Inoki et al., 2003]. Accordingly, we assessed whether TCS2 is regulated by AMPK following PRAS40 KD or EtOH treatment. Figure 8A shows that PRAS40 KD increased the phosphorylation of TSC2 at T1462 by 30%, when compared to the values of scrambled controls. In contrast, EtOH did not alter TSC2 phosphorylation at this site. To examine whether TSC2 is directly regulated by AMPK, an in vitro AMPK activity assay was performed using TSC2 as a substrate. AMPK activity was increased, as judged by its ability to phosphorylate TSC2 (Fig. 8B). Thus, in myocytes where PRAS40 was knocked down, our data suggest that AMPK may partially mediate its effect on mTOR via its action on TSC2.
Fig. 8. PRAS40 knockdown increases AMPK activity towards TSC2.
Panel A, cell extracts were collected and analyzed via Western blotting using an antibody that recognizes phosphorylated TSC2. Panel B, AMPK kinase activity was examined using an in vitro AMPK activity assay where TSC2 was utilized as the substrate in the presence of AMP. Cells were lysed in 1% NP-40, and AMPK was immunoprecipitated from 150 μg of lysate. The ability of AMPK to phosphorylate TSC2 (T1462) was determined as described under “Materials and Methods.” Results were normalized with immunoprecipitated AMPK which was assessed by immunoblotting. Results are expressed as a percentage of basal scrambled control levels. Each bar graph represents mean ± SE of 3 independent experiments consisting of 3 replicate samples per experiment. (* P< 0.05).
DISCUSSION
The ability of EtOH to decrease protein synthesis in myocytes is associated with a concomitant decrease in mTOR, S6K1, and 4EBP1 phosphorylation, suggesting a role for these kinases in mediating the EtOH effect [Hong-Brown et al., 2006]. The present study extends these findings by examining the post-translational modifications and interactions of various members of the mTORC1 complex. In addition, we observed that AMPK plays a key role in this process. Remarkably, many of the effects of EtOH were mimicked by the knockdown of PRAS40, including changes in protein synthesis and signaling events.
Herein, we report that EtOH increased the phosphorylation of raptor. This is in agreement with studies which reported an elevation in phosphorylated raptor under energy stress conditions [Gwinn et al., 2008]. EtOH also enhanced T246 phosphorylation of PRAS40. This was unexpected, as we (Fig. 1C) and others [Sancak et al., 2007; Vander Haar et al., 2007] have also detected an increase following insulin treatment of cells. The induced phosphorylation of PRAS40 has been posited to increase mTOR activity [Vander Haar et al., 2007; Zhang et al., 2009]. These previous data suggest that PRAS40 functions as a negative regulator of mTORC1, and that phosphorylation of PRAS40 relieves this inhibition. Therefore, based on this model, we expected a decreased phosphorylation of PRAS40 following exposure to EtOH. When viewed in this light, our results suggest that phosphorylation of PRAS40 at T246 may not be essential, or at least not sufficient to positively regulate mTOR activity and protein synthesis. Indeed, recent studies reported that phorbol esters can activate mTORC1 signaling without inducing PRAS40 phosphorylation at T246 [Fonseca et al., 2008]. However, this does not exclude the possibility that other phosphorylation sites such as S221 or S212 may be responsible for regulating mTOR activity under our experimental conditions.
The role PRAS40 plays in affecting mTORC1 activity may be more complicated than expected. Surprisingly, PRAS40 KD inhibited protein synthesis, and this decrease was associated with a suppression of mTORC1 activity. Thus, in C2C12 myocytes, the knockdown of PRAS40 did not reduce its function as a mTORC1 inhibitor. Instead, the effects were similar to those seen after the addition of EtOH. Reduction in PRAS40 was associated with decreased levels of GβL. This could potentially have a negative effect on mTOR activity, because GβL is important for the full activity of mTOR kinase [Kim et al., 2003; Wullschleger et al., 2005]. PRAS40 KD also increased the phosphorylation of raptor and enhanced the interaction with its binding partners, while levels of p-mTOR were decreased. Collectively, these findings may explain the observed decrease in mTOR activity.
Changes in protein phosphorylation can trigger alterations in existing protein-protein interactions. In the presence of EtOH, there was an increased interaction between PRAS40 and mTOR. This is consistent with reports in which stressors such as amino acid deprivation or the glycolytic inhibitor 2-deoxyglucose increased the PRAS40-mTOR interaction [Vander Haar et al., 2007]. On the other hand, this increased stability was unexpected, based on the phophorylation status of PRAS40 at T246. As described above, the phosphorylation of other PRAS40 residues may be decreased by EtOH, and if so, this may account for the increased PRAS40-mTOR association. We also observed an increased interaction between raptor and mTOR, in agreement with previous reports [Kim et al., 2002; Zhang et al., 2009]. Phosphorylation of raptor at S792 was apparently not responsible for the increased mTOR-raptor association, since mutation of this residue does not affect the association of raptor-mTOR [Gwinn et al., 2008]. However, it is possible that other phosphorylation sites may also be responsible for this change.
Previous studies reported that phosphorylation of PRAS40 and raptor increased the association of these proteins with 14-3-3 [Gwinn et al., 2008; Kovacina et al., 2003; Vander Haar et al., 2007; Yu et al., 2008]. 14-3-3 regulates the activity of its binding partners by inducing changes in the proteins catalytic activity, or by triggering a disruption of existing protein-protein interactions [Bridges and Moorhead, 2005; Yaffe, 2002]. In the current study, EtOH increased raptor phosphorylation and enhanced its binding to 14-3-3. Hence, it is possible this interaction may account, in part, for the decreased mTOR activity. In contrast to these results, the level of PRAS40 and 14-3-3 association was unchanged, even though PRAS40 was hyperphosphorylated at residue T246 following EtOH exposure. One possible explanation for our result is that other PRAS40 phosphorylation sites are responsible for the interaction. Indeed, it has been reported that phosphorylation of PRAS40 at S221 plays a role in the binding of this protein to 14-3-3 [Wang et al., 2008]. Regardless, these data are consistent with reports showing that induction of mTORC1 activity does not necessarily require the binding of PRAS40 to 14-3-3 [Fonseca et al., 2008; Sancak et al., 2007]. Again, these results, as well as our own, are in contrast to studies in which insulin increased the interaction between PRAS40 and 14-3-3 [Kovacina et al., 2003; Vander Haar et al., 2007]. Hence, our data suggest that the changes in affinity of various components of the mTORC1, and/or enhanced raptor and 14-3-3 interaction play an important role in reducing mTOR activity in response to EtOH.
In our work, the increased association between proteins was not limited to treatment with EtOH, as a number of similar results were observed in PRAS40 KD cells. For example, there were increased levels of mTOR/raptor and raptor/14-3-3 complexes. However, in contrast to EtOH, knockdown decreased the interaction between GβL and mTOR. Thus, the independent factors EtOH and PRAS40 KD each decreased mTOR activity and protein synthesis. Furthermore, this effect was mediated by changes in the affinity of protein-protein interactions.
EtOH can potentially influence the activity of mTOR via the actions of a number of upsteam regulators. Phospholipase D, for example, signals to mTOR, and EtOH has been shown to have an antagonistic effect on this process [Fujita et al., 2008]. AMPK and IRS-1 also signal to mTOR, although they target different components of the mTORC1 complex. Changes in the cellular energy status activate AMPK [Memmott and Dennis, 2009], thereby down-regulating energy expensive processes such as protein synthesis. In our study, EtOH increased AMPK activity in concert with a decrease in mTOR activity. Two possible mechanisms could account for the signaling of AMPK to mTOR. AMPK could either directly phosphorylate raptor, or it could act indirectly, via its action on TSC2. In the present study, AMPK increased the phosphorylation of the mTORC1 component raptor, and enhanced its interaction with 14-3-3. This is in agreement with results reported by Gwinn et al. (2008). In contrast, AMPK did not increase the phosphorylation of TSC2 following EtOH exposure. This is similar to results from Smith et al. (2005) who observed that various cellular stressors impair mTOR signaling in a TSC2-independent manner. Finally, EtOH may also signal to mTOR via the IRS-1 pathway, as EtOH significantly increased total IRS-1. This change was accompanied by increased phosphorylation of its downstream target Akt. The increased phosphorylation of Akt could account for the observed increased phosphorylation of PRAS40 [Andrabi et al., 2007; Miller et al., 2008; Nascimento et al., 2006; Vander Haar et al., 2007].
PRAS40 KD increased AMPK activity and raptor phosphorylation in a manner similar to EtOH. In contrast to EtOH, there was an increase in TSC2 phosphorylation following PRAS40 knockdown. TSC2 has been shown to be regulated by Akt and AMPK [Inoki et al., 2002; Inoki et al., 2003; Manning et al., 2002]. However, the observed increase of TSC2 phosphorylation in the current study does not appear to result from increased Akt activity, because Akt phosphorylation was decreased in knockdown cells. On the other hand, our in vitro kinase results indicate that PRAS40 knockdown increases AMPK activity. This, in turn, directly phosphorylates both raptor and TSC2, a result that is in agreement with previous studies [Gwinn et al., 2008; Inoki et al., 2003]. Thus, it appears that raptor and TSC2 represent major targets of AMPK, and as such, they may be required for the suppression of mTOR kinase activity in response to PRAS40 KD.
Based on our result and information from the literature, we propose the following model for how EtOH and PRAS40 KD impair mTORC1 function and protein synthesis in C2C12 myocytes (Fig. 9). EtOH appears to signal to the mTORC1 via the action of AMPK. AMPK can directly regulate raptor at S792, and AMPK activity may also indirectly increase PRAS40 phosphorylation, most likely through its actions on the regulators IRS-1, PDK1 and Akt. Changes in protein interactions appear to play a major role in the inhibitory effects of EtOH on protein synthesis. For example, PRAS40 showed an increased interaction with mTOR, consistent with its role as an inhibitor. Raptor also exhibited an enhanced binding to mTOR, as well as an increased binding with 14-3-3. This latter observation may partially explain the suppression of mTOR activity, since 14-3-3 may block the ability of raptor to recruit downstream target proteins. The knockdown of PRAS40 had similar effects as EtOH, with certain exceptions. PRAS40 knockdown activated AMPK, and this kinase was shown to directly phosphorylate both raptor and TSC2. The activation of TSC2 may play a role in reducing mTOR activity. In agreement with the effects of EtOH, there was an increased interaction between raptor, mTOR and 14-3-3. However, the level of the GβL and mTOR interaction decreased. This reduction may partially account for the decline in mTOR activity, since GβL is important for this activity [Wullschleger et al., 2005]. Taken together, our data suggest a mechanism by which EtOH or PRAS40 KD may suppress the activity of mTORC1 by increasing the affinity among the mTORC1 components and 14-3-3, thereby decreasing protein synthesis.
Fig. 9. Model describing possible mechanisms by which EtOH and PRAS40 knockdown regulate mTORC1 phosphorylation and protein-protein interactions.
EtOH signals mTORC1 via both the AMPK and IRS-1/PDK1/Akt pathways. The activation of AMPK results in the increased phosphorylation of raptor. EtOH also increases raptor/mTOR and raptor/14-3-3 binding, as well as increasing the GβL-mTOR interaction. Likewise, there is an enhanced binding of PRAS40 to mTOR, and this is independent of changes in the PRAS40/14-3-3 association (panel A). In general, PRAS40 knockdown elicits similar effects as EtOH. However, PRAS40 knockdown decreases the GβL-mTOR interaction, while increasing the levels of p-TSC2 in an Akt-independent manner (panel B). Taken together, these changes in protein-protein interactions may partially account for the decrease in mTORC1 activity and protein synthesis.
ACKNOWLEDGEMENTS
This study was supported by National Institute of Health Grant AA11209.
LITERATURE CITED
- Andrabi S, Gjoerup OV, Kean JA, Roberts TM, Schaffhausen B. Protein phosphatase 2A regulates life and death decisions via Akt in a context-dependent manner. Proc Natl Acad Sci U S A. 2007;104:19011–6. doi: 10.1073/pnas.0706696104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avruch J, Long X, Ortiz-Vega S, Rapley J, Papageorgiou A, Dai N. Amino acid regulation of TOR complex 1. Am J Physiol Endocrinol Metab. 2009;296:E592–602. doi: 10.1152/ajpendo.90645.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges D, Moorhead GB. 14-3-3 proteins: a number of functions for a numbered protein. Sci STKE 2005. 2005:re10. doi: 10.1126/stke.2962005re10. [DOI] [PubMed] [Google Scholar]
- Fonseca BD, Lee VH, Proud CG. The binding of PRAS40 to 14-3-3 proteins is not required for activation of mTORC1 signalling by phorbol esters/ERK. Biochem J. 2008;411:141–9. doi: 10.1042/BJ20071001. [DOI] [PubMed] [Google Scholar]
- Fonseca BD, Smith EM, Lee VH, MacKintosh C, Proud CG. PRAS40 is a target for mammalian target of rapamycin complex 1 and is required for signaling downstream of this complex. J Biol Chem. 2007;282:24514–24. doi: 10.1074/jbc.M704406200. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Hiroyama M, Sanbe A, Yamauchi J, Murase S, Tanoue A. ETOH inhibits embryonic neural stem/precursor cell proliferation via PLD signaling. Biochem Biophys Res Commun. 2008;370:169–73. doi: 10.1016/j.bbrc.2008.03.060. [DOI] [PubMed] [Google Scholar]
- Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006;11:859–71. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–26. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, Tokunaga C, Avruch J, Yonezawa K. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–89. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- Hong-Brown LQ, Brown CR, Huber DS, Lang CH. Alcohol and indinavir adversely affect protein synthesis and phosphorylation of MAPK and mTOR signaling pathways in C2C12 myocytes. Alcohol Clin Exp Res. 2006;30:1297–307. doi: 10.1111/j.1530-0277.2006.00157.x. [DOI] [PubMed] [Google Scholar]
- Hong-Brown LQ, Brown CR, Huber DS, Lang CH. Alcohol regulates eukaryotic elongation factor 2 phosphorylation via an AMP-activated protein kinase-dependent mechanism in C2C12 skeletal myocytes. J Biol Chem. 2007;282:3702–12. doi: 10.1074/jbc.M606593200. [DOI] [PubMed] [Google Scholar]
- Hong-Brown LQ, Brown CR, Huber DS, Lang CH. Lopinavir impairs protein synthesis and induces eEF2 phosphorylation via the activation of AMP-activated protein kinase. J Cell Biochem. 2008;105:814–23. doi: 10.1002/jcb.21882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong-Brown LQ, Frost RA, Lang CH. Alcohol impairs protein synthesis and degradation in cultured skeletal muscle cells. Alcohol Clin Exp Res. 2001;25:1373–82. [PubMed] [Google Scholar]
- Huang B, Porter G. Expression of proline-rich Akt-substrate PRAS40 in cell survival pathway and carcinogenesis. Acta Pharmacol Sin. 2005;26:1253–8. doi: 10.1111/j.1745-7254.2005.00184.x. [DOI] [PubMed] [Google Scholar]
- Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–57. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–90. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, Hall MN. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6:1122–8. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–75. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- Kim DH, Sarbassov DD, Ali SM, Latek RR, Guntur KV, Erdjument-Bromage H, Tempst P, Sabatini DM. GbetaL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol Cell. 2003;11:895–904. doi: 10.1016/s1097-2765(03)00114-x. [DOI] [PubMed] [Google Scholar]
- Kovacina KS, Park GY, Bae SS, Guzzetta AW, Schaefer E, Birnbaum MJ, Roth RA. Identification of a proline-rich Akt substrate as a 14-3-3 binding partner. J Biol Chem. 2003;278:10189–94. doi: 10.1074/jbc.M210837200. [DOI] [PubMed] [Google Scholar]
- Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, Oppliger W, Jenoe P, Hall MN. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell. 2002;10:457–68. doi: 10.1016/s1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell. 2002;10:151–62. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- Memmott RM, Dennis PA. Akt-dependent and -independent mechanisms of mTOR regulation in cancer. Cell Signal. 2009;21:656–64. doi: 10.1016/j.cellsig.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AM, Brestoff JR, Phelps CB, Berk EZ, Reynolds THt. Rapamycin does not improve insulin sensitivity despite elevated mammalian target of rapamycin complex 1 activity in muscles of ob/ob mice. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1431–8. doi: 10.1152/ajpregu.90428.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento EB, Fodor M, van der Zon GC, Jazet IM, Meinders AE, Voshol PJ, Vlasblom R, Baan B, Eckel J, Maassen JA, Diamant M, Ouwens DM. Insulin-mediated phosphorylation of the proline-rich Akt substrate PRAS40 is impaired in insulin target tissues of high-fat diet-fed rats. Diabetes. 2006;55:3221–8. doi: 10.2337/db05-1390. [DOI] [PubMed] [Google Scholar]
- Oshiro N, Takahashi R, Yoshino K, Tanimura K, Nakashima A, Eguchi S, Miyamoto T, Hara K, Takehana K, Avruch J, Kikkawa U, Yonezawa K. The proline-rich Akt substrate of 40 kDa (PRAS40) is a physiological substrate of mammalian target of rapamycin complex 1. J Biol Chem. 2007;282:20329–39. doi: 10.1074/jbc.M702636200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E, Carr SA, Sabatini DM. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25:903–15. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Sato T, Nakashima A, Guo L, Tamanoi F. Specific Activation of mTORC1 by Rheb G-protein in Vitro Involves Enhanced Recruitment of Its Substrate Protein. J Biol Chem. 2009;284:12783–91. doi: 10.1074/jbc.M809207200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol. 2007;9:316–23. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- Wang L, Harris TE, Lawrence JC., Jr. Regulation of proline-rich Akt substrate of 40 kDa (PRAS40) function by mammalian target of rapamycin complex 1 (mTORC1)-mediated phosphorylation. J Biol Chem. 2008;283:15619–27. doi: 10.1074/jbc.M800723200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Harris TE, Roth RA, Lawrence JC., Jr. PRAS40 regulates mTORC1 kinase activity by functioning as a direct inhibitor of substrate binding. J Biol Chem. 2007;282:20036–44. doi: 10.1074/jbc.M702376200. [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Oppliger W, Hall MN. Molecular organization of target of rapamycin complex 2. J Biol Chem. 2005;280:30697–704. doi: 10.1074/jbc.M505553200. [DOI] [PubMed] [Google Scholar]
- Yaffe MB. How do 14-3-3 proteins work?-- Gatekeeper phosphorylation and the molecular anvil hypothesis. FEBS Lett. 2002;513:53–7. doi: 10.1016/s0014-5793(01)03288-4. [DOI] [PubMed] [Google Scholar]
- Yu F, Narasimhan P, Saito A, Liu J, Chan PH. Increased expression of a proline-rich Akt substrate (PRAS40) in human copper/zinc-superoxide dismutase transgenic rats protects motor neurons from death after spinal cord injury. J Cereb Blood Flow Metab. 2008;28:44–52. doi: 10.1038/sj.jcbfm.9600501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Beharry ZM, Harris TE, Lilly MB, Smith CD, Mahajan S, Kraft AS. PIM1 protein kinase regulates PRAS40 phosphorylation and mTOR activity in FDCP1 cells. Cancer Biol Ther. 2009;8 doi: 10.4161/cbt.8.9.8210. [DOI] [PubMed] [Google Scholar]
- Zhang L, Kimball SR, Jefferson LS, Shenberger JS. Hydrogen peroxide impairs insulin-stimulated assembly of mTORC1. Free Radic Biol Med. 2009;46:1500–9. doi: 10.1016/j.freeradbiomed.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]