Abstract
Genetically modified (GM) strains now exist for many organisms, producing significant promise for agricultural production. However, if these organisms have some fitness advantage, they may also pose an environmental harm when released. High mating success of GM males relative to WT males provides such an important fitness advantage. Here, we provide documentation that GM male medaka fish modified with salmon growth hormone possess an overwhelming mating advantage. GM medaka offspring possess a survival disadvantage relative to WT, however. When both of these fitness components are included in our model, the transgene is predicted to spread if GM individuals enter wild populations (because of the mating advantage) and ultimately lead to population extinction (because of the viability disadvantage). Mating trials indicate that WT males use alternative mating tactics in an effort to counter the mating advantage of GM males, and we use genetic markers to ascertain the success of these alternative strategies. Finally, we model the impact of alternative mating tactics by WT males on transgene spread. Such tactics may reduce the rate of transgene spread, but not the outcome.
Keywords: genetically modified organism, alternative mating tactics, sperm competition, medaka
The production of genetically modified (GM) organisms (GMOs) continues at a rapid pace, prompting concerns about undesirable ecological consequences if these organisms enter natural communities (1–3). Based on six major components of an organism's fitness (i.e., juvenile viability, adult viability, age at sexual maturity, female fecundity, male fertility, and male mating advantage), we have recently provided a framework to predict possible risks of ecological harm associated with the spread of transgenes after a GMO release. One risk, extinction, results in the local elimination of conspecific populations (both WT and GM individuals); the other risk, invasion, involves ecosystem disruption as GM individuals replace their WT counterparts (4, 5).
Harm to wild populations resulting from either extinction or invasion risk requires that transgenes of GMOs can spread in nature, which in turn requires that GMOs have an advantage over their WT counterparts in at least one fitness component. An extinction risk is predicted when a transgene produces conflicting effects on different fitness components. As a result of an advantage in one component, GMOs replace WT genotypes in a naturally occurring population of conspecifics, whereas a disadvantage in another fitness component reduces population size, ultimately resulting in population extinction. We refer to this scenario as a Trojan gene effect (4, 5).
One scenario in which opposing pleiotropic effects of transgenes are predicted to produce a Trojan gene effect is when GM males have a mating advantage relative to WT males, but the GM offspring they produce have reduced viability relative to WT offspring (4, 5). The predicted extinction outcome resulting from these opposing effects has recently been confirmed with a deterministic model (6). Previous research in our laboratory has shown that GM lines of our study organism have reduced juvenile viability relative to WT controls (4, 7). Here, we investigate whether the GM male mating advantage required for a Trojan gene effect is also satisfied for one GM line.
To assess the mating success of GM males relative to WT males, we created a GM line of Japanese medaka (Oryzias latipes) by inserting a salmon growth hormone gene driven by a metallothionine promoter (8) into eggs just after fertilization (9). Subsequently, we reared both GM and WT medaka under similar conditions in the laboratory and conducted a series of breeding trials designed to assay both mating success (based on direct observations) and reproductive success (based on molecular assays of paternity). We found that GM males possess a significant mating advantage relative to WT males. We also discovered that the competitively disadvantaged WT males combat the mating advantage of GM males by using alternative mating tactics to sire offspring. As a consequence, we extend our original model to explore how, in general, alternative mating tactics might influence the timeline of population extinction.
Materials and Methods
Husbandry. Details of the production of the transgenic line (MtsGH67)¶ and general rearing methods (9) have been reported elsewhere. Populations of both GM and WT lines were maintained in the laboratory by maximizing reproductive productivity and minimizing all external sources of mortality. For the present study, both the GM and WT males used in mating trials were haphazardly chosen from a pool of at least 100 males that originated from 100 different families. All GM and WT males were reared at the same density and were similar in age before mating trials. To accomplish these conditions, we transferred recently hatched fry to 4.5-liter tanks at a density of no more than 35 fry per tank. Each tank was equipped with a sponge filter. We fed fry live Artemia once a day and larval AP100 diet (Zeigler Brothers, Gardners, PA) twice a day. At 3 weeks of age, we transferred the fry to 35-liter tanks and fed them flake food until 8–10 weeks of age. At 8 weeks, the number of fry per tank was reduced to six, and fry were fed Artemia once a day and flake food twice a day.
Mating Trials. We used five GM males and five WT males in the mating trials; all females were WT and were replaced during the course of the trials only if they stopped breeding. Five 4.5-liter tanks were used as breeding observation tanks; each tank contained one GM male, one WT male, and a female. All five tanks were monitored simultaneously at lights on to obtain mating data. After at least five matings were observed in all five tanks, both the GM and WT male were transferred to one of the other five tanks, each with a different competing male of the other genotype. Thus, each of the five GM males eventually competed with each of the five WT males, and vice versa; only females always remained resident in their initial tank.
Molecular Assays of Sperm Competition. Males used in mating trials were genotyped before forming mating sets to ensure that parentage could be assessed on the basis of one locus. After fertilization, eggs were collected from each female and reared until hatching. Medaka fry were then collected, preserved in 100% ethanol, and subsequently dissected to remove the eyes (which often inhibit PCR); their DNA was extracted according to conventional procedures (11). For the parentage analyses, a tetranucleotide microsatellite locus (designated OL1) was developed from a medaka sequence found in the GenBank database (accession no. AB010101) (12). We designed PCR primers (OL1 forward, 5′-GGTCACTCATTACAGTTCTC-3′ and OL1 reverse, 5′-CATTTCTGTAATGAATCTGTGG-3′) that amplified the microsatellite in the presence of BSA (final concentration of 200 ng/μl) by using a thermal profile of 3 s at 95°C, 3 s at 57°C, and 12 s at 72°C for 35 cycles in an MJ 200 thermal cycler. Fluorescently labeled reactions (6FAM) were subsequently loaded into 4.75% polyacrylamide gels and electrophoresed by using an ABI 377 sequencer (Applied Biosystems). Genotypes were assigned by using genotyper software, and paternity was determined by subtraction (13) because the maternity of each clutch was known.
The Trojan Gene Model. We have provided the general details of our basic model elsewhere (4, 5, 9). Here, we concentrate on two fitness components, the mating success of GM males relative to WT males and relative juvenile daily viability (5), and extend the model to include trio matings (i.e., matings in which both competing males simultaneously attempt to fertilize the same eggs of a female). Although both GM and WT males could either initiate a mating (i.e., use the primary mating tactic) or join a mating in progress (i.e., use the joining mating tactic), GM males rarely join matings initiated by WT males (see below). Thus, in our model, only WT males are allowed to be joiners.
The model begins with an arbitrary population composed of 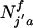 females and
females and 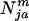 males of genotype j′ and j, where j′ or j = 1 (aa), 2 (Aa), and 3 (AA) in the ath age class, where aa is WT. The initial age distribution could be arbitrary or some specified distribution. Here, we use a negative exponential age distribution with parameters chosen by trial and error such that a stable age distribution resulted after several thousand iterations of the model with only the WT genotype present in the population. The frequencies of mating and offspring production determine the number of ages to monitor. For species such as medaka, which mate and produce offspring every day after attaining sexual maturity, time (t) is measured in days. The age at sexual maturity for the jth genotype is sj. The maximum longevity of any genotype is d. Consequently, the population size at time t is:
males of genotype j′ and j, where j′ or j = 1 (aa), 2 (Aa), and 3 (AA) in the ath age class, where aa is WT. The initial age distribution could be arbitrary or some specified distribution. Here, we use a negative exponential age distribution with parameters chosen by trial and error such that a stable age distribution resulted after several thousand iterations of the model with only the WT genotype present in the population. The frequencies of mating and offspring production determine the number of ages to monitor. For species such as medaka, which mate and produce offspring every day after attaining sexual maturity, time (t) is measured in days. The age at sexual maturity for the jth genotype is sj. The maximum longevity of any genotype is d. Consequently, the population size at time t is:
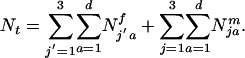 |
Note that estimation of d is not necessary and simply denotes that sums are taken over all age classes until the last age class dies off. To determine gene frequency changes it is necessary to ascertain the number of sexually mature individuals of each genotype and sex. The total number of sexually mature females and males in the population is:
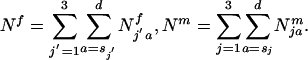 |
The relative frequency of sexually mature females and males of genotype j′ or j at time t is:
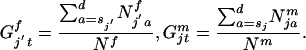 |
The total number of sexually mature females and males in the population is:
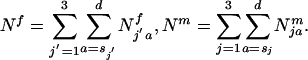 |
Let mj be relative mating advantage of jth male genotype. We assume that all three female genotypes have equal mating success; that is, there is always an excess of males and all females mate. Then the probability that a male of genotype j will initiate a mating is
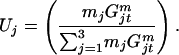 |
The probability of a trio mating is the probability that a GM male will initiate a mating Uj × the probability a WT male will join the mating. The latter is determined by the frequency of WT males (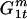 ) × the conditional probability that he will join the mating given that he is present (h).
) × the conditional probability that he will join the mating given that he is present (h).
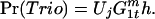 |
Thus, the probability of a mating occurring without a joining male is
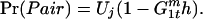 |
The effectiveness of mating is the probability that an egg is fertilized either without a joining male (1) or with a joining male (k). We assume that trio matings do not occur between a female and two WT males because they (i) are similar in size and (ii) do not spread the transgene. Thus, the effectiveness of mating for each genotype is as follows:
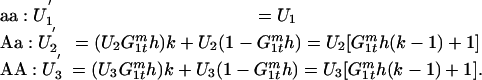 |
The relative effective frequency of matings of genotype j males in the presence of trio matings is
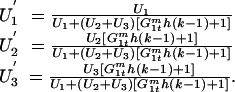 |
Note that if either h = 0 (no trio matings) or k = 1 (GM male fertilizes all of the eggs in a trio mating), then U′j = Uj for all j.
Results and Discussion
The insertion of growth hormone genes accelerates growth (14) and produces larger adults in several fish species (15, 16). Our MtsGH67 medaka line possessed a growth advantage over their WT counterparts that persisted throughout adulthood. Five adult GM males and five WT males were haphazardly chosen from a larger source population for mating trials. The GM males were 83% heavier than the WT adult males (mean ± SD, 563.0 ± 80.1 mg and 307.6 ± 53.7 mg, respectively; t = 5.92; P < 0.001; df = 7.0).
Larger adult body size provides mating advantages for males in a diverse array of species (17) including fish (14, 18, 19). Previous studies in our laboratory (using only WT medaka) show that larger males have a 4-fold mating advantage over smaller males (20). Here, we demonstrate that a similar size advantage occurs when GM males compete with WT males for mating opportunities. The observation of mating activity in medaka is facilitated by several features of their natural history (20): mating usually occurs at dawn in nature or within an hour after lights come on when fish are maintained on a controlled photoperiod in the laboratory (the matings reported in this study occurred in a median of 12 min after lights on), females mate nearly every day, and, despite external fertilization, eggs remain attached to a female's vent and are easily collected for rearing.
We observed 168 matings in 25 sets of mating trials in which each of the five GM males competed with each of the five WT males to mate with a WT female. A minimum of 5 matings and an average 6.8 matings were observed for each set of two males. We calculated the percentage of matings by each male in each set. GM males obtained 69.3–88.9% of the matings when averaged across their five competitors. Overall, GM males averaged 75.6% of all matings, resulting in a significant mating advantage over WT males (t = 8.29; P <0.001; df = 8).
During the mating trials, we observed that GM males physically dominated their smaller, WT competitors and controlled access to sexually receptive females. With nearly continuous access to females, GM males courted them frequently; however, the vast majority of courtship attempts ended in female rejection displays. Matings typically lasted <30 s, ending when the female swam away with eggs clearly visible on her vent. Only male–female interactions that culminated in eggs visibly extruding from females were counted as matings; courtships followed by mating activity without egg release were considered to be only pairings.
Despite the dominance of GM males, WT males were relentless in approaching females and attempting to mate. Most such approaches were aborted when the larger GM male would chase and nip the WT male. Nevertheless, successful mating by WT males occurred at a low frequency, usually when GM males were temporarily away from females. When these opportunities arose, WT males either quickly courted females or attempted to mate without courtship. Although most of these attempts were also rejected by females, persistence by WT males eventually resulted in some matings.
In addition to typical courtship and mating behavior, WT medaka males used two alternative mating tactics (disruption and joining) to increase their chances of siring young. Although alternative mating tactics have not been previously reported for medaka, they are common in many other taxa (17), including fish (21–23). In most species, males use alternative tactics because their low social status or body condition reduces success in obtaining mates (21). Such was the case in medaka, as the smaller WT males were at a distinct physical disadvantage. When WT males used the mating disruption tactic, they would first swim under a pair as they were mating then dart upward between the paired individuals to separate the female from the male. Of 211 observed pairings, 58 disruptions were attempted (27%). Matings were successfully disrupted 47 times, and the disrupting male later mated with the female 7 times (14.9%).
When WT males used the joining tactic, they would swim under a mating pair and then float upward alongside one of the mating individuals. The joining male would then perform all of the behaviors typical of a male during mating. Thus, the joining tactic provided the opportunity for sperm competition (24, 25) as sperm from each male vied to fertilize the same set of ova. WT males joined 54 of the 127 matings (42.5%) initiated by GM males. In contrast, GM males joined only 7 of the 41 matings initiated by WT males (17.1%). Thus, WT males used the joining tactic significantly more often than GM males did (χ2 = 8.68; P = 0.003).
We conducted a second study to assess paternity of young produced in trio matings. This study differed from our initial study in that competing males in each tank were selected by using the following two criteria: they had to differ sufficiently in size to be distinguished visually, and they also had to differ in both alleles of the microsatellite locus used to genotype them and their progeny. Thus, in this second study, WT males could compete against either GM males or other WT males. Fertilization success of joining males did not differ as a function of the genotype of the primary mating male (t = 1.70; P = 0.11; df = 14.3); thus, data were pooled for paternity analyses. We observed 199 matings in these trials. Again, larger males possessed a distinct mating advantage, obtaining 138 of the 199 matings (69.3%). Smaller males joined 57 of the 138 matings (41.3%) initiated by larger males, whereas larger males joined only 2 of the 60 matings (3.3%) initiated by smaller males (χ2 = 28.82; P <0.001).
We conducted a paternity analysis of 394 fry from 32 of the 59 trio matings. On average, 22.9% (±28.9%) of the fry sired in these matings had genotypes consistent with paternity by joining males. Thus, smaller, competitively disadvantaged males used two routes to sire offspring: mating with females by using the primary mating tactic, albeit with minimal success, and joining matings initiated by competitively superior males, although siring far fewer young in these matings than primary males did (77 compared with 317; χ2 = 146.2; P <0.001). Males of other species that use alternative mating tactics may rarely obtain matings by using the primary mating tactic of their species (but see ref. 26). The relative fertilization success of males using an alternative mating tactic varies widely within (26) and among (26, 27) species, with one report showing that as many as 65% of the young in a population may be sired by males using an alternative tactic (26).
Because alternative mating tactics involving sperm competition are common in many fish species, particularly in several commercially important species for which GM lines have been created (26), we modeled the interaction between sperm competition and the Trojan gene effect. Specifically, we considered how common and successful an alternative mating tactic used by WT males had to be to delay the predicted time to population extinction compared with a population that lacked alternative tactics by WT males. To address this issue, we extended our general model to include two additional variables: the percentage of matings by GM males that are joined by WT males and the percentage of young sired by WT males as a result of joining. Because joining by GM males was much less common than joining by WT males, we did not include this in the model.
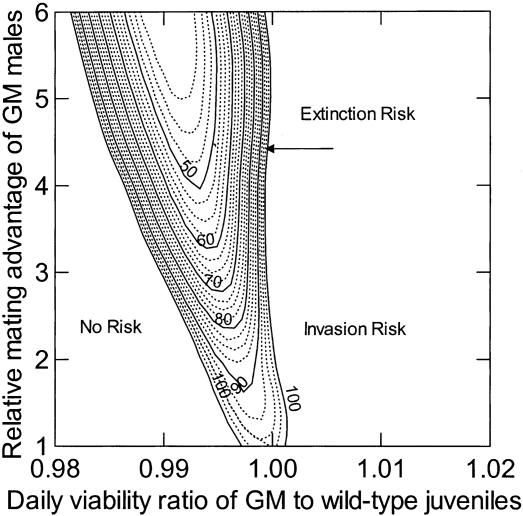
In the absence of sperm competition, our model predicted that population extinction should occur in 49.4 generations if GM medaka males possessed at least a 4-fold mating advantage and their offspring had a relative daily viability of 0.994 (ratio of GM to WT daily juvenile viability; Fig. 1). In this analysis, we used a range of relative daily viability and relative GM mating advantage values for two reasons: even using the same gene construct to produce different GM lines from the same WT stock may result in lines with different fitness component values, because the transgenes could insert in different locations in the genome and copy number could vary, and because the same GM line may show different fitness component values in different environments (as a result of genotype × environment interactions). The level of sperm competition that we observed in our medaka trials (42.5% trio matings with joining males fertilizing 22.9% of the fry) only resulted in a very modest delay in the predicted time to population extinction: 51.4 generations, assuming the same values of GM male mating advantage and GM juvenile viability.
Fig. 1.
Predicted time to extinction in generations (contour lines) as a function of the ratio of GM to WT daily juvenile viability and GM male mating advantage relative to WT males when no trio matings occur. Region of no risk results because low GM juvenile viability prevents transgene spread; region of invasion risk results when transgene spreads, but high GM juvenile viability does not cause population extinction.
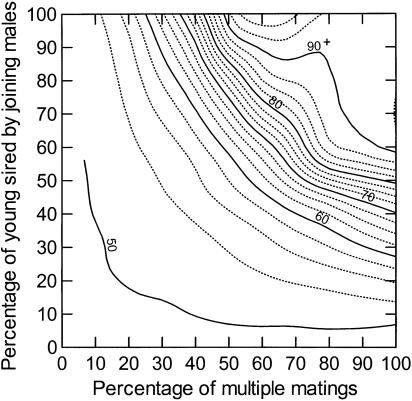
To examine how variation in the intensity of alternative mating tactics by WT males could generally influence a predicted Trojan gene effect, we considered a range of values for percent of trio matings and percent of fertilization success by joining males. Predictions pertain to our medaka population in that we assumed a particular level of GM male mating advantage (4-fold) and relative GM juvenile daily viability (0.994). However, our model could be applied to any species with appropriate changes in parameter values. Because joining behaviors in some species can involve multiple males joining in a mating rather than just one (22, 27–29), we replace the term “trio matings” with “multiple male matings.” Our model predicts that extinction times can be increased by 10 or more generations only when multiple matings are very common (i.e., >50% of the matings) and joining males are successful in sperm competition (i.e., sire >40% of the young) (Fig. 2).
Fig. 2.
Predicted time to extinction in generations (contour lines) as a function of the percentage of multiple male matings and percentage of young sired by joining WT males. Relative mating success of GM male was set to 4.0; relative juvenile GM viability was set to 0.994 per day. Note that, as both variables approach 100%, there is no spread of the transgene, and thus the predicted time to extinction approaches infinity.
Differential mating success resulting from within-sex competitive advantages and between-sex mating preferences (sexual selection) has long been considered a potent evolutionary force (17). Recent empirical studies have revealed that the intensity of sexual selection can often exceed that of viability selection in nature (30). Most studies on sexual selection have concentrated on its effects on the evolution of secondary sexual characters and patterns of mate choice (17). We extend this arena to the potential influence of sexual selection on population extinction when GM traits (such as larger body size) influence mating success. Our general model predicts an extinction outcome when GM males have a 4-fold mating advantage and their young have a survival disadvantage relative to WT (4, 5). However, as we report here, alternative male mating tactics can alter this outcome.
In extending our model to incorporate alternative mating tactics, two issues become apparent. First, many species already possess some type of alternative mating tactic, presumably as a result of a history of intense sexual selection. Second, any current increase in the intensity of sexual selection caused by the introduction of competitively superior GM males also should select for greater use (and increased fertilization success in sperm competition) of alternative mating tactics by disadvantaged males. Thus, our modeling of the effects of sperm competition (Fig. 2) considers an array of multiple mating percentages and success potentials by joining males. These components of reproductive success for males using alternative tactics should not be affected by only selection, but also are likely to vary as a function of factors such as overall male density, relative frequency of males using alternative tactics in a population, and habitat type. Published studies of sperm competition in fish, for example, have already revealed that sperm competition can be intense (22) when multiple male matings occur; however, the frequency of such matings in nature is known for only a few species (10, 11, 29). As predicted by our model, an increased incidence of multiple male matings should act to delay a Trojan gene effect. This is not to say that GM individuals will be eliminated from a WT population, but that population extinction would take much longer or might even be deferred completely. In the latter case, an invasion outcome (5) may be the end result of a GMO release, producing a population with a different behavioral profile and potential for novel ecological effects on other species.
Acknowledgments
The MtsGH67 construct used to make transgenic lines was generously provided by R. H. Devlin of Fisheries and Oceans Canada. We thank O. Larson and K. Mounts for assistance in collecting behavioral data; T. Kruer for care and PCR testing of fish; S. Eddy for help with microsatellite analyses; and P. Waser, K. Larson, and the DeWoody laboratory for comments on a previous draft of the manuscript. This research was supported by Grant 2000-04052 from the U.S. Department of Agriculture National Biological Risk Assessment Program.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: GM, genetically modified; GMO, GM organism.
Footnotes
Kruer, T. L., Peck, S. L., Hostetler, H. A., Devlin, R. H. & Muir, W. M. (2002) Transgenic Res. 11, 83 (abstr.).
References
- 1.National Research Council (2002) Animal Biotechnology: Science-Based Concerns (Natl. Acad. Press, Washington, DC). [PubMed]
- 2.Reichhardt, T. (2000) Nature 406, 10-12. [DOI] [PubMed] [Google Scholar]
- 3.Stokstad, E. (2002) Science 297, 1797-1799. [DOI] [PubMed] [Google Scholar]
- 4.Muir, W. M. & Howard, R. D. (1999) Proc. Natl. Acad. Sci. USA 96, 13853-13856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muir, W. M. & Howard, R. D. (2002) Transgenic Res. 11, 101-114. [DOI] [PubMed] [Google Scholar]
- 6.Hedrick, P. W. (2001) Can. J. Fisheries Aquatic Sci. 58, 841-844. [Google Scholar]
- 7.Jimenez, L. V. (2000) Ph.D. thesis (Purdue University, West Lafayette, IN).
- 8.Devlin, R. H., Yesaki, T. Y., Biagi, C. A., Donaldson, E. M., Swanson, P. & Chan, W.-K. (1994) Nature 371, 209-210. [Google Scholar]
- 9.Muir, W. M. & Howard, R. D. (2001) Am. Nat. 158, 1-16. [DOI] [PubMed] [Google Scholar]
- 10.Neff, B. D. & Gross, M. R. (2001) Proc. R. Soc. London Ser. B 268, 1559-1563. [Google Scholar]
- 11.DeWoody, J. A., Fletcher, D., Wilkins, S. D., Mackiewicz, M. & Avise, J. C. (2000) Mol. Ecol. 9, 2119-2128. [DOI] [PubMed] [Google Scholar]
- 12.Inagaki, H., Koga, A., Bessho, Y. & Hoir, H. (1998) Pigment Cell Res. 11, 283-290. [DOI] [PubMed] [Google Scholar]
- 13.DeWoody, J. A., Walker, D. & Avise, J. C. (2000) Genetics 154, 1907-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muir, W. M. & Howard, R. D. (2001) in Genetically Engineered Organisms: Assessing Environmental and Human Health Effects, eds. Letourneau, D. K. & Burrows, B. E. (CRC, Boca Raton, FL), pp. 355-384.
- 15.Devlin, R. H., Biagi, C. A., Yesaki, T. Y., Smailus, D. E. & Byatt, J. C. (2001) Nature 409, 781-782. [DOI] [PubMed] [Google Scholar]
- 16.Rahman, M. A., Mak, R., Ayad, H., Smith, A. & Maclean, N. (1998) Transgenic Res. 7, 357-369. [DOI] [PubMed] [Google Scholar]
- 17.Andersson, M. (1994) Sexual Selection (Princeton Univ. Press, Princeton).
- 18.Jones, A. G. & Avise, J. C. (1997) Evolution 51, 1611-1622. [DOI] [PubMed] [Google Scholar]
- 19.Jones, A. G., Walker, D., Kvarnemo, C., Lindström, K. & Avise, J. C. (2001) Proc. Natl. Acad. Sci. USA 98, 9151-9156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howard, R. D., Martens, R. S., Innis, S. A., Drnevich, J. M. & Hale, J. (1998) Anim. Behav. 55, 1151-1163. [DOI] [PubMed] [Google Scholar]
- 21.Gross, M. R. (1996) Trends Ecol. Evol. 11, 92-98. [DOI] [PubMed] [Google Scholar]
- 22.Fu, P., Neff, B. D. & Gross, M. R. (2001) Proc. R. Soc. London Ser. B 268, 1106-1112. [Google Scholar]
- 23.Taborsky, M. (2001) J. Hered. 92, 100-110. [DOI] [PubMed] [Google Scholar]
- 24.Parker, G. A. (1970) Biol. Rev. 45, 525-567. [Google Scholar]
- 25.Birkhead, T. R. & Moller, A. P. (1998) Sperm Competition and Sexual Selection (Academic, London).
- 26.Fleming, I. A. & Reynolds, J. D. (2004) in Evolution Illuminated: Salmon and Their Relatives, eds. Hendry, A. P. & Stearns, S. C. (Oxford Univ. Press, Oxford), pp. 264-294.
- 27.Neff, B. D. (2001) J. Hered. 92, 111-119. [DOI] [PubMed] [Google Scholar]
- 28.Taborsky, M. (1994) Adv. Study Behav. 23, 1-100. [Google Scholar]
- 29.Garant, D., Fleming, I. A., Einum, S. & Bernatchez, L. (2003) Ecol. Lett. 6, 541-549. [Google Scholar]
- 30.Kingsolver, J. G., Hoekstra, H. E., Hoekstra, J. M., Berrigan, D., Vignieri, S. N., Hill, C. E., Gilbert, P. & Beerli, P. (2001) Am. Nat. 157, 245-261. [DOI] [PubMed] [Google Scholar]