Abstract
Background:
Diabetes mellitus (DM) is a highly prevalent disease. Atrophy and spontaneous ulcers are the most common cutaneous manifestation of diabetic dermopathy (DD). Before spontaneous ulcers, we believe there is an underlying damage stage although the mechanism is unknown.
Aims:
To explore the expression of extracellular signal-regulated kinase1/2 (ERK1/2), its correlated upstream protein epidermal growth factor receptor (EGFR) and its downstream transcription factor E twenty-six (ETS)-like 1(ELK-1)in the damage of the diabetic rat skin, and to explore the role of ERK1/2 on the recessive damage to diabetic rat skin.
Materials and Methods:
Eighty Sprague-Dawley (SD) rats weighing 260-300 g were randomly divided into control and streptozotocin (STZ)-induced diabetes groups. After 0.5, 2, 4, and 8 weeks, the shaved skin specimens from the back of rats in both groups were collected to observe the histological characteristics of the skin, to measure the thickness of the epidermis and the dermis, and to observe the ultrastructure. Immunohistochemistry (IHC) and Western blot techniques were used to detect the expression and activation of ERK1/2, EGFR, ELK-1 in the skin of the rats. Results: There are ultrastructural changes in the DM skin. With the continuance of the diabetes course, the thicknesses of the epidermis and dermis decreased, and the expression of phospho-ERK1/2 (P-ERK1/2), EGFR, and ELK-1 was decreased gradually in the back skin of the diabetes rats. It was significantly lower in 4 and 8 week DM than that of the normal control (P < 0.05). The expression of P-EGFR and P-ERK1/2 in the back skin of the diabetes rats was positively correlated (r = 0.572 P < 0.05), and the positive correlation was also obtained between P-ERK1/2 and P-ELK-1 (r = 0.715, P < 0.05).
Conclusion:
The phenomenon of recessive damage exists in the skin of diabetes rats, which probably may relate to the weakness of the signal transduction: P-EGFR → ERK1/2 → ELK-1.
Keywords: Activating transcription factors, diabetes, epidermal growth factor receptors, extracellular signal-regulated mitogen-activated protein kinases, skin
Introduction
What was known?
It is known that recombinant human platelet-derived rowth factor enhanced dermal wound healing by a pathway involving ERK and c-fos in diabetic rats.
Diabetes mellitus (DM) is a highly prevalent disease. An estimated 48.3 million Americans are expected to have diabetes by 2050, a 19.8% increasing compared with 2005.[1] Worldwide, diabetes is estimated to affect 151 million people, with a projected increasing to 324 million by the year 2025.[2] with approximately 30% and 70% of patients experiencing some skin involvement during the course of their illness, and these can be the first presenting sign of diabetes.[3] Diabetic dermopathy (DD) is the most common cutaneous manifestation of DM.[4] DD refers to atrophic, spontaneous ulcers, sclerosis, diabetic blisters, and diabetic foot, etc., and particularly, atrophy and spontaneous ulcers are the most common cutaneous manifestation of DD. There is no doubt that atrophy and spontaneous ulcers of DD may relate to the inhibited of the epidermal cell proliferation.
The epidermal growth factor receptor (EGFR) is involved in many cellular processes including cell proliferation, motility, adhesion, and angiogenesis via the activation of principally extracellular signal-regulated kinase (ERK) pathway. ELK-1 are important downstream signaling molecules of ERK. The signal transduction pathway phospho-EGFR → ERK1/2 → ELK-1 plays an important role in maintaining the normal epidermis cell proliferation. However, there have been few studies on the correlation between the biophysical characteristics of skin and the P-EGFR → ERK1/2 → ELK-1 signal transduction pathway in patients with diabetes.
The aim of this study was to evaluate pathological and physiological changes in DM dermal tissue and to explore the correlation between EGFR/ERK/ELK-1 mitogen-activated protein kinase (MAPK) pathway and the underlying skin damage in diabetes using a streptozotocin (STZ)-induced rat model of diabetes experiments.
Materials and Methods
Experimental animals
Eighty specific-pathogen-free male Sprague-Dawley (SD) (F) rats, weighing 260-300 g, supplied by the Laboratory Animal Center of Ningxia Medical University were used in these studies.
Reagents and equipment
STZ (Sigma Company, St. Louis, MO, USA); anti-phospho-ERK1/2 monoclonal antibody (#4376, Cell Signaling Technologies Inc., USA); anti-phospho-ELK-1 monoclonal antibody (#9186, Cell Signaling Technologies Inc., USA); anti-phospho-EGFR monoclonal antibody (#2236, Cell Signaling Technologies Inc., USA); streptavidin-peroxidase (S-P) immunohistochemistry testing kit (SPN-9001, 9002), (Zymed Company, USA); microscope micrometer (Shanghai 3rd Optical Instrument Company, Qing Pu, Shanghai).
Treatment
All rats were housed in a climatically controlled room with free access to water and food under a 12-h light ⁄ 12-h dark cycle. The 80 SD rats were randomized into two groups, Group DM (60 rats) and Group normal control (NC) (20 rats). After a 24-h fast for all animals, rats in the DM group were given a single intraperitoneal injection containing 55 mg/kg STZ in citrate buffer to induce DM. Group NC rats were injected with the same concentration of citric acid buffer. Diabetes was confirmed 72 h after STZ injection from reading specific blood glucose (blood glucose >16 mmol/l). 0.5-week (DM0.5), 2-week (DM2), 4-week (DM4) and 8-week (DM8) after the STZ treatment killed 15 DM rats and 5 NC rats, respectively, then the full-thickness shaved skin of the back were removed for research. Skin was acquired from the back of each rat and divided into three parts. One part was fixed in 2.5% pentobarbital sodium, one part was fixed in 4% formaldehyde solution, and one portion was frozen at −70°C. All procedures were carried out in accordance with the guidelines for animal experiments, and the protocol was approved by the local Animal Ethics Committee.
Histological observation and skin thickness measurement
Dorsal skin from rats in each of the groups was isolated and fixed in formalin and embedded in paraffin prior to sectioning. Paraffin sections of 5 μm were deparaffinized in xylene, 3 × 7 min, room temperature, and rehydrated by stepwise washes in decreasing ethanol/H2O ratio (100-50%, followed by soaking in water). Sections were cut and stained with hematoxylin and eosin (H and E), and the cutaneous histological characteristics were observed under a light microscope.
The thickness of epidermal and the dermal was measured according to the method described by Hwang, et al.,[5] using a micrometer under a microscope. Each sample was measured five times at intervals of 40 mm. The average value was calculated and reported as the thickness of the epidermal and dermal of the sample.
Immunohistochemical examination of mouse skin
For immunohistochemical staining, sections were incubated in Superblock (Pierce Biotechnology Inc., Rockford, IL, USA) blocking reagent for 1 h at room temperature to block non-specific antigen sites. After washing × 3 in PBS, slides were incubated overnight at 4°C with phospho-specific antibodies against EGFR, ERK1/2, and ELK-1 (Cell Signaling Technology Incorporation) at 1:100, Slides were photographed using an Olympus DF plan microscope at × 400 magnification. Images were processed with Image Plus 6.0 software (Media Cybernetics Incorporation, USA). Choose integrated optical density (IOD) as the evaluation parameters and analyzed ten images of each slice. The average value was calculated and reported.
Tissue lysates and western blot analyses
Mouse epidermis was scraped from isolated dorsal skin on ice and homogenized with a Polytron for three 10 s bursts in RIPA lysis buffer (150 mM NaCl, 50 mM Tris-HCl pH 7.5, 1 mM EDTA, 1% NP-40, 1 mM PMSF, 1 mMNa-orthovanadate) supplemented with 40 μl complete protease inhibitor cocktail tablets (Roche Ltd) according to manufacturer-provided instructions. Extracted protein was quantified using the Bio-Rad Protein Assay kit (Bio-Rad Lab., Hercules, CA). Proteins were separated by SDS acrylamide gel electrophoresis (12%) and transferred to nitrocellulose membranes (Schleicher and Schuell, Dassel, Germany). Blots were blocked with 5% BSA for 1 h at room temperature, followed by incubation overnight with antibodies against phosphorylated forms of EGFR, ERK1/2 and ELK-1 (all from Cell Signaling Technology). The antibodies were used at 1:400. Blots were washed with TBS/0.1% Tween 20 and incubated with horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature, followed by three additional washes with TBS/0.1% Tween 20. Chemiluminescence detection was performed according to the manufacturer's instructions (Pierce, Rockford, IL, USA), followed by exposure to X-ray film.
Statistical analysis
All data are expressed as mean ± SEM. Data were analyzed by ANOVA or by Kruskal-Wallis ANOVA for testing data in ranks when data were not normally distributed or the variance differed between groups. Differences between specific groups were analyzed by Tukey's test for multiple comparisons or using the Holm-Sidak method. For all analyses, P < 0.05 was considered statistically significant.
Results
General observation
In terms of the appearance, the rats’ fur in Group DM became dark and dull after 2 weeks of the STZ treatment. After 4 weeks treatments, compared with normal rats, it was observed that the experiment group showed visible weight loss, a quick temper, thinned skin and significantly reduced Activity.
Thickness of the skin
Through measuring the thickness of epidermal and the dermal of the sample, it was found that the average thickness of normal rat's skin epidermal and dermal was 21.4 ± 1.51 μm, and 1444.72 ± 126.11 μm, respectively and there was no obvious difference in DM0.5 groups. However, with the onset time, the thickness of the epidermis and dermis of the back decreased gradually. The epidermis and dermis were significantly thinner in skin sections from the DM4 and DM8 groups compared with sections from NC groups (P < 0.05) [Table 1].
Table 1.
Measurement of the thickness of the skin
Skin morphology observation
The histopathology observation and immunohistochemical examination of normal rats skin
NC rats showed intact skin tissue structure, with clear epidermal layers characterized by the typical multilayer epithelium structure and an abundant number of epidermal cells. Cuticle was thin and uniform, and spinous layer cells relatively were of uniform size, basal cells were in the high columnar shape. The dermal papilla was clear. Collagen fibers arranged uniformly. There was no inflammatory cell infiltration in dermal.
To investigate the expression of P-EGFR, P-ERK1/2, and P-ELK-1 in normal rats skin, IHC staining of paraffin sections from rat's skin with phosphorylation site-specific antibodies to P-EGFR, P-ERK1/2, and P-ELK-1 was performed. P-EGFR was localized predominantly to the surface of the cell membrane. Some were expressed in the nucleus. The IOD value was 133.09 ± 34.45. P-ERK1/2 and P-ELK-1 were localized predominantly to the nucleus and cytoplasm of the epidermal cells. The IOD value was 287.41 ± 57.16 and 257.99 ± 56.19 [Figure 1a].
Figure 1.
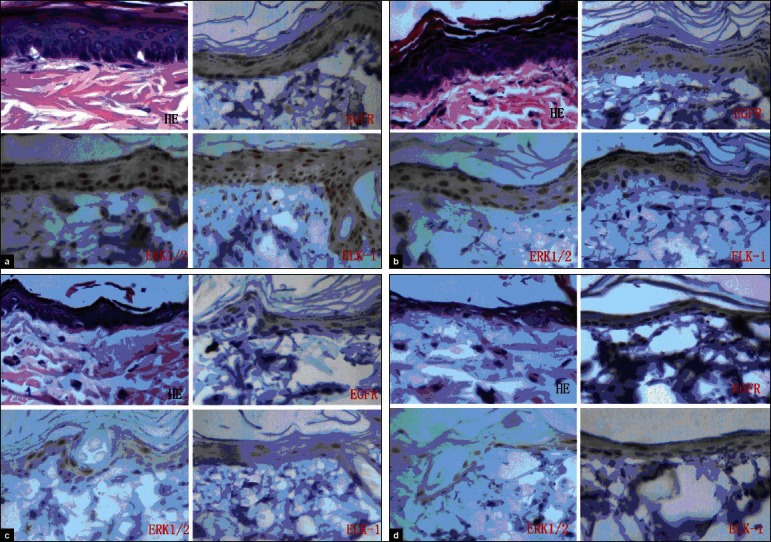
The Histopathology observation and immunohistochemical examination of normal (a) and diabetes (b: DM2, c: DM4, d: DM8) rats skin
The histopathology observation and immunohistochemical examination of diabetes rats skin
After 0.5 weeks of the treatment, it was found that the epidermis and dermis of the DM has not changed in histopathology observation and immunohistochemical examination. After 2 weeks of the treatment, the rat's skin tissue in Group DM was not found to have obvious histological changes in the structure of the epidermal cells. Most epidermal cells remained in the 3-4 levels, but some areas reduced. In IHC examination, the expression of phospho-ERK1/2, EGFR, and ELK-1 decreased slightly [Figure 1b]. After 4 weeks, rats in Group DM had unclear epidermal layers, and an absence of the multilayer epithelium structure in most of the skin was observed. In IHC and Western blot, the expression of phospho-ERK1/2, EGFR, and ELK-1 was decreased [Figure 1c]. After 8 weeks, rats in Group DM had a significant decrease in the number of epidermal cells and significant histological changes. Additionally, portions of the collagen fibers became atrophic, swollen, and degenerated, and it was accompanied by an inflammation. Subcutaneous fat displayed progressive atrophy or was absent. The expression of phospho-EGFR, ERK1/2, and ELK-1 decreased significantly. The IOD values were 86.76 ± 25.8, 139.71 ± 27.82, and 106.99 ± 16.47 to the phospho-EGFR, ERK1/2, and ELK-1, respectively, and they were significantly lower than that of group NC (P < 0.05) [Figure 1d].
Western blot
To confirm the immunohistochemical examination results, the Western blot was performed using the same phospho-specific antibodies. It was found that with the continuance of the diabetes course, the expressions of phospho-ERK1/2, EGFR, and ELK-1 was decreased gradually in the back skin of the diabetes rats. Especially in 4 and 8 weeks DM groups, these expressions were significantly lower than in the normal groups.(P < 0.05) [Figure 2]. Interestingly it was observed that the expressions of P-EGFR and P-ERK1/2 in the back skin of the diabetes rats was positively correlated (r = 0.572, P < 0.05), so was the expression of P-ERK1/2 and P-ELK-1 (r = 0.715, P < 0.05).
Figure 2.
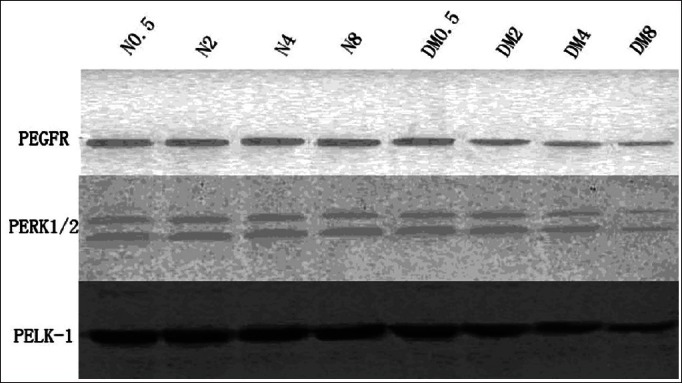
The expression of phospho-EGFR, phospho-ERK1/2, and phospho-ELK-1 in the normal, DM2, DM4, and DM8 rat's back skin by Western blotting: With the continuance of the diabetes course, the expression of P-EGFR, P-ERK1/2, and ELK-1 decreased gradually
Discussion
The steady proliferation rate of normal skin tissue and cells is important for maintaining the normal metabolism and structural integrity. Through proliferation, differentiation and continually upward moving, the basement epidermal cells in epidermal tissues eventually become horny cells finally and slough off the surface of the skin.[6,7]
The normal process requires healthy skin structures and the physiological functions of the skin, while in the diabetic patients skin, the atrophy and spontaneous ulcers are a common clinical phenomenon.[8] Before spontaneous ulcers, we believe there is an underlying damage stage.
To prove this underlying damage stage exists, we detected the back skin from diabetic rats and found that the thickness of the epidermis and dermis of diabetic skin was thinner than the control group, and there was an obvious diminution in the number of epidermal cells compared with control tissue. These general and histological observations both suggest that a dysfunction of epidermal cell proliferation may exist in DM skin.
Although now the mechanism for many diabetes-associated skin conditions remains unknown, it is thought that DD is developed from the vascular complications of diabetes.[9] As the proliferation of epidermal cells is related to the cells themselves, as well as to signals in their microenvironment, changes in the cells and the extracellular microenvironment jointly lead to a different proliferation pattern.[10–12]
Many epidermal abnormalities may be related to alterations in cell signaling, especially to EGFR-mediated cell responses to environmental challenges. EGFR signaling is needed for maintaining the proliferation, differentiation, and survival of epidermal cells.[10] We know ligands for EGFR include EGF, heparin binding EGF-like growth factor (HB-EGF), and transforming growth factor. Controlling a proper level of EGFR signaling is critical for the physiological state of epidermal tissues, and long-term exposure to hyperglycemia may inevitably affect EGFR signaling pathway, leading to the cell dysfunction, including compromised barrier function and delayed wound healing. It has been reported that a dramatic decrease in hepatic EGFR transcripts was observed during the progression of diabetes,[13] and type 2 diabetic mice show that the EGFRs are upregulated and the phosphorylation of them are also elevated, which were suspected to play a role in kidney enlargement in diabetes.[14] However, there are very few reports about the EGFR signaling in diabetic dermopathy. In this study, we found the phosphorylation of EGFR decreased in the diabetic rat's skin.
ERK1/2 is a member of MAPK family. They have a central function in the intracellular signal transduction initiated by extracellular stimuli, including growth factors and hormones.[15] There are sufficient data that support MAPK signaling pathway is closely related to the formation of diabetic complications. It has been implicated in the pathogenesis of diabetic nephropathy,[16] retinopathy,[17] angiopathy[18] and apoptosis and atrophy in skeletal muscle.[19] It has been reported that insulin-resistant and hyperinsulinemic states cause enhanced activity in the MEK/ERK pathway,[20,21] and Cheng, et al.[22] reported recombinant human platelet-derived growth factor (PDGF) enhanced dermal wound healing by a pathway involving ERK and c-fos in diabetic rats.
ELK-1 is a member of the ternary complex factor (TCF) subfamily of E twenty-six (ETS)-domain transcription factors.[23] It is targeted in the nucleus by three major MAP kinase pathways as well as the CaMKII pathway.[24] Phosphorylation of nuclear ELK-1 enhances its binding affinity to the DNA at serum response elements and stimulate transcription of immediate early genes.[25] Within this functional context, nuclear ELK-1 has been implicated in processes such as neuronal differentiation,[26] cellular proliferation,[27] and tumorigenesis.[28] Phosphorylation of p42/p44 MAP kinase can activate the transcription activation domain (TAD) of ELK-1.[29]
In this study, we found that the thickness of the epidermis and dermis of the back in the DM groups started thinning from 2-weeks, and ingravescenced after 2 weeks of the treatment. In 4-week DM and 8-week DM, there were significant differences compared with normoglycemia groups. With the continuance of the diabetes course the expression of phospho-ERK1/2 and ELK-1 kept decreasing in the back skin of the diabetes rats. The expression of phospho-ERK1/2, the downstream signaling pathway of EGFR consists of phospho-EGFR. It was found that the expression of P-EGFR and P-ERK1/2 in the back skin of the diabetes rats was positively correlated, and the positive correlation was also obtained between P-ERK1/2 and P-ELK-1.
Therefore, this study suggests there is a direct correlation between hyperglycemia and phospho-EGFR-ERK1/2-ELK-1 expression in the diabetic rats skin. These results may be because increase of glucose in blood causes increase in the skin glucose.[30] Under high glucose concentrations, the accumulation of toxic substances, such as advanced glycation endoproducts (AGEs), induces EGFR glycosylation, reduces EGFR phosphorylation, and downregulates ERK1/2 and ELK-1 activities. On the other hand, high glucose and AGEs inhibit the serine/threonine kinases activation of ERK1/2 in diabetic skin, and downregulates ELK-1 activities. Therefore, reduced ERK1/2 signaling pathway activity caused by hyperglycemia may contribute to the abnormalities, such as basal cell degeneration and decreased cell proliferation, which cause underlying damage. More importantly, such abnormalities cause spontaneous ulcers or delayed Epithelial wound healing in diabetic skin.
What is new?
This study explored the expression of ERK1/2, its correlated upstream protein EGFR, and its downstream transcription factor ELK-1 in the damage of the diabetic rat skin, and explored the role of ERK1/2 on the recessive damage to diabetic rat skin firstly.
Footnotes
Source of support: This work was supported by Grant-in-Aid for National Natural Science Foundation of China (No.81060181) and Natural Science Foundation of Ningxia of China (NZ1222).
Conflict of Interest: Nil.
References
- 1.Narayan KM, Boyle JP, Geiss LS, Saaddine JB, Thompson TJ. Impact of recent increase in incidence on future diabetes burden: U.S., 2005-2050. Diabetes Care. 2006;29:2114–6. doi: 10.2337/dc06-1136. [DOI] [PubMed] [Google Scholar]
- 2.Segars LW, Lea AR. Assessing prescriptions for statins in ambulatory diabetic patients in the United States: A national, cross-sectional study. Clin Ther. 2008;30:2159–66. doi: 10.1016/j.clinthera.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Morgan AJ, Schwartz RA. Diabetic dermopathy: A subtle sign with grave implications. J Am Acad Dermatol. 2008;58:447–51. doi: 10.1016/j.jaad.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Shemer A, Bergman R, Linn S, Kantor Y, Friedman-Birnbaum R. Diabetic dermopathy and internal complications in diabetes mellitus. Int J Dermatol. 1998;37:113–5. doi: 10.1046/j.1365-4362.1998.00273.x. [DOI] [PubMed] [Google Scholar]
- 5.Hwang K, Kim DJ, Hwang SH. Thickness of Korean upper eyelid skin at different levels. J Craniofac Surg. 2006;17:54–6. doi: 10.1097/01.scs.0000188347.06365.a0. [DOI] [PubMed] [Google Scholar]
- 6.Greig AV, James SE, McGrouther DA, Terenghi G, Burnstock G. Purinergic receptor expression in the regeneration epidermis in a rat model of normal and delayed wound healing. Exp Dermatol. 2003;12:860–71. doi: 10.1111/j.0906-6705.2003.00110.x. [DOI] [PubMed] [Google Scholar]
- 7.Szegedi A, Páyer E, Czifra G, Tóth BI, Schmidt E, Kovács L, et al. Protein kinase C isoenzymes differentially regulate the differentiation-dependent expression of adhesion molecules in human epidermal keratinocytes. Exp Dermatol. 2009;18:122–9. doi: 10.1111/j.1600-0625.2008.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang CM, Lincoln J, Cook JE, Becker DL. Abnormal connexin expression underlies delayed wound healing in diabetic skin. Diabetes. 2007;56:2809–17. doi: 10.2337/db07-0613. [DOI] [PubMed] [Google Scholar]
- 9.Goyal A, Raina S, Kaushal SS, Mahajan V, Sharma NL. Pattern of cutaneous manifestations in diabetes mellitus. Indian J Dermatol. 2010;55:39–41. doi: 10.4103/0019-5154.60349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sibilia M, Kroismayr R, Lichtenberger BM, Natarajan A, Hecking M, Holcmann M. The epidermal growth factor receptor: From development to tumorigenesis. Differentiation. 2007;75:770–87. doi: 10.1111/j.1432-0436.2007.00238.x. [DOI] [PubMed] [Google Scholar]
- 11.Harel A, Bloch O, Vardi P, Bloch K. Sensitivity of HaCat keratinocytes to diabetogenic toxins. Biochem Pharmacol. 2002;63:171–8. doi: 10.1016/s0006-2952(01)00847-4. [DOI] [PubMed] [Google Scholar]
- 12.Shen S, Wertheimer E, Sampson SR, Tennenbaum T. Characterization of glucose transport system in keratinocytes: Insulin and IGF-1 differentially affect specific transporters. J Invest Dermatol. 2000;115:949–54. doi: 10.1046/j.1523-1747.2000.00161.x. [DOI] [PubMed] [Google Scholar]
- 13.Chua BH, Chua CC, Zhao ZY, Krebs CJ. Estrone modulates the EGF receptor in the liver of db/db mouse. J Recept Res. 1991;11:941–57. doi: 10.3109/10799899109064689. [DOI] [PubMed] [Google Scholar]
- 14.Wassef L, Kelly DJ, Gilbert RE. Epidermal growth factor receptor inhibition attenuates early kidney enlargement in experimental diabetes. Kidney Int. 2004;66:1805–14. doi: 10.1111/j.1523-1755.2004.00955.x. [DOI] [PubMed] [Google Scholar]
- 15.Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, et al. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr Rev. 2001;22:153–83. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 16.Ma J, Phillips L, Wang Y, Dai T, LaPage J, Natarajan R, et al. Curcumin activates the p38MPAK-HSP25 pathway in vitro but fails to attenuate diabetic nephropathy in DBA2J mice despite urinary clearance documented by HPLC. BMC Complement Altern Med. 2010;10:67. doi: 10.1186/1472-6882-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Y, Feng B, Chen S, Zuo Y, Chakrabarti S. Glucose-induced endothelin-1 expression is regulated by ERK5 in the endothelial cells and retina of diabetic rats. Can J Physiol Pharmacol. 2010;88:607–15. doi: 10.1139/Y10-033. [DOI] [PubMed] [Google Scholar]
- 18.Liu Z, Cao W. p38 mitogen-activated protein kinase: A critical node linking insulin resistance and cardiovascular diseases in type 2 diabetes mellitus. Endocr Metab Immune Disord Drug Targets. 2009;9:38–46. doi: 10.2174/187153009787582397. [DOI] [PubMed] [Google Scholar]
- 19.Sishi B, Loos B, Ellis B, Smith W, du Toit EF, Engelbrecht AM. Diet-induced obesity alters signalling pathways and induces atrophy and apoptosis in skeletal muscle in a prediabetic rat model. Exp Physiol. 2011;96:179–93. doi: 10.1113/expphysiol.2010.054189. [DOI] [PubMed] [Google Scholar]
- 20.Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: Molecular and pathophysiological mechanisms. Circulation. 2006;113:1888–904. doi: 10.1161/CIRCULATIONAHA.105.563213. [DOI] [PubMed] [Google Scholar]
- 21.Potenza MA, Marasciulo FL, Chieppa DM, Brigiani GS, Formoso G, Quon MJ, et al. Insulin resistance in spontaneously hypertensive rats is associated with endothelial dysfunction characterized by imbalance between NO and ET-1 production. Am J Physiol Heart Circ Physiol. 2005;289:H813–22. doi: 10.1152/ajpheart.00092.2005. [DOI] [PubMed] [Google Scholar]
- 22.Cheng B, Liu HW, Fu XB, Sun TZ, Sheng ZY. Recombinant human platelet-derived growth factor enhanced dermal wound healing by a pathway involving ERK and c-fos in diabetic rats. J Dermatol Sci. 2007;45:193–201. doi: 10.1016/j.jdermsci.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 23.Sharrocks AD. The ETS-domain transcription factor family. Nat Rev Mol Cell Biol. 2001;2:827–37. doi: 10.1038/35099076. [DOI] [PubMed] [Google Scholar]
- 24.Yordy JS, Muise-Helmericks RC. Signal transduction and the Ets family of transcription factors. Oncogene. 2000;19:6503–13. doi: 10.1038/sj.onc.1204036. [DOI] [PubMed] [Google Scholar]
- 25.Yang SH, Shore P, Willingham N, Lakey JH, Sharrocks AD. The mechanism of phosphorylation-inducible activation of the ETS-domain transcription factor Elk-1. EMBO J. 1999;18:5666–74. doi: 10.1093/emboj/18.20.5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanhoutte P, Nissen JL, Brugg B, Gaspera BD, Besson MJ, Hipskind RA, et al. Opposing roles of Elk-1 and its brain-specific isoform, short Elk-1, in nerve growth factor-induced PC12 differentiation. J Biol Chem. 2001;276:5189–96. doi: 10.1074/jbc.M006678200. [DOI] [PubMed] [Google Scholar]
- 27.Sharrocks AD. Complexities in ETS-domain transcription factor function and regulation: Lessons from the TCF (ternary complex factor) subfamily. The Colworth Medal Lecture. Biochem Soc Trans. 2002;30:1–9. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 28.Chai Y, Chipitsyna G, Cui J, Liao B, Liu S, Aysola K, et al. c-Fos oncogene regulator Elk-1 interacts with BRCA1 splice variants BRCA1a/1b and enhances BRCA1a/1b-mediated growth suppression in breast cancer cells. Oncogene. 2001;20:1357–67. doi: 10.1038/sj.onc.1204256. [DOI] [PubMed] [Google Scholar]
- 29.Marais R, Wynne J, Treisman R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell. 1993;73:381–93. doi: 10.1016/0092-8674(93)90237-k. [DOI] [PubMed] [Google Scholar]
- 30.Chen XF, Lin WD, Lu SL, Xie T, Ge K, Shi YQ, et al. Mechanistic study of endogenous skin lesions in diabetic rats. Exp Dermatol. 2010;19:1088–95. doi: 10.1111/j.1600-0625.2010.01137.x. [DOI] [PubMed] [Google Scholar]


