Abstract
Acute myocardial infarction (AMI) triggers mobilization of stem cells from bone marrow (BM) into peripheral blood (PB). Based on our observation that the bioactive sphingophospholipids, sphingosine-1 phosphate (S1P), and ceramide-1 phosphate (C1P) regulate trafficking of hematopoietic stem cells (HSCs), we explored whether they also direct trafficking of non-hematopoietic stem cells (non-HSCs). We detected a 3–6-fold increase in circulating CD34+, CD133+, and CXCR4+ lineage-negative (Lin−)/CD45− cells that are enriched in non-HSCs [including endothelial progenitors (EPCs) and very small embryonic-like stem cells (VSELs)] in PB from AMI patients (P<0.05 vs. controls). Concurrently, we measured a ∼3-fold increase in S1P and C1P levels in plasma from AMI patients. At the same time, plasma obtained at hospital admission and 6 h after AMI strongly chemoattracted human BM-derived CD34+/Lin− and CXCR4+/Lin− cells in Transwell chemotaxis assays. This effect of plasma was blunted after depletion of S1P level by charcoal stripping and was further inhibited by the specific S1P1 receptor antagonist such as W146 and VPC23019. We also noted that the expression of S1P receptor 1 (S1P1), which is dominant in naïve BM, is reduced after the exposure to S1P at concentrations similar to the plasma S1P levels in patients with AMI, thus influencing the role of S1P in homing to the injured myocardium. Therefore, we examined mechanisms, other than bioactive lipids, that may contribute to the homing of BM non-HSCs to the infarcted myocardium. Hypoxic cardiac tissue increases the expression of cathelicidin and β-2 defensin, which could explain why PB cells isolated from patients with AMI migrated more efficiently to a low, yet physiological, gradient of stromal-derived factor-1 in Transwell migration assays. Together, these observations suggest that while elevated S1P and C1P levels early in the course of AMI may trigger mobilization of non-HSCs into PB, cathelicidin and β-2 defensin could play an important role in their homing to damaged myocardium.
Introduction
Acute myocardial infarction (AMI) leads to ischemic heart disease (IHD) and is one of the most prevalent causes of death and morbidity in the United States and worldwide [1–3]. Despite significant advances in medical therapy and interventional strategies, the prognosis of millions of patients with AMI and IHD remains dismal [4,5]. Currently, there are no therapies that can replace the infarcted myocardium, and therapies are mostly symptomatic. The shortage of donor hearts available for transplantation creates a need to develop new myocardial regenerative strategies, including stem cell therapies.
Cardiomyocytes undergo continuous renewal, maintained at least in part by bone marrow (BM)-derived cells [6–8]. We focused our studies on non-hematopoietic stem cells (non-HSCs) that include populations such as CD34+, CD133+, and CXCR4+ lineage-negative (Lin−)/CD45− cells, as they are enriched in very small embryonic-like stem cells (VSELs) [9–11]. While the mechanisms of cardiomyocyte renewal are poorly understood, this process is capable of renewing up to half of the cardiomyocytes during the normal life span [12]. In rodents, this phenomenon is dynamic, responds to myocardial injury [13], and is maintained, at least in part, by BM-derived cells [14]. AMI initiates innate reparatory mechanisms through which non-HSCs are mobilized from BM into peripheral blood (PB) and chemoattracted to the ischemic myocardium, a process that can potentially contribute to myocardial regeneration as we and others have demonstrated [15–20]. Nevertheless, very little is known about the underlying mechanism and clinical significance of this mobilization phenomenon. Clinical studies investigating stem cell mobilization as a strategy to augment repair of the infarcted myocardium have achieved limited success, probably as result of the low number of mobilized non-HSCs homing to damaged heart tissue [21–24].
Similarly, the process of recruitment of stem cells from BM into PB itself is still not fully understood. The α-chemokine stromal derived factor-1 (SDF-1) has been identified as a potent stem cell chemoattractant present in PB plasma [25]. Recently, however, other factors, notably bioactive lipids—sphingosine-1 phosphate (S1P) and ceramide-1 phosphate (C1P), have been identified in PB as major HSCs chemoattractants that enhance their egress from BM into PB [26–29]. SDF-1 has been also reported to become u-pregulated in hypoxia inducible factor-1α (HIF-1α) manner at sites of organ tissue injury (eg, in infarcted myocardium) [30–32]. While a role for the SDF-1/CXCR4 axis in stem cell trafficking is undisputed, its exclusive role in homing to a highly proteolytic microenvironment, such as the ischemic/infarcted myocardium, is less established, and redundant mechanisms may exist [27,33,34]. The limited contribution of SDF-1 to myocardial regeneration may be explained by its active degradation at the sites of inflammation and myocardial infarction by metalloproteinases [33,35,36]. However, as recently demonstrated, despite SDF-1 degradation by proteases, the chemotactic responsiveness of HSCs to even low SDF-1 gradient could be significantly enhanced by members of the family of cationic antimicrobial peptides (CAMPs), including products of complement cascade (CC) activation—anaphylatoxin C3a [37–40] and fibroblast- and leukocyte-derived-cathelicidin and β-2 defensin [41]. Thus, an increase in the level of CAMPs at sites of injury enhances the responsiveness of stem cells to even very low levels of SDF-1.
Based on the above literature, we hypothesized and show for the first time that bioactive lipids (S1P and C1P) and elements of the innate immune system (cathelicidin and β-2 defensin) are up-regulated during AMI and can potentially contribute to mobilization of non-HSCs from BM into PB followed by their homing to the ischemic myocardium.
Materials and Methods
The study population consisted of 40 patients with acute ST-elevation myocardial infarction (STEMI). We enrolled 30 age- and sex-matched subjects to the study population into the control (CTRL) group. The CTRL group is asymptomatic with no history of caronary artery disease but with a similar risk factor profile to the STEMI group. Patients with STEMI were referred within 12 h of symptom onset for primary percutaneous coronary intervention (PPCI). Patients were excluded if they had a systemic inflammatory process, cancer, recent motor vehicle accident, recent surgery, active infection, history of MI or revascularization [coronary artery bypass graft, percutaneous coronary intervention (PCI)], unsuccessful revascularization, or onset of the symptoms >12 h. PB samples (3 mL of PB each) were obtained at presentation in all patients baseline denoting the time of arrival to the hospital (BSL) followed by samples at 6, 12, 24, and 48 after PCI, and only PPCI patients were enrolled. BM samples were obtained from normal individuals, and the BM cells were examined by smear and flow cytometry for any pathological findings before being utilized in the chemotaxis experiments. The study protocol complies with the Declaration of Helsinki and was approved by the institutional ethics committee. All patients provided written informed consent.
Quantitation of C1P and S1P
PB samples were obtained in ethylenediaminetetraacetic acid tubes, and plasma was isolated by centrifuging whole blood for 10 min at 800g. Supernatant was then removed and centrifuged at 9,400g for 10 min to remove platelets, and the supernatant was then used for lipid measurements. RBCs were isolated using the leukocyte depletion kit (Pall, Inc., East hills, NY) and purified by centrifuging at 600g for 10 min followed by washing in normal saline at the same speed. To asses the effect of activated complement on S1P and C1P release, RBCs were incubated for 3 h at 37°C with saline, an antibody against RBCs alone (BD Biosciences, San Jose, CA), normal human serum complement alone at a 1:5 dilution (Quidel, Santa Clara, CA), or antibody and complement together. Lipids were extracted from plasma, supernatant, and RBCs using acidified organic solvents, as previously described [42]. An analysis of S1P and C1P was carried out using a Shimadzu UFLC coupled with an AB Sciex 4000-Qtrap hybrid linear ion trap triple quadrupole mass spectrometer in multiple reaction monitoring mode as previously described [43]. The mobile phase consisted of 75/25 of methanol/water with formic acid (0.5%) and 5 mM ammonium formate (0.1%) as solvent A and 99/1of methanol/water with formic acid (0.5%) and 5 mM ammonium formate (0.1%) as solvent B. For the analysis of various C1P species, the separation was achieved by maintaining 75% of solvent B for 3 min, then increasing to 100% B over the next 3 min, and maintaining at 100% B for the last 18 min. The column was equilibrated back to the initial conditions in 3 min. The flow rate was 0.5 mL/min with a column temperature of 60°C. The sample injection volume was 10 μL. The mass spectrometer was operated in the positive electrospray ionization mode with optimal ion source settings determined by synthetic standards with a declustering potential of 46 V, entrance potential of 10 V, collision energy of 19 V, collision cell exit potential of 14 V, curtain gas of 30 psi, ion spray voltage of 5,500 V, ion source gas1/gas2 of 40 psi, and temperature of 550°C.
Flow cytometric analysis and fluorescence-activated cell sorting sorting of circulating primitive stem cells from PB
Erythrocytes were lysed twice using BD Pharm Lyse lysing buffer (BD Biosciences) at room temperature for 10 min and subsequently washed in phosphate-buffered saline (PBS) to yield total nucleated cells (TNCs). TNCs were subsequently stained for hematopoietic lineages markers (Lin) using the following fluorescein isothiocyanate-conjugated antibodies (Abs) against humans: CD2 (clone RPA-2.10); CD3 (clone UCHT1); CD14 (clone M5E2); CD16 (clone 3G8); CD19 (clone HIB19); CD24 (clone ML5); CD56 (clone NCAM16.2); CD66b (clone G10F5); and CD235a (clone GA-R2). These Abs were purchased from BD Biosciences. The cells were simultaneously stained for the panleukocytic marker—CD45 (PE-Cy7conjugated Abs, clone HI30; BD Biosciences) and one of the following antigens: CXCR4 (APC-conjugated Abs, clone 12G5; BD Biosciences), CD34 (APC-conjugated Abs, clone 581; BD Biosciences), CD133 (CD133/1, APC-conjugated Abs; Miltenyi Biotec, Auburn, CA), S1P receptor-1 (PE-conjugated Abs, clone 218713; R&D Systems, Minneapolis, MN), and S1P3 (primary antibody followed by a secondary antibody labeled with PE-Cy7; Santa Cruz Biotechnology, Santa Cruz, CA). Staining was performed in PBS with 2% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA) at 4°C for 30 min. Cells were subsequently washed, re-suspended, and analyzed using an LSR II (BD Biosciences). At least 106 events were acquired from each sample. The absolute numbers of circulating stem cells were calculated (individually for each patient) per 1 μL of PB based on the percentage content of these cells detected by flow cytometry and the absolute number of white blood cells per 1 μL of PB. FlowJo software was used for the analysis (Tree Star, Ashland, OR).
Chemotaxis assays
Cell migration assays were performed using the chemotactic (Boyden) chamber (Neuroprobe, Gaithersburg, MD). BM- and PB-derived cells were lysed as described earlier. Cells were then suspended in S1P free medium (RPMI with 0.1% FBS) for 3 h before the migration assays. The lower chambers were loaded with CTRLs or the testing agents. Cell suspension (1×106 cells/100 μL) was loaded into the upper chambers on a 5 μm membrane; the chambers were incubated (37°C, 95% humidity, and 5% CO2) for 3 h; and subsequently, cells in the lower chambers were harvested, stained against the lineage markers CD34 and CXCR4 as detailed earlier, and counted by fluorescence-activated cell sorting. The lower chambers contained no chemoattractant medium-Vehicle (RPMI medium with 0.1% FBS, ie, CTRL) or plasma isolated from STEMI patients during the peak mobilization of stem cells. To examine the role of bioactive lipids in inducing BM-derived stem cell migration, simultaneous experiments utilizing charcoal-stripped plasma in the lower chamber were performed. Similar to examining the role of S1P1 in this mobilization, BM-derived cells were incubated with 10 μM of the selective S1P1 receptor antagonist W146 at 10 μM (Cayman Chemicals, Ann Arbor, MI) or VPC23019 at 10 μM (Avanti Polar Lipids, Alabaster, AL) for 1 h before the migration assay. To examine the role of LL37 in PBCs migration, PB cells isolated from patients with AMI (1×106 cells/100 μL) were loaded in the upper chambers. The lower chamber was loaded with RPMI medium with 0.1% FBS alone or supplemented with SDF-1 at 2 ng/mL (PeproTech, Rocky Hills, NJ) alone, LL37 at 2.5 ng/mL (AnaSpec, Fremont, CA) alone, or the combination of SDF-1 (2 ng/mL) and LL37 (2.5 ng/mL). Chemotaxis in these experiments was conducted as detailed earlier. All migration results are reported as fold migration compared with CTRLs.
Myocardial ischemia and cardiac fibroblast isolation experiments
Six C57/B6 mice were utilized in the myocardial hypoxia experiments. Hearts were excised and canulated for the Langendorff apparatus, using perfusion buffer containing 4.7 mM K+ and 1.8 mM Ca++. The CTRLs were allowed to beat for 50 min in warmed and oxygenated perfusion buffer and then removed, and left ventricles were flash frozen in liquid nitrogen. The ischemic hearts were hung and allowed to beat for 5 or 6 min with flow in the warmed and oxygenated perfusion buffer; then, the flow was stopped for 30 min, and the hearts were bathed in the warmed buffer. After ischemia, flow was restarted and the hearts started beating in about 0.5–1 min after onset of flow and continued beating for 20 min to simulate reperfusion injury. The left ventricles from ischemic hearts were then flash frozen as detailed earlier. Frozen myocardial samples were utilized for the real time-polymerase chain reaction (RQ-PCR) experiments.
Cardiac fibroblasts were isolated from C57/B6 mice after sacrificing mice and isolating the hearts. Left ventricular tissues were minced into small pieces (less than 1 mm in diameter) using razor blades. The minced left ventricular tissues were incubated with HBSS solution (Invitrogen) containing glucose, NaCl, KCl, and NaHCO3; and supplemented with diaspase and collagenase B (Roche, Indiannapolis, IN) for 30 min followed by washing twice. Cell pellets were incubated overnight in Dulbecco's modified Eagle medium (Invitrogen) supplemented with 10% FBS (Thermoscientific, Waltham, MA) overnight. Floating cells were washed, and adherent cells were allowed to grow to 70%–80% confluence. Hypoxia experiments were conducted in hypoxia incubators, and cells were maintained at <1% O2, 5% CO2, and 37°C for either 2 h followed by 1 h reperfusion or 72 h hypoxia followed by reperfusion. Cells were then harvested and flash frozen for RQ-PCR.
Statistical analysis
Data are expressed as mean±standard error of the means. Differences were analyzed using the unpaired Student t-test or analysis of variance (one way or multiple comparisons) as appropriate. Post hoc multiple comparison procedures were performed using 2-sided Dunnett or Dunn tests as appropriate with CTRL samples as the CTRL category. The significance level throughout the analyses was chosen to be 0.05. All statistical analyses were performed using the SPSS (version 16) statistical software (SPSS, Inc., Chicago, IL). All authors had full access to and took full responsibility for the integrity of the data. All authors have read and agreed with the article as written.
Results
Concurrent temporal elevation of plasma S1P and C1P levels and non-HSCs numbers in PB after AMI
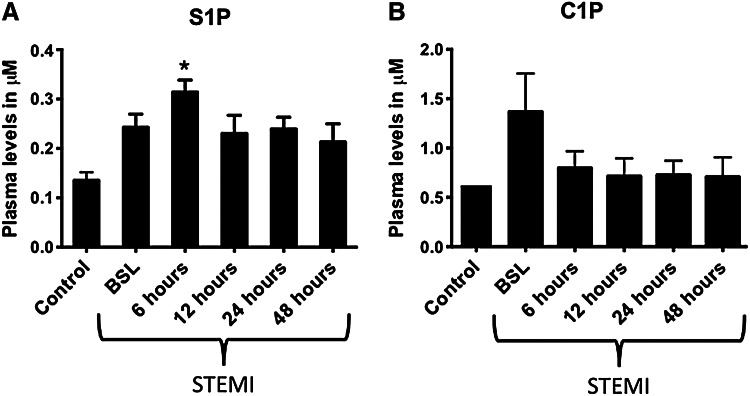
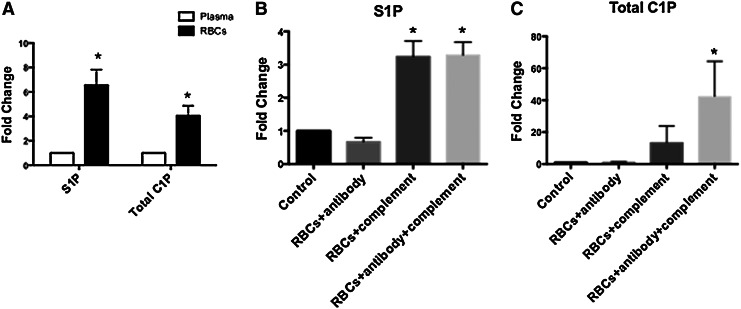
Our recent studies demonstrated the critical role of bioactive lipids such as S1P ands C1P in the mobilization and homing of BM-derived HSCs [27,28]. Since we expect that similar mechanisms are involved in the release of non-HSCs from their BM niches in AMI, we examined the changes in S1P levels in PB plasma after acute myocardial injury and as compared with CTRLs. Figure 1 shows that, in comparison to CTRLs, the levels of the bioactive lipid S1P were elevated after AMI, particularly in the early phases after myocardial ischemia and started decreasing after successful revascularization. The level of S1P increased 3 fold to 0.31±0.02 μM as compared with 0.14±0.02 in CTRLs (P<0.01). S1P levels then decreased after 6 h from the acute event but continued to be elevated at 48 h compared with CTRLs (Fig. 1). In whole blood, RBCs and platelets contain significantly higher levels of S1P and C1P compared with plasma (Fig. 2A). Release of S1P and CIP from RBCs could, therefore, account for higher plasma levels in the setting of AMI. Activation of CC and the resultant generation of C5b-C9 (membrane attack complex) may enhance release of S1P from RBCs. The CC was activated in AMI patients [44–48], and in our AMI population, C5b-C9 levels were elevated in serum (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/scd). On incubation with activated CC components, RBCs released S1P and C1P in a manner that could account for the elevated plasma levels of S1P and C1P in plasma of AMI patients (Fig. 2B, C). The higher plasma levels at BSL may also reflect the release of C1P from damaged cardiomyocytes as recently shown in the murine model of acute heart ischemia [48]. However, the role of C1P in non-HSC mobilization requires further studies, given the limited and transient increase in its levels and somehow the lack of temporal correlation with the number of mobilized non-HSCs. It is important to mention that there are no receptors identified for C1P, and, thus, receptor knockout or receptor-blocking experiments are not possible at this time. Nonetheless, our recent data in mice suggest an important cooperative role of bioactive lipids in the egress from BM of endothelial progenitor cells (EPCs), mesenchymal stem cells (MSCs), and VSELs and the subsequent homing of these circulating cells to the damaged heart tissue [26].
FIG. 1.
Elevated levels of bioactive lipids at early times after AMI. Bar graphs showing the plasma levels of sphingosine-1 phosphate (A) and ceramide-1 phosphate (B) in patients with STEMI and controls; showing peak levels early in STEMI patients. (*P<0.05 as compared with controls). AMI, acute myocardial infarction; BSL, baseline denoting the time of arrival to the hospital; C1P, ceramide-1 phosphate; S1P, sphingosine-1 phosphate; STEMI, ST-elevation myocardial infarction.
FIG. 2.
RBCs contain high concentrations of bioactive lipids that are released on complement activation. Bar graphs showing the content of S1P in the plasma and purified RBCs (A). Content of S1P and C1P is significantly higher in the RBCs compartment compared with plasma. On incubation with activated complement and activated complement together with antibodies against RBCs, cells release both S1P (B) and C1P (C). (*P<0.05 as compared with plasma or control). C1P, ceramide-1 phosphate; RBCs, red blood cells; S1P, sphingosine-1 phosphate.
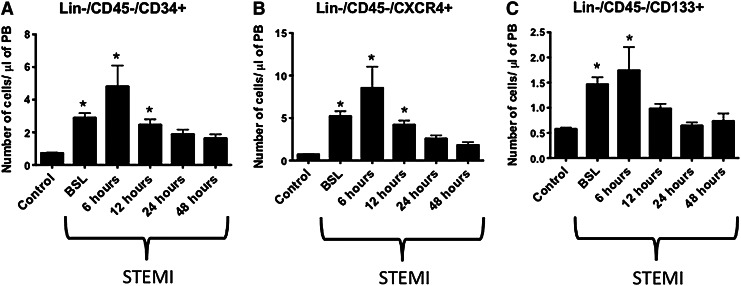
We observed a temporal correlation between the elevation in plasma levels of bioactive lipids and the peak mobilization of non-HSCs after acute myocardial injury (Fig. 3A–C). The absolute numbers of non-HSCs populations such as Lin−/CD45−/CD34+, Lin−/CD45−/CXCR+, and Lin−/CD45−/CD133+ cells peaked at the early stages after myocardial ischemia (4.8±1.3 vs. 0.7±0.04 cells/μL of PB, 8.6±2.5 vs. 0.7±0.06 cells/μL of PB, and 1.7±0.5 vs. 0.6±0.03 cells/μL of PB 6 h after presentation in STEMI patients vs. CTRLs respectively, P<0.05). This mobilization correlated with the early elevation of plasma levels of S1P and total C1P, thus suggesting a chemotactic role for bioactive lipids.
FIG. 3.
Temporal increase in number of circulating BMSPCs at early times after AMI. Bar graphs showing the absolute numbers of circulating non-hematopeitic stem cells (Lin−/CD45−/CD34+ (A), CXCR4+ (B), and CD133+ (C)) enriched in very small embryonic-like stem cells in the PB of AMI patients and controls; showing a peak mobilization early in STEMI patients. (*P<0.05 as compared with controls). PB, peripheral blood; BMSPCs, bone marrow stem and progenitor cells.
BM-derived stem cells express S1P receptors
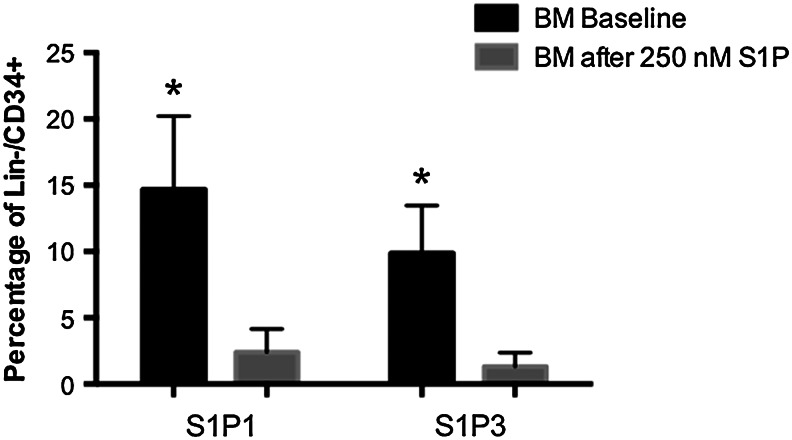
Given the potential role of bioactive lipids in the mobilization of non-HSCs, we examined the expression of various S1P receptors (S1Prs) on the surface of bone marrow stem and progenitor cells (BMSPCs) isolated from normal individuals and found high expression of type 1 and type 3 S1P receptors—S1Pr1 and S1Pr3 respectively (Fig. 4). These receptors play an important role in trafficking of lympho/hematopoietic cells [49,50].
FIG. 4.
S1P receptors are expressed in naïve BMSPCs, and surface expression is reduced after exposure to S1P. Bar graphs showing the expression of S1P receptor 1 and 3 on the surface of BM-derived Lin−/CD34+ stem cells. The graphs show a relatively higher expression of S1P receptors in the BM stem cells that are reduced significantly after 2 h exposure to S1P (250 nM) (*P<0.05 as compared with controls). BM, bone marrow.
We focused on the subset of BM-derived cells Lin−/CD34+ cells that are enriched in stem and progenitor cells, and flow cytometry analysis revealed that Lin−/CD34+ express both S1Pr1 (14.7%±1.4%) and S1Pr3 (9.9%±0.9%) (Fig. 4). Furthermore and as described with lympho/hematopoietic cells [51,52], this expression was dynamic, and both receptors were internalized in the presence of S1P (Fig. 4). The expression of S1Prs on PB lineage-negative cells in the early phases after AMI was significantly lower than in their counterparts isolated from the BM (data not shown). The increased level of plasma S1P could explain the possible internalization of S1Prs on circulating peripheral blood stem and progenitor cells (PBSPCs). Of note, since C1P receptors are not yet identified, we could not perform similar receptor expression studies for this bioactive lipid.
BM-derived stem cells migrate toward plasma isolated from acute myocardial ischemia patients in an S1P-dependent manner
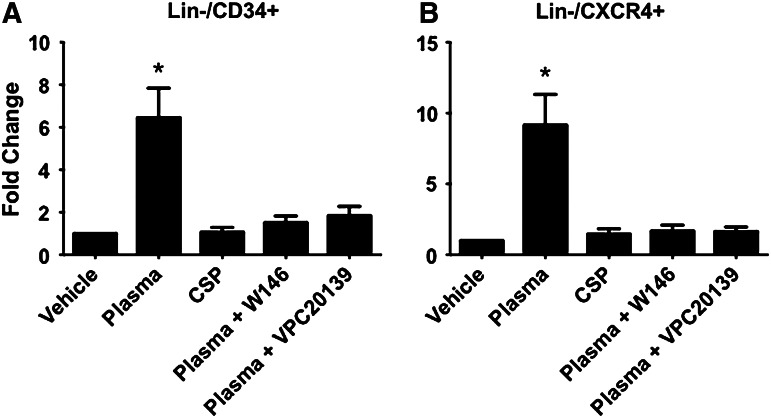
Since the plasma levels of S1P were elevated simultaneously with the peak mobilization of non-HSCs at 6 h after revascularization, we sought to examine the role of bioactive lipids in the migration of BMSPCs using in vitro migration assays. Figure 5A and B shows that Lin−/CD34+ and Lin−/CXCR4+ BMSCs migrate toward intact plasma isolated from STEMI patients at the peak mobilization of non-HSCs. This migration was inhibited by delipidation of the plasma (charcoal stripping), which removed >90% of the S1P (Supplementary Fig. S2). In addition, pretreatment of BM cells with the selective S1Pr1 antagonist, W146 (10 μM), or the S1Pr1/S1Pr3 receptor antagonist, VPC23019 (10 μM) also significantly reduced the migration of BM cells toward plasma from AMI patients. Neither W146 nor VC23019 altered cell viability (Supplementary Fig. S3). These data in total support a potential role for bioactive lipids present in the plasma of STEMI patients in the mobilization and egress of stem cells from the BM to the PB. Our data demonstrate multiple mechanisms responsible for stem cell mobilization and homing in ischemic heart injury. For instance, we noted increased expression of CXCR4 on mobilized PB endothelial cells and cardiac fibroblasts under hypoxic conditions that may facilitate these phenomena (data not shown). Furthermore, exposure to S1P at 250 nM increases the expression of CXCR4 on circulating mobilized EPCs, which suggest an important interplay between bioactive lipids and CXCR4 that can orchestrate stem cell mobilization and homing in ischemic conditions as has been recently shown [53].
FIG. 5.
BMSPCs migrate toward plasma from AMI patients in an S1P/S1P receptor-dependent fashion. Bar graphs showing the migration of lineage-negative (Lin−)/CD34+ and CXCR4-positive cells toward plasma isolated from patients with AMI. As shown, Lin−/CD34+ (A) and Lin−/CXCR4+ (B) cells migrate toward plasma isolated from AMI patients at peak stem cell mobilization (*P<0.05 as compared with controls). This mobilization was blunted by CSP and the use of selective S1P receptor 1 blocker (W146) and S1P receptor 1 and 3 selective blocker (VPC20139), where the number of migrated BM stem cells was not significantly different from vehicle. CSP, charcoal stripped plasma; Lin−, lineage-negative cells.
Ischemic cardiac tissues express antimicrobial cationic peptides cathelicidin and β-2 defensin that enhance responsiveness of circulating in PB cells to SDF-1 gradient
Our data highlighted the potential role of S1P and the dynamic expression of its receptors in the chemotaxis of non-HSCs from the BM to the PB in the setting of AMI (Figs. 1–5). However, the reduced expression of S1Pr1 and S1Pr3 after exposure to S1P levels similar to those encountered in the PB (∼250 μM) in the early phases after AMI (Fig. 4) suggest that other mechanisms than those involving bioactive lipids may be involved in their homing from PB to the myocardium. To support this further, previous experiments have indicated that S1P lyase activity is increased in the ischemic myocardium, thus leading to S1P degradation, lower local levels of S1P, and myocardial injury [54]. Therefore, it is unlikely that S1P will play a major role in the homing of circulating non-HSCs to myocardium.
Several reports suggest the involvement of the SDF-1 that is up-regulated in HIF-1α manner in infarcted myocardium in homing of stem cells to damaged heart tissues [55]. However, since infracted myocardium is enriched in proteolytic enzymes [eg, metalloproteinases—matrix metalloproteinases (MMPs)] [56] that degrade SDF-1 [35,36], its actual chemotactic gradient is usually low. As we have recently demonstrated, despite SDF-1 degradation by MMPs, the chemotactic responsiveness of stem cells to even low SDF-1 gradient could be significantly enhanced by members of the family of CAMPs, including products of CC activation–anaphylatoxin C3a [57] and fibroblast- and leukocyte-derived cathelicidin and β-2 defensin [41]. Given the above data, we investigated the expression and potential role of CAMPs in homing of non-HSCs into infracted myocardium.
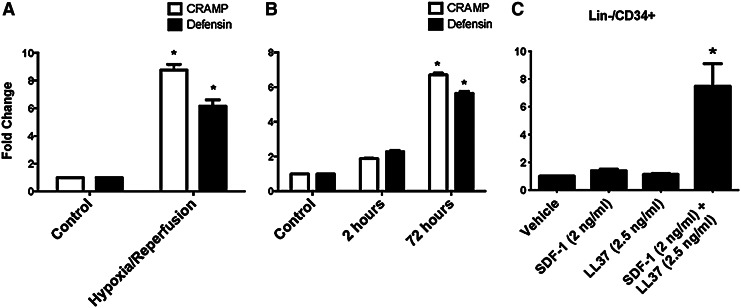
Our assessment of the CC activation indicates that the time course of this process correlated with our observation of the peak elevation of S1P (Supplementary Fig. S1). Thus, activation of CC may simultaneously release C3a in the plasma that enhances responsiveness of BM cells to low SDF-1 gradient. Next, we investigated whether the myocardium expresses 2 other CAMPs (cathelicidin and β-2 defensin) whose expression is regulated by hypoxia. To address this question, we first employed the langendorff apparatus, and murine cardiac ventricular tissues were subjected to ischemic/reperfusion injury (30 min ischemia followed by 20 min reperfusion). Figure 6A shows increased expression of both CAMPs in hypoxia/reperfusion as compared with CTRL myocardium. We then examined cardiac fibroblasts subjected to hypoxia using a hypoxic chamber, and the expression of cathelicidin and β-2 defensin was measured in parallel with the expression of HIF-1α. Figure 6B shows that both CAMPs were up-regulated in hypoxia. Finally, we employed Transwell chemotactic assays to examine the hypothesis that human PBSPCs respond to a low gradient of SDF-1 in the presence of cathelicidin, which was similar to our previous observations with HSCs [41]. Figure 6C shows that LL37 enhanced the migration of PB-derived Lin−/CD34+ cells toward low SDF-1 gradient that is ineffective in inducing this migration by itself. Interestingly, neither C3a alone as shown in the past [39] nor LL37 alone, as demonstrated here, was effective in inducing measurable migration of PB-derived stem cells.
FIG. 6.
Cardiomyocytes increase the expression of cathelicidins after ischemic injury, and cathelicidins prime BMSPCs to lower levels of SDF1. Bar graphs showing the increased mRNA expression of murine CRAMP and Defensin in murine cardiac tissues subjected to 30 min hypoxia followed by 20 min reperfusion as compared with controls (A). The expression of CRAMP and Defensin was also increased in cardiac fibroblasts subjected to 72 h of hypoxia in hypoxic chambers followed by reperfusion as compared with 2 h of hypoxia and controls (B) (*P<0.05 as compared with controls). (C) Demonstrates bar graphs for the migration of lineage-negative (Lin−)/CD34+-positive cells toward RPMI medium supplemented with 0.1% fetal bovine serum alone (Vehicle), supplemented with SDF-1 (2 ng/mL) alone, LL37 (2.5 ng/mL) alone, or SDF-1 in addition to LL37. As shown, Lin−/CD34+ cells migrate in significantly higher numbers toward SDF-1 in the presence of the priming factor LL37 (*P<0.05 as compared with controls). CRAMP, cathelicidins related antimicrobial protein; SDF-1, stromal derived factor-1.
Discussion
AMI initiates multiple innate reparatory mechanisms, including the activation of the CC that is responsible for the release of bioactive lipids such as S1P and C1P from their natural reservoirs in RBCs, platelets, and local endothelial cells. In this study, we identify an important role of bioactive lipids in the mobilization of BMSPCs into the PB after AMI. We also demonstrate that AMI results in increased expression of cathelicidins in the myocardium, resulting in a potential role in priming circulating mobilized PBSPCs to physiological SDF-1 levels and, hence, potentially improving their homing to the ischemic myocardium. Taken together, these data suggest that while the levels of SDF-1 in the myocardial tissue may be influenced by the elevated levels of proteases, an increase in CAMPs level enhances the responsiveness of non-HSCs to SDF-1 gradient and may potentially be aiding in their homing. These findings underscore the therapeutic potential of strategies targeting the modulation of bioactive lipids, cathelicidins, and their receptors in BMSPCs-based myocardial regenerative studies.
It is well known that S1P is transported in PB mainly by erythrocytes and is also associated with albumin and high-density lipoprotein [58,59]. Our data show a 4–7-fold higher content of S1P and C1P in erythrocytes compared with plasma (Fig. 2). Erythrocytes can take up and release S1P, and this buffering function likely explains the ∼×25 higher concentration of S1P in PB as compared with tissues. Innate immune system activation after ischemic myocardial injury, including the CC [44–48], may play an important role in the release of S1P from blood cells such as activated platelets [60–62], RBCs [58,63], and endothelial cells [28,64]. We demonstrate for the first time that plasma levels of S1P and C1P are significantly elevated after AMI (Fig. 1). The elevated plasma levels of these bioactive lipids correlated with the activation of the CC as evidenced by the elevated plasma levels of C5b-9 (Supplementary Fig. S1). Furthermore, exposure of erythrocytes to activated complement ex-vivo results in the release of bioactive lipids (Fig. 2), which could explain the correlation between elevated plasma levels of S1P in the setting of AMI and systemic complement activation.
The number of circulating hematopoietic stem and progenitor cells increases in PB in response to systemic or local inflammation, strenuous exercise and stress, tissue/organ injury, and pharmacological agents. We and others have demonstrated the mobilization of BMSPCs, including VSELs, in PB shortly after acute myocardial injury [17–19,65–67]. The utilization of BM-derived populations enriched in VSELs has been previously examined in animal and human studies [68–70]. During these experiments, the regenerative capacity of VSELs has been shown to improve the left ventricular function after myocardial infarction. In the human study, the benefit was observed mostly in patients with reduced left ventricular function at baseline. Furthermore, in our initial studies characterizing VSELs, we demonstrated the cardiac differentiation capacity of BM-derived VSELs in vitro [10,71].
It is, however, important to mention that the surface markers outlined in our study are not unique to any given population of BM- or PB-derived stem cells. CD34+ and CD133+ cells isolated from the PB have been shown to differentiate into endothelial cells in vitro and home to ischemic limb in animal in vivo models [72–74]. EPCs are mobilized in the setting of ischemic cardiac injury in correlation with elevated levels of vascular endothelial growth factor (VEGF) and express CD34, CD133, VEGF receptor 2, and CXCR4 [17,18,75]. Thus, these non-HSCs represent multiple overlapping subpopulations of stem/progenitor cells such as EPCs, MSCs, and VSELs that are capable of repopulating the injured heart and aid directly or indirectly [76,77] in its regeneration.
Overall, the mobilization process has been postulated to be directed by a decrease in SDF-1–CXCR4 and VLA-4–VCAM-1 interactions in BM, reversal of the trans-endothelial chemotactic gradient between the BM microenvironment and plasma, activation of the coagulation cascade, and, finally, as recently postulated, activation of CC [27,28,40]. Interestingly, many of the earlier mentioned mechanisms and cells are activated in the setting of AMI. The CC is activated locally at sites of myocardial infarction with elevated levels of C5b-9 both in the myocardium and plasma [48]. Recent evidence suggests the role of C5b-9 and other members of the CC in the mobilization and homing of BM stem cells [28,38,39]. We demonstrate the activation of the CC in the plasma after AMI. Our experiments also indicate that the exposure of peripheral RBCs to activated complement results in the release of bioactive lipids and, thus, can explain the temporal correlation between the elevated levels of C5b-9 and bioactive lipids in the plasma of patients with AMI (Fig. 2). Taken together, these data support our hypotheses that AMI activates the CC, which, in turn, activates the release of bioactive lipids from RBCs.
Recently, S1P and C1P have been shown to be important mediators in the signaling cascades involved in apoptosis/survival, proliferation, stress responses, and cell trafficking [78,79]. However, there are no data on the role of bioactive lipids in the mobilization of BMSPCs in IHD. The data shown here for the first time support an important role of bioactive lipids in the mobilization of BMSPCs after MI. We noted an elevated level of S1P and C1P in the plasma of MI patients shortly after the onset of myocardial ischemia. These levels showed a temporal correlation with the increased numbers of circulating BMSPCs, suggesting a role of bioactive lipids in this mobilization (Figs. 1 and 3). Furthermore, plasma samples isolated from AMI patients at peak BMSPCs mobilization were capable of chemoattracting BMSPCs in migration assays, a phenomenon that was blocked by delipidation of the plasma and selective S1Pr1 antagonists (Fig. 5).
On the other hand, assessing the role of C1P in this phenomenon will be more challenging, as there are no known receptor knockout or blocking agents for C1P. However, our recent data from murine models demonstrate an increase in C1P level in the myocardium after hypoxic injury and chemotactic responsiveness of BM-derived EPCS, MSCs, and VSELs to C1P gradient. These data support an involvement of this bioactive lipid in regeneration of damaged tissues [48]. Thus, our findings extend the role of bioactive lipids in myocardial ischemia and mobilization of BMSPCs into PB. However, we are aware that more work is needed to demonstrate in in vivo models that damaged myocardium may chemoattract circulating PB non-HSC in a bioactive lipid-dependent manner. To address this question, our previous data as well as the literature demonstrate a reduction in S1P in the myocardium after AMI secondary to the up-regulation of S1P lyase (data not shown). On the other, since C1P is elevated in the myocardium after ischemia/reperfusion [48], this suggests its potential role in homing of circulating BMSPCs to the ischemic myocardium [26,80].
Studies mobilizing BM derived cells using granulocyte colony stimulating factor or transplanting BM-derived cells after ischemia-induced damage have faced limited engraftment and modest success [21,24,81–85]. Evidence from animal studies demonstrate that BM cells mobilized in the setting of AMI home to the myocardium but differentiate at very low rates to cardiomyocytes [86]. Further evidence suggests that paracrine factors released from BMSCs such as cKit+ cells recruit and stimulate resident cardiac stem cells to proliferate, differentiate, and repair the myocardium after ischemic injury [87]. Regardless of the mechanisms of benefit, better engraftment of the transplanted cells is needed. After myocardial infarction, there is an elevation in the MMPs at the site of infarction as early as few hours after the acute event [56]. Elevated MMPs have been shown to degrade traditional chemokines such as SDF-1 [35] and monocyte chemoattractant protein [36] among others, thus lowering their chemoattractant activity. Recent evidence suggests the role of cathelicidins in priming BM-derived HSCs migration to lower levels of SDF-1 and their contribution to HSCs homing to the BM after irradiation injury [41]. We demonstrate that CAMPs are overexpressed after myocardial ischemia in cardiac tissues as well as cardiac fibroblasts. On the same note, the human CAMP-LL37 primes mobilized PBSPCs isolated from patients following AMI to low, yet physiological, levels of SDF-1 (2 ng/mL) (Fig. 6). This is in agreement with other studies which showed that pre-incubating endothelial progenitor cells with LL37 enhanced their homing and recruitment to areas of hind limb ischemia and the process of neorevascularization [88]. Taken together, these data support a potential novel role for CAMPs in the homing of BMSPCs to the ischemic myocardium. These findings may have important therapeutic implications in planning future BMSPCs-based myocardial regenerative studies. Since the members of the innate immune system have been successfully employed to enhance homing of HSCs into BM [37,89,90], we are currently examining similar pathways in enhancing the homing of transplanted BM-derived stem cells into the ischemic myocardium after AMI in animal models.
In conclusion, this study highlights the potential role of bioactive lipids and cathelicidin in the mobilization and homing of BM derived cells to the ischemic myocardium with their potential role in cardiomyocyte chimerism. Multiple new therapies that modulate the plasma levels of S1P or S1Pr expression are approved by the FDA and can be utilized in improving the mobilization of BM-derived stem cells in myocardial ischemia. Similarly, priming of BM-derived cells with LL37 or a product of the activated CC such as C3a can be used to improve their homing to the ischemic myocardium and, thus, overcome a major hurdle in stem cell regenerative myocardial therapies. We are currently examining both strategies in our laboratory to improve the mobilization and homing of BMSPCs to the ischemic myocardium.
Supplementary Material
Acknowledgments
Funding Sources—Drs. Abdel-Latif, Karapetyan and Ziada and their work are supported in part by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1TR000117. Dr. Smyth is supported by the NIH grant (UL1TR000117), the Heart Lung and Blood Institute (R01HL078663). This material is also based on work supported in part by resources at the Lexington VA Medical Center. Dr. Ratajczak is supported by NIH 2R01 DK074720, Maestro grant 2011/02/A/NZ4/00035 and Stella and Henry Hoenig Endowment. Dr. Morris is supported by NIH grants HL078663, GM050388, 1P20RR021954, and UL1RR033173.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Roger VL. Go AS. Lloyd-Jones DM. Adams RJ. Berry JD. Brown TM. Carnethon MR. Dai S. de Simone G, et al. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braunwald E. Bristow M. Congestive heart failure: fifty years of progress. Circulation. 2000;102:IV14–IV23. doi: 10.1161/01.cir.102.suppl_4.iv-14. [DOI] [PubMed] [Google Scholar]
- 3.McMurray J. Pfeffer M. Heart failure. Lancet. 2005;365:1877–1889. doi: 10.1016/S0140-6736(05)66621-4. [DOI] [PubMed] [Google Scholar]
- 4.Levy D. Kenchaiah S. Larson MG. Benjamin EJ. Kupka MJ. Ho KK. Murabito JM. Ramachandran VS. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1442–1444. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 5.Roger VL. Weston SA. Redfield MM. Hellermann-Homan JP. Killian J. Yawn BP. Jacobsen SJ. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 6.Deb A. Wang S. Skelding KA. Miller D. Simper D. Caplice NM. Bone marrow-derived cardiomyocytes are present in adult human heart: a study of gender-mismatched bone marrow transplantation patients. Circulation. 2003;107:1247–1249. doi: 10.1161/01.cir.0000061910.39145.f0. [DOI] [PubMed] [Google Scholar]
- 7.Quaini F. Urbanek K. Beltrami AP. Finato N. Beltrami CA. Nadal-Ginard B. Kajstura J. Leri A. Anversa P. Chimerism of the transplanted heart. N Engl J Med. 2002;346:5–15. doi: 10.1056/NEJMoa012081. [DOI] [PubMed] [Google Scholar]
- 8.Rupp S. Koyanagi M. Iwasaki M. Bauer J. von Gerlach S. Schranz D. Zeiher AM. Dimmeler S. Characterization of long-term endogenous cardiac repair in children after heart transplantation. Eur Heart J. 2008;29:1867–1872. doi: 10.1093/eurheartj/ehn223. [DOI] [PubMed] [Google Scholar]
- 9.Kucia M. Halasa M. Wysoczynski M. Baskiewicz-Masiuk M. Moldenhawer S. Zuba-Surma E. Czajka R. Wojakowski W. Machalinski B. Ratajczak M. Morphological and molecular characterization of novel population of CXCR4+ SSEA-4+ Oct-4+ very small embryonic-like cells purified from human cord blood: preliminary report. Leukemia. 2007;21:297–303. doi: 10.1038/sj.leu.2404470. [DOI] [PubMed] [Google Scholar]
- 10.Kucia M. Reca R. Campbell FR. Zuba-Surma E. Majka M. Ratajczak J. Ratajczak MZ. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006;20:857–869. doi: 10.1038/sj.leu.2404171. [DOI] [PubMed] [Google Scholar]
- 11.Kucia M. Wysoczynski M. Ratajczak J. Ratajczak M. Identification of very small embryonic like (VSEL) stem cells in bone marrow. Cell Tissue Res. 2008;331:125–134. doi: 10.1007/s00441-007-0485-4. [DOI] [PubMed] [Google Scholar]
- 12.Bergmann O. Bhardwaj RD. Bernard S. Zdunek S. Barnabe-Heider F. Walsh S. Zupicich J. Alkass K. Buchholz BA, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsieh PC. Segers VF. Davis ME. MacGillivray C. Gannon J. Molkentin JD. Robbins J. Lee RT. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13:970–974. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Weger RA. Verbrugge I. Bruggink AH. van Oosterhout MM. de Souza Y. van Wichen DF. Gmelig-Meyling FH. de Jonge N. Verdonck LF. Stem cell-derived cardiomyocytes after bone marrow and heart transplantation. Bone Marrow Transplant. 2008;41:563–569. doi: 10.1038/sj.bmt.1705939. [DOI] [PubMed] [Google Scholar]
- 15.Abdel-Latif A. Zuba-Surma EK. Ziada KM. Kucia M. Cohen DA. Kaplan AM. Van Zant G. Selim S. Smyth SS. Ratajczak MZ. Evidence of mobilization of pluripotent stem cells into peripheral blood of patients with myocardial ischemia. Exp Hematol. 2010;83:1131–1142. doi: 10.1016/j.exphem.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lev E. Kleiman N. Birnbaum Y. Harris D. Korbling M. Estrov Z. Circulating endothelial progenitor cells and coronary collaterals in patients with non-ST segment elevation myocardial infarction. J Vasc Res. 2005;42:408–414. doi: 10.1159/000087370. [DOI] [PubMed] [Google Scholar]
- 17.Massa M. Rosti V. Ferrario M. Campanelli R. Ramajoli I. Rosso R. De Ferrari G. Ferlini M. Goffredo L. et al A. Increased circulating hematopoietic and endothelial progenitor cells in the early phase of acute myocardial infarction. Blood. 2005;105:199–206. doi: 10.1182/blood-2004-05-1831. [DOI] [PubMed] [Google Scholar]
- 18.Shintani S. Murohara T. Ikeda H. Ueno T. Honma T. Katoh A. Sasaki K. Shimada T. Oike Y. Imaizumi T. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103:2776–2779. doi: 10.1161/hc2301.092122. [DOI] [PubMed] [Google Scholar]
- 19.Wojakowski W. Tendera M. Kucia M. Zuba-Surma E. Paczkowska E. Ciosek J. Halasa M. Krol M. Kazmierski M, et al. Mobilization of bone marrow-derived Oct-4+ SSEA-4+ very small embryonic-like stem cells in patients with acute myocardial infarction. J Am Coll Cardiol. 2009;53:1–9. doi: 10.1016/j.jacc.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wojakowski W. Tendera M. Zebzda A. Michalowska A. Majka M. Kucia M. Maslankiewicz K. Wyderka R. Król M, et al. Mobilization of CD34(+), CD117(+), CXCR4(+), c-met(+) stem cells is correlated with left ventricular ejection fraction and plasma NT-proBNP levels in patients with acute myocardial infarction. Eur Heart J. 2006;27:283–289. doi: 10.1093/eurheartj/ehi628. [DOI] [PubMed] [Google Scholar]
- 21.Abdel-Latif A. Bolli R. Zuba-Surma EK. Tleyjeh IM. Hornung CA. Dawn B. Granulocyte colony-stimulating factor therapy for cardiac repair after acute myocardial infarction: a systematic review and meta-analysis of randomized controlled trials. Am Heart J. 2008;156:216–226. doi: 10.1016/j.ahj.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valgimigli M. Rigolin G. Cittanti C. Malagutti P. Curello S. Percoco G. Bugli A. Della P. Bragotti L, et al. Use of granulocyte-colony stimulating factor during acute myocardial infarction to enhance bone marrow stem cell mobilization in humans: clinical and angiographic safety profile. Eur Heart J. 2005;26:1838–1845. doi: 10.1093/eurheartj/ehi289. [DOI] [PubMed] [Google Scholar]
- 23.Zohlnhofer D. Dibra A. Koppara T. de Waha A. Ripa RS. Kastrup J. Valgimigli M. Schomig A. Kastrati A. Stem cell mobilization by granulocyte colony-stimulating factor for myocardial recovery after acute myocardial infarction: a meta-analysis. J Am Coll Cardiol. 2008;51:1429–1437. doi: 10.1016/j.jacc.2007.11.073. [DOI] [PubMed] [Google Scholar]
- 24.Zohlnhöfer D. Ott I. Mehilli J. Schömig K. Michalk F. Ibrahim T. Meisetschläger G. von Wedel J. Bollwein H, et al. Stem cell mobilization by granulocyte colony-stimulating factor in patients with acute myocardial infarction: a randomized controlled trial. JAMA. 2006;295:1003–1010. doi: 10.1681/01.asn.0000926832.82033.c5. [DOI] [PubMed] [Google Scholar]
- 25.Kucia M. Jankowski K. Reca R. Wysoczynski M. Bandura L. Allendorf DJ. Zhang J. Ratajczak J. Ratajczak MZ. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol Histol. 2004;35:233–245. doi: 10.1023/b:hijo.0000032355.66152.b8. [DOI] [PubMed] [Google Scholar]
- 26.Kim C. Schneider G. Abdel-Latif A. Mierzejewska K. Sunkara M. Borkowska S. Ratajczak J. Morris AJ. Kucia M. Ratajczak MZ. Ceramide-1-phosphate regulates migration of multipotent stromal cells (MSCs) and endothelial progenitor cells (EPCs) - implications for tissue regeneration. Stem Cells. 2012 doi: 10.1002/stem.1291. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ratajczak MZ. Kim CH. Abdel-Latif A. Schneider G. Kucia M. Morris AJ. Laughlin MJ. Ratajczak J. A novel perspective on stem cell homing and mobilization: review on bioactive lipids as potent chemoattractants and cationic peptides as underappreciated modulators of responsiveness to SDF-1 gradients. Leukemia. 2012;26:63–72. doi: 10.1038/leu.2011.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ratajczak MZ. Lee H. Wysoczynski M. Wan W. Marlicz W. Laughlin MJ. Kucia M. Janowska-Wieczorek A. Ratajczak J. Novel insight into stem cell mobilization-plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex. Leukemia. 2010;24:976–985. doi: 10.1038/leu.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Juarez JG. Harun N. Thien M. Welschinger R. Baraz R. Dela Pena A. Pitson SM. Rettig M. Dipersio JF. Bradstock KF. Bendall LJ. Sphingosine-1-phosphate facilitates trafficking of hematopoietic stem cells and their mobilization by CXCR4 antagonists in mice. Blood. 2011;119:707–716. doi: 10.1182/blood-2011-04-348904. [DOI] [PubMed] [Google Scholar]
- 30.Ceradini DJ. Kulkarni AR. Callaghan MJ. Tepper OM. Bastidas N. Kleinman ME. Capla JM. Galiano RD. Levine JP. Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 31.Lakkisto P. Kyto V. Forsten H. Siren JM. Segersvard H. Voipio-Pulkki LM. Laine M. Pulkki K. Tikkanen I. Heme oxygenase-1 and carbon monoxide promote neovascularization after myocardial infarction by modulating the expression of HIF-1alpha, SDF-1alpha and VEGF-B. Eur J Pharmacol. 2010;635:156–164. doi: 10.1016/j.ejphar.2010.02.050. [DOI] [PubMed] [Google Scholar]
- 32.Youn SW. Lee SW. Lee J. Jeong HK. Suh JW. Yoon CH. Kang HJ. Kim HZ. Koh GY, et al. COMP-Ang1 stimulates HIF-1alpha-mediated SDF-1 overexpression and recovers ischemic injury through BM-derived progenitor cell recruitment. Blood. 2011;117:4376–4386. doi: 10.1182/blood-2010-07-295964. [DOI] [PubMed] [Google Scholar]
- 33.Agarwal U. Ghalayini W. Dong F. Weber K. Zou YR. Rabbany SY. Rafii S. Penn MS. Role of cardiac myocyte CXCR4 expression in development and left ventricular remodeling after acute myocardial infarction. Circ Res. 2010;107:667–676. doi: 10.1161/CIRCRESAHA.110.223289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim CH. Wu W. Wysoczynski M. Abdel-Latif A. Sunkara M. Morris A. Kucia M. Ratajczak J. Ratajczak MZ. Conditioning for hematopoietic transplantation activates the complement cascade and induces a proteolytic environment in bone marrow: a novel role for bioactive lipids and soluble C5b-C9 as homing factors. Leukemia. 2012;26:106–116. doi: 10.1038/leu.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McQuibban GA. Butler GS. Gong JH. Bendall L. Power C. Clark-Lewis I. Overall CM. Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J Biol Chem. 2001;276:43503–43508. doi: 10.1074/jbc.M107736200. [DOI] [PubMed] [Google Scholar]
- 36.McQuibban GA. Gong JH. Wong JP. Wallace JL. Clark-Lewis I. Overall CM. Matrix metalloproteinase processing of monocyte chemoattractant proteins generates CC chemokine receptor antagonists with anti-inflammatory properties in vivo. Blood. 2002;100:1160–1167. [PubMed] [Google Scholar]
- 37.Lee HM. Wysoczynski M. Liu R. Shin DM. Kucia M. Botto M. Ratajczak J. Ratajczak MZ. Mobilization studies in complement-deficient mice reveal that optimal AMD3100 mobilization of hematopoietic stem cells depends on complement cascade activation by AMD3100-stimulated granulocytes. Leukemia. 2010;24:573–582. doi: 10.1038/leu.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jalili A. Marquez-Curtis L. Shirvaikar N. Wysoczynski M. Ratajczak M. Janowska-Wieczorek A. Complement C1q enhances homing-related responses of hematopoietic stem/progenitor cells. Transfusion. 2010;50:2002–2010. doi: 10.1111/j.1537-2995.2010.02664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jalili A. Shirvaikar N. Marquez-Curtis L. Qiu Y. Korol C. Lee H. Turner AR. Ratajczak MZ. Janowska-Wieczorek A. Fifth complement cascade protein (C5) cleavage fragments disrupt the SDF-1/CXCR4 axis: further evidence that innate immunity orchestrates the mobilization of hematopoietic stem/progenitor cells. Exp Hematol. 2010;38:321–332. doi: 10.1016/j.exphem.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ratajczak MZ. Reca R. Wysoczynski M. Kucia M. Baran JT. Allendorf DJ. Ratajczak J. Ross GD. Transplantation studies in C3-deficient animals reveal a novel role of the third complement component (C3) in engraftment of bone marrow cells. Leukemia. 2004;18:1482–1490. doi: 10.1038/sj.leu.2403446. [DOI] [PubMed] [Google Scholar]
- 41.Wu W. Kim CH. Liu R. Kucia M. Marlicz W. Greco N. Ratajczak J. Laughlin MJ. Ratajczak MZ. The bone marrow-expressed antimicrobial cationic peptide LL-37 enhances the responsiveness of hematopoietic stem progenitor cells to an SDF-1 gradient and accelerates their engraftment after transplantation. Leukemia. 2012;26:736–745. doi: 10.1038/leu.2011.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mathews TP. Kennedy AJ. Kharel Y. Kennedy PC. Nicoara O. Sunkara M. Morris AJ. Wamhoff BR. Lynch KR. Macdonald TL. Discovery, biological evaluation, and structure-activity relationship of amidine based sphingosine kinase inhibitors. J Med Chem. 2010;53:2766–2778. doi: 10.1021/jm901860h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Selim S. Sunkara M. Salous AK. Leung SW. Berdyshev EV. Bailey A. Campbell CL. Charnigo R. Morris AJ. Smyth SS. Plasma levels of sphingosine 1-phosphate are strongly correlated with haematocrit, but variably restored by red blood cell transfusions. Clin Sci (London) 2011;121:565–572. doi: 10.1042/CS20110236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arumugam TV. Shiels IA. Woodruff TM. Granger DN. Taylor SM. The role of the complement system in ischemia-reperfusion injury. Shock. 2004;21:401–409. doi: 10.1097/00024382-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 45.Bjerre M. Hansen TK. Flyvbjerg A. Complement activation and cardiovascular disease. Horm Metab Res. 2008;40:626–634. doi: 10.1055/s-0028-1083786. [DOI] [PubMed] [Google Scholar]
- 46.Ren G. Dewald O. Frangogiannis NG. Inflammatory mechanisms in myocardial infarction. Curr Drug Targets Inflamm Allergy. 2003;2:242–256. doi: 10.2174/1568010033484098. [DOI] [PubMed] [Google Scholar]
- 47.Riedemann NC. Ward PA. Complement in ischemia reperfusion injury. Am J Pathol. 2003;162:363–367. doi: 10.1016/S0002-9440(10)63830-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sumitra M. Manikandan P. Nayeem M. Manohar BM. Lokanadam B. Vairamuthu S. Subramaniam S. Puvanakrishnan R. Time course studies on the initiation of complement activation in acute myocardial infarction induced by coronary artery ligation in rats. Mol Cell Biochem. 2005;268:149–158. doi: 10.1007/s11010-005-3856-8. [DOI] [PubMed] [Google Scholar]
- 49.Mandala S. Hajdu R. Bergstrom J. Quackenbush E. Xie J. Milligan J. Thornton R. Shei GJ. Card D. Keohane C. Rosenbach M. Hale J. Lynch CL. Rupprecht K. Parsons W. Rosen H. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 50.Sanna MG. Liao J. Jo E. Alfonso C. Ahn MY. Peterson MS. Webb B. Lefebvre S. Chun J. Gray N. Rosen H. Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem. 2004;279:13839–13848. doi: 10.1074/jbc.M311743200. [DOI] [PubMed] [Google Scholar]
- 51.Sensken SC. Graler MH. Down-regulation of S1P1 receptor surface expression by protein kinase C inhibition. J Biol Chem. 2010;285:6298–6307. doi: 10.1074/jbc.M109.049692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thangada S. Khanna KM. Blaho VA. Oo ML. Im DS. Guo C. Lefrancois L. Hla T. Cell-surface residence of sphingosine 1-phosphate receptor 1 on lymphocytes determines lymphocyte egress kinetics. J Exp Med. 2010;207:1475–1483. doi: 10.1084/jem.20091343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Golan K. Vagima Y. Ludin A. Itkin T. Cohen-Gur S. Kalinkovich A. Kollet O. Kim C. Schajnovitz A, et al. S1P promotes murine progenitor cell egress and mobilization via S1P1-mediated ROS signaling and SDF-1 release. Blood. 2012;119:2478–2488. doi: 10.1182/blood-2011-06-358614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bandhuvula P. Honbo N. Wang GY. Jin ZQ. Fyrst H. Zhang M. Borowsky AD. Dillard L. Karliner JS. Saba JD. S1P lyase: a novel therapeutic target for ischemia-reperfusion injury of the heart. Am J Physiol Heart Circ Physiol. 2011;300:H1753–H1761. doi: 10.1152/ajpheart.00946.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karshovska E. Zernecke A. Sevilmis G. Millet A. Hristov M. Cohen CD. Schmid H. Krotz F. Sohn HY, et al. Expression of HIF-1alpha in injured arteries controls SDF-1alpha mediated neointima formation in apolipoprotein E deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:2540–2547. doi: 10.1161/ATVBAHA.107.151050. [DOI] [PubMed] [Google Scholar]
- 56.Peterson JT. Li H. Dillon L. Bryant JW. Evolution of matrix metalloprotease and tissue inhibitor expression during heart failure progression in the infarcted rat. Cardiovasc Res. 2000;46:307–315. doi: 10.1016/s0008-6363(00)00029-8. [DOI] [PubMed] [Google Scholar]
- 57.Ratajczak J. Reca R. Kucia M. Majka M. Allendorf DJ. Baran JT. Janowska-Wieczorek A. Wetsel RA. Ross GD. Ratajczak MZ. Mobilization studies in mice deficient in either C3 or C3a receptor (C3aR) reveal a novel role for complement in retention of hematopoietic stem/progenitor cells in bone marrow. Blood. 2004;103:2071–2078. doi: 10.1182/blood-2003-06-2099. [DOI] [PubMed] [Google Scholar]
- 58.Hanel P. Andreani P. Graler MH. Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J. 2007;21:1202–1209. doi: 10.1096/fj.06-7433com. [DOI] [PubMed] [Google Scholar]
- 59.Okajima F. Plasma lipoproteins behave as carriers of extracellular sphingosine 1-phosphate: is this an atherogenic mediator or an anti-atherogenic mediator? Biochim Biophys Acta. 2002;1582:132–137. doi: 10.1016/s1388-1981(02)00147-6. [DOI] [PubMed] [Google Scholar]
- 60.Schaphorst KL. Chiang E. Jacobs KN. Zaiman A. Natarajan V. Wigley F. Garcia JG. Role of sphingosine-1 phosphate in the enhancement of endothelial barrier integrity by platelet-released products. Am J Physiol Lung Cell Mol Physiol. 2003;285:L258–L267. doi: 10.1152/ajplung.00311.2002. [DOI] [PubMed] [Google Scholar]
- 61.Yatomi Y. Ohmori T. Rile G. Kazama F. Okamoto H. Sano T. Satoh K. Kume S. Tigyi G. Igarashi Y. Ozaki Y. Sphingosine 1-phosphate as a major bioactive lysophospholipid that is released from platelets and interacts with endothelial cells. Blood. 2000;96:3431–3438. [PubMed] [Google Scholar]
- 62.Yatomi Y. Ruan F. Hakomori S. Igarashi Y. Sphingosine-1-phosphate: a platelet-activating sphingolipid released from agonist-stimulated human platelets. Blood. 1995;86:193–202. [PubMed] [Google Scholar]
- 63.Pappu R. Schwab SR. Cornelissen I. Pereira JP. Regard JB. Xu Y. Camerer E. Zheng YW. Huang Y. Cyster JG. Coughlin SR. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 64.Venkataraman K. Lee YM. Michaud J. Thangada S. Ai Y. Bonkovsky HL. Parikh NS. Habrukowich C. Hla T. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ Res. 2008;102:669–676. doi: 10.1161/CIRCRESAHA.107.165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grundmann F. Scheid C. Braun D. Zobel C. Reuter H. Schwinger R. Müller-Ehmsen J. Differential increase of CD34, KDR/CD34, CD133/CD34 and CD117/CD34 positive cells in peripheral blood of patients with acute myocardial infarction. Clin Res Cardiol. 2007;96:621–627. doi: 10.1007/s00392-007-0543-7. [DOI] [PubMed] [Google Scholar]
- 66.Kucia M. Dawn B. Hunt G. Guo Y. Wysoczynski M. Majka M. Ratajczak J. Rezzoug F. Ildstad ST. Bolli R. Ratajczak MZ. Cells expressing early cardiac markers reside in the bone marrow and are mobilized into the peripheral blood following myocardial infarction. Circ Res. 2004;95:1191–1199. doi: 10.1161/01.RES.0000150856.47324.5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leone A. Rutella S. Bonanno G. Abbate A. Rebuzzi A. Giovannini S. Lombardi M. Galiuto L. Liuzzo G, et al. Mobilization of bone marrow-derived stem cells after myocardial infarction and left ventricular function. Eur Heart J. 2005;26:1196–1204. doi: 10.1093/eurheartj/ehi164. [DOI] [PubMed] [Google Scholar]
- 68.Dawn B. Tiwari S. Kucia MJ. Zuba-Surma EK. Guo Y. Sanganalmath SK. Abdel-Latif A. Hunt G. Vincent RJ, et al. Transplantation of bone marrow-derived very small embryonic-like stem cells attenuates left ventricular dysfunction and remodeling after myocardial infarction. Stem Cells. 2008;26:1646–1655. doi: 10.1634/stemcells.2007-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tendera M. Wojakowski W. Ruzyllo W. Chojnowska L. Kepka C. Tracz W. Musialek P. Piwowarska W. Nessler J, et al. Intracoronary infusion of bone marrow-derived selected CD34+ CXCR4+ cells and non-selected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction: results of randomized, multicentre Myocardial Regeneration by Intracoronary Infusion of Selected Population of Stem Cells in Acute Myocardial Infarction (REGENT) Trial. Eur Heart J. 2009;30:1313–1321. doi: 10.1093/eurheartj/ehp073. [DOI] [PubMed] [Google Scholar]
- 70.Zuba-Surma EK. Guo Y. Taher H. Sanganalmath SK. Hunt G. Vincent RJ. Kucia M. Abdel-Latif A. Tang XL, et al. Transplantation of expanded bone marrow-derived very small embryonic-like stem cells (VSEL-SCs) improves left ventricular function and remodelling after myocardial infarction. J Cell Mol Med. 2011;15:1319–1328. doi: 10.1111/j.1582-4934.2010.01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wojakowski W. Tendera M. Kucia M. Zuba-Surma E. Milewski K. Wallace-Bradley D. Kazmierski M. Buszman P. Hrycek E, et al. Cardiomyocyte differentiation of bone marrow-derived Oct-4+CXCR4+SSEA-1+ very small embryonic-like stem cells. Int J Oncol. 2010;37:237–247. doi: 10.3892/ijo_00000671. [DOI] [PubMed] [Google Scholar]
- 72.Asahara T. Kawamoto A. Endothelial progenitor cells for postnatal vasculogenesis. Am J Physiol Cell Physiol. 2004;287:C572–C579. doi: 10.1152/ajpcell.00330.2003. [DOI] [PubMed] [Google Scholar]
- 73.Asahara T. Murohara T. Sullivan A. Silver M. van der Zee R. Li T. Witzenbichler B. Schatteman G. Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 74.Peichev M. Naiyer AJ. Pereira D. Zhu Z. Lane WJ. Williams M. Oz MC. Hicklin DJ. Witte L. Moore MA. Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 75.Brehm M. Ebner P. Picard F. Urbien R. Turan G. Strauer BE. Enhanced mobilization of CD34(+) progenitor cells expressing cell adhesion molecules in patients with STEMI. Clin Res Cardiol. 2009;98:477–486. doi: 10.1007/s00392-009-0021-5. [DOI] [PubMed] [Google Scholar]
- 76.Ratajczak J. Kucia M. Mierzejewska K. Marlicz W. Pietrzkowski Z. Wojakowski W. Greco NJ. Tendera M. Ratajczak MZ. Paracrine proangiopoietic effects of human umbilical cord blood-derived purified CD133(+) cells-implications for stem cell therapies in regenerative medicine. Stem Cells Dev. 2013;22(3):422–430. doi: 10.1089/scd.2012.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ratajczak MZ. Kucia M. Jadczyk T. Greco NJ. Wojakowski W. Tendera M. Ratajczak J. Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies? Leukemia. 2012;26:1166–1173. doi: 10.1038/leu.2011.389. [DOI] [PubMed] [Google Scholar]
- 78.Arana L. Gangoiti P. Ouro A. Trueba M. Gomez-Munoz A. Ceramide and ceramide 1-phosphate in health and disease. Lipids Health Dis. 2010;9:15. doi: 10.1186/1476-511X-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chalfant CE. Spiegel S. Sphingosine 1-phosphate and ceramide 1-phosphate: expanding roles in cell signaling. J Cell Sci. 2005;118:4605–4612. doi: 10.1242/jcs.02637. [DOI] [PubMed] [Google Scholar]
- 80.Cui J. Engelman RM. Maulik N. Das DK. Role of ceramide in ischemic preconditioning. J Am Coll Surg. 2004;198:770–777. doi: 10.1016/j.jamcollsurg.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 81.Abdel-Latif A. Bolli R. Tleyjeh I. Montori V. Perin E. Hornung C. Zuba-Surma E. Al-Mallah M. Dawn B. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 82.Assmus B. Honold J. Schachinger V. Britten M. Fischer-Rasokat U. Lehmann R. Teupe C. Pistorius K, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 83.Engelmann M. Theiss H. Hennig-Theiss C. Huber A. Wintersperger B. Werle-Ruedinger A. Schoenberg S. Steinbeck G. Franz W. Autologous bone marrow stem cell mobilization induced by granulocyte colony-stimulating factor after subacute ST-segment elevation myocardial infarction undergoing late revascularization: final results from the G-CSF-STEMI (granulocyte colony-stimulating factor st-segment elevation myocardial infarction) trial. J Am Coll Cardiol. 2006;48:1712–1721. doi: 10.1016/j.jacc.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 84.Fan L. Chen L. Chen X. Fu F. A meta-analysis of stem cell mobilization by granulocyte colony-stimulating factor in the treatment of acute myocardial infarction. Cardiovasc Drugs Ther. 2007;22:45–54. doi: 10.1007/s10557-007-6072-9. [DOI] [PubMed] [Google Scholar]
- 85.Perin EC. Silva GV. Zheng Y. Gahremanpour A. Canales J. Patel D. Fernandes MR. Keller LH. Quan X, et al. Randomized, double-blind pilot study of transendocardial injection of autologous aldehyde dehydrogenase-bright stem cells in patients with ischemic heart failure. Am Heart J. 2012;163:415–421e1. doi: 10.1016/j.ahj.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 86.Fukuhara S. Tomita S. Nakatani T. Yutani C. Kitamura S. Endogenous bone-marrow-derived stem cells contribute only a small proportion of regenerated myocardium in the acute infarction model. J Heart Lung Transplant. 2005;24:67–72. doi: 10.1016/j.healun.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 87.Loffredo FS. Steinhauser ML. Gannon J. Lee RT. Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell Stem Cell. 2011;8:389–398. doi: 10.1016/j.stem.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pfosser A. El-Aouni C. Pfisterer I. Dietz M. Globisch F. Stachel G. Trenkwalder T. Pinkenburg O. Horstkotte J, et al. NF kappaB activation in embryonic endothelial progenitor cells enhances neovascularization via PSGL-1 mediated recruitment: novel role for LL37. Stem Cells. 2010;28:376–385. doi: 10.1002/stem.280. [DOI] [PubMed] [Google Scholar]
- 89.Lee HM. Wu W. Wysoczynski M. Liu R. Zuba-Surma EK. Kucia M. Ratajczak J. Ratajczak MZ. Impaired mobilization of hematopoietic stem/progenitor cells in C5-deficient mice supports the pivotal involvement of innate immunity in this process and reveals novel promobilization effects of granulocytes. Leukemia. 2009;23:2052–2062. doi: 10.1038/leu.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wysoczynski M. Reca R. Lee H. Wu W. Ratajczak J. Ratajczak MZ. Defective engraftment of C3aR−/− hematopoietic stem progenitor cells shows a novel role of the C3a-C3aR axis in bone marrow homing. Leukemia. 2009;23:1455–1461. doi: 10.1038/leu.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.