Abstract
Background
Inpatient aggression is a serious challenge in pediatric psychiatry.
Methods
A chart review study in adolescent psychiatric inpatients consecutively admitted over 24 months was conducted, to describe aggressive events requiring an intervention (AERI) and to characterize their management. AERIs were identified based on specific institutional event forms and/or documentation of as-needed (STAT/PRN) medication administration for aggression, both recorded by nursing staff.
Results
Among 408 adolescent inpatients (age: 15.2±1.6 years, 43.9% male), 1349 AERIs were recorded, with ≥1 AERI occurring in 28.4% (n=116; AERI+). However, the frequency of AERIs was highly skewed (median 4, range: 1–258). In a logistical regression model, the primary diagnosis at discharge of disruptive behavior disorders and bipolar disorders, history of previous inpatient treatment, length of hospitalization, and absence of a specific precipitant prior to admission were significantly associated with AERIs (R2=0.32; p<0.0001). The first line treatment of patients with AERIs (AERI+) was pharmacological in nature (95.6%). Seclusion or restraint (SRU) was used at least once in 59.4% of the AERI+ subgroup (i.e., in 16.9% of all patients; median within-group SRU frequency: 3). Treatment and discharge characteristics indicated a poorer prognosis in the AERI+ (discharge to residential care AERI+: 22.8%, AERI−: 5.6%, p<0.001) and a greater need for psychotropic polypharmacy (median number of psychotropic medications AERI+: 2; AERI−: 1, p<0.001).
Conclusions
Despite high rates of pharmacological interventions, SRU continue to be used in adolescent inpatient care. As both of these approaches lack a clear evidence base, and as adolescents with clinically significant inpatient aggression have increased illness acuity/severity and service needs, structured research into the most appropriate inpatient aggression management is sorely needed.
Introduction
Aggression on psychiatric inpatient wards is a frequent problem, in particular on child and adolescent units (Barzmann et al. 2011; Cornaggia et al. 2011). Acute aggressive outbreaks characterize disruptive behavioral disorders, but also occur in other adolescent psychiatric conditions, such as attention-deficit/hyperactivity disorder (ADHD), mood disorders (in particular bipolar disorders [BP]), psychotic disorders, mental retardation, and autism spectrum disorders. Aggression may be the cause for hospitalization, or, conversely, be provoked by the conditions of the hospitalization (Bowers 2011) and can prolong the inpatient stay. While most agree that a balance between staff and patient safety as well as patients' autonomy is needed, the exact way to best achieve this balance is highly controversial. Various inpatient aggression management and prevention techniques have been suggested, with some advocating for a complete elimination of pharmacological sedation and mechanical restraints (Donat 2005; Ashcraft and Anthony 2008).
Prevalence rates of aggressive incidents on inpatient wards vary with age and range between 3 and 30% in adults (for review see Bowers et al. 2011; Cornaggia 2011), 23–50% in cohorts including adolescents (Barton et al. 2001; Ryan et al. 2004; Dean et al. 2008; Barzmann et al. 2011), being as high as 58–76% in prepubertal children (Garrison et al. 1995; Crocker et al. 2010). Aggressive incidents in child and adolescent inpatients have been associated with male sex (Gabel and Shindledecker 1990; Garrison et al. 1995; Barton et al. 2001), disruptive behavioral disorders (Barton et al. 2001, Croker et al. 2010), and mental retardation (Sukhodolsky et al. 2005), as well as with parental substance use disorders (Gabel and Shindlehecker 1991) and history of parental violence (Gabel and Shindledecker 1991). Aggressive behavior during child and adolescent hospitalization has also been related to an increasing length of stay (Dean et al. 2008) and to poor discharge prognoses (Gabel and Shindledecker 1991; Blader 2006). In addition, aggressive incidents have a strong negative impact on the physical and psychological integrity of staff. For example, 30% of aggressive events on adult inpatient wards resulted in staff injuries (Bowers et al. 2011). Moreover, reduced work performance and satisfaction have been reported in staff experiencing persistent violence (Delaney and Hardy 2008; Dean et al. 2010; Kulkarni et al. 2011).
Given this impact of aggressive behavior on the course of patient recovery and on staff and patient safety, it is important to identify potentially aggressive adolescents early during hospitalization and to develop effective management strategies. To date, the management of inpatient aggressive behaviors differs across institutions, but typically includes some behavioral measures, seclusion, mechanical restraints, and/or pharmacological interventions (Desmukh 2010). Pharmacological responses to aggression are common across all age groups (Dean et al. 2006; Stein-Parbury 2008), even though the slim systematic empirical data for these interventions may contradict this practice (Vitiello 1991). A recent meta-analysis on seclusion and/or restraint use (SRU) in children and adolescents, reports widely variable SRU (affecting 26–29% of pediatric inpatients, De Hert et al. 2011), even though there is no empirical evidence for a therapeutic efficacy of these interventions (Sailas and Fenton 2000). These rates highly exceed those reported for adults. A recent international review on coercive measures in adult psychiatric inpatients presents nationally varying rates ranging from 0 to 36%, with the majority of studies showing SRU incidences <10% (Steinert et al. 2010). Interestingly, SRU has completely been replaced by 1:1 nursing in Iceland (Steinert et al. 2010).
As children and adolescents are particularly vulnerable regarding the potential side effects of pharmacological interventions (Correll et al. 2008, 2011) and the psychological effects of coercive measures (Mohr et al. 2003, Hammer 2011), data on these interventions are sorely needed to inform most adequate treatment strategies.
Therefore, the purpose of this study was to 1) describe frequencies and qualities of aggressive events on an adolescent inpatient unit that prompted a specific intervention; 2) characterize the management of these aggressive events; and 3) compare the characteristics of patients with or without aggressive behavior in that sample.
Methods
This chart review study was conducted in accordance with and approved by the North Shore-Long Island Jewish Health Center Institutional Review Board.
Subjects and setting
Medical charts of 450 patients consecutively admitted between January 1, 2001 and December 31, 2002 to the Adolescent Pavilion at The Zucker Hillside Hospital, a suburban, tertiary care, academic teaching hospital, were reviewed. The Adolescent Pavilion is a 23 bed, acute psychiatric care unit admitting patients between 12 and 19 years of age who are referred from the outpatient clinic and the emergency room of The Zucker Hillside Hospital. A total of 42 inpatient stays (9.5%) were excluded from this study, either because patients were readmitted during the study period (n=24, 5.3%) or because data on aggressive events were insufficient (n=19, 4.2%).
Data sources
Based on hospital policy, aggressive events requiring an intervention (AERIs) were defined as situations in which patients posed a significant danger to themselves or others and that were documented by nursing staff in the patient charts in two operationalized ways: 1) pharmacological intervention documented on a specific STAT/PRN (i.e., as needed medication) sheet; and 2) nonpharmacological intervention specifying the use of seclusion or restraint, documented on a semi-structured, institutional incident form.
These nonpharmacological interventions included:
-
I. Seclusions:
1. Strict seclusion (further coined only “seclusion”): Patients were confined to a room furnished with a mattress only with the door locked.
2. Quiet room: Patients were confined to a room furnished with a mattress only with the door unlocked.
-
II. Mechanical restraints: Any type of restriction of a patient's physical freedom.
1. Sheet restraint: Body and limbs fastened with a sheet fixated to the frame of the bed.
2. Four-point restraint: Arms and legs fastened to the frame of the bed.
3. Wrist restraint: Both wrists fastened to one another.
4. Mittens: Compulsory use of mittens.
Furthermore, the documentation of the type of aggression (i.e., verbal, physical against objects, physical against self or against other people, and combinations thereof) was also required. Presence or absence of AERI and the number of AERIs was recorded for each patient. Moreover, the types of mechanical or pharmacological responses to these events were recorded for the entire time of hospitalization (present/absent).
Clinical and demographic information was extracted from the “Initial Clinical Examination Form,” a 12 page semistructured intake form completed by the admitting physician, the psychosocial history form that is filled out by parents/caregivers upon admission, the intake and progress notes of the treating attending psychiatrist, the discharge summary note, the medication order sheets, and medical notes section. The comprehensive institutional intake form includes in-depth data on age; sex; race; insurance status; current and past psychiatric history and treatment; family psychiatric history; clinical American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV) diagnoses; current, highest, and lowest Global Assessment of Functioning (GAF) score in the past year; and discharge disposition/recommendation. The 10 page social and developmental history questionnaire includes additional data on demographics, past and current psychiatric history and treatment, family psychiatric history, recent family stressors, and a parental rating of the impact their child's illness has had on the family. Psychiatric diagnoses were made clinically based on DSM-IV by board-eligible or board-certified child and adolescent psychiatrists (American Psychiatric Association 1994).
Admission, discharge, and medication information was extracted from all charts and coded as medication classes and number of doses.
Data analysis
Primary diagnoses and chief complaints were grouped into hierarchical categories to reduce the number of factors for statistical analyses. Patients with ≥1 AERI were categorized as AERI+; all others were categorized as AERI−. Frequencies were described according to data distribution using means and standard deviation or standard error for normally distributed data, and median, 10th, 25th, 75th, and 90th percentile (as needed) for non-normally distributed data.
Group comparisons were performed using χ2 tests, nominal logistical fits, and t tests or Wilcoxon rank sum tests as per data type and distribution. For rare events, Fisher's exact test was used. Two sided tests with α=0.05 were used in all comparisons without correction for multiple comparisons, because of the descriptive nature of the analyses. To construct a model of demographic and clinical variables associated with the presence of AERIs, we conducted two separate nominal logistical regression analyses. In the first model, all variables that had shown group separating effects (p>0.05; Table 1) were entered into a nominal logistical regression model. In the second model, only those variables known to healthcare practitioners at the time of admission were entered (e.g., excluding length of stay or disposition) to identify only variables predicting future inpatient aggression. Stepwise elimination excluded those variables that did not significantly (p>0.05) contribute to the group separation, thus reducing the model to only include significantly contributing factors. Both models were run as forward stepwise regression as well as a backward stepwise regression. Statistical calculations used JMP 5.0.1, 1989-2003, SAS Institute Inc.
Table 1.
Complete Demographic and Clinical Characteristics in 408 Adolescent Inpatients with or without Aggressive Episodes Requiring an Intervention (AERI+ [n=116] vs. AERI− [n=292])
| Total | AERI+ | AERI− | F/χ2 | p value | |
|---|---|---|---|---|---|
| Male gender (n, %) n=408 | 179 (43.9) | 52 (44.8) | 127 (43.5) | χ2=0.60 | 0.806 |
| Age (years±SD) | 15.6±1.6 | 15.2±1.6 | 15.8±1.6 | F=9.57 | 0.002 |
| Ethnicity (n, %)n=403 | χ2=13.1 | 0.01 | |||
| Caucasian | 252 (62.5) | 64 (55.2) | 188 (66.5) | ||
| African-American | 62 (15.4) | 30 (25.9) | 32 (11.2) | χ2=10.7 | 0.001 |
| Hispanic | 44 (10.9) | 11 (9.5) | 33 (11.5) | ||
| Asian | 20 (5) | 4 (3.5) | 16 (5.6) | ||
| Other | 25 (6.2) | 7 (6.0) | 18 (6.3) | ||
| Insurance status,n=407 | χ2=10.6 | 0.005 | |||
| Private n (%) | 289 (71.0) | 69 (60.0) | 220 (75.3) | ||
| Medicaid n (%) | 112 (27.5) | 45 (39.1) | 67 (23.0) | χ2=2.85 | 0.09 |
| None n (%) | 6 (1.5) | 1 (0.9) | 5 (1.7) | ||
| Number of previous inpatient treatments | 0.7±1.1 | 1.1±1.4 | 0.5±1.0 | F=21.50 | <0.0001 |
| Previous inpatient treatment,n=407 | 148 (36.3) | 62 (53.9) | 86 (29.4) | χ2=21.3 | <0.0001 |
| Previous outpatient treatment, n=395 | 323 (81.8) | 95 (85.6) | 228 (80.3) | 0.211 | |
| Positive psychiatric family history | 250 (77.9) | 69 (77.5) | 181 (78.0) | χ2=0.009 | 0.9247 |
| Chief complaintn=408 | χ2=19.6 | 0.0004 | |||
| Depression, suicidal ideation, suicidal attempt | 192 (47.1) | 38 (32.8) | 154 (52.7) | χ2=3.79 | 0.0516 |
| Aggression or Impulsivitiy/Oppositionality | 128 (31.4) | 53 (45.7) | 75 (25.7) | χ2=5.7 | 0.0164 |
| Psychotic symptoms | 55 (13.5) | 18 (15.5) | 37 (12.7) | ||
| Mania | 8 (2.0) | 3 (2.6) | 5 (1.7) | ||
| Other complaints | 25 (6.1) | 4 (3.5) | 21 (7.2) | ||
| Precipitant of hospitalization | χ2=10.7 | 0.01 | |||
| No specific precipitant | 173 (42.5) | 63 (54.3) | 110 (37.8) | χ2=7.78 | 0.005 |
| Specific conflict | 145 (35.3) | 33 (28.4) | 112 (38.4) | ||
| Other/unknown | 73 (17.9) | 19 (16.4) | 54 (18.6) | ||
| Trauma | 16 (3.9) | 1 (0.9) | 15 (15.2) | ||
| Mean number of psychiatric diagnoses | 1.8 (1) | 2.0 (1) | 1.8 (1) | F=3.39 | 0.0662 |
| Main psychiatric diagnosis at discharge | χ2=30.72 | <0.0001 | |||
| Depressive disordersa | 143 (35.1) | 22 (19.1) | 121 (41.4) | χ2=12.74 | 0.0004 |
| Bipolar disordersb | 87 (21.4) | 36 (31.3) | 51 (17.6) | χ2=6.14 | 0.0132 |
| Psychotic disordersc | 47 (11.5) | 14 (12.2) | 33 (11.3) | ||
| Mood disorder not otherwise specified | 51 (12.3) | 17 (14.8) | 34 (11.6) | ||
| Disruptive behavior disorderd | 42 (10.3) | 20 (17.4) | 22 (7.5) | χ2=7.9 | 0.0049 |
| Other | 37 (9.1) | 6 (5.2) | 31 (10.6) | ||
| GAF at admission (mean, SE) | 31.9±0.40 | 30.6±0.67 | 32.4±0.42 | F=5.7 | 0.01 |
| Suicidal idea(s) or attempt(s) at admissione | 172 (42.1) | 33 (28.5) | 139 (47.6) | χ2=12.5 | 0.0004 |
Major depressive disorder, depressive disorder NOS.
Bipolar I disorder, bipolar II disorder, bipolar disorder not otherwise specified (NOS).
Schizophrenia, schizophreniform disorder, schizoaffective disorder, psychotic disorder NOS.
Oppositional defiant disorder, conduct disorder, attention-deficit/hyperactivity disorder.
The patients are a subgroup of the pooled group of “chief complaint: depression, suicidal ideation, suicidal attempt.”
GAF, Global Assessment of Functioning.
Results
At least one AERI was recorded in 116 (28.4%) of the 408 patients with data.
Sociodemographic and clinical characteristics
The AERI+ subgroup was slightly younger (AERI+: 15.2±1.6 years, AERI−: 15.8±1.6 years, p=0.002) and included more African-American patients (AERI+: 25.9%, AERI−: 11.2%, p=0.001; Table 1).
A history of previous inpatient treatment was more prevalent in the AERI+ subgroup (AERI+: 53.9%; AERI−: 29.4%; p<0.0001; Table 1). These subgroups also differed regarding the chief complaints at admission, with a lower proportion of depressive complaints or suicidal ideas/attempts in the AERI+ subgroup (AERI+: 32.8%, AERI−: 52.2%; p=0.05, see also Table 1) and a higher proportion of complaints of aggression, impulsivity or oppositionality in the AERI+ subgroup (AERI+: 45.7%, AERI−: 25.7%; p=0.02). Absence of a specific precipitant prior to admission was significantly more frequent in the AERI+ subgroup (AERI+: 54.3%, AERI−: 37.8%; p=0.005).
The strongest discriminator between AERI+ and AERI– patients was the main diagnosis at discharge (p<0.0001, Table 1). Disruptive behavioral disorders (AERI+: 17.5%; AERI−: 7.5%; χ2=7.9; p=0.0049) and bipolar disorders (AERI+: 31.3%; AERI−: 17.5%; χ2=6.14; p=0.0132) were more prevalent in AERI+. By contrast, depressive disorders were significantly less frequent in AERI+ patients (AERI+: 19.1%; AERI−: 41.4%; χ2=12.74; p=0.0004).
Moreover, among comorbid conditions AERIs were more frequent in ADHD (AERI+: n=34 [29.6%]; AERI−: n=38 [13.0%]; χ2=15.5; p<0.0001) and in pervasive developmental disorders (PDD) and autism (AERI+: n=7 [1.72%]; AERI−: n=3 [0.74%]; Fisher's exact p=0.007). By contrast, eating disorders were underrepresented in AERI+ (n=1 [0.87%]; AERI−: n=17 [5.8%]; Fisher's exact p=0.03). AERI+ and AERI− subgroups did not differ regarding comorbidity with the following disorders: substance abuse, adjustment disorders, anxiety disorders, obsessive-compulsive disorder (OCD), learning disorders, mental retardation, personality disorders, psychotic disorder not otherwise specified (NOS), and schizophrenia (Fisher's exact p>0.1 for all comparisons). Homicidal ideas at admission were extremely rare and statistically not associated with AERIs during hospitalization (AERI+: 6.0%; ARI−: 2.8%, p=0.14). Patients with suicidal ideas/attempts at admission (pooled with all patients with depressive complaints as chief complaint at admission) were unlikely to be involved in AERIs (Table 1, AERI+: 28.5%; ARI−: 47.6%, p=0.0004).
To control for the interaction of factors showing univariate associations, factors associated with AERI+ subgroup designation were modeled in a multivariate approach. Forward and backward logistic regression did not differ for the primary analysis. The final logistic regression model of significant factors included the primary diagnosis at discharge, history of previous inpatient treatment, length of hospitalization, and absence of a specific precipitant of admission, (R2=0.32; p<0.0001, Table 2).
Table 2.
Factors Associated with Aggressive Episodes Requiring an Intervention
| A. Variables associated with aggressive episodes requiring an intervention; nominal logistic fit, R2=0.32; p<0.0001 | χ2 | p value |
|---|---|---|
| Duration of hospitalization | 38.9072501 | 0.0000 |
| No specific precipitant of hospitalization | 2.93260766 | 0.0868 |
| Previous inpatient treatment | 2.84941101 | 0.0914 |
| Age at admission <15.5 years | 0.6912455 | 0.4057 |
| GAF at admission | 1.98230579 | 0.1591 |
| Primary discharge diagnosis (other vs. specific categories) | 1.13252857 | 0.2872 |
| Primary discharge diagnosis (depression vs. all other categories) | 1.13252857 | 0.2872 |
| Primary discharge diagnosis (psychosis vs. all other categories) | 2.86669842 | 0.0904 |
| Primary discharge diagnosis (mood & disruptive behavior d/o vs. all other categories) | 2.08762 | 0.1485 |
| B. Clinical predictors for aggressive episodes requiring an intervention; Nominal logistic fit, R2=0.11; p<0.0001 | ||
| No specific precipitant of hospitalization | 3.78228449 | 0.0518 |
| Previous inpatient treatment | 12.4994245 | 0.0004 |
| Age at admission <15.5 years | 6.38079395 | 0.0115 |
| GAF at admission | 8.60462904 | 0.0034 |
| Caucasian | 0.0788026 | 0.7789 |
| Asian | 0.58311728 | 0.4451 |
| Hispanic | 0.40636066 | 0.5238 |
| African-American | 3.20352861 | 0.0735 |
| Insurance, Medicaid vs. others | 2.40263985 | 0.1211 |
A: Testing all characteristics; B: Testing only factors available at intake.
GAF, Global Assessment of Functioning.
As the duration of hospitalization and the primary diagnosis at discharge are not foreseeable at admission, we constructed a second model considering only those factors that would be known by day 1 of hospitalization (R2=0.11; p<0.0001, Table 2). For these models, a backward stepped regression model could not be constructed, as the chief complaint at admission yielded unstable values as a result of high variation; therefore, the forward stepped model is presented. As seven patients accounted for >50% of the AERIs (see subsequent text), we repeated the models, excluding these patients, yielding significant results for both model 1 (R2=0.29 p<0.0001) and model 2 (R2=0.10 p<0.0001).
Specific characteristics of aggressive patients with >50 AERIs
Seven (1.7%) patients accounted for 56.6% of AERIs. These patients included four males (mean age: 14.7±1.5 years SD, median 14.5). Six patients had multiple psychiatric diagnoses at discharge (four with three diagnoses, and one each with one, four, and five diagnoses; the single diagnosis was bipolar disorder). Diagnoses included: Four with ADHD, four with PDD/autism spectrum, three with mood disorder NOS, three with oppositional defiant disorder (ODD)/impulse control disorder (ICD)/conduct disorder, two with combined OCD and Tourette's disorder and two with bipolar disorder, one with comorbid substance abuse. All seven patients experienced relatively long hospitalizations (138±63 days, median 128), earlier hospitalizations were frequent (mean 1.2±1.2, median 0.5), and the mean GAF at admission was as low as 26.4±6.9. Two of these patients had expressed homicidal ideas/attempts at admission (of note is that only five patients of the entire sample had expressed homicidal ideas).
The patient, who experienced 258 AERIs, was diagnosed with Asperger's syndrome, disruptive behavior disorder and OCD, was admitted for self-injurious behavior, and showed predominantly repeated self-injurious behavior, but also outwardly directed aggression during hospitalization. AERIs were managed with antipsychotics (Aps), compulsory use of mittens (243/258 times), and wrist restraints (15/258 times).
Characteristics of AERIs
During the 2 year observation period, a total of 1349 AERIs was documented. The majority of patients expressed both verbal and physical aggression (n=88/116, 75.9%), whereas only a few used either only verbal (n=18/116, 15.5%) or physical aggression (n=10/116, 8.6%). Most often, patients directed the physical aggression concomitantly toward external targets (objects and people) and self (n=48/116; 41.4%). In the remaining physically aggressive patients, aggression was directed against people and objects (n=25/116; 21.6%), against people only (n=13/116; 11.2 %), against objects only (n=8/116; 6.9 %) and four patients (3.4%) showed self-injurious behavior only.
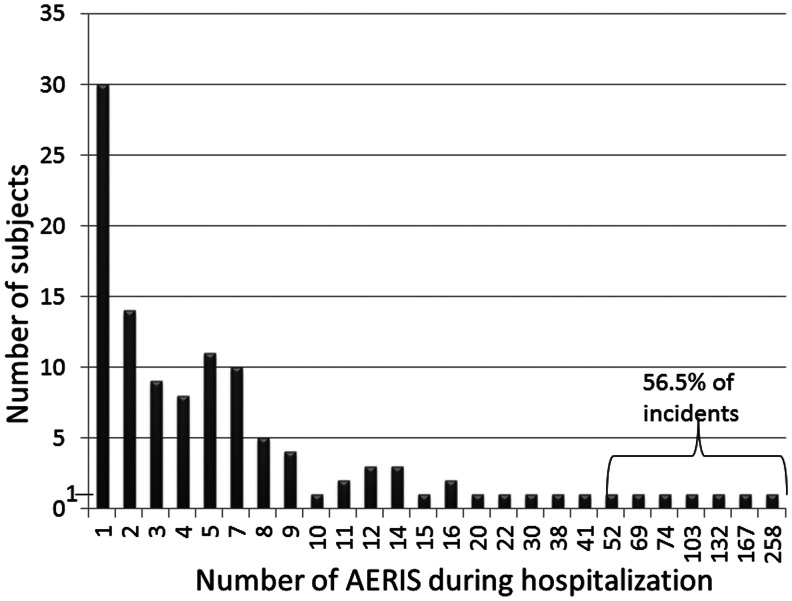
The frequency of AERIs was highly skewed (Fig. 1), with a median of 4 (range: 1–258; 25th percentile: 1, 75th percentile: 8.75); corresponding to an individual daily rate of 0.0977 (range: 0.006–3.9; 25th percentile: 0.06, 75th percentile: 0.2). The majority of aggressive patients showed at maximum five AERIs during their hospitalization (n=72/116; 62.0%). Conversely, out of the 1349 incidents, more than half, that is, 855 incidents (56.6%) were accounted for by only seven patients (1.7%), with individual cumulative frequencies in these patients ranging from 52 to 258 AERIs (for details see previous section: Specific characteristics of aggressive patients with >50 AERIs). This group included four patients with disruptive behavior disorders and three with bipolar/mood disorders as the primary diagnosis; plus comorbid Tourette's disorder in two, and PDD in two others. For the entire cohort, the frequency of AERIs per 1000 inpatient days was 56.3 on average, with a median of 0 (range: 0–3909, 75th percentile: 27.5, 90th percentile: 153.8).
FIG. 1.
Column diagram plotting the individual cumulative frequency of aggressive events requiring an intervention (AERI) during the adolescents' hospitalization (for all patients with at least one AERI, AERI+, n=116; see text). Note that most frequently, patients experienced only one AERI. By contrast, >50% of incidents related to seven patients only.
Within the AERI+ subgroup, the total number of AERIs (but not the daily frequency of AERIs) was significantly associated with the primary diagnosis at discharge (F=2.279, p=0.05; increased in disruptive behavior disorder, decreased in depression). By contrast, none of the demographic and other clinical factors that discriminated between the AERI+ and AERI− subgroups related significantly to the individual number of AERIs within the AERI+ subgroup, or to the individual daily frequency of AERIs within the AERI+ subgroup.
Responses to aggressive episodes: Types and frequencies of interventions
Pharmacological interventions were the first line response in all aggressive patients: 95.6% of AERI+ patients received an as-needed medication (Table 3). Mechanical interventions were used additionally at least once in 59.4 % (n=69/116) of AERI+ patients, that is, in 16.9% of the entire cohort. No death was reported as a result of mechanical restraint use.
Table 3.
As-Needed (PRN) Medication Use in Adolescent Inpatients with Aggressive Episodes Requiring an Intervention (AERI+)
| Patients receiving prn medication: | n (%) |
|---|---|
| Any prn medication | 111 (95.6) |
| Antipsychotics | 79 (68.1) |
| First-generation antipsychotics | 77 (66.4) |
| Second-generation antipsychotics | 12 (10.3) |
| Sedatives | 51 (43.9) |
| Antihistamines | 27 (23.3) |
| Median number of as-needed doses: | Median (25th, 75th, 90th percentile) |
| Antipsychotics | 3 (0; 13; 26.5) |
| First-generation antipsychotic | 3 (0; 12; 23.6) |
| Second-generation antipsychotics | 0 (0; 0; 1) |
| Sedatives | 0 (0; 4; 10.3) |
| Antihistamines | 7 (2; 18; 33.8) |
STAT/PRN medication use
The majority of AERI+ were treated with APs as STAT/PRN medication (n=79; 68.1%; Table 3). In these cases, APs were used alone (n=34; 29.3%) or in combination/alternating with sedatives (n=45; 38.8%). Sedatives alone were used in only 6 patients (5.6%) and 27 (23.3%) received antihistamines. Typically, first-generation injectable APs (FGAs) were used (n=77; 66.4%), rather than second-generation APs (SGAs; n=12; 10.3%; Table 3). Repeated doses of these medications were administered throughout the patients' hospitalizations (Table 3).
Mechanical interventions
During the 2 year observation, 69 patients (59.4% of AERI+) were subjected to mechanical interventions at least once during their hospitalization. This group is designated SRU+(Table 4). Although the proportion of aggressive patients exposed to SRU is substantial, the frequency of SRU per SRU+ patient for the entire time of hospitalization was limited, with a median of three SRUs (range: 0–258; 25th percentile: 1; 75th percentile: 8.5). The median frequency of SRU per AERI+ patient for the entire time of hospitalization was 1 (range: 0–258; 25th percentile: 0; 75th percentile: 4).
Table 4.
Demographic and Clinical Factors: Seclusion and Restraint Use (SRU+vs. SRU− within the Subgroup Requiring an Intervention for Aggression AERI+)
| Total AERI+ | SRU+ (n=69) | SRU− (n=47) | p value | |
|---|---|---|---|---|
| Age (years±SD), n=116 | 15.2±1.6 | 15.25±1.75 | 15.26±1.44 | p=0.9 |
| Male gender (n, %), n=116 | 52 (44.8) | 30 (43.5) | 22 (46.8) | p=0.7 |
| Ethnicity (n, %) n=116 | p=0.4 | |||
| Caucasian | 64 (55.2) | 34 (49.3) | 30 (63.8) | |
| African-American | 30 (25.9) | 18 (26.1) | 12 (25.5) | |
| Hispanic | 11 (9.5) | 8 (12.0) | 3 (6.4) | |
| Asian and other | 11 (9.5) | 11 (13.1) | 2 (4.2) | |
| Previous inpatient treatment n=115 | 62 (54.0) | 36 (53.0%) | 26 (55.3%) | p=0.8 |
| GAF at admission, mean±SE | 30.6±0.67 | 30.0±0.85 | 31.2±1.02 | p=0.4 |
| Verbal aggression only, n=114 | 18 (15.5) | 1 (1.5) | 17 (36.2) | p<0.0001 |
| Physical aggressiona (n, %) | 48 (41.4) | 40 (58.0) | 8 (17.0) | p=0.002 |
| Main psychiatric diagnosis at discharge | p=0.8 | |||
| Bipolar /affective disorders | 36 (31.3) | 23 (33.8) | 13 (27.7) | |
| Depressive disorders | 22 (19.1) | 10 (14.8) | 12 (25.5) | |
| Disruptive behavior disorder | 20 (17.4) | 12 (17.7) | 8 (17.0) | |
| Mood disorders | 17 (14.8) | 11 (16.2) | 6 (12. 8) | |
| Psychotic disorders | 14 (12.2) | 8 (11.8) | 6 (12.8) | |
| Other | 6 (5.2) | 4 (5.9) | 2 (4.3) | |
| Median length of stay (days; 25th, 75th percentile) | 49.5 (17.3; 96.5) | 68.0 (28.5; 105.5) | 24.0 (13.0; 75.0) | p=0.0004 |
| # of AERIs per day median (25th, 75th percentile) | 0.1 (0.05; 0.2) | 0.1 (0.05; 0.2) | 0.1 (0.06; 0.2) | p=0.6 |
| # of as needed medication doses per day Median (25th, 75th percentile) | 0.33 (0.17; 0; 6) | 0.36 (0.23; 0.70) | 0.25 (0.11; 0.53) | p=0.01 |
Combined outwardly directed physical aggression and self-injury.
GAF, Global Assessment of Functioning.
The time sequence of pharmacological intervention and mechanical intervention was not clearly documented in our database; however, only focusing on those patients who experienced a single AERI (n=30, see Fig. 1), we found that a combined pharmacological and mechanical intervention was used in 15 (50%) of these patients.
Restraints were the most frequently used mechanical intervention (n=54; i.e., 46.6% of AERI+, 13.2% of the entire cohort). However, the frequencies of restraint use were very limited, both for the entire unit as well as for SRU+ (n=69). Within this small subgroup, the individual median frequencies for specific restraint types during the entire hospitalization were as follows. Sheet restraint: 1 (range: 0–22; 25th percentile: 0; 75th percentile: 2; 90th percentile: 4); four point restraint: 0 (range: 0–17; 75th percentile: 1; 90th percentile: 6); wrist restraint: 0 (range: 0–15 times; 75th percentile: 1; 90th percentile: 4).
The median incidences of the specific restraint types per 100 inpatient days for the entire cohort were as follows. Four point restraint: 0 (90th percentile: 0; 97.5th percentile: 4.8); sheet restraint: 0 (90th percentile: 0; 97.5th percentile: 6.8); and wrist restraint 0 (90th percentile: 0; 97.5th percentile: 3.1).
Seclusion was used less frequently, with 42 patients (36.3% of AERI+) experiencing this intervention at least once. In SRU+, the median individual frequency of seclusion was 1 (range: 0–17; 25th percentile: 0; 75th percentile: 1.5; 90th percentile: 5). The median incidence of seclusion per 100 inpatient days was 0 (90th percentile: 0.44; 97.5th percentile: 6.9).
The least frequent mechanical interventions were the use of mittens (n=18/116; 15.5%) and the quiet room (n=16/116; 14.3%).
Factors associated with SRU
Age, sex, and race were not associated with SRU (p>0.2 for all group comparisons, Table 4). Similarly, neither the primary diagnosis at discharge, previous inpatient treatment, or GAF at admission showed any association with SRU, although these factors were found to predict aggressive inpatient behavior in general, as mentioned previously. Surprisingly, the daily frequency of AERIs was also not associated with SRU (p>0.2; Table 4).
SRU was nearly exclusively associated with the type of aggression, and, therefore, with physically aggressive acts. SRU occurred only once in a purely verbally aggressive patient (p>0.0001; Table 4). Moreover, SRU was significantly higher in patients showing combined self-injury and outwardly directed aggression, than in all other types of physical aggression combined (p=0.002; Table 4). However, the daily rate of AERIs was comparable in both subgroups (SRU+ vs. SRU−; p=0.6; Table 4). The duration of hospitalizations was significantly longer in SRU+ patients (p=0.0004; Table 4).
Patients experiencing SRU had a higher frequency of pharmacological interventions (Table 4). Moreover, compared with those AERI+ patients without any SRU, SRU+ patients were more likely to have been exposed to all classes of as-needed medications (p<0.01 for all medication classes described previously).
Outcome: GAF, disposition and pharmacological treatment at discharge
As during admission, GAF at discharge was slightly lower in the AERI+ subgroup than in the AERI− subgroup (p=0.04); however, the relative clinical improvement was equal across groups. Importantly, the length of stay was much longer in the AERI+ subgroup (median: 49.5 days; range: 3–293 days) than in the AERI– subgroup (median: 10 days; range: 1–158 days; F=157.31, p<0.0001, Table 5).
Table 5.
Outcome in 408 Adolescent Inpatients with or without Aggressive Episodes Requiring an Intervention (AERI+ [n=116] vs. AERI− [n=292])
| Total | AERI+ | AERI− | p value | |
|---|---|---|---|---|
| GAF at discharge (mean, SD) | 50.6±9.8 | 48.9±9.9 | 51.2±9.8 | 0.0407 |
| Change in GAF (admission to discharge; mean, SD) | 18.6±11.1 | 18.1±11.0 | 18.7±11.1 | 0.6167 |
| Median length of stay (days; 25th, 75th percentile) | 13.0 (8; 34.75) | 49.5 (17.3; 96.5) | 10.0 (7.0; 18.0) | <0.0001 |
| Perspective at discharge (n, %) | ||||
| Outpatient care | 269 (67.3) | 53 (46.5) | 216 (75.5) | <0.0001 |
| Day hospital | 62 (15.5) | 23 (20.2) | 39 (13.6) | 0.1028 |
| Residential treatment facility | 42 (10.5) | 26 (22.8) | 16 (5.6) | <0.0001 |
| Inpatient facility | 27 (6.8) | 12 (10.5) | 15 (5.2) | 0.0574 |
| Pharmacological treatment at discharge | ||||
| Median # of medications (25th, 75th percentile) | 2 (1; 2) | 2 (2; 3) | 1 (1; 2) | <0.0001 |
| Change # of medications, baseline to discharge (±SD) | 0.50±1.1 | 0.72±1.2 | 0.41±0.95 | 0.0079 |
| Antipsychotics (n, %) | 200 (49.0) | 83 (71.6) | 117 (40.1) | <0.0001 |
| First-generation antipsychotics (n, %) | 12 (2.9) | 7 (6.0) | 5 (1.7) | 0.0198 |
| Second-generation antipsychotics (n, %) | 190 (46.6) | 77 (66.4) | 113 (38.7) | <0.0001 |
| Antidepressants (n, %) | 190 (46.6) | 44 (37.9) | 146 (50.0) | 0.0275 |
| Mood stabilizers (n, %) | 189 (46.3) | 78 (67.2) | 111 (38.0) | <0.0001 |
| Lithium (n, %) | 71 (17.4) | 29 (25.0) | 42 (14.4) | 0.01 |
| Anxiolytics/Hypnotics (n, %) | 42 (10.3) | 13 (11.2) | 29 (9.9) | 0.7022 |
| Stimulants (n, %) | 37 (9.1) | 17 (14.7) | 20 (6.9) | 0.0133 |
| Anticholinergics (n, %) | 19 (4.7) | 10 (8.6) | 9 (3.1) | 0.02 |
| Alpha 2 agonists (n, %) | 9 (2.2) | 4 (3.5) | 5 (1.7) | 0.2815 |
GAF, Global Assessment of Functioning.
Regarding their discharge disposition, fewer patients in the AERI+ subgroup could participate in the regular outpatient care (χ2=31.20, p<0.0001) and more had to be transferred to residential treatment facilities (χ2=25.70, p<0.0001).
Pharmacological treatment at discharge also differed between subgroups. The median medication number was significantly higher in AERI+patients (p<0.0001). Specifically, the proportion of patients receiving mood stabilizers (χ2=28.52, p<0.0001) and APs (χ2=32.93, p<0.0001; predominantly SGAs [χ2=25.57, p<0.0001]) was higher in the AERI+ subgroup (Table 5).
Discussion
To the best of our knowledge, this is the first report to consider pharmacological and mechanical interventions in aggressive adolescent inpatient behavior. At least one AERI was recorded in 28.4% of adolescent inpatients. The majority of these events involved physical aggression and were counteracted with a pharmacological intervention in 69.3% and/or a mechanical intervention in 58.7%, corresponding to an incidence of seclusion and restraint use of 16.9% for the entire ward population.
Adolescents involved in AERIs were slightly younger, and differed mostly from their peers regarding their psychiatric history, with a higher representation of disruptive behavioral and bipolar disorders, lower general functioning, and a more complicated history with a higher frequency of prior psychiatric hospitalizations. This profile, except for a lack of male preponderance, is in line with earlier publications on aggressive inpatient behavior in the acute care setting in adolescents (Barton et al. 2001; Dean et al. 2008; Barzmann et al. 2011) and prepubertal children (Garrison et al. 1995; Carlson et al. 2009; Potegal et al. 2009; Crocker et al. 2010; Phillips et al. 2011). While longer hospitalizations and younger age are associated with higher frequencies of inpatient aggression in adults as well (for review see Bowers 2011), bipolar disorder has not consistently been related to increased rates of adult inpatient aggression. However, it has to be noted that the diagnoses in this study were based on clinical assessment. In recent years, a debate has arisen criticizing a potential overdiagnosis of bipolar disorders in children and adolescents with chronic irritability (Blader and Carlson 2007; Moreno et al. 2007; Carlson et al. 2009; Leibenluft 2011). However, on the other hand, our results could also be related to the loss of behavioral control that is associated with immature and hyperactive emotional processes underlying bipolar disorder (Kalmar et al. 2009). Studies in adults have frequently failed to relate inpatient aggression to psychiatric diagnoses and among those that have found diagnostic differences between aggressive and nonaggressive inpatients, schizophrenia has been associated most frequently with aggressive inpatient incidents (Bowers et al. 2011). Some other factors that may be of particular importance in adolescents, such as parental violence, parental substance abuse, staff attitudes, and other specific ward conditions may have played a role also, but these factors were, unfortunately, not recorded in our retrospective chart review.
In line with earlier research, the frequency of aggressive events was highly skewed (compare Bowers et al. 2011). Out of the 1349 AERIs, >50% were accounted for by only seven patients. This finding is in line with studies in adults (for review, see Bowers et al. 2011); and even higher skewedness has been reported in youth, where 7.4% of patients accounted for 81% of all seclusions (Angold and Pickles 1993), 15% of patients accounted for 73% of all seclusions (Atkins and Ricciuti 1992), or 7% of patients accounted for 50% of all seclusions (Earle and Forquer 1995). Therefore, it is important to acknowledge that aggressive inpatient incidents are regular events only to a limited extent, often happening in clusters, relating to the presence of particularly problematic patients. It is possible that experienced clinicians can identify these outstandingly aggressive patients as high-risk patients at admission, as suggested earlier (Phillips et al. 2011). However, the likelihood of aggressive behavior in the remainder of inpatients was difficult to predict based on sociodemographic and clinical variables available at admission, as even a multivariate predictor model accounted for only 11% of the variance in patients with at least one AERI in our study.
Surprisingly, and in contrast to earlier research in adults (meta-analysis of adults in Bowers et al. 2011) and prebubertal children (Carlson et al. 2009; Potegal et al. 2009), a chief complaint of aggression, oppositionality, or impulsivity at admission was only weakly associated with subsequent inpatient aggression requiring an intervention in our study. Carlson and colleagues found a strong predictive value of reported rages prior to admission for subsequent rages during admission (Carlson et al. 2009), with rages defined as “agitated/angry behaviors requiring seclusion or medication because the child could not be verbally redirected to ‘time out’.” Notably, this definition differs from the one used in our sample, which was clearly restricted to subjects posing a danger to others or themselves. In a mixed sample of pre- and postpubertal youth in a secured facility (Tompsett et al. 2011) absence/presence of aggression against adults was the most predictive indicator of events requiring restraint. Notably, the negative predictive value of prior behavior (i.e., the association of no aggressive behavior prior to admission with no need for intervention for aggression) was very high in both studies (Carlson et al. 2009; Tompsett et al. 2011), whereas the positive predictive value was only moderate. Possibly, the lack of a strong association is the result of the different social framework of the hospital as opposed to family life, such that for some patients being taken out of the family context may decrease aggressive behavior (Carlson et al. 2010), whereas for others, conditions on the ward may even trigger or sustain aggressive behavior. It has to be noted, however, that in this retrospective chart review, only complaints brought forth by the adolescent patients and caretakers were recorded during the intake procedure, certainly yielding much less information on the diversity of an individual's aggression history. Moreover, rather than being noted as a specific complaint, the compiled information on an individual's aggression history certainly influenced the admission and discharge diagnosis, and these diagnoses were significantly associated with subsequent inpatient aggression.
Our multivariate models only explained the presence or absence of at least one AERI. By contrast, none of the variables relating to at least one AERI was associated with the total number or the frequency of aggressive events, or with SRU. Rather, only the primary diagnosis at discharge was associated with the frequency of aggressive events. However, this association is partly redundant, as the behavior during hospitalization influences the discharge diagnosis. Therefore, structured risk assessment tools are required to identify high-risk patients with a special need for anti-aggressive strategies early during their hospitalization.
In this regard, two rating scales have been tested specifically in adolescent inpatients (Barzmann et al. 2011; Tompsett et al. 2011). Both tools assessed multiple, combined factors of a detailed history of aggression, but the one that additionally covered exposure to violence, intellectual functioning, and psychiatric diagnosis among other clinical and demographic factors (Tompsett et al. 2011), found that the history of aggression against adults remained as the most significant factor predicting clinically relevant aggression. It has been argued that risk assessments based solely on patient factors are doomed to fail, as these ignore the contextual aspect of ward and staff influence (Steinert 2002). In this regard, short-term risk assessment strategies that have demonstrated efficient reductions in violence rates in adult psychiatry, may also improve aggression management in adolescent psychiatry (Van de Sande 2011): Daily, formalized monitoring of precursors of aggressive behavior, of impairment in social or psychological functioning in daily routines, combined with weekly in-depth monitoring of aggression precursors that were subsequently discussed with the unit staff, led to a 50% reduction of inpatient violence and to a significant reduction in seclusion and coercive medication use.
Our criteria for aggressive patients requiring an intervention were based on the recording of either pharmacological restraint use and/or SRU. Therefore, purely behavioral interventions, which were regularly used on the ward as preventive measures and in response to lower level aggression, were not captured in this study and are, therefore, not the topic of this discussion. This implies also that the prevalence of mild to moderate aggressive behavior that can be de-escalated without a mechanical or pharmacologic intervention is likely higher than the numbers recorded in our retrospective chart review.
STAT/PRN medication was used as the first line management in aggressive inpatients. FGAs were used for this purpose most frequently, followed by benzodiazepines. The general STAT/PRN frequency compares well with the total frequency of STAT/PRN psychotropic medication in adult psychiatric wards (i.e., regardless of aggression), where the most frequent reasons for STAT/PRN prescriptions were agitation, threatening behavior, and mood disturbances (for review see Stein-Parbury et al. 2008). There are only few studies on this topic in children and adolescents, showing STAT/PRN rates of as much as 50% (Dean et al. 2006) to 86% in unselected cases (Vitiello 1987; Kaplan and Busner 1997), with aggressive or disruptive behavior named as the most frequent indication. The use of short-acting intramuscular APs and benzodiazepines for the STAT/PRN treatment of agitation (Citrome 2007; Srivastava 2009), with an emphasis of efficacy of the treatment of psychotic agitation and some additional data in agitation associated with bipolar mania (Citrome 2007), is based on evidence from controlled and registration studies in adults, but not in children or adolescents. By contrast, antihistamines were not used as anti-aggression intervention in an Australian study (Dean 2008). There is no controlled evidence supporting the prescription of antihistamines for agitation or aggressive behavior. To the contrary, the only study addressing this issue has failed to show any superiority over placebo for this medication class when used in short-acting injectable form, versus saline injection (Vitiello 1991).
Contrary to the lack of evidence for acute pharmacological interventions for aggression, evidence exists for standing pharmacological treatment of aggression (Pappadopulous et al. 2006; Zuddas 2011). In our study, SGAs and mood stabilizers were the most frequently used standing medications at discharge in the AERI+ subgroup. The use of mood stabilizers reflects the high prevalence of bipolar spectrum disorders in this subgroup, and is in line with treatment guidelines (Kowatch et al. 2005), although mood stabilizers had significantly lower effect sizes in pediatric mania than did SGAs (Correll et al. 2010). Similarly, APs have demonstrated clear efficacy with numbers needed to treat (NNTs) of 2–5 for the treatment of aggression across diagnostic categories (Pappadopulous et al. 2006), and are recommended by experts for aggression if other, less restrictive measures fail, despite lack of regulatory approval for this purpose (Pappadopulos et al. 2011). Moreover, SGAs are indicated for both adolescent schizophrenia and pediatric bipolar disorder (Correll et al. 2011). We also noted a slight predominance of FGAs and concomitant anticholinergic prescription in the aggressive subgroup, but our data were not detailed enough to understand the specifics of this prescription pattern. As a consequence of the increased comorbidity of ADHD in AERI+, the prescription of stimulants was also more frequent in this subgroup.
Almost half of AERIs were manageable with a pharmacological intervention alone; however, additional use of containment was needed in 58% of aggressive patients, translating into a unit-wide frequency of SRU of 16.9%. Although this ratio is higher than that in most adult psychiatric settings, where the majority of studies report SRU<10% (for review see Steinert et al. 2010), this number compares with that in earlier studies in adolescent acute inpatient care, where rates ranged from 17 to 22% (Miller et al. 1989; Angold and Pickles 1993; Dean et al. 2008; Larson et al. 2008; Azeem et al. 2011). By contrast, higher rates have been reported in other settings, such as forensic institutions (Donovan et al. 2003; Tompsett 2011) and in residential care (LeBel 2004; Leidy 2006), as well as in prepubertal children (Delaney 2001; Sourander et al. 2002; Crocker 2010), or in highly selected cohorts, such as youth with conduct disorder (Swett et al. 1989). The latter factors are known to increase the incidence of aggression per se, and as SRU is rarely reported as a proportion of aggressive patients, it is possible that the rates differ relative to the incidence of aggression in these populations. Nonetheless, this appears not to be the case in the adult psychiatric setting. A recent international review on SRU in adults (16,000 admissions, 12 countries) reported considerable differences in utilization rates of coercive measures despite comparable compositions of patient cohorts (Steinert et al. 2010). Even though national or statewide specific legislation sets a different frame for the management of inpatient aggression, culture, traditions, and institutional policies, rather than clearly defined medical or safety requirements, appeared to have driven the high variability of responses to aggressive behavior (Steinert et al. 2010). This conclusion could only be drawn, based on the high number of psychiatric units reviewed. By contrast, data for SRU in child and adolescent psychiatric care institutions are still limited (Delaney 2001; De Hert et al. 2011), so that such conclusions cannot currently be drawn. Still, the shaping of these “traditions” lacks an empirical basis. For example, whereas seclusion as opposed to restraint is illegal in Massachusetts, no differences in patients' perception regarding these different coercive measures were found in a recent randomized study on this topic (Bergk et al. 2011).
It is noteworthy that no sociodemographic characteristics were related to SRU, dismissing the suspicion of racial profiling, raised by some human rights activists. By contrast, a clustering of physical aggression during the hospitalization was associated with SRU, justifying the aim of staff security, which is often named as the principal reason for SRU. Furthermore, patients experiencing mechanical restraint had already received a higher frequency of as-needed medications, indicating that pharmacological interventions had been used to address the patients' behavior, and that those strategies alone had had limited success.
Limitations
The results of this study have to be interpreted within its limitations, including: 1) Retrospective design, 2) lack of data on the time course of individual responses and effects of interventions for AERI, 3) lack of data on the sequential use of oral followed by i.m. PRN/STAT medication use for patients who were offered and accepted oral PRN/STAT medication during less than serious/dangerous behaviors and subsequently worsened despite oral PRN/STAT treatment, 4) lack of data on nonmedical behavioral interventions other than the quiet room, 5) lack of history on previous exposure to violence, and 6) lack of proportional calculation on the availability of child and adolescent inpatient services relative to the needed capacity in the catchment area of our hospital that could affect the severity of admitted youth.
Conclusions
Further research on the efficacy and safety of STAT/PRN medications and structured models of aggression management, such as the use of early identification of aggressive patients, or the use of rapid intervention teams, is sorely needed.
Clinical Significance
The management of aggressive behaviors in youth is still a challenging issue, both from the nursing and from the medical perspective. Despite the high prevalence of STAT/PRN pharmacological interventions and the use of standing medication with anti-aggressive potential, physical interventions were still used in this acute inpatient sample. Structured preventive measures may be needed to further reduce the need of ad hoc interventions. These measures need to be developed as an interdisciplinary effort involving the entire medical team, with adequate support from clinical administration.
Disclosures
Drs. Amanbekova, Baeza, Chekuri, Kapoor, Saito, and Ramani have nothing to disclose. Dr. Correll has been a consultant and/or advisor to or has received honoraria from: Actelion, Alexza; AstraZeneca, Biotis, Bristol-Myers Squibb, Cephalon, Desitin, Eli Lilly, GSK, IntraCellular Therapies, Lundbeck, Medavante, Medscape, Merck, Novartis, Ortho-McNeill/Janssen/J&J, Otsuka, Pfizer, ProPhase, and Sunovion and Takeda. He has received grant support from Bristol-Myers Squibb, Feinstein Institute for Medical Research, Janssen/J&J, National Institute of Mental Health (NIMH), National Alliance for Research in Schizophrenia and Depression (NARSAD), and Otsuka. Dr de Hert has been a consultant for, received grant/research support and honoraria from, and been on the speakers/advisory boards of Astra Zeneca, Bristol-Myers Squibb, Eli Lilly, Janssen-Cilag, Lundbeck JA, Pfizer, and Sanofi Aventis. Dr. Carbon, as a family member, has the same conflicts as Dr. Correll.
References
- American Psychiatric Association. 4th. Washington, DC: American Psychiatric Association; 1994. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Angold A. Pickles A. Seclusion on an adolescent unit. J Child Psychol Psychiatry. 1993;34:975–989. doi: 10.1111/j.1469-7610.1993.tb01102.x. [DOI] [PubMed] [Google Scholar]
- Ashcraft L. Anthony W. Eliminating seclusion and restraint in recovery-oriented crisis services. Psychiatr Serv. 2008;59:1198–1202. doi: 10.1176/ps.2008.59.10.1198. [DOI] [PubMed] [Google Scholar]
- Atkins MS. Ricciuti A. The disproportionate use of seclusion in a children's psychiatric state hospital. Resid Treat Child Youth. 1992;10:23–33. [Google Scholar]
- Azeem MW. Aujla A. Ramerth M. Binsfeld G. Jones RB. Effectiveness of six core strategies based on trauma reducing seclusions and restraints at a child and adolescent psychiatric hospital. J Child Adolesc Psychiatr Nurs. 2011;24:11–15. doi: 10.1111/j.1744-6171.2010.00262.x. [DOI] [PubMed] [Google Scholar]
- Barton G. Rey JM. Simpson P. Denshire E. Patterns of critical incidents and their effect on outcome in an adolescent inpatient service. Aust N Z J Psychiatry. 2001;35:155–159. doi: 10.1046/j.1440-1614.2001.00872.x. [DOI] [PubMed] [Google Scholar]
- Barzman DH. Brackenbury L. Sonnier L. Schnell B. Cassedy A. Salisbury S. Sorter M. Mossman D. Brief Rating of Aggression by Children and Adolescents (BRACHA): Development of a tool for assessing risk of inpatients' aggressive behavior. J Am Acad Psychiatry Law. 2011;39:170–179. [PubMed] [Google Scholar]
- Bergk J. Einsiedler B. Flammer E. Steinert T. A randomized controlled comparison of seclusion and mechanical restraint in inpatient settings. Psychiatr Serv. 2011;62:1310–1317. doi: 10.1176/ps.62.11.pss6211_1310. [DOI] [PubMed] [Google Scholar]
- Blader, JC: Pharmacotherapy and postdischarge outcomes of child inpatients admitted for aggressive behavior. J Clin Psychopharmacol. 2006;4:419–425. doi: 10.1097/01.jcp.0000227356.31203.8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blader JC. Carlson GA. Increased rates of bipolar disorder diagnoses among U.S. child, adolescent, and adult inpatients, 1996–2004. Biol Psychiatry. 2007;62:107–114. doi: 10.1016/j.biopsych.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers L. Stewart D. Papadopoulos C. Dack C. Ross J. Khanom H. Jeffrey D. Inpatient violence, aggression: A literature review. Section of Mental Health Nursing Health Service and Population Research Institute of Psychiatry Kings College London. 2011. http://www.kcl.ac.uk/iop/depts/hspr/research/ciemh/mhn/projects/litreview/LitRevAgg.pdf. [Apr 23;2013 ]. http://www.kcl.ac.uk/iop/depts/hspr/research/ciemh/mhn/projects/litreview/LitRevAgg.pdf
- Carlson GA. Potegal M. Margulies D. Basile J. Gutkovich Z. Liquid risperidone in the treatment of rages in psychiatrically hospitalized children with possible bipolar disorder. Bipolar Disord. 2010;12:205–212. doi: 10.1111/j.1399-5618.2010.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson GA. Potegal M. Margulies D. Gutkovich Z. Basile J. Rages—what are they and who has them? J Child Adolesc Psychopharmacol. 2009;19:281–288. doi: 10.1089/cap.2008.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citrome L. Comparison of intramuscular ziprasidone, olanzapine, or aripiprazole for agitation: a quantitative review of efficacy and safety. J Clin Psychiatry. 2007;68:1876–1885. doi: 10.4088/jcp.v68n1207. [DOI] [PubMed] [Google Scholar]
- Cornaggia CM. Beghi M. Pavone F. Barale F. Aggression in psychiatry wards: A systematic review. Psychiatry Res. 2011;189:10–20. doi: 10.1016/j.psychres.2010.12.024. [DOI] [PubMed] [Google Scholar]
- Correll, CU, Kratochvil, CJ, March, JS: Developments in pediatric psychopharmacology: focus on stimulants, antidepressants, and antipsychotics. J Clin Psychiatry. 2011;72:655–670. doi: 10.4088/JCP.11r07064. [DOI] [PubMed] [Google Scholar]
- Correll CU. Sheridan EM. DelBello MP. Antipsychotic and mood stabilizer efficacy and tolerability in pediatric and adult patients with bipolar I mania: A comparative analysis of acute, randomized, placebo-controlled trials. Bipolar Disord. 2010;12:116–141. doi: 10.1111/j.1399-5618.2010.00798.x. [DOI] [PubMed] [Google Scholar]
- Crocker JH. Stargatt R. Denton C. Prediction of aggression and restraint in child inpatient units. Aust N Z J Psychiatry. 2010;44:443–449. doi: 10.3109/00048670903489825. [DOI] [PubMed] [Google Scholar]
- De Hert M. Dirix N. Demunter H. Correll CU. Prevalence, correlates of seclusion, restraint use in children, adolescents: a systematic review. Eur Child Adolesc Psychiatry. 2011;20:221–230. doi: 10.1007/s00787-011-0160-x. [DOI] [PubMed] [Google Scholar]
- Dean AJ. Duke SG. Scott J. Bor W. George M. McDermott BM. Physical aggression during admission to a child and adolescent inpatient unit: Predictors and impact on clinical outcomes. Aust N Z J Psychiatry. 2008;42:536–543. doi: 10.1080/00048670802050587. [DOI] [PubMed] [Google Scholar]
- Dean AJ. Gibbon P. McDermott BM. Davidson T. Scott J. Exposure to aggression and the impact on staff in a child and adolescent inpatient unit. Arch Psychiatr Nurs. 2010;24:15–26. doi: 10.1016/j.apnu.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Dean AJ. McDermott BM. Marshall RT. PRN sedation-patterns of prescribing, administration in a child, adolescent mental health inpatient service. Eur Child Adolesc Psychiatry. 2006;15:277–281. doi: 10.1007/s00787-006-0532-9. [DOI] [PubMed] [Google Scholar]
- Delaney KR. Hardy L. Challenges faced by inpatient child/adolescent psychiatric nurses. J Psychosoc Nurs Ment Health Serv. 2008;46:21–24. doi: 10.3928/02793695-20080201-04. [DOI] [PubMed] [Google Scholar]
- Delaney, KR: Developing a restraint- reaction program for child/adolescent inpatient treatment. J Child Adolesc Psychiatr Nurs. 2001;14:128–140. doi: 10.1111/j.1744-6171.2001.tb00304.x. [DOI] [PubMed] [Google Scholar]
- Deshmukh P. Kulkarni G. Barzman D. Recommendations for pharmacological management of inpatient aggression in children, adolescents. Psychiatry (Edgmont) 2010;7:32–40. [PMC free article] [PubMed] [Google Scholar]
- Donat DC. Encouraging alternatives to seclusion, restraint, and reliance on PRN drugs in a public psychiatric hospital. Psychiatr Serv. 2005;56:1105–1108. doi: 10.1176/appi.ps.56.9.1105. [DOI] [PubMed] [Google Scholar]
- Donovan A. Plant R. Peller A. Siegel L. Martin A. Two-year trends in the use of seclusion and restraint among psychiatrically hospitalized youth. Psychiatr Serv. 2003;54:987–993. doi: 10.1176/appi.ps.54.7.987. [DOI] [PubMed] [Google Scholar]
- Earle KA. Forquer SL. Use of seclusion with children and adolescents in public psychiatric hospitals. Am J Orthopsychiatry. 1995;65:238–244. doi: 10.1037/h0079621. [DOI] [PubMed] [Google Scholar]
- Gabel S. Shindledecker R. Aggressive behavior in youth: Characteristics, outcome, and psychiatric diagnoses. J Am Acad Child Adolesc Psychiatry. 1991;30:982–988. doi: 10.1097/00004583-199111000-00017. [DOI] [PubMed] [Google Scholar]
- Gabel S. Shindledecker R. Parental substance abuse and suspected child abuse/maltreatment predict outcome in children's inpatient treatment. J Am Acad Child Adolesc Psychiatry. 1990;29:919–924. doi: 10.1097/00004583-199011000-00014. [DOI] [PubMed] [Google Scholar]
- Garrison WT. Ecker B. Friedman M. Davidoff R. Haeberle K. Wagner M. Aggression and counteraggression during child psychiatric hospitalization. J Am Acad Child Adolesc Psychiatry. 1990;29:242–250. doi: 10.1097/00004583-199003000-00013. [DOI] [PubMed] [Google Scholar]
- Hammer JH. Springer J. Beck NC. Menditto A. Coleman J. The relationship between seclusion and restraint use and childhood abuse among psychiatric inpatients. J Interpers Violence. 2011;26:567–579. doi: 10.1177/0886260510363419. [DOI] [PubMed] [Google Scholar]
- Kalmar JH. Wang F. Chepenik LG. Womer FY. Jones MM. Pittman B. Shah MP. Martin A. Constable RT. Blumberg HP. Relation between amygdala structure and function in adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2009;48:636–642. doi: 10.1097/CHI.0b013e31819f6fbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan SL. Busner J. The use of prn and stat medication in three child psychiatric inpatient settings. Psychopharmacol Bull. 1997;33:161–164. [PubMed] [Google Scholar]
- Kowatch RA. Fristad M. Birmaher B. Wagner KD. Findling RL. Hellander M. Child Psychiatric Workgroup on Bipolar Disorder. Treatment guidelines for children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:213–235. doi: 10.1097/00004583-200503000-00006. [DOI] [PubMed] [Google Scholar]
- Kulkarni G. Deshmukh P. Barzman D. Collaborative problem solving (CPS) as a primary method of addressing acute pediatric pathological aggression along with other modalities. Psychiatr Q. 2011;81:167–175. doi: 10.1007/s11126-010-9126-2. [DOI] [PubMed] [Google Scholar]
- Larson TC. Sheitman BB. Kraus JF. Mayo J. Leidy LA. Managing treatment resistant violent adolescent: A step forward by substituting seclusion for mechanical restraint? Adm Policy Ment Health. 2008;35:198–203. doi: 10.1007/s10488-007-0156-5. [DOI] [PubMed] [Google Scholar]
- LeBel J. Stromberg N. Duckworth K. Kerzner J. Goldstein R. Weeks M. Harper G. LaFlair L. Sudders M. Child and adolescent inpatient restraint reduction: a state initiative to promote strength-based care. J Am Acad Child Adolesc Psychiatry. 2004;43:37–45. doi: 10.1097/00004583-200401000-00013. [DOI] [PubMed] [Google Scholar]
- Leibenluft E. Severe mood dysregulation, irritability, and the diagnostic boundaries of bipolar disorder in youths. Am J Psychiatry. 2011;168:129–142. doi: 10.1176/appi.ajp.2010.10050766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidy BD. Haugaard JJ. Nunno MA. Kwartner JK. Review of restraint data in residential treatment center for adolescent females. Child Youth Care Forum. 2006;35:339–352. [Google Scholar]
- Leigh E. Smith P. Milavic G. Stringaris A. Mood regulation in youth: research findings and clinical approaches to irritability and short-lived episodes of mania-like symptoms. Curr Opin Psychiatry. 2012;25:271–6. doi: 10.1097/YCO.0b013e3283534982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. Walker MC. Friedman D. Use of a holding technique to control the violent behavior of seriously disturbed adolescents. Hosp Community Psychiatry. 1989;40:520–524. doi: 10.1176/ps.40.5.520. [DOI] [PubMed] [Google Scholar]
- Mohr WK. Petti TA. Mohr BD. Adverse effects associated with physical restraint. Can J Psychiatry. 2003;48:330–337. doi: 10.1177/070674370304800509. [DOI] [PubMed] [Google Scholar]
- Moreno C. Laje G. Blanco C. Jiang H. Schmidt AB. Olfson M. National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Arch Gen Psychiatry. 2007;64:1032–1039. doi: 10.1001/archpsyc.64.9.1032. [DOI] [PubMed] [Google Scholar]
- Pappadopulos E. Rosato NS. Correll CU. Findling RL. Lucas J. Crystal S. Jensen PS. Experts' recommendations for treating maladaptive aggression in youth. J Child Adolesc Psychopharmacol. 2011;21:505–515. doi: 10.1089/cap.2010.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappadopulos E. Woolston S. Chait A. Perkins M. Connor DF. Jensen PS. Pharmacotherapy of aggression in children and adolescents: Efficacy and effect size. J Can Acad Child Adolesc Psychiatry. 2006;15:27–39. [PMC free article] [PubMed] [Google Scholar]
- Phillips NL. Stargatt R. Fisher L. Risk assessment: predicting physical aggression in child psychiatric inpatient units. Aust N Z J Psychiatry. 2011;45:638–645. doi: 10.3109/00048674.2011.587396. [DOI] [PubMed] [Google Scholar]
- Potegal M. Carlson G. Margulies D. Gutkovitch Z. Wall M. Rages or temper tantrums? The behavioral organization, temporal characteristics, and clinical significance of angry-agitated outbursts in child psychiatry inpatients. Child Psychiatry Hum Dev. 2009;40:621–636. doi: 10.1007/s10578-009-0148-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan EP. Sparrow V. Messick D. Aaron J. Burnette M. A prospective study of assault against staff by youths in a pediatric state hospital. Psychiatr Serv. 2004;55:665–670. doi: 10.1176/appi.ps.55.6.665. [DOI] [PubMed] [Google Scholar]
- Sailas E. Fenton M. Seclusion, restraint for people with serious mental illnesses. Cochrane Database Syst Rev. 2000;2:CD001163. doi: 10.1002/14651858.CD001163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourander A. Ellilä H. Välimäki M. Piha J. Use of holding, restraints, seclusion and time-out in child and adolescent psychiatric in-patient treatment. Eur Child Adolesc Psychiatry. 2002;11:162–167. doi: 10.1007/s00787-002-0274-2. [DOI] [PubMed] [Google Scholar]
- Srivastava A. Limited evidence for the effectiveness of p.r.n. medications among psychiatric inpatients. J Psychiatr Pract. 2009;15:193–201. doi: 10.1097/01.pra.0000351879.52883.10. [DOI] [PubMed] [Google Scholar]
- Steinert T. Lepping P. Bernhardsgrütter R. Conca A. Hatling T. Janssen W. Keski–Valkama A. Mayoral F. Whittington R. Incidence of seclusion and restraint in psychiatric hospitals: a literature review and survey of international trends. Soc Psychiatry Psychiatr Epidemiol. 2010;45:889–897. doi: 10.1007/s00127-009-0132-3. [DOI] [PubMed] [Google Scholar]
- Steinert T. Prediction of inpatient violence. Acta Psychiatr Scand. 2002;106(Suppl 412):133–141. doi: 10.1034/j.1600-0447.106.s412.29.x. [DOI] [PubMed] [Google Scholar]
- Stein–Parbury J. Reid K. Smith N. Mouhanna D. Lamont F. Use of pro re nata medications in acute inpatient care. Aust N Z J Psychiatry. 2008;42:283–292. doi: 10.1080/00048670701881553. [DOI] [PubMed] [Google Scholar]
- Sukhodolsky DG. Cardona L. Martin A. Characterizing aggressive and noncompliant behaviors in a children's psychiatric inpatient setting. Child Psychiatry Hum Dev. 2005;36:177–193. doi: 10.1007/s10578-005-3494-0. [DOI] [PubMed] [Google Scholar]
- Swett C. Michaels AS. Cole JO. Effects of a state law on rates of restraint on a child and adolescent unit. Bull Am Acad Psychiatry Law. 1989;17:165–169. [PubMed] [Google Scholar]
- Tompsett CJ. Domoff S. Boxer P. Prediction of restraints among youth in a psychiatric hospital: Application of translational action research. J Clin Psychol. 2011;67:368–382. doi: 10.1002/jclp.20772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Sande R. Nijman HL. Noorthoorn EO. Wierdsma AI. Hellendoorn E. van der Staak C. Mulder CL. Aggression and seclusion on acute psychiatric wards: effect of short-term risk assessment. Br J Psychiatry. 2011;199:473–478. doi: 10.1192/bjp.bp.111.095141. [DOI] [PubMed] [Google Scholar]
- Vitiello B. Hill JL. Elia J. Cunningham E. McLeer SV. Behar D. P.r.n. medications in child psychiatric patients: a pilot placebo-controlled study. J Clin Psychiatry. 1991;52:499–501. [PubMed] [Google Scholar]
- Zuddas A. Zanni R. Usala T. Second generation antipsychotics (SGAs) for non–psychotic disorders in children and adolescents: A review of the randomized controlled studies. Eur Neuropsychopharmacol. 2011;21:600–620. doi: 10.1016/j.euroneuro.2011.04.001. [DOI] [PubMed] [Google Scholar]