Abstract
Objective
Significant evidence supports a genetic contribution to the development of posttraumatic stress disorder (PTSD). Three previous studies have demonstrated an association between PTSD and the nine repeat allele of the 3′ untranslated region (3′UTR) variable number tandem repeat (VNTR) in the dopamine transporter (DAT, rs28363170). Recently a novel, functionally significant C/T single-nucleotide polymorphism (SNP) in the 3′UTR (rs27072) with putative interactions with the 3′VNTR, has been identified. To provide enhanced support for the role of DAT and striatal dopamine regulation in the development of PTSD, this study examined the impact of a haplotype defined by the C allele of rs27072 and the nine repeat allele of the 3′VNTR on PTSD diagnosis in young trauma-exposed children.
Methods
DAT haplotypes were determined in 150 trauma-exposed 3–6 year-old children. PTSD was assessed with a semistructured interview. After excluding double heterozygotes, analysis was performed on 143 total subjects. Haplotype was examined in relation to categorical and continuous measures of PTSD, controlling for trauma type and race. Additional analysis within the two largest race categories was performed, as other means of controlling for ethnic stratification were not available.
Results
The number of haplotypes (0, 1, or 2) defined by the presence of the nine repeat allele of rs28363170 (VNTR in the 3′UTR) and the C allele of rs27072 (SNP in the 3′UTR) was significantly associated with both the diagnosis of PTSD and total PTSD symptoms. Specifically, children with one or two copies of the haplotype had significantly more PTSD symptoms and were more likely to be diagnosed with PTSD than were children without this haplotype.
Conclusions
These findings extend previous findings associating genetic variation in the DAT with PTSD. The association of a haplotype in DAT with PTSD provides incremental traction for a model of genetic vulnerability to PTSD, a specific underlying mechanism implicating striatal dopamine regulation, and insight into potential future personalized interventions.
Introduction
Unfortunately, exposures to traumatic events in childhood are not rare, and often not random. It is estimated that one in four children will experience at least one traumatic event before adulthood, and that many children experience both multiple types of trauma as well as repeated exposure to the same traumatic event (Cohen 2010). Whereas some children seem resilient to traumatic experiences, a significant proportion of children, including very young children, develop posttraumatic stress disorder (PTSD).
Although family and twin studies have established a substantial genetic contribution to PTSD (True et al. 1993; Sack et al. 1995; Xian et al. 2000; Yehuda et al. 2001; Stein, et al. 2002) studies examining the impact of specific genes on the development of PTSD are limited. The majority of genes examined to date are associated with the hypothalamic pituitary adrenal axis (HPA), dopaminergic, and serotonergic neurotransmission (reviewed in Koenen 2007; Cornelis et al. 2010; Skelton et al. 2012). Replication studies of the association between PTSD and specific genes have been reported with four genes: the serotonin (SLC6A4), forkhead binding protein 5 (FKBP5), dopamine receptor 2 (DRD2), and the dopamine transporter (SLC6A3) (Comings et al. 1996; Segman et al. 2002; Lee et al. 2006; Kilpatrick et al. 2007; Binder et al. 2008; Drury et al. 2009; Koenen et al. 2009; Xie, et al. 2009; Voisey et al. 2009; Kolassa et al. 2010; Xie et al. 2010; Valente et al. 2011; Wang et al. 2011; Boscarino et al. 2012; Chang et al. 2012; Pietrzak et al. 2012). Failed replications also exist for these genes (Gelernter et al. 1999; Bailey et al. 2010; Goenjian et al. 2012) and potential genetic heterogeneity as a function of trauma type or ethnicity may exist (Xie et al. 2009).
Studies involving genes known to regulate dopamine metabolism may represent the strongest empirical candidates, as these associations are complimented by the evidence documenting alterations in dopamine and dopamine metabolism in individuals with PTSD (Segman, et al. 2000; Goldstein, et al. 2007; Koenen et al. 2011; Schulz-Heik et al. 2011). Research further suggests that cognitive processes, associated with dopaminergic function, specifically attention and executive function, are implicated in PTSD (Kimble, et al. 2000; Hopper et al. 2006; Gunnar and Quevedo 2007; Geuze et al. 2008; De Bellis et al. 2009; Bar-Haim, et al. 2010; Hauschildt, et al. 2011). Attentional hypervigilance for threat has been demonstrated experimentally in both adults and youths with PTSD (Bryant and Harvey 1997; Dalgleish et al. 2001). The mesocortical/mesoprefrontal dopaminergic system (from midbrain to prefrontal cortex) is preferentially activated by stress exposure, and is hypothesized to be related to the symptom of increased vigilance found in PTSD (Vermetten and Bremner 2002). Taken together, the evidence linking PTSD symptoms to alterations in circulating dopamine levels and associated deficits in executive function and attentional processes, provide a consistent neurogenetically informed model that supports further exploration of the association between PTSD and genes such as DAT that are known to regulate dopamine neurotransmission and similar neurocognitive processes (Ehlers and Clark 2000; Meiser-Stedman 2002; Aupperle et al. 2012).
The dopamine transporter (DAT) regulates the reuptake of dopamine into the presynaptic terminals, particularly in mesocorticolimbic and nigrostriatal pathways. A variable number tandem repeat (VNTR) polymorphism (rs28363170) is found in the 3′ untranslated region (3′UTR) of DAT. Individuals can have alleles ranging in length from 3 to 12 repeats, although the majority of individuals have either 9 or 10 repeats (Min Kang et al. 1999). The nine repeat allele has been associated with increased approach-avoidance behaviors and it is hypothesized that this allele may result in an elevated sensitivity, or detection, of emotional contextual cues (Enter et al. 2012). Four studies have now demonstrated an association between PTSD and the nine repeat allele of the DAT 3′VNTR (Segman et al. 2002; Drury et al. 2009; Valente et al. 2011; Chang et al. 2012). One additional study failed to replicate the association with DAT 3′VNTR; however, genotype was characterized differently in that study than in previous research, thus limiting the ability to compare results (Bailey et al. 2010).
The single-nucleotide polymorphism (SNP) rs27072 (C/T), with a minor allele (T) in high linkage disequilibrium with the 10 repeat allele of the 3′UTR, appears to impact DAT function, both independently and in conjunction with the 3′VNTR. Pinsonneault and colleagues demonstrated that this SNP influenced DAT mRNA levels in human tissue from the substantia nigra and impacted DAT mRNA levels in vitro when transfected in cis (i.e., on the same chromosome) with the 3′VNTR (Pinsonneault et al. 2011). The T (minor) allele has previously been associated with bipolar disorder, and two other studies have examined the rs27072 SNP and attention-deficit/hyperactivity disorder (ADHD) (Feng et al. 2005; Ouellet-Morin et al. 2008; Pinsonneault et al. 2011). No previous studies have examined this SNP, or any DAT haplotypes, in relation to PTSD. Given the in vitro and in vivo evidence cited, a DAT haplotype defined by both the 3′VNTR and rs27072 may more precisely reflect functional differences in DAT than the VNTR alone. If functional differences in DAT are involved in the pathophysiology of PTSD, this haplotype would be expected to more accurately predict PTSD than either variant alone (Heinz et al. 2000; Cheon et al. 2005), particularly as the functional significance of VNTR alleles other than the 9 or 10 are unknown.
We tested whether a functional haplotype, defined by the nine repeat allele of the 3′VNTR and the C allele of rs27072, is associated with PTSD in trauma-exposed, preschool-age children. We have previously reported an association between the nine repeat allele and PTSD in a subset of these same children who were exposed to Hurricane Katrina (Drury et al. 2009). In this report, we extend our findings in two ways. First, we examined whether a haplotype defined by these two polymorphisms, with putative functional significance, is associated with PTSD. Specifically, we hypothesized that individuals with two copies of the 9/C haplotype (i.e., C/C and 9/9) would have the highest risk for PTSD and the greatest number of total symptoms. Individuals with one copy of the haplotype would have an intermediate risk, and those lacking this haplotype would demonstrate the lowest risk. Second, we included children with different types of traumatic experiences (i.e., single event injuries and repeated domestic violence exposure), as our previous study was limited only to children exposed to Hurricane Katrina. We then tested whether the previous associations between DAT and PTSD, found in preschool-age survivors of Hurricane Katrina (Drury et al. 2009), adult survivors of combat (Segman et al. 2002) and adults exposed to urban violence and other traumas Valente et al. 2011; (Chang et al. 2012) could be replicated with this haplotype in preschool age children exposed to different traumatic experiences. The established association of DAT with increased risk for PTSD in multiple studies, across age and trauma exposure, combined with increasing evidence that suggests that genotype-based differences influence treatment response, underscores the need for an enhanced understanding of the role of DAT and dopamine neurotransmission in the etiology and manifestation, particularly the co-morbidity, of PTSD (Roessner et al. 2010; Froelich et al. 2011; Abraham et al. 2012).
Methods
Participants and procedure
We recruited 150 3–6 year-old children from an on-going study (R01 MH65884-01A1), 1 year after the study began (genetic data was not originally proposed). DNA was collected on consecutively enrolled subjects from January 2005 until enrollment ended in April 2008. Analysis was performed on 143 participants with appropriate haplotype and complete PTSD data (see Genotyping section for further details).
Inclusion criteria for the entire study were: 1) Having experienced at least one type of life-threatening trauma. Data were collected on traumatic exposure using 12 events from the Life Events section of the Preschool Age Psychiatric Assessment (PAPA) (Egger et al. 2006). Medical events counted if they involved major surgery or were invasive beyond a blood draw (e.g., voiding cystourethrogram or lumbar puncture). 2) Age between 36 and 83 months at the time of most recent trauma and time of enrollment. Exclusion criteria were: 1) Head trauma with Glasgow Coma Scale score ≤7 in the emergency room. 2) Mental retardation, autistic disorder, blindness, deafness, and coming from foreign language-speaking families.
Participants had experienced a variety of traumatic events. Twenty-four were identified from the registry of a level 1 trauma center and had experienced single trauma events such as motor vehicle accidents and accidental injuries (single trauma group). Twenty-two children, recruited from three battered women's programs in the New Orleans metropolitan area, had witnessed severe domestic violence (domestic violence). Ninety-seven children had experienced the Hurricane Katrina disaster (Katrina). Many children in the study had experienced more than one type of traumatic event and/or had had multiple exposures to particular types of trauma. One child had experienced both domestic violence and Hurricane Katrina; therefore, he was not included in either group. We had previously reported the association between carriers of the nine repeat allele of the DAT 3′VNTR and PTSD in subset of 88 children exposed to Hurricane Katrina (Drury et al. 2009).
The study was approved by the Tulane University Committee on Use of Human Subjects. Written informed consent was obtained from primary caregivers. Signed assent was not obtained from children because of their young age. Participants were monetarily compensated for their participation.
Genotyping
DNA was extracted from MasterAmp buccal swabs using Epicentre Biotechnologies MasterAmp DNA extraction solution (Illumina Inc., Madison, WI), following manufacturer's recommendations. Samples were run in duplicate with known controls. All genotyping was done blind to all outcomes (Heils et al. 1996). Genotyping for the DAT 3′VNTR was performed using polymerase chain reaction (PCR). PCR products were then electrophoresed through a 2% agarose gel using standard protocols. Allele determination was made based on the size of fragments compared with known genotypes and size standards (Michelhaugh et al. 2001). PCR was performed on a Bio-Rad C1000 thermal cycler (Bio-Rad Laboratories, Hercules, CA) using the 5' primer (forward): 5'-TGTGG TGTAGGGAACGGCCTGAG-3', 3' primer (reverse): 5'-CTTCC TGGAGGTCACGGCTCAAGG-3'. PCR was performed in a 50 μL reaction with 10 pmol of each primer, 1.25 U of Ex Taq™ DNA Polymerase (TaKara Bio Inc., Otsu, Shiga, Japan), 1x Ex Taq™ Buffer, 200 μmdNTPs. Thermal cycling conditions were an initial denaturation for 5 minutes followed by 40 cycles of 94°C for 30 seconds, 58°C for 30 seconds, and 72°C for 45 seconds. 10 μl of the final reaction was size fractionated on a 2% agarose gel with a standard 1 kb DNA ladder for size determination. Alleles ranged from 3 repeats to 12 repeats. Samples that failed to amplify fragment size consistently or failed to amplify were eliminated from further analysis. Genotype for rs27072 was determined using TaqMan SNP assay c___2396868_10 (Life Technologies, Carlsbad, CA). PCR was performed in 10 μl reactions, in triplicate on a Bio-Rad C1000 thermal cycler (Bio-Rad Laboratories, Hercules, CA) and thermal cycling conditions were 95°C for 10 minutes then 40 cycles of 92°C for 15 seconds and 60°C for 60 seconds. All samples were run with known controls. Genotypes were in Hardy–Weinberg equilibrium and consistent with previous studies.
Haplotypes were constructed based on nine repeat allele status of the 3′VNTR and C allele of the rs27072 (C/T) because of known functional interactions. The number of haplotypes was assigned based on the number of homozygote patterns. Specifically, individuals who were homozygous for both 9/9 and C/C were categorized as having two copies of the haplotype. Individuals who were heterozygous for the nine repeat allele and homozygous C/C were categorized as having one copy of the haplotype. Individuals who were heterozygous C/T and homozygous 9/9 were also categorized as having one copy of the haplotype. Double heterozygotes (9/* and C/T) were excluded because haplotype status could not be unambiguously assigned because parental DNA data were lacking (n=7). This resulted in 143 individuals for the final analysis. All remaining genotype combinations were categorized as 0 copies of the haplotype (Min Kang et al. 1999).
PTSD diagnosis and symptoms
PTSD diagnosis and symptom counts were assessed using the Preschool Age Psychiatric Assessment (PAPA). Test–retest reliability of the PAPA is comparable to structured psychiatric interviews used to assess older children and adults, with an intraclass correlation coefficient of 0.56 and a κ of 0.73 for the PTSD module (Egger et al. 2006). The PAPA interview was performed by trained research assistants who were blind to genotyping information. Initial interviewers were trained on the content and scoring of the PAPA by a trainer from Duke University, where the PAPA was created. Subsequent interviewers were trained on content and scoring rules by one of the local investigators (MS). New interviewers who joined the study first observed experienced interviewers give three interviews, then observed and coded two interviews given by experienced interviewers with whom they compared coding following the interviews. Then, they administered their first interview while being observed by a trainer, and were given immediate feedback. Next, the coding of every symptom of their next three interviews was completed with the advice of an experienced interviewer. Finally, throughout the study, the lead investigator (MS) met individually with interviewers weekly to watch their most symptomatic interviews on videotape in order to prevent drift, critique technique, and correct coding errors, with special attention focused on the criterion B, re-experiencing symptoms, and the two avoidance symptoms.
Data analysis
PTSD diagnosis by haplotype status was tested with χ2 tests. Number of PTSD symptoms by haplotype was tested using PROC GLM in SAS 9.2 (SAS Institute Inc., Cary, NC) controlling for trauma group and race. Gender, race, and age were examined for association with genotype. Analysis of variance examining symptoms specific to each PTSD criterion were also examined for association with haplotype. Post-hoc pairwise analysis was performed comparing the three haplotype groupings (0, 1, and 2 copies). Further analysis within the two largest race categories (black and white) was performed to examine potential confounding resulting from population stratification. Number of symptoms by genotype within the black and white subgroups was tested with nonparametric Wilcoxon rank sum test. Finally, analysis within the previously unexamined subjects with non-hurricane-related trauma was explored. Consistent with other exploratory studies, and the limited by the sample size when stratified by ethnic group, we did not correct for multiple comparisons.
Results
PTSD diagnosis was not associated with race, trauma group, age, or gender. Haplotype was not associated with trauma group, age, or gender. The frequency of the 9 repeat allele was 20% and the frequency of the 10 repeat allele was 80%. The allele frequency for the rs27072 was 88% for the C allele and 12% for the T allele. Haplotype frequencies are presented in Table 1. Race was significantly associated with haplotype (χ2 [df=4, n=143]=11.8, p=.02) and, therefore, all subsequent analysis controlled for race. Post-hoc analysis was also performed within each race subgroup, recognizing the impact on power and the influence of sample size.
Table 1.
Haplotype Distribution and Allele Frequency Across Demographic Categories
| 2 haplotypes n (%) | 1 haplotype n (%) | 0 haplotypes n (%) | p value | |
|---|---|---|---|---|
| Total, n=143 | 14 (10%) | 31 (22%) | 98 (69%) | |
| Race | ||||
| Black | 3 (2%) | 20 (14%) | 64 (45%) | 0.02 |
| White | 9 (6%) | 9 (6%) | 23 (16%) | |
| Other/Mixed | 2 (1%) | 2 (1%) | 11 (8%) | |
| Age in years | ||||
| Mean (SD) | 5.39 (1.21) | 5.20 (1.15) | 5.16 (1.03) | ns |
| Gender | ||||
| Male | 8 (6%) | 22 (15%) | 56 (39%) | |
| Female | 6 (4%) | 9 (6%) | 42 (29%) | ns |
Genetic association with PTSD diagnosis and symptoms
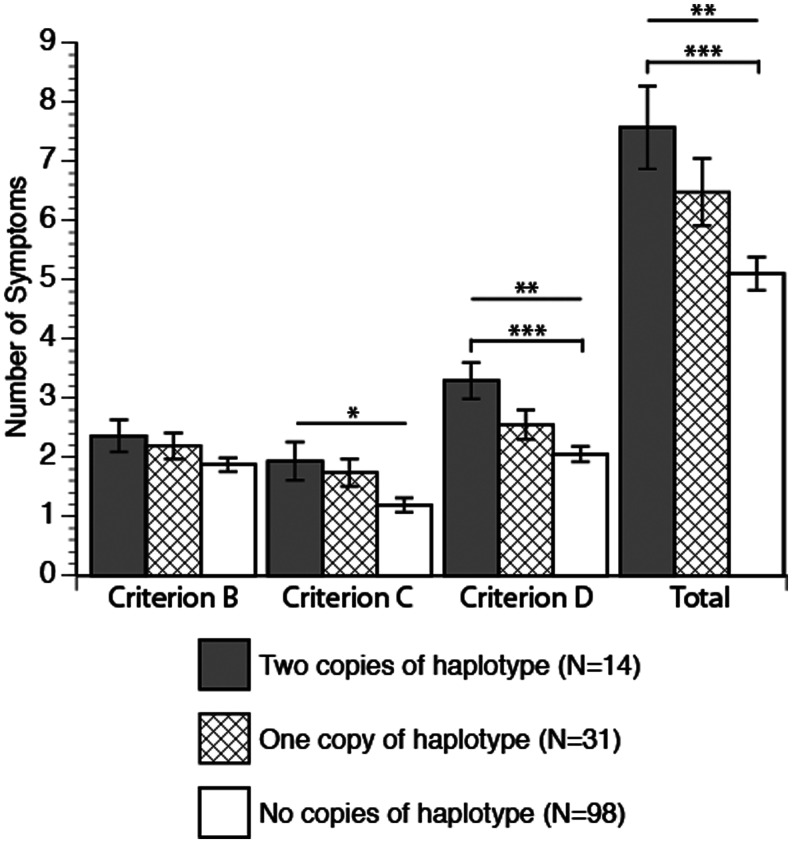
Haplotype status significantly predicted PTSD diagnosis. Individuals with two copies of the haplotype had the highest risk of diagnosis (79%), individuals with one haplotype had intermediate risk (58%), and individuals with no copies of the haplotype had the least risk (38%) (χ2 [df=2, n=143]=10.47, p=0.005) (Table 2). Haplotype was also significantly associated with the continuous measures (number of symptoms) of criterion C (r2=0.07, f[4]=2.56, p<0.05), criterion D (r2=0.09, f(4)=3.6, p<.008), and total PTSD symptoms (r2=.1, f [4]=3.81, p<0.006) (Fig. 1).
Table 2.
Haplotype Association with PTSD Diagnosis
| 2 haplotypes n (%) | 1 haplotype n (%) | 0 haplotypes n (%) | p value | |
|---|---|---|---|---|
| Total, n=143 | ||||
| Yes PTSD | 11 (8%) | 18 (13%) | 37 (26%) | 0.005 |
| No PTSD | 3 (2%) | 13 (9%) | 61 (43%) | |
| Black, n=87 | ||||
| Yes PTSD | 3 (2%) | 13 (9%) | 23 (16%) | 0.011 |
| No PTSD | 0 (0%) | 7 (5%) | 41 (29%) | |
| White, n=41 | ||||
| Yes PTSD | 6 (4%) | 4 (3%) | 8 (6%) | 0.263 |
| No PTSD | 3 (2%) | 5 (3%) | 15 (10%) | |
PTSD, posttraumatic stress disorder.
FIG. 1.
Posttraumatic stress disorder (PTSD) symptoms by DAT haplotype dosage. Significance indicated by ***p<0.004, **p<0.008, *p<0.04. Pairwise correlations indicated by capped bars.
As a significant difference in haplotype frequencies was detected between black and white race categories, analyses were also performed within each race category, black (n=87) and white (n=41). Other race categories did not contain sufficient numbers of individuals for meaningful analysis (n=15). Analysis within the black race category continued to find a statistically significant relationship between PTSD diagnosis and allele status (χ2 df=2, n=87=9.02, p=0.01). The diagnosis was present in 100% of those with two haplotypes, in 65% of those with one haplotype, and in 36% of those with no copies of the haplotype (Table 2). Analysis within the smaller white race subgroup did not find a statistically significant association between haplotype and PTSD diagnosis. Although the rates of diagnosis by haplotype were consistent with a pattern of increased risk in the white subgroup, with 67% diagnosed in those with two copies of the haplotype, 44% in those with one copy, and 35% in those with no haplotypes.
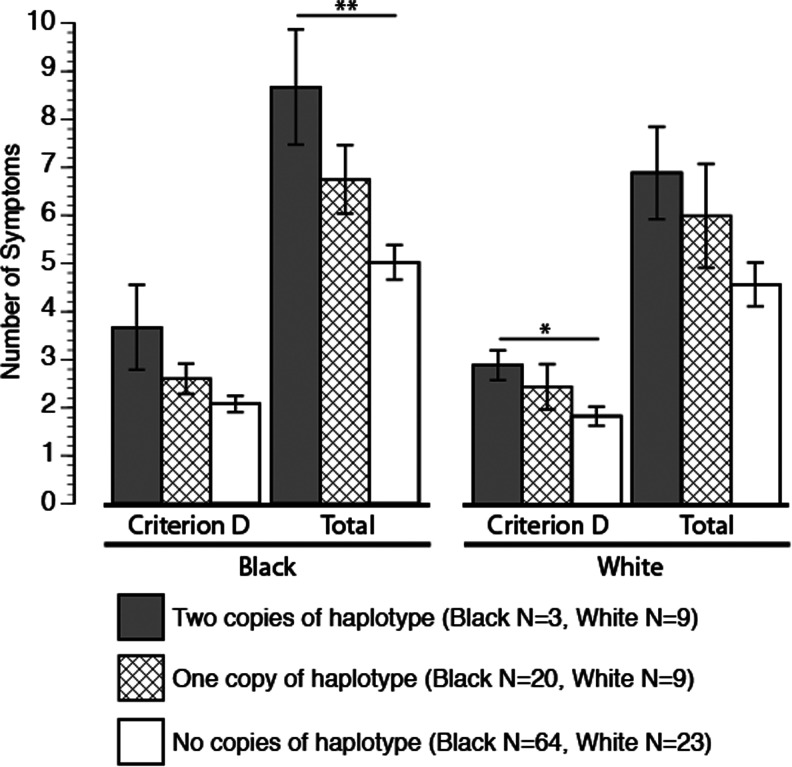
Number of haplotypes significantly predicted the total number of PTSD symptoms within the black subgroup (r2=0.09, f (2)=4.42, p<0.01) and approached significance with the total number of PTSD symptoms within the white subgroup (r2=0.13, f (2)=2.9, p<0.07) (Fig. 2). Haplotype was significantly associated with the number of criterion D symptoms within the white subgroup (r2=0.15, f(2)=3.59, p<0.04) and approached significance within the black subgroup (r2=0.07, f (2)=2.75, p<0.06).
FIG. 2.
Posttraumatic stress disorder (PTSD) criterion D and total symptoms within black and white racial groupings. Significance indicated by **p<0.02, *p<0.04.
As we have previously demonstrated an association between the DAT 9 repeat allele and PTSD in a subset of this sample exposed only to Hurricane Katrina (Drury et al. 2009), we sought to determine if this association would be maintained in children exposed to other trauma types who were not previously included in our initial study. In this subset of children, who were exposed to either repeated trauma as a result of witnessing domestic violence or a single incident traumatic event, we tested the association between DAT haplotype and PTSD diagnosis, controlling for ethnicity. In this subset (n=46), PTSD diagnosis continued to be significantly associated with DAT haplotype (χ2 [df=2, n=44]=7.5, p=0.02). In this subsample, 100% of children with two copies of the haplotype had PTSD, 71% of those with one copy had PTSD, and 37% of those without the haplotype had PTSD. Further stratification by ethnicity was not performed, because of the limited sample size.
Discussion
In this study, we replicate previous studies finding an association between allelic variation in DAT and the development of PTSD in trauma-exposed individuals. Further, the demonstration of this association with a putative functional haplotype provides increased evidence implicating functional differences in DAT, and subsequent aberrant dopaminergic signaling, in the phenotypic manifestations of PTSD. Although four previous studies have reported associations between the nine repeat allele of the 3′VNTR (rs28363170) and PTSD, conclusive evidence of the functional significance of this 3′VNTR remains elusive. Our findings replicate previous studies in both adults and children linking DAT and PTSD, extend the genetic relevance by utilizing a haplotype, and confirm that the association between PTSD and DAT crosses types of traumatic experiences. The consistency of this association between genetic variation in DAT and PTSD combined with the link to a neurobiologically informed hypothesis, represents a novel approach to countering the prevalence of failed replication studies in psychiatric genetic research. DAT haplotype accounted for <10% of the variance in PTSD symptoms consistent with most genetic studies of psychiatric illnesses, which suggests that single genes likely account for only a small amount of the variance in the development of psychopathology. This also suggests, however, that there remains a considerable amount of unaccounted variance. More complex models that incorporate cumulative genetic vulnerability or intermediate phenotypes may, therefore, be appropriate avenues of future exploration (Boscarino et al. 2011, 2012).
The 3′UTR, where both of these polymorphisms reside, is associated with subcellular localization and posttranslational modification of mRNA. The regulation of DAT expression levels by the 3′UTR is expected to be regulated in part by microRNAs (miRNA), noncoding RNAs that serve as rapid modifiers of mRNA function. Several miRNA seed sites, highly conserved DNA sequences critical for miRNA function, are located in the sequence surrounding the rs27072 (Pinsonneault et al. 2011). Studies examining functional differences between the 9 and 10 alleles of the 3′UTR VNTR alone have been inconsistent, suggesting that more complex regulatory processes controlling DAT protein levels and transporter function may be involved (Heinz et al. 2000, Van Dyck et al. 2005, van de Giessen et al. 2009). Most recently, Chang et al. demonstrated an association between PTSD and the 5′methylation status and nine allele of the 3′UTR VNTR in a large case control study suggesting that alternative regulatory mechanisms that influence gene expression may be critical for defining the association between DAT and PTSD (Chang et al. 2012). As the molecular evidence surrounding the regulation of DAT expression accumulates, more complex haplotype analysis, and the inclusion of epigenetic modifications including methylation and miRNA regulation, may provide even greater insight into this increasingly well-established relationship between DAT and PTSD.
Although the association between the number of DAT haplotypes was significant with total PTSD symptoms and criterion C symptoms, the association appears strongest with criterion D symptoms. Criterion D symptoms are conceptualized as increased arousal phenomena that include hypervigilance, concentration difficulties, irritability, exaggerated startle, and sleep disturbance. The underlying disturbance of these symptoms is thought to be due to individuals' preoccupations with trauma-related internalizations that preclude normative orienting, attention-shifting, and self-regulation. This model is consistent with research that has found increased attention to threat stimuli in individuals with PTSD compared to individuals without PTSD in samples of children and adolescents 9–17 years-old (Dalgleish et al. 2001) and adults (Bryant and Harvey 1997). As PTSD is associated with heightened attentional bias to threat, it follows that alterations in striatal dopamine levels, as a result of functional DAT polymorphic variants, would result in increased risk for the development of PTSD. However, as regulation of attention is caused by pulsatile releases of dopamine following exposure to a relevant stimuli, it is plausible that a more accurate model of the dopaminergic deficit in PTSD is not caused by too high or too low striatal dopamine, but rather by a deficiency in the control of the fluctuation of dopamine levels, which is needed as one discriminates between relevant and irrelevant stimuli. This model is consistent with the dopamine hypothesis in relation to prefrontal cortex functioning (Diamond et al. 2004; Diamond 2007) and is consistent with a model implicating miRNA regulation.
The impact of dopamine and DAT on attentional systems is also relevant for treatment considerations in PTSD with comorbid syndromes or problems, specifically ADHD. For example, it has been shown that ADHD, in which attention difficulties are paramount, is comorbid with PTSD in very young children 38% of the time (Scheeringa et al. 2003), and concern has been expressed that externalizing disruptive behaviors may often be misdiagnosed as ADHD when PTSD is the true underlying syndrome (Madras et al. 2006). Interestingly, methylphenidate, through its direct effect on striatal dopamine, via DAT, combined with the established role of striatal function in attention orienting and disengaging, may have a specific role, particularly during exposure treatment, in enhancing attentional disengagement needed for effective cognitive behavioral therapy (CBT) (Abraham et al. 2012). Further, as recent studies suggest that the response to stimulants, which primarily act on DAT, is impacted by genotype (Joober et al. 2006; Szobot et al. 2011), understanding how these genetic variants impact DAT function, dopamine regulation, and the mechanism of action for specific stimulants may identify not only a subset of individuals more likely to respond to a particularly treatment modality but also identify novel targets for PTSD treatment, including miRNAs. Whereas genetic studies of psychological disorders have previously been limited to identifying risk and resilience, the use of genetic studies to define underlying neurobiological mechanisms and inform treatment approaches represents a somewhat underutilized advantage of genetic studies, particularly those able to leverage existing pre-clinical and translational molecular genetic studies.
Limitations
Several limitations to this study exist. First, we a priori assigned haplotype status of the 9 and C alleles. Because we lacked parental DNA, we were unable to classify double heterozygotes without ambiguity. To account for this challenge, analysis was performed excluding double heterozygotes (n=7). Linkage disequilibrium between the T allele and the 10 repeat allele of the 3′VNTR does exist. Although alternative approaches for estimating haplotype status exist, we utilized this approach based on the known biological interaction, and believe it represents the most conservative estimation. Future studies with parental DNA that permit the accurate construction of haplotype status, as well as consideration of haplotypes spanning the entire gene, not simply the 3′UTR, are needed. A second limitation is the relatively small sample size of each race subgroup and the existence of statistically significant differences in haplotype frequency between the white and black race categories. Statistical analysis to adjust for race stratification has been performed in past studies to prevent false positive findings in studies when allele frequencies differ normatively between race groups. However, we did not detect differences in PTSD diagnosis or symptoms between our race categories. Additionally, analysis within the black subgroup continued to demonstrate a significant association with PTSD diagnosis. Although we did not obtain significant values with the PTSD diagnosis within the white subgroup, total symptoms approached significance, and criterion D symptoms remained significant, even given the small sample size (Hutchison et al. 2005), offering evidence that our results are not caused by population stratification. Although the determination of genetic admixture would have been ideal, additional genotyping information was not available in this sample. We acknowledge that stratification by ethnicity significantly decreased our power, but suggest that the continued significance of our findings strengthens our conclusions rather than detracting from them. Finally, this study includes both new children, exposed to single or repeated trauma, and the sample of preschool children, exposed to Hurricane Katrina, in which we previously reported an association between PTSD and only the nine repeat. However, analysis using only the previously unanalyzed children (n=46) continued to find a significant association between DAT haplotype status and PTSD. Analysis in this small subset did not permit stratification by ethnicity, but analysis was performed controlling for ethnicity. Finally, even though we tested the association between DAT haplotype and both PTSD diagnosis and symptoms clusters, we did not correct for multiple comparisons. Had Bonferoni corrections been applied, our initial findings, not stratified by ethnicity, would remain significant except for the association with cluster C symptoms. However, the smaller subsets (i.e., within each race group) would fail to reach statistical significance. Certainly, larger studies replicating these findings in preschool children, and those of other ages, is warranted.
Conclusions
A neurogenetic approach to psychiatric illnesses that leverages the power of replication in other samples, in vitro molecular studies; structural and functional imaging; and carefully defined phenotypes, race groups, and developmental ages may answer the challenges facing psychiatric genetics currently. Additionally, studies using this approach have significant potential to inform treatment options and begin to give neurobiological guidance to the selection of therapies and pharmacological agents.
Clinical Significance
Clinical applications of genetic findings in this early stage are somewhat remote. However, it is likely that the future of genetic studies will be found in the ability to ultimately identify vulnerable individuals and guide targeted interventions or preventive approaches for those most at risk following trauma exposure. Given the increasing numbers of studies demonstrating association between PTSD and DAT, traction is building in at least four areas related to PTSD, the underlying mechanisms leading to the development of psychopathology, and personalized approaches to interventions. First, the role of DAT in the regulatory control of dopamine, and the empirical relations between attentional control and PTSD, suggests that DAT variants may represent plausible biological indicators of both PTSD development and treatment response. Second, DAT is the main site of action of stimulant medications and the main regulator of dopamine levels in the striatum. Given the association of the alternative 10/10 genotype of DAT with ADHD (Shook et al. 2011) and the significant symptom overlap between ADHD and PTSD (i.e., difficulty concentrating, sleep disturbances, hypervigilant motor activities), the clarification of trauma exposure and consideration of the presence of PTSD in children unresponsive to stimulant treatment for ADHD is prudent, particularly in young children, in whom differentiating these symptoms may be most challenging (Heinz et al., 2000; Purper-Ouakil, et al. 2005; van de Giessen et al. 2009). Third, as stimulant medications and atomoxetine appear to alter dopamine transmission via different mechanisms, and may be differentially impacted by DAT polymorphisms, randomized trials examining differential response to these medications in children with comorbid PTSD and ADHD are warranted. Fourth, as DAT variants have the potential to identify one neurobiological path in the etiology, course, and treatment response of PTSD, these findings would be expected to contribute to the eventual development and implementation of genetically based personalized interventions.
Acknowledgments
We are grateful to the children and their parents who participated in this study.
Disclosures
No competing financial interests exist.
References
- Abraham A. Cunningham C. Lattal M. Methyphenidate enhances extinction of contextual fear. Learn Mem. 2012;19:67–72. doi: 10.1101/lm.024752.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aupperle R. Allard C. Grimes E. Simmons A. Flagan T. Behrooznia M. Cissell S. Twamley E. Thorp S. Norman S. Paulus M. Stein M. Dorsolateral prefrontal cortex activation during emotional anticipation and neuropsychological performance in posttraumatic stress disorder. Arch Gen Psychiatry. 2012;69:360–371. doi: 10.1001/archgenpsychiatry.2011.1539. [DOI] [PubMed] [Google Scholar]
- Bailey JN. Goenjian AK. Noble EP. Walling DP. Ritchie T. Goenjian HA. PTSD and dopaminergic genes, DRD2 and DAT, in multigenerational families exposed to the Spitak earthquake. Psychiatry Res. 2010;178:507–510. doi: 10.1016/j.psychres.2010.04.043. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y. Holoshitz Y. Eldar S. Frenkel TI. Muller D. Charney DS. Pine DS. Fox NA. Wald I. Life-threatening danger and suppression of attention bias to threat. Am J Psychiatry. 2010;167:694–698. doi: 10.1176/appi.ajp.2009.09070956. [DOI] [PubMed] [Google Scholar]
- Binder E. Bradley R. Kiu W. Epstein M. Deveau T. Mercer K. Tang Y. Gillespie C. Heim C. Nemeroff C. Schwartz A. Cubells J. Ressler K. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscarino J. Erlich P. Hoffman S. Rukstalis M. Stewart W. Association of FKBP5, COMT and CHRNA5 polymorphisms with PTSD among outpatients at risk for PTSD. Psychiatry Res. 2011;188:173–174. doi: 10.1016/j.psychres.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscarino J. Erlich P. Hoffman S. Zhang X. Higher FKBP5, COMT, CHRNA5, and CRHR1 allele burdens are associated with PTSD and interact with trauma exposure: Implications for neuropsychiatric research and treatment. Neuropsychiatr Dis Treat. 2012;8:131–139. doi: 10.2147/NDT.S29508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant R. Harvey A. Attentional bias in posttraumatic stress disorder. J Trauma Stress. 1997;10:635–644. doi: 10.1023/a:1024849920494. [DOI] [PubMed] [Google Scholar]
- Chang S-C. Koenen KC. Galea S. Aiello AE. Soliven R. Wildman DE. Uddin M. Molecular variation at the SLC6A3 locus predicts lifetime risk of PTSD in the Detroit Neighborhood Health Study. PLos ONE. 2012;7:e39184. doi: 10.1371/journal.pone.0039184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheon K. Ryu Y. Kim J. Cho D. The homozygosity for 10-repeat allele at dopamine transporter gene and dopamine transporter density in Korean children with attention deficit hyperactivity disorder: Relating to treatment response to methylphenidate. Eur Neuropsychopharmacol. 2005;15:95–101. doi: 10.1016/j.euroneuro.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Cohen J. Practice parameter for the assessment and treatment of children and adolescents with posttraumatic stress disorder. J Am Acad Child Adolesc Psychiatry. 2010;49:414–430. [PubMed] [Google Scholar]
- Comings D. Muhleman D. Gysin R. Dopamine D2 (DRD2) gene and susceptibility to posttraumatic stress disorder: A study and a replication. Biol Psychiatry. 1996;40:368–372. doi: 10.1016/0006-3223(95)00519-6. [DOI] [PubMed] [Google Scholar]
- Cornelis M. Nugent N. Amstadter A. Koenen K. Genetics of post-traumatic stress disorder: Review and recommendations for genome-wide association studies. Curr Psychiatry Rep. 2010;12:313–326. doi: 10.1007/s11920-010-0126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish T. Moradi A. Taghavi M. Neshat-doost H. Yule W. An experimental investigation of hypervigilance for threat in children and adolescents with post-traumatic stress disorder. Psychol Med. 2001;31:541–547. doi: 10.1017/s0033291701003567. [DOI] [PubMed] [Google Scholar]
- De Bellis MD. Hooper SR. Spratt EG. Woolley DP. Neuropsychological findings in childhood neglect and their relationships to pediatric PTSD. J Int Neuropsychol Soc. 2009;15:868–878. doi: 10.1017/S1355617709990464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. Consequences of variations in genes that affect dopamine in prefrontal cortex. Cereb Cortex. 2007;17:i161–170. doi: 10.1093/cercor/bhm082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. Briand L. Fossella J. Gehlbach L. Genetic and neurochemicl modulation of prefrontal cognitive functions in children. Am J Psychiatry. 2004;161:125–132. doi: 10.1176/appi.ajp.161.1.125. [DOI] [PubMed] [Google Scholar]
- Drury S. Theall K. Keats B. Scheeringa M. The role of the dopamine transporter (DAT) in the development of PTSD in preschool children. J Trauma Stress. 2009;22:534–539. doi: 10.1002/jts.20475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger H. Erkanli A. Keeler G. Potts E. Walter B. Angold A. Test-retest reliability of the Preschool Age Psychiatric Assessment (PAPA) J Am Acad Child Adolesc Psychiatry. 2006;45:538–549. doi: 10.1097/01.chi.0000205705.71194.b8. [DOI] [PubMed] [Google Scholar]
- Ehlers A. Clark D. A cognitive model of posttraumatic stress disorder. Behav Res Ther. 2000;38:319–345. doi: 10.1016/s0005-7967(99)00123-0. [DOI] [PubMed] [Google Scholar]
- Enter D. Colzato L. Roelofs K. Dopamine transporter polymorphisms affect social approach-avoidance tendencies. Genes Brain Behav. 2012;11:671–676. doi: 10.1111/j.1601-183X.2012.00791.x. [DOI] [PubMed] [Google Scholar]
- Feng Y. Wigg K. Makkar R. Ickowicz A. Pathare T. Tannock R. Roberts W. Malone M. Kennedy JL. Schachar R. Barr CL. Sequence variation in the 30-untranslated region of the dopamine transporter gene, attention-deficit hyperactivity disorder (ADHD) Am J Med Genet B Neuropsychiatr Genet. 2005;139B:1–6. doi: 10.1002/ajmg.b.30190. [DOI] [PubMed] [Google Scholar]
- Froelich T. Epstein J. Nick T. Melguizo Castro M. Stein M. Brinkman M. Graham A. Langberg J. Kahn R. Pharmacogenetic predictors of methylphenidate dose-response in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2011;50:1129–1139. doi: 10.1016/j.jaac.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelernter J. Southwick S. Goodson S. Morgan A. Nagy L. Charney D. No association between D2 dopamine receptor (DRD2) “A” system alleles, or DRD2 haplotypes, and posttraumatic stress disorder. Biol Psychiatry. 1999;45:620–625. doi: 10.1016/s0006-3223(98)00087-0. [DOI] [PubMed] [Google Scholar]
- Geuze E. Vermetten E. Ruf M. de Kloet CS. Westenberg HGM. Neural correlates of associative learning and memory in veterans with posttraumatic stress disorder. J Psychiatr Res. 2008;42:659–669. doi: 10.1016/j.jpsychires.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Goenjian A. Bailey J. Walling D. Steinberg A. Schmidt D. Dandekar U. Noble E. Association of TPH1, TPH2, and 5HTTLPR with PTSD and depressive symptoms. J Affect Disord. 2012;140:244–252. doi: 10.1016/j.jad.2012.02.015. [DOI] [PubMed] [Google Scholar]
- Goldstein L. Rasmusson A. Bunney B. Roth R. Dopamine beta-hydroxylase (DBH) activity and prefrontal cortical monoamine response to psychological stress in the rat. J Neurosci. 2007;16:1087–1089. doi: 10.1523/JNEUROSCI.16-15-04787.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR. Quevedo KM. Progress in Brain Research. Vol. 167. Elsevier; 2007. Early care experiences, HPA axis regulation in children: a mechanism for later trauma vulnerability; pp. 137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauschildt M. Peters MJV. Moritz S. Jelinek L. Heart rate variability in response to affective scenes in posttraumatic stress disorder. Biol Psychology. 2011;88:215–222. doi: 10.1016/j.biopsycho.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Heils A. Treufel A. Petri S. Stober G. Riederer P. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Heinz A. Goldman D. Jones D. Palmour R. Hommer D. Gorey J. Lee K. Linnoila M. Weinberger D. Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology. 2000;22:133–138. doi: 10.1016/S0893-133X(99)00099-8. [DOI] [PubMed] [Google Scholar]
- Hopper JW. Spinazzola J. Simpson WB. van der Kolk BA. Preliminary evidence of parasympathetic influence on basal heart rate in posttraumatic stress disorder. J Psychosom Res. 2006;60:83–90. doi: 10.1016/j.jpsychores.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Hutchison K. Stallings M. McGeary J. Bryan A. Population stratification in the candidate gene study: fatal threat or red herring? Psychol Bull. 2005;130:66–79. doi: 10.1037/0033-2909.130.1.66. [DOI] [PubMed] [Google Scholar]
- Joober R. Grizenko N. Sengupta S. Amor LB. Schmitz N. Schwartz G. Karama S. Lageix P. Fathalli F. Torkaman–Zehi A. Stepanian MT. Dopamine transporter 3[prime]-UTR VNTR genotype and ADHD. A pharmaco-behavioural genetic study with methylphenidate. Neuropsychopharmacology. 2006;32:1370–1376. doi: 10.1038/sj.npp.1301240. [DOI] [PubMed] [Google Scholar]
- Kilpatrick D. Koenen K. Ruggiero K. Acierno R. Galea S. Resnick H. Roitzsch J. Boyle J. Gelernter J. The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults. Am J Psychiatry. 2007;164:1693–1699. doi: 10.1176/appi.ajp.2007.06122007. [DOI] [PubMed] [Google Scholar]
- Kimble M. Kaloupek D. Kaufman M. Deldin P. Stimulus novelty differentially affects attentional allocation in PTSD. Biol Psychiatry. 2000;47:880–890. doi: 10.1016/s0006-3223(99)00258-9. [DOI] [PubMed] [Google Scholar]
- Koenen K. Genetics and posttraumatic stress disorder: Review and recommendations for future studies. J Trauma Stress. 2007;20:737–750. doi: 10.1002/jts.20205. [DOI] [PubMed] [Google Scholar]
- Koenen KC. Aiello A. Bakshis E. Amstadter A. Ruggiero K. Acierno R. Kilpatrick D. Gelernter J. Galea S. Modification of the association between the serotonin transporter genotype and risk of posttraumatic stress disorder in adults by county-level social environment. Am J Epidemiol. 2009;169:704–711. doi: 10.1093/aje/kwn397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen KC. Uddin M. Chang S-C. Aiello AE. Wildman DE. Goldmann E. Galea S. SLC6A4 methylation modifies the effect of the number of traumatic events on risk for posttraumatic stress disorder. Depress Anxiety. 2011;28:639–647. doi: 10.1002/da.20825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolassa I. Ertl V. Eckart M. Glochner F. Kolassa S. Papassotiropoulos A. de Quervain D. Elbert T. Association study of trauma load and SLC6A4 promoter polymorphism in PTSD: Evidence from survivors of the Rwanda genocide. J Clin Psychiatry. 2010;71:543–547. doi: 10.4088/JCP.08m04787blu. [DOI] [PubMed] [Google Scholar]
- Lee H. Lee M. Kang R. Kim H. Kim S. Kee B. Kim Y. Kim Y. Kim J. Yeon B. Oh K. Oh B. Yoon J. Lee C. Jung H. Chee I. Paik I. Influence of the serotonin transporter promoter gene polymorphism on susceptibility to posttraumatic stress disorder. Depress Anxiety. 2006;21:135–139. doi: 10.1002/da.20064. [DOI] [PubMed] [Google Scholar]
- Madras B. Miller G. Fishman A. The dopamine transporter and attention-deficit/ hyperactivity disorder. Biol Psychiatry. 2006;57:1397–1409. doi: 10.1016/j.biopsych.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Meiser–Stedman R. Towards a cognitive-behavioral model of PTSD in children and adolescents. Clin Child Fam Psychol Rev. 2002;5:217–232. doi: 10.1023/a:1020982122107. [DOI] [PubMed] [Google Scholar]
- Michelhaugh S. Fiskerstrand C. Lovejoy E. Bannon M. Quinn J. The dopamine transporter gene (SLC6A3) variable number tandem repeats domain enhances transcription in dopamine neurons. J Neurochem. 2001;79:1033–1038. doi: 10.1046/j.1471-4159.2001.00647.x. [DOI] [PubMed] [Google Scholar]
- Min Kang A. Palmatier M. Kidd K. Global variation of a 40-bp VNTR in the 3′-untranslated region of the dopamine transporter gene (SLC6A3) Biol Psychiatry. 1999;46:151–160. doi: 10.1016/s0006-3223(99)00101-8. [DOI] [PubMed] [Google Scholar]
- Ouellet-Morin I. Wigg K. Feng Y. Dionne G. Robaey P. Brendgen M. Vitaro F. Simard L. Schachar R. Tremblay RE. Pérusse D. Boivin M. Barr CL. Association of the dopamine transporter gene, ADHD symptoms in a Canadian population-based sample of same-age twins. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1442–1449. doi: 10.1002/ajmg.b.30677. [DOI] [PubMed] [Google Scholar]
- Pietrzak RH. Galea S. Southwick SM. Gelernter J. Examining the relation between the serotonin transporter 5-HTTPLR genotype x trauma exposure interaction on a contemporary phenotypic model of posttraumatic stress symptomatology: A pilot study. J Affect Disord. 2012 Nov 23; doi: 10.1016/j.jad.2012.11.003. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsonneault JK. Han DD. Burdick KE. Kataki M. Bertolino A. Malhotra AK. Gu HH. Sadee W. Dopamine transporter gene variant affecting expression in human brain is associated with bipolar disorder. Neuropsychopharmacology. 2011;36:1644–1655. doi: 10.1038/npp.2011.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purper-Ouakil D. Wohl M. Mouren M. Verpillat P. Adès J. Gorwood P. Meta-analysis of family-based association studies between the dopamine transporter gene and attention deficit hyperactivity disorder. Psychiatr Genet. 2005;15:53–59. doi: 10.1097/00041444-200503000-00009. [DOI] [PubMed] [Google Scholar]
- Roessner V. Sagvolden T. DasBanerjee T. Middleton FA. Faraone SV. Walaas SI. Becker A. Rothenberger A. Bock N. Methylphenidate normalizes elevated dopamine transporter densities in an animal model of the attention-deficit/hyperactivity disorder combined type, but not to the same extent in one of the attention-deficit/hyperactivity disorder inattentive type. Neuroscience. 2010;167:1183–1191. doi: 10.1016/j.neuroscience.2010.02.073. [DOI] [PubMed] [Google Scholar]
- Sack W. Clarke G. Seeley J. Posttraumatic stress disorders across two generations of Cambodian refugees. J Am Acad Child Adolesc Psychiatry. 1995;34:1160–1166. doi: 10.1097/00004583-199509000-00013. [DOI] [PubMed] [Google Scholar]
- Schulz–Heik RJ. Schaer M. Eliez S. Hallmayer JF. Lin X. Kaloupek DG. Woodward SH. Catechol-O-methyltransferase Val158Met polymorphism moderates anterior cingulate volume in posttraumatic stress disorder. Biol Psychiatry. 2011;70:1091–1096. doi: 10.1016/j.biopsych.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Segman R. Cooper–Kazaz R. Macciardi F. Goltser T. Halfon Y. Dobroboski T. Association between the dopamine transporter gene and post-traumatic stress disorder. Mol Psychiatry. 2002;7:903–907. doi: 10.1038/sj.mp.4001085. [DOI] [PubMed] [Google Scholar]
- Segman R. McInerney S. Macciardi F. Lasko N. Orra S. Pitman R. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: A functional MRI study. Biol Psychiatry. 2000;40:368–372. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Shook D. Brady C. Lee PS. Kenealy L. Murphy ER. Gaillard WD. VanMeter JW. Cook EH. Stein M. Vaidya CJ. Effect of dopamine transporter genotype on caudate volume in childhood ADHD and controls. Am J Med Genet Part B: Neuropsychiatr Genet. 2011;156:28–35. doi: 10.1002/ajmg.b.31132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton K. Ressler K. Norrholm S. Jovanovic T. Bradley–Davino B. PTSD, gene variants: New pathways and new thinking. Neuropsychopharmacology. 2012;62:628–637. doi: 10.1016/j.neuropharm.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M. Jang K. Taylor S. Vernon P. Livesly WJ. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder: A twin study. Am J Psychiatry. 2002;159:1675–1681. doi: 10.1176/appi.ajp.159.10.1675. [DOI] [PubMed] [Google Scholar]
- Szobot CM. Roman T. Hutz MH. Genro JP. Shih MC. Hoexter MQ. Júnior N. Pechansky F. Bressan RA. Rohde LAP. Molecular imaging genetics of methylphenidate response in ADHD and substance use comorbidity. Synapse. 2011;65:154–159. doi: 10.1002/syn.20829. [DOI] [PubMed] [Google Scholar]
- True W. Rice J. Eisen S. Heath A. Goldberg J. Lyons M. Nowak J. Twin study of genetic and environmental contributions to liability for symptoms of posttraumatic stress symptoms. Arch Gen Psychiatry. 1993;50:257–264. doi: 10.1001/archpsyc.1993.01820160019002. [DOI] [PubMed] [Google Scholar]
- Valente NLM. Vallada H. Cordeiro Q. Miguita K. Bressan RA. Andreoli SB. Mari JJ. Mello MF. Candidate-gene approach in posttraumatic stress disorder after urban violence: association analysis of the genes encoding serotonin transporter, dopamine transporter, and BDNF. J Mol Neurosci. 2011;44:59–67. doi: 10.1007/s12031-011-9513-7. [DOI] [PubMed] [Google Scholar]
- van de Giessen EM. de Win MML. Tanck MWT. van den Brink W. Baas F. Booij J. Striatal dopamine transporter availability associated with polymorphisms in the dopamine transporter gene SLC6A3. J Nucl Med. 2009;50:45–52. doi: 10.2967/jnumed.108.053652. [DOI] [PubMed] [Google Scholar]
- Van Dyck CH. Malison RT. Jacobsen LK. Seibyl JP. Staley JK. Laruelle M. Baldwin RM. Innis RB. Gelernter J. Increased dopamine transporter availability associated with the 9-repeat allele of the SLC6A3 Gene. J Nucl Med. 2005;46:745–751. [PubMed] [Google Scholar]
- Vermetten E. Bremner J. Circuits and systems in stress: II. Applications to neurobiology and treatment in posttraumatic stress disorder. Depress Anxiety. 2002;16:14–38. doi: 10.1002/da.10017. [DOI] [PubMed] [Google Scholar]
- Voisey J. Swagell C. Hughes I. Morris C. van Daal A. Noble E. Kann B. Heslop K. Hons McD Young R. Lawford B. The DRD2 gene 957C> T polymorphism is associated with posttraumatic stress disorder in war veterans. Depress Anxiety. 2009;26:28–33. doi: 10.1002/da.20517. [DOI] [PubMed] [Google Scholar]
- Wang Z. Baker DG. Harrer J. Hamner M. Price M. Amstadter A. The relationship between combat-related posttraumatic stress disorder and the 5-HTTLPR/rs25531 polymorphism. Depress Anxiety. 2011;28:1067–1073. doi: 10.1002/da.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian H. Chantarujikapong S. Scherrer J. Eisen SL. MJ Goldberg J. Tsuang M. True WR. Genetic and environmental influences on PTSD, alcohol and drug dependence in twin pairs. Drug Alcohol Depend. 2000;61:95–102. doi: 10.1016/s0376-8716(00)00127-7. [DOI] [PubMed] [Google Scholar]
- Xie P. Kranzler H. Poling J. Stein M. Anton R. Brady K. Weiss R. Farrer L. Gelernter J. Interactive effect of stressful life events and the serotonin transporter 5–HTTLPR genotype on posttraumatic stress disorder diagnosis in 2 independent populations. Arch Gen Psychiatry. 2009;66:1201–1209. doi: 10.1001/archgenpsychiatry.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie P. Kranzler H. Poling J. Stein M. Anton R. Farrer L. Gelernter J. Interaction of FKBP5 with childhood adversity on risk for post-traumatic stress disorder. Neuropsychopharmacology. 2010;35:1684–1692. doi: 10.1038/npp.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R. Halligan S. Beirer L. Relationship of parental trauma exposure and PTSD to PTSD, depressive and anxiety disorders in offspring. J Psychiatr Res. 2001;35:261–270. doi: 10.1016/s0022-3956(01)00032-2. [DOI] [PubMed] [Google Scholar]