Abstract
Bone marrow has proved to be an adequate source of stem cells for the treatment of numerous disorders, including neurodegenerative diseases. Bone marrow can be easily and relatively painlessly extracted from a patient or allogenic donor and then transplanted into the degenerative area. Here, the grafted cells will activate a number of mechanisms in order to protect, repair, and/or regenerate the damaged tissue. These properties make the bone marrow a feasible source for cell therapy. In this work, we transplanted bone marrow cells into a mouse model of motoneuron degeneration, with the particularity of placing the cells in the hindlimb muscles rather than in the spinal cord where neuronal degeneration occurs. To this end, we analyze the possibility for the transplanted cells to increase the survival rate of the spinal cord motoneurons by axonal-guided retrograde neurotrophism. As a result, the mice significantly improved their motor functions. This coincided with an increased number of motoneurons innervating the treated muscle compared with the neurons innervating the non-treated contralateral symmetric muscle. In addition, we detected an increase in glial-derived neurotrophic factor in the spinal cord, a neurotrophic factor known to be involved in the rescue of degenerating motoneurons, exerting a neuroprotective effect. Thus, we have proved that bone marrow injected into the muscles is capable of rescuing these motoneurons from death, which may be a possible therapeutic approach for spinal cord motoneuron degenerative diseases, such as amyotrophic lateral sclerosis.
Introduction
Motoneuron diseases (MND) and amyotrophic lateral sclerosis (ALS) are neurodegenerative disorders that are characterized by progressive motoneuron degeneration. With the exception of some familiar cases (∼20% of cases in MND and 10% in ALS), the cause for this degeneration is still unknown. It has been suggested that local toxic damage and/or reduction of trophic support from a diseased cell seem to be involved [1,2]. Ultimately, neuron death and axonal degeneration denervate motoneurons and muscles. However, neuronal and axonal degeneration do not necessarily occur together and in some situations, may occur due to separate and independent mechanisms [3].
In animal models of ALS, the loss of neuromuscular junctions occurs before motoneurons degenerate and well before the symptoms appear, leading to the hypothesis that ALS may also be a “dying back” axonopathy [4]. Indeed, recent studies have shown that protecting the neurons from death is not sufficient to avoid the development of the disease, whereas targeting its degenerating axon seems to be a more efficient therapeutic approach [5].
There are a number of trophic factors that induce neuronal survival both in vitro and in vivo, some of which have been used in various neurodegenerative diseases, including ALS. These include insulin-like growth factor (IGF-1) [6], vascular endothelial growth factor (VEGF) [7,8], and glial-derived neurotrophic factor (GDNF). In the case of GDNF, it has been shown that this factor supports the survival of motoneurons in vitro more efficiently than other neurotrophic factors [9]. In vivo, GDNF prevents programmed cell death of motoneurons during ontogenesis [10], axotomy-induced degeneration of motoneurons [11], and genetically determined progressive motoneuron degeneration [12]. On the other hand, Suzuki et al. reported that GDNF secreted by human neural progenitor cells protects motoneuronsomata but not their projections to the skeletal muscles in an ALS rat model [4]. Interestingly, spinal motoneurons express the GFRα1/GDNF receptor [13], and GDNF can be retrogradely transported using a receptor-mediated mechanism [11]. GDNF rescues axotomized motoneurons by inducing antiapoptotic proteins (neuronal-apoptosis-inhibitory protein and X-linked inhibitor of apoptosis) [14,15]. Due to their bioavailability and potential toxicity, the use of neurotrophic factors requires the development of suitable modes of delivery, in order to develop more efficient neuroprotective methods.
Therefore, we explored the effect of transplanting bone marrow cells (BMCs) into the skeletal muscles of muscle deficient osteocondrodystrophy (mdf/ocd) mice, a motoneuron degenerative mouse model. Inspired by our previous results on cellular neurotrophism [12,16,17], our aim was to use BMCs as GDNF secreting pumps that release in a paracrine manner the physiological concentrations of this factor, and possibly trigger other cell-derived trophic mechanisms. We believe that GDNF acts on the neuromuscular junctions, activating mechanisms that prevent axonal terminal degeneration and promote retrograde neurotrophism, thus acting against neuronal cell death. We observed that BMCs, once transplanted in the muscles, expressed GDNF and produced a trophic effect in spinal motoneurons, promoting motoneuron survival and avoiding muscular denervation.
Materials and Methods
Animals
All animal experiments have been performed in compliance with the Spanish and European Union laws on animal care in experimentation (Council Directive 86/609/EEC), and have been analyzed and approved by the Animal Experimentation Committee of our university. The mouse model used in this work is the mdf/ocd mutation, as in our previous work [18]. This model was initially discovered by Blot et al. [19], with Poirier et al. confirming the motoneuron defect of the mice [20]. Recently, this model has been shown to be affected by a loss of function in Scyl1 [21]. A total of 66 males and females of 10 weeks of age mdf/ocd mice were used distributed for enzyme-linked immunosorbent assay (ELISA), quantitative-polymerase chain reaction (Q-PCR), immunohistochemistry, motoneuron count, and behavior tests. Ten C57BL/6 mice were used as controls for the ELISA tests.
The green fluorescent protein (GFP) mutant mice [C57Bl/6-Tg(ACTB-EGFP)1Osb/J] used were bred in our animal facilities [22]. GDNF/LacZ knockout mice were bred in our animal facilities. Homozygous mice die at birth, while heterozygous ones are viable and used for colony maintenance [23].
Isolation of BMCs
Femurs were dissected from 6- to 8-week-old GFP+ mice, sacrificed by cervical dislocation. Bone marrow was extracted, and single-cell suspensions were obtained by mechanical dissociation. The cells were then counted and resuspended in standard basal medium (Dulbecco's modified Eagle medium [DMEM]; Invitrogen) at the necessary concentration for the transplantation procedures.
Isolation of bone marrow and spleen cells from GDNF knockout mice
This procedure was reported in our previous work [17]. Briefly, pregnant heterozygous GDNF knockout mice were sacrificed 21 days post conception, and the embryos were extracted. Embryos lacking kidneys, corresponding to homozygous mutants, were selected [23]. Femur bone and spleen were extracted by mincing, centrifugation, and re-suspension in DMEM for transplantation.
Behavior assays
In order to compare these results with our previous work [17], the mice were submitted to the same 3 tests: footprint (FP) analysis to study stride length, accelerating rota-rod (RR) analysis, and maximum speed test in a treadmill (TM). Tests were performed on a weekly basis for 3 weeks before the transplantation process. After the transplant, the mice were allowed to recover for 1 week, and then submitted again to the weekly behavior tests during at least another 3 weeks. Mice in which only standard culture medium was injected were used as controls, and were submitted to the same tests applied to the experimental mice.
Footprint
Before analysis, the mice were trained for 4 days. The hindlimb paws of the mice were painted with non-toxic, washable paints. Then, they were placed in an opaque methacrylate tube with a black cardboard box attached to one of its ends. The tube and box were placed over a 60 cm long by a 10 cm-wide strip of paper, where the mouse was expected to walk toward the darkness of the cardboard box, leaving its FPs on the paper. In this manner, the stride length could be measured, as it has been previously shown that mice with affected motor functions have a decreased stride length [17,24].
Rota-rod
The RR test was performed on an 8500 Rota-rod (Leica Scientific Instruments). The lane is 500 mm wide, and the rod has a diameter of 30 mm. Before analysis, the mice were trained on the rod for 4 days. Afterward, the mice were tested on a weekly basis, placing each mouse 8 times on the accelerating RR cylinder, which uniformly increased speed from 4 to 40 rpm over a 5 min period, until the mouse fell. Both the time of fall and the speed reached were recorded and averaged per session [12,17,25].
Treadmill
An LE 8700 treadmill model was used (Leica Scientific Instrument). The lane is 41 mm long and 10 mm wide. The runway, when in movement, pushes the animal to the shock grid, set at 0.4 mA. In this manner, it was possible to measure the maximum possible speed that each mouse could attain. The TM accelerated at a constant rate, and the maximum speed reached was noted. Before analysis, the mice were trained for 4 days with the apparatus. Then, each mouse was placed 8 times in the TM per session, and the average maximum speed attained was calculated. With this assay, it is possible to measure the maximum speed attained by the mice, including in neuro-degenerative models [17,26].
Cell transplantation
Half-an-hour before performing the surgical procedure, the mice were injected intraperitoneally with 0.1 mg/kg of buprenorphine (Buprex; Schering-Plough). Then, they were anesthetized with isofluorane gas (Esteve Veterinary) and placed on the surgery table backside up. Skin was dissected, and quadriceps muscle was exposed. Either 1 million bone marrow-derived cells or fresh culture medium (DMEM) was injected, all in a maximum volume of 5 μL.
After the injection, the incision was sutured, and the mice were monitored for several days in order to ensure that the operation did not cause any additional motor damage, such as paralysis or tremors in the hind legs.
Tissue preparation and immunohistochemistry
Five weeks after injection/grafting, the mice were anesthetized with isofluorane and fixed by intra-cardiac perfusion with 4% paraformaldehyde (PFA) in phosphate buffer (pH 7.4). The spinal cord was carefully excised and kept in 4% PFA overnight. After fixation, the spinal cords, brains, and quadriceps muscles were cryoprotected by increasing concentrations of sucrose; finally embedded in Tissue-Tek (Electron Microscopy Science); and frozen. Transverse sections of 20 μm were obtained with a cryostat and mounted on slides. The sections were treated with 1% hydrogen peroxide for 30 min and then incubated for 60 min at room temperature in 0.1% lysine in phosphate-buffered saline to block non-specific binding. The following primary antibodies were used: anti-GFP (rabbit or mouse, 1:200; Molecular Probes), anti-GDNF (rabbit, 1:200; Santa Cruz Biotechnology), and anti-ChAT (goat, 1:200; Chemicon International, Inc.). The sections were incubated overnight at room temperature with the primary antibody. Afterward, in all cases except for GFP staining, biotinylated secondary antibodies were used (1:200; Vector Laboratories), and in the last step, streptavidin conjugated with either Cy3 (1:500; GE Healthcare) or Alexa Fluor 488 in the case of GFP (mouse or rabbit, 1:500; Molecular Probes) was used. Histological samples were observed in a fluorescence microscope (Leica DMR; Leica Microsystems), and micrographs were taken with a confocal microscope (Leica DMR).
Motor neuron counting
Cell counting was performed using 6 bone marrow-grafted and 5 culture media-injected [sham operated (SH)] symptomatic mice and 3 non-treated (NT) mice. Four weeks after surgery, the mice were taken to the surgical room, the quadriceps muscle was exposed, and 10 μL of 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanineperchlorate (DiI) was injected, dissolved in 80% ethanol. After a survival period of 5 days, which is required for complete retrograde motoneuron labeling, the animals were sacrificed and processed histologically.
To compare the number of motoneurons in the lumbosacral enlargement of the spinal cord in bone marrow-grafted and non-grafted mice, we counted only neurons located in the ventral horn that were DiI+ and showed a distinct nucleus.
Enzyme-linked immunosorbent assay
A total of 17 mice were used to study the possible differences in GDNF levels between the treated and non-treated muscles. Four weeks after surgery, the mice were anesthetized with an overdose of sodium pentobarbital (100 mg/kg) and decapitated. Different groups of animals were used: wild-type (WT) mice C57 (n=4), NT symptomatic mdf/ocd mice (n=6), and whole bone marrow-grafted mdf/ocd mice (n=7). The quadriceps muscle was removed within 3–5 min after decapitation. Tissue was stored at −80°C. All samples were used within 15 days after freezing. The tissue levels of GDNF were measured with an ELISA kit (GDNF EmaxImmuno Assay System; Promega), according to the protocol of the supplier.
RNA isolation and Q-PCR
Total RNA was isolated from quadriceps femoris (QF) muscle using the RNeasy Fibrous Tissue Mini Kit (Qiagen) following the manufacturer's protocol, and total RNA was assessed for purity and then quantified with a Nanodrop® ND-1000 spectrophotometer. For spinal cord RNA, the same process was performed using the RNeasy Lipid Tissue Mini Kit (Qiagen).
Total RNA (2 μg) was reverse transcribed to cDNA by AMW-RT (Finnzymes) of total RNA using oligo-dT primers. Two hundred-fifty nanograms (250 ng) of cDNA was used for pre-amplification with TaqMan® PreAmp Master Mix Kit protocol (Applied Biosystems), following the kit's instructions. The pooled assay mix was prepared by combining the commercially available probe set (TaqMan Gene Expression Assays) with GDNF (Mm00599849_m1*) and GAPDH asendogenous control (Mm99999915_g1*) using the TaqMan Gene Expression Assay kit into a single tube and using TE buffer to dilute the pooled assays to a final concentration of 0.2×. The 50 μL of pre-amplification reaction included 25 μL of TaqMan PreAmp Master Mix, 12.5 μL of pooled assay mix, and 12.5 μL of 20 ng/μL cDNA sample. The reactions were incubated in an Applied Biosystems Thermocycler for 10 min at 95°C followed by 14 cycles of 95°C for 15 s and 60°C for 4 min. The pre-amplification product was then diluted 1:20 and analyzed using the TaqMan real-time PCR on a StepOnePlus (Applied Biosystems) instrument with an initial denaturation step for 10 min at 95°C, followed by 40 cycles at 92°C for 15 s, 60°C for 1 min with 5 μL cDNA PreAmplified per reaction (20 μL: Taqman Universal PCR Mastermix; Applied Biosystems).
The 2−ΔΔCT method was used for quantification. Data are represented as mean±standard error of the mean.
Relative differences between treated (TR) and NT control samples were analyzed by Student's t-test, where a P-value of <0.05 was considered statistically significant.
Neuromuscular junction analysis
The motor plates were immunostained with anti-Chat (see “Tissue Preparation and Immunohistochemistry”). Fourteen histological sections were analyzed (23 muscle fibers), totaling 22 motor plates, separated in 15 motor plates in bone marrow-treated muscles and 7 plates in non-treated muscles. In order to calculate the motor plate area, images were analyzed with the Leica software, using a fluorescence microscope, and the junctional area was measured with the help of a microscope ruler. Since the motor plates have an ellipsoid shape, the ellipse area formula was used (π×A×B, where A and B are the major and minor diameters). The muscle fiber width was also measured.
Statistical analysis
Statistical analysis was performed using SigmaPlot 10.0 software. Both paired t-Student tests (for behavior assay) and t-Student (for protein quantification) were used with a P<0.05 significance criteria.
Results
Bone marrow TR symptomatic mice improve in the behavior tests analyzed
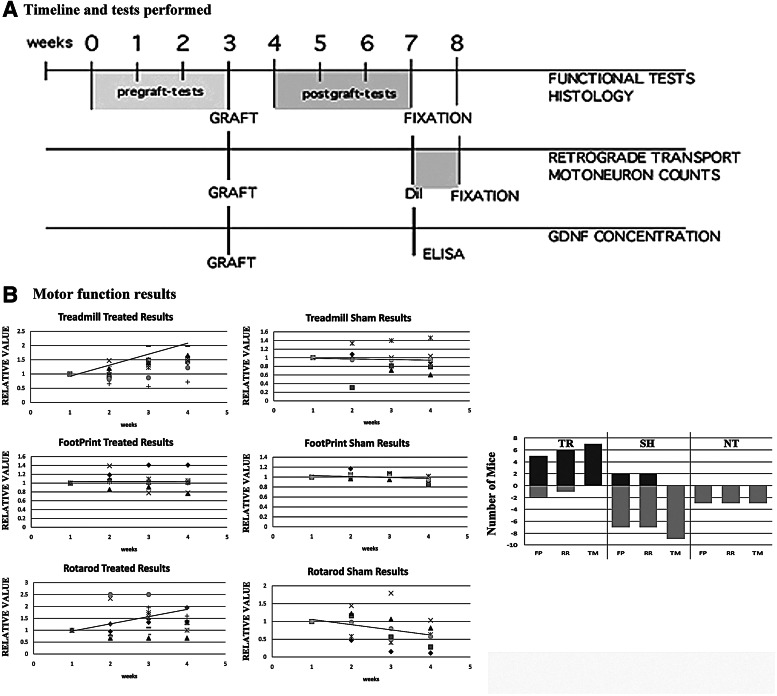
A total of 19 symptomatic mdf/ocd mice were used, separated in 3 groups. In the first group (n=7), whole bone marrow was injected into the left quadriceps (TR). The second group consisted of a control group where only DMEM medium was injected (SH, n=9). Finally, the third group (n=3) consisted of NT mice. All behavior tests were performed in the 3 groups before and after grafting (Fig. 1A). As a result, 5 out of 7 of the TR mice significantly improved in the FP assay, 6 out of 7 in the RR, and all 7 in the TM. In the sham group, only 2 out of 9 improved in FP and RR (2 in each), and none in TM. None of the NT mice improved in any of the 3 tests (Fig. 1B).
FIG. 1.
Behavior tests performed in the TR and NT mice. (A) Timeline of the experimentation protocol. (B) The first 3 tables (in vertical) represent the different behavior tests performed by the bone marrow TR group along with the tendency line, whereas the second 3 tables are the values of the SH group. The last table (far right) depicts the number of mice that significantly improved in the different tests. TR, indicates a treated mouse transplanted with bone marrow cells (BMCs); SH, indicates a control mouse where culture medium was injected; NT, indicates a non-treated animal; FP, indicates footprint test; TM, treadmill; RR, rotarod.
In the TR group, there is a clear tendency to improve in the TM and RR tests 1 week after treatment, while the FP performance is maintained; that is, the mice neither improve nor worsen in the following weeks. As for the control groups, the mice tend to worsen in all 3 tests.
Bone marrow-treated muscles have increased muscle fiber size and motor plate area
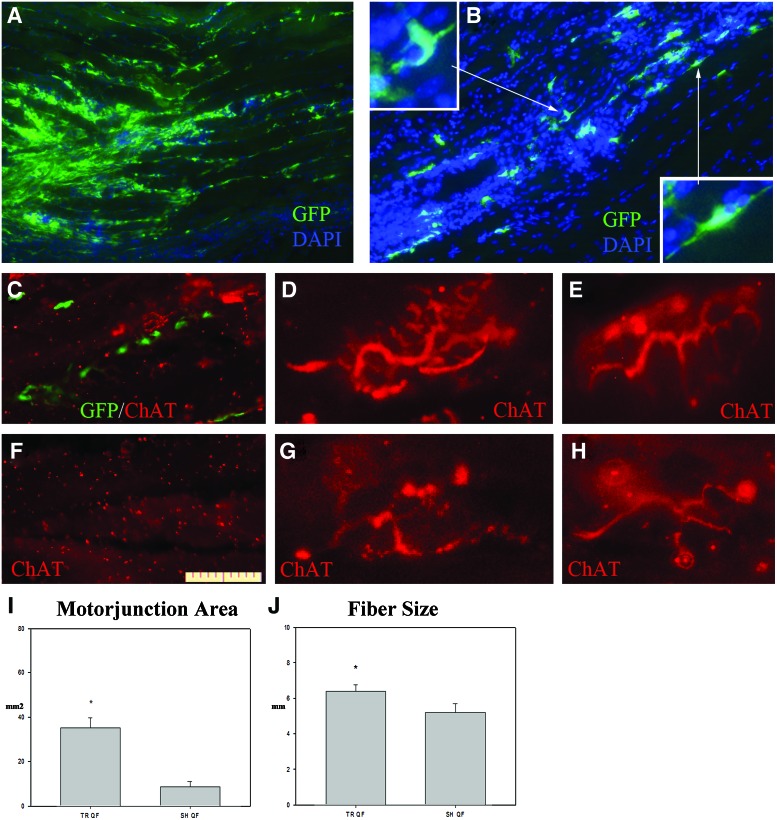
The experimental mice were sacrificed 26–27 days after bone marrow transplantation, and their tissues (muscles, spinal cord, and brain) were processed for histological analysis. Immunohistochemical analysis of the grafted muscle resulted in the presence of many GFP+ cells being disseminated between the basal membrane and the muscle fibers (Fig. 2A, B). The majority of these cells presented an elongated morphology, while others were polygonal shaped and had dendrite-like appendages (Fig. 2B).
FIG. 2.
Bone marrow transplantation in muscle tissue. (A) Immunohistochemical image of the transplanted area. The cells are located in a large cluster and spread throughout the muscle tissue, with the majority located in between the muscle fibers. (B) Transplanted cells were detected between the muscle fibers. The cells present an elongated morphology, with multiple prolongations (insets). Green staining is GFP, and blue is 4′,6-diamidino-2-phenylindole (DAPI). (C) Muscular longitudinal section showing GFP-grafted BMC and ChAT-positive neuromuscular terminals in the grafted territory. (D, E) Neuromuscular junctions and motor end plates in the grafted muscles. (F) Muscular longitudinal section of contralateral non-grafted muscles processed by anti-ChAT immunoreactivity, where motor nerve terminals are absent. (G, H) Atrophic motor terminal and neuromuscular junctions in the contralateral non-grafted muscles. Scale bar in (F): 100 μm in (A, B); 50 μm in (C, F); 25 μm in (D, E, G–I). Histogram depicting the values of motor endplate area obtained in the TR and SH groups. (J) Histogram depicting muscle fiber width in the SH and TR groups. GFP, green fluorescent protein. *P<0.05. Color images available online at www.liebertpub.com/scd
To explore the effect of BMCs grafts in neuromuscular junctions, we analyzed ChAT expression on immunohistological sections of grafted and control muscles. ChAT-positive nerve terminals and neuromuscular junctions, with numerous motor end plates, were abundant in experimental muscles around the grafted BMCs (Fig. 2C–E), but very few atrophic nerve terminals, showing scattered motor end plates, were detected in control muscles (Fig. 2F–H). Furthermore, the experimental motor plate area as well as the muscle fiber width were significantly larger than in the NT group (P<0.005 and P<0.05, respectively) (Fig. 2I, J respectively).
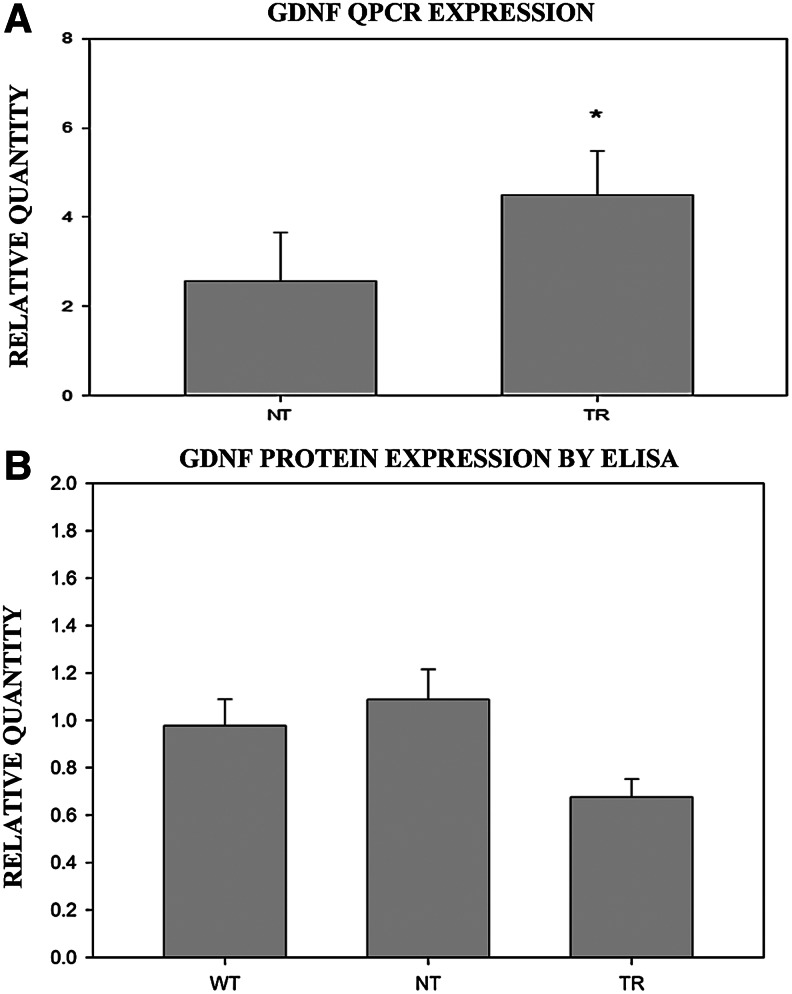
Transplanted cells increase GDNF transcription, but decrease GDNF protein concentration, in the muscle of mdf/ocd mice
Q-PCR showed that the bone marrow TR group presented a significantly higher transcription of GDNF compared with SH mice (sham controls 4.49±1.86; bone marrow TR 7.85±1.69; n=4 in each case, P=0.007) (Fig. 3A). GDNF protein concentration was analyzed by ELISA in each QF of TR (n=7), NT (n=9) and WT (n=10) mice (Fig. 3B). In the bone marrow-grafted group, the treated muscles presented lower concentrations of GDNF compared with the contralateral muscle and the other control groups (P<0.01). There was no significant difference between the hindlimb muscles of the control groups (WT 0.98±0.11 n=4; NT 1.09±0.13; TR 0.68±0.07) (Fig. 3C).
FIG. 3.
(A) Q-PCR analysis of GDNF in the TR and NT groups. (B) Histogram depicting the average values of GDNF content of the left versus right hindlimb muscles in the WT, NT, and TR mice. In the TR group, the left hindlimb muscle corresponds to the treated side. Values above or below 1 would indicate that there is more or less, respectively, GDNF in the left hindlimb muscle than in the right. Q-PCR, quantitative-polymerase chain reaction; GDNF, glial-derived neurotrophic factor; ELISA, enzyme-linked immunosorbent assay; WT, wild-type. *P<0.05.
Bone marrow transplantation in the muscle increases the survival of motoneurons in the spinal cord
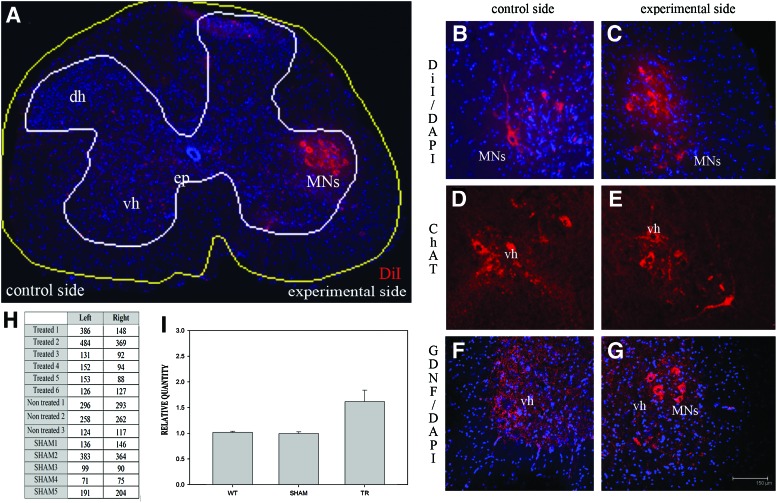
Four weeks after the cell injection (or medium in the case of sham controls), DiI was injected into the left and right quadriceps of the transplanted mice and controls. After 7 days, the spinal cord (as well as the muscle tissue as previously seen) was processed and analyzed by fluorescence microcopy (Fig. 4A–C). As a result, the treated left limb coincided with a higher number of retrograde-labeled motoneurons innervating the treated muscles, compared with the non-transplanted side (Fig. 4H, I). Interestingly, fluorescent cellular debris was abundant in the non-transplanted side, in some cases around the remaining fluorescent motoneurons (Fig. 4B). This debris may be remnants of retrogradely labeled motoneurons that have suffered cell death during the 5 days after DiI labeling. In this context, in the experimental side, almost no fluorescent cellular debris was observed, suggesting a much less active degenerative process (Fig. 4C). There were no significant differences in the number of DiI-labeled motoneurons in either side of the spinal cord of the control mice (Fig. 4H, I). These data were corroborated by anti-ChAT immunostaining, detecting a lower number of ChAT-positive motoneurons and a higher number of cellular debris in the right side of the spinal cord compared with the left (Fig. 4D, E). Thus, BMCs, when grafted into the hindlimb muscles, are capable of increasing the survival of motoneurons in the spinal cords of mdf/ocd mice, which correlates with the maintenance of neuromuscular junctions in the muscles and an improvement in the behavior tests performed in the mdf/ocd mice. A histological analysis of the spinal cord revealed no GDNF immunoreactivity in neurons of the non-treated side (Fig. 4F), whereas GDNF was detected in the cytoplasm of motoneurons in the motor ventro-lateral columns, where the neurons innervating the grafted muscle were localized (Fig. 4G).
FIG. 4.
DiI injected in the muscle retrogradely stains motoneurons in the spinal cord. (A) Image taken at 10×showing a transversal section of the lumbar spinal cord. The BMCs were transplanted in the muscles of one limb, whereas DiI was injected in both limbs. There is a clear increase in the number of stained motoneurons innervating the limb in which the BMCs were injected. (B, C) Close-up of the stained motoneurons in right (B) and left (C) lumbosacral spinal cord ventral horns. (D, E) Anti-ChAT immunoreactive motoneurons in right (D) and left (E) lumbosacral spinal cord ventral horns. (F, G) GDNF expression (in red) in the motoneurons of the anterior horn of the spinal cord (L3–L5) in the control side (F) and TR side (G). Scale bar in (G) is the same for (B–G). (H) Number of DiI-stained motoneurons in the spinal cord in both the grafted and control mice. In the TR mice, there was an increase in the number of motoneurons innervating the grafted limb. (I) Histogram depicting the average values of the number of motoneurons in the left versus the right ventral horns of the spinal cord in the WT, sham control (SHAM), and TR mice. In the TR group, the left ventral horn of the spinal cord corresponds to the motoneurons that innervate the treated muscles. Values above or below 1 would indicate that there is more or less, respectively, surviving motorneurons in the left ventral horn than in the right. DiI, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanineperchlorate; vh, ventral horn; dh, dorsal horn; MNs, motorneurons; ep, ependyma. Color images available online at www.liebertpub.com/scd
The region of the spinal cord innervating the treated muscle presents increased GDNF concentration, but not increased GDNF transcription
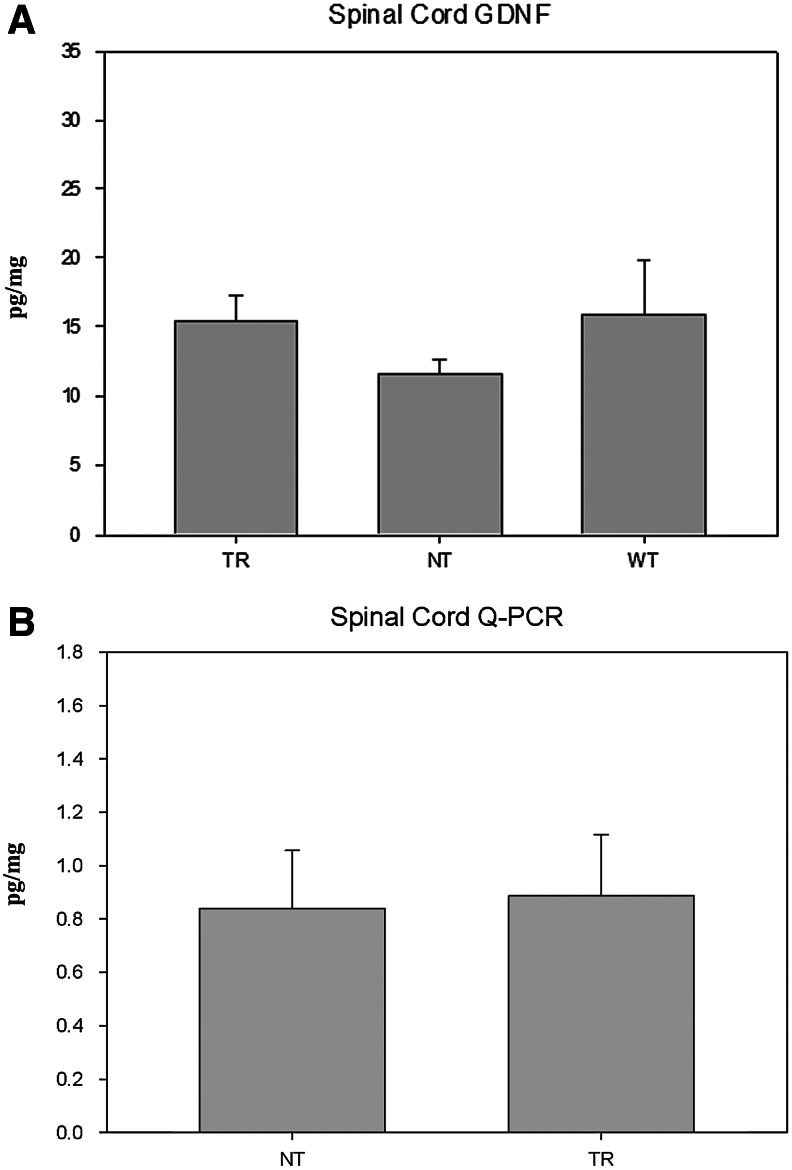
The increased GDNF immunoreactivity observed in the lumbar region of the spinal cord in the TR group coincided with a significantly higher concentration of GDNF (analyzed by ELISA) (P<0.05) (TR 15.49±1.78; NT 11.66±0.93; WT 15.92±3.87) (Fig. 5A). This concentration increase does not coincide, however, with increased GDNF transcription (measured by Q-PCR), obtaining similar results between the TR and NT groups (NT 0.83±0.21; TR 0.88±0.23).
FIG. 5.
(A) GDNF content as measured by ELISA in the lumbosacral segments of spinal cord of TR, NT mutant mice, and WT mice. (B) Q-PCR analysis of GDNF in the spinal cord of TR and NT mice.
No increased GDNF expression in the spinal cord with GDNF-knock out BMCs transplantation
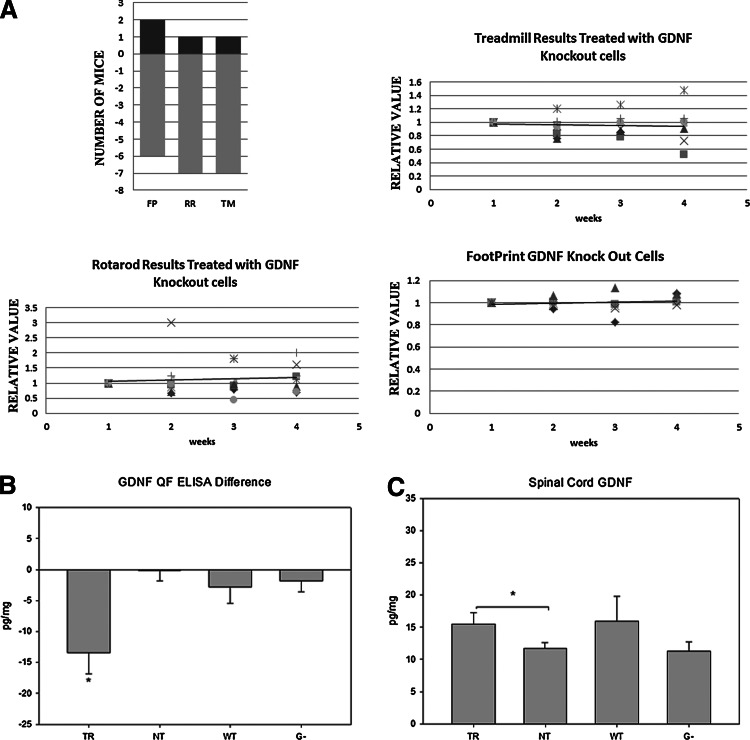
To further prove that GDNF secretion by the grafted BMCs is implicated in the results obtained, BMCs isolated from GDNF knockout (G−) mice were grafted. As a result, the TR mice did not significantly improve in the behavior tests, as we obtained similar results in the sham controls (Fig. 6A), with only 2 out of 8 mice improving in FP and 1 out of 8 in the RR and TM test. Furthermore, there was no difference in GDNF concentration either in the hindlimb muscle or in the spinal cord (G− 1.85±1.78 and G− 11.29±1.45; Fig. 6B, C respectively).
FIG. 6.
(A) Behavior tests performed in the mice injected with GDNF knockout BMCs. FP is footprint, RR is rotarod, and TM is treadmill. The next 3 histograms are the values obtained weekly, and the tendency line. (B) GDNF concentration difference between treated and non-treated muscles in TR, NT, WT, and G− (mice injected with GDNF knockout BMCs). (C) GDNF concentration in the spinal cord in TR, NT, WT, and G− (mice injected with GDNF knockout BMCs). *P<0.05 and different symbols reflect different individual animals.
The cerebral cortex of the TR mice shows increased GDNF expression in the contralateral side corresponding to the treated muscle
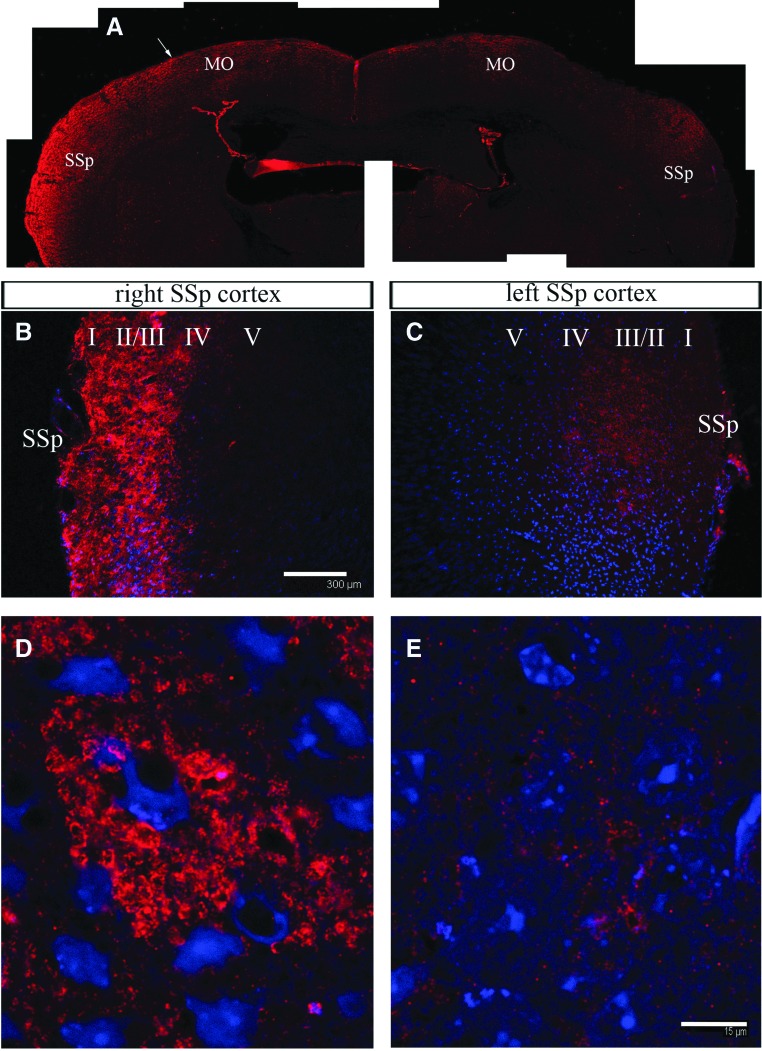
The cerebral cortex of the brain was analyzed in both the control and TR mice. As a result, an increased GDNF immunoreactivity was detected in the TR mice, specifically in the caudal area of primary motor and somatosensory areas of the side contralateral to the grafted muscle (Fig. 7A, B). The motor area corresponds to the area of hindlimb cortical pyramids, which innervate the anterior horn of the contralateral side. GDNF immunoreactivity was distributed in all cortical layers, although it was more abundant in the superficial layers, in a granular extracellular or microcellular pattern, while the surrounding cellular profiles were detected in negative or lower fluorescence (Fig. 7D). This increase was not observed in the contralateral side (Fig. 7A, C, E).
FIG. 7.
Increased GDNF immunoreactivity in the cortex of TR mice. (A) GDNF in the cortex corresponding to the primary motor and sensorial areas, where there is a clear difference in GDNF expression in the contralateral (left) compared with the ipsilateral (right) sides to the grafted left hindlimb muscles. (B, C) Close-up image of the left (B) and right (C) transition between sensorial and motor primary cortex areas [arrow in (A)]. Scale bar in (B) is the same for (C, D, E) GDNF immunoreaction is accumulated in the interneuronal spaces between cortical neurons of the left (D) and right (E) cortex. Scale bar in (E) is the same for (D). MO, motor primary cortex area; SSp, somatosensorial primary area. Color images available online at www.liebertpub.com/scd
Discussion
In this article, we injected whole bone marrow isolated from the femur of GFP mice into the quadriceps of mdf/ocd mice. This surgical procedure is simple, clean, and the animal rapidly recovers, with no further damage. Various authors report that, to increase the survival of the transplanted cells into the muscle tissue, it is necessary to produce a focal injury [27]. In our case, despite the cell death process in the grafted cell population, affecting differentiated blood cells and possibly to some extent, marrow progenitor cells [28], the remaining cells are sufficient to produce a significant increase in the survival of the spinal motoneurons. In addition. the increase in motoneuron survival coincides with the maintenance of neuromuscular junctions and an improvement in the behavior tests, indicating a recovery of lost function. This recovery suggests that non-functional motoneurons maintain for some time muscular end plates and are still capable of re-establishing functional activity at early stages of the degenerative process. This opens the possibility of at least some functional recovery, as well as a deceleration of the progression of the disease. In addition, since this work can be applied as a possible therapeutic approach for ALS patients, it is important to note that the method used causes minimum tissue damage to the patient.
The mdf mouse model is a suitable model for motor neuron degeneration [12,17,19], showing a progression of the degeneration similar to that in human disease. Recently, it has been proved that this mouse model is caused by a loss-of-function mutation in the Scyl1gene [21]. However, significant cerebellar atrophy occurs at a more advanced age than the animals used in this study. Using young animals allows us to avoid this degeneration, and, thus, only motoneuron degeneration may be observed.
We have previously demonstrated that transplanted BMCs are capable of improving the survival and motor functions of mdf mice through the production and secretion of trophic factors such as GDNF [12,17] when transplanted into the spinal cord. However, axonal degeneration is a major clinical and pathological feature of ALS, which is considered a distal axonopathy [5,18,29], that disconnects the motoneuron from the muscle, and ultimately results in the death of the neuron as well. Two intracellular pathways have been suggested to coexist in motoneurons: one that is necessary for the survival of the cell body and a second which is required for axonal survival [30].
Besides GDNF, other factors have been considered as being capable of promoting motoneuron survival in the spinal cord, such as IGF-1 [31,32], fibroblast growth factor-9 [33], cilliary neurotrophic factor with NT-3 [34,35], cardiotrophin-1 [36], or VEGF [37,38].
Of these trophic factors, the most significant with regard to promoting motoneuron survival and overall improvement in the development of ALS is GDNF [39]. In adult muscle tissue, GDNF activates terminal axonal branching and neuromuscular synapseformation in physiological conditions [40]; denervation induces its increase [41]. This has also been observed in humans, both in post-mortem studies [42] and in biopsies [43], noting that this increase only occurs in the affected muscles, and not in the non-affected muscles. This increase could be a response of the muscle trying to generate new motor plates, as GDNF is implicated in this process. This neurotrophic factor is transported retrogradely through the axon from the muscle tissue to the soma of the motoneuron [44]. The transplantation of BMCs accordingly should give better results if it is applied in the area where the motoneurons capture GDNF: the target muscle. In this manner, the grafted cells would secrete growth factors, such as GDNF, and these would be captured by the neighboring axons. Consequently, the growth factor may act locally by promoting neuromuscular junction recovery and travel toward the neuron cell body and prevent its denervation and cell death.
Previous studies have shown that it is possible to improve motoneuron survival using growth factors. However, the majority of these reports used viral transfection of the GDNF gene, obtaining very diverse results. For example, by injecting GDNF gene-expressing lentivirus into the spinal cord, the authors reported a survival of the motoneuron, but not of its axon [45]. When the injection was administered in the muscle, GDNF delayed the onset of the disease and improved locomotor performance, although the effect was only temporary [46]. Other laboratories have reported an increase in motoneuron number, but no improvement in the behavior tests [47]. An intramuscular injection of a plasmid containing GDNF did not improve motoneuron survival or the behavior tests [48]. Lastly, an injection of GDNF protein directly into the muscle resulted in retrograde transport of the factor into the motoneurons, preventing cell death, but did not result in clinical improvement [49].
In this work, we demonstrated that BMCs survive within muscular tissue where they are grafted and significantly increase motoneuron survival. Interestingly, when the BMCs were injected into the muscle, there was an increase in GDNF mRNA levels, albeit protein concentration was decreased. This decrease coincided with an increased number of motor end plates in the muscle, corroborated by an increased number of motoneurons retrogradely stained when DiI was injected into the muscle. Furthermore, motor plate and muscle fiber sizes were increased in the TR mice. This increase in innervation may also increase the GDNF uptake by the motoneurons and be transported from the muscles to the spinal cord, resulting in a higher concentration of GDNF in the spinal cord, despite having lower mRNA levels. The fact that GDNF is taken from the muscle into the spinal cord implicates that the motoneurons, which were in the process of degeneration, have prevented their fate and maintained their function, indicated by the significant improvements in behavior tests. Thus, bone marrow transplantation in the muscle prevents motoneuron degeneration.
In our case, the increase in surviving motoneurons correlates with an improvement in the behavior tests, and this improvement is statistically higher than that obtained by our group using bone marrow graft in the spinal cord [12,17]. Moreover, this is consistent with the improvement obtained by GDNF in RR tests used in other articles [50,46]. In addition, the increase of intracellular GDNF in the motoneurons of the anterior horn of the spinal cord could be related to the increase of this trophic factor in the cortex innervating these motor neurons. Indeed, this mechanism of retrograde spino-cortical interaction may be underlying the observed plasticity changes in cortical function that allowed recovery of motor function after peripheral neurectomy in cats [51,52]. Thus, the observed increase of GDNF in the motor cortex may trigger plastic changes in the corticospinal system that finally have the capability to produce major changes in the efficacy of this pathway in modulating motor activity and functional recovery in transplanted mice. Further work should be performed at this level to confirm this hypothesis.
In conclusion, an intramuscular injection without a previous lesion of BMCs can be used in neurodegenerative diseases affecting motoneurons and their axons. Due to the almost innocuous nature of the procedure and the fast recovery of the experimental animals, this procedure can be contemplated as a therapeutic option in clinical trials.
Acknowledgments
The authors would like to thank Roberto Gallego for the inspiration and stimuli to develop this work. This work was supported by Ministerio Ciencia E Innovacion (MICINN BFU2010-27326), DIGESIC-MEC BFU2008-00588, Ingenio 2010 MEC-CONSOLIDER CSD2007-00023, from the GVA Prometeo grant 2009/028, and ISCIII Cibersam and Tercel (RD06/0010/0023), as well as ELA Foundation, Fundación Diogenes-Elche City Government, Rotary Club Elche-Illice, and Fundació Gent per Gent grant 24 NEURO.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Turner BJ. Murray SS. Piccenna LG. Lopes EC. Kilpatrick TJ. Cheema SS. Effect of p75 neurotrophin receptor antagonist on disease progression in transgenic amyotrophic lateral sclerosis mice. J Neurosci Res. 2004;78:193–199. doi: 10.1002/jnr.20256. [DOI] [PubMed] [Google Scholar]
- 2.Phukan J. Hardiman O. The management of amyotrophic lateral sclerosis. J Neurol. 2009;256:176–186. doi: 10.1007/s00415-009-0142-9. [DOI] [PubMed] [Google Scholar]
- 3.Coleman MP. Perry VH. Axon pathology in neurological disease: a neglected therapeutic target. Trends Neurosci. 2002;25:532–537. doi: 10.1016/s0166-2236(02)02255-5. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki M. McHugh J. Tork C. Shelley B. Klein SM. Ebischer PA. Svendsen CN. GDNF secreting human neural progenitor cells protect dying motor neurons, but not their projection to muscle, in a rat model of familial ALS. PLoS One. 2007;2:e689. doi: 10.1371/journal.pone.0000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischer LR. Culver DG. Tennant P. Davis AA. Wang M. Castellano-Sanchez A. Khan J. Polak MA. Glass J. D. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol. 2004;185:232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Nagano I. Shiote M. Murakami T. Kamada H. Hamakawa Y. Matsubara E. Yokoyama M. Moritaz K. Shoji M. Abe K. Beneficial effects of intrathecal IGF-1 administration in patients with amyotrophic lateral sclerosis. Neurol Res. 2005;27:768–772. doi: 10.1179/016164105X39860. [DOI] [PubMed] [Google Scholar]
- 7.Storkebaum E. Lambrechts D. Dewerchin M. Moreno-Murciano MP. Appelmans S. Oh H. Van Damme P. Rutten B. Man WY, et al. Treatment of motoneurondegeneration by intracerebroventricular delivery of VEGF in a rat model of ALS. Nat Neurosci. 2005;8:85–92. doi: 10.1038/nn1360. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y. Mao XO. Xie L. Banwait S. Marti HH. Greenberg DA. Jin K. Vascular endothelial growth factor overexpression delays neurodegeneration and prolongs survival in amyotrophic lateral sclerosis mice. J Neurosci. 2007;27:304–307. doi: 10.1523/JNEUROSCI.4433-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henderson CE. Phillips HS. Pollock RA. Davies AM. Lemeulle C. Armanini M. Simmons L. Moffet B. Vandlen RA. Koliatsov VE. Rosenthal A. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science. 1994;266:1062–1064. doi: 10.1126/science.7973664. [DOI] [PubMed] [Google Scholar]
- 10.Oppenheim RW. Houenou LJ. Johnson JE. Lin LF. Li L. Lo AC. Newsome AL. Prevette DM. Wang S. Developing motor neurons rescued from programmed and axotomy-induced cell death by GDNF. Nature. 1995;373:344–346. doi: 10.1038/373344a0. [DOI] [PubMed] [Google Scholar]
- 11.Yan Q. Matheson C. Lopez OT. In vivo neurotrophic effects of GDNF on neonatal and adult facial motor neurons. Nature. 1995;373:341–344. doi: 10.1038/373341a0. [DOI] [PubMed] [Google Scholar]
- 12.Cabanes C. Bonilla S. Tabares L. Martinez S. Neuroprotective effect of adult hematopoietic stem cells in a mouse model of motoneuron degeneration. Neurobiol Dis. 2007;26:408–418. doi: 10.1016/j.nbd.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Treanor JJ. Goodman L. de Sauvage F. Stone DM. Poulsen KT. Beck CD. Gray C. Armanini MP. Pollock RA, et al. Characterization of a multicomponent receptor for GDNF. Nature. 1996;382:80–83. doi: 10.1038/382080a0. [DOI] [PubMed] [Google Scholar]
- 14.Airaksinen MS. Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- 15.Perrelet D. Ferri A. Liston P. Muzzin P. Korneluk RG. Kato AC. IAPs are essential for GDNF-mediated neuroprotective effects in injured motor neurons in vivo. Nat Cell Biol. 2002;4:175–179. doi: 10.1038/ncb751. [DOI] [PubMed] [Google Scholar]
- 16.Jones J. Jaramillo-Merchan J. Bueno C. Pastor D. Viso-Leon M. Martinez S. Mesenchymal stem cells rescue Purkinje cells and improve motor functions in a mouse model of cerebellar ataxia. Neurobiol Dis. 2010;40:415–423. doi: 10.1016/j.nbd.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Pastor D. Viso-Leon MC. Jones J. Jaramillo-Merchan J. Toledo-Aral JJ. Moraleda JM. Martinez S. Comparative effects between bone marrow and mesenchymal stem cell transplantation in GDNF expression and motor function recovery in a motorneuron degenerative mouse model. Stem Cell Rev Rep. 2011;8:445–458. doi: 10.1007/s12015-011-9295-x. [DOI] [PubMed] [Google Scholar]
- 18.Hall ED. Oostveen JA. Gurney ME. Relationship of microglial and astrocytic activation to disease onset and progression in a transgenic model of familial ALS. Glia. 1998;23:249–256. doi: 10.1002/(sici)1098-1136(199807)23:3<249::aid-glia7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 19.Blot S. Poirier C. Dreyfus PA. The mouse mutation muscle deficient (mdf) is characterized by a progressive motoneuron disease. J Neuropathol Exp Neurol. 1995;54:812–825. [PubMed] [Google Scholar]
- 20.Poirier C. Blot S. Fernandes M. Carle GF. Stanescu V. Stanescu R. Guenet JL. A high-resolution genetic map of mouse chromosome 19 encompassing the muscle-deficient osteochondrodystrophy (mdf-ocd) region. Mamm Genome. 1998;9:390–391. doi: 10.1007/s003359900777. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt WM. Kraus C. Hoger H. Hochmeister S. Oberndorfer F. Branka M. Bingemann S. Lassmann H. Muller M, et al. Mutation in the Scyl1 gene encoding amino-terminal kinase-like protein causes a recessive form of spinocerebellar neurodegeneration. EMBO Rep. 2007;8:691–697. doi: 10.1038/sj.embor.7401001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okabe M. Ikawa M. Kominami K. Nakanishi T. Nishimune Y. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez MP. Silos-Santiago I. Frisen J. He B. Lira SA. Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996;382:70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- 24.McGavern DB. Zoecklein L. Drescher KM. Rodriguez M. Quantitative assessment of neurologic deficits in a chronic progressive murine model of CNS demyelination. Exp Neurol. 1999;158:171–181. doi: 10.1006/exnr.1999.7082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaspar BK. Frost LM. Christian L. Umapathi P. Gage FH. Synergy of insulin-like growth factor-1 and exercise in amyotrophic lateral sclerosis. Ann Neurol. 2005;57:649–655. doi: 10.1002/ana.20451. [DOI] [PubMed] [Google Scholar]
- 26.Kayatekin BM. Gonenc S. Acikgoz O. Uysal N. Dayi A. Effects of sprint exercise on oxidative stress in skeletal muscle and liver. Eur J Appl Physiol. 2002;87:141–144. doi: 10.1007/s00421-002-0607-3. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki M. McHugh J. Tork C. Shelley B. Hayes A. Bellantuono I. Aebischer P. Svendsen CN. Direct muscle delivery of GDNF with human mesenchymal stem cells improves motor neuron survival and function in a rat model of familial ALS. Mol Ther. 2008;16:2002–2010. doi: 10.1038/mt.2008.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonilla S. Silva A. Valdes L. Geijo E. Garcia-Verdugo JM. Martinez S. Functional neural stem cells derived from adult bone marrow. Neuroscience. 2005;133:85–95. doi: 10.1016/j.neuroscience.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 29.Fischer LR. Glass JD. Axonal degeneration in motor neuron disease. Neurodegener Dis. 2007;4:431–442. doi: 10.1159/000107704. [DOI] [PubMed] [Google Scholar]
- 30.Sagot Y. Rosse T. Vejsada R. Perrelet D. Kato AC. Differential effects of neurotrophic factors on motoneuron retrograde labeling in a murine model of motoneuron disease. J Neurosci. 1998;18:1132–1141. doi: 10.1523/JNEUROSCI.18-03-01132.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaspar BK. Llado J. Sherkat N. Rothstein JD. Gage FH. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2003;301:839–842. doi: 10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]
- 32.Ozdinler PH. Macklis JD. IGF-I specifically enhances axon outgrowth of corticospinal motor neurons. Nat Neurosci. 2006;9:1371–1381. doi: 10.1038/nn1789. [DOI] [PubMed] [Google Scholar]
- 33.Kanda T. Iwasaki T. Nakamura S. Ueki A. Kurokawa T. Ikeda K. Mizusawa H. FGF-9 is an autocrine/paracrine neurotrophic substance for spinal motoneurons. Int J Dev Neurosci. 1999;17:191–200. doi: 10.1016/s0736-5748(99)00026-x. [DOI] [PubMed] [Google Scholar]
- 34.Haase G. Kennel P. Pettmann B. Vigne E. Akli S. Revah F. Schmalbruch H. Kahn A. Gene therapy of murine motor neuron disease using adenoviral vectors for neurotrophic factors. Nat Med. 1997;3:429–436. doi: 10.1038/nm0497-429. [DOI] [PubMed] [Google Scholar]
- 35.Haase G. Pettmann B. Bordet T. Villa P. Vigne E. Schmalbruch H. Kahn A. Therapeutic benefit of ciliary neurotrophic factor in progressive motor neuronopathy depends on the route of delivery. Ann Neurol. 1999;45:296–304. [PubMed] [Google Scholar]
- 36.Bordet T. Lesbordes JC. Rouhani S. Castelnau-Ptakhine L. Schmalbruch H. Haase G. Kahn A. Protective effects of cardiotrophin-1 adenoviral gene transfer on neuromuscular degeneration in transgenic ALS mice. Hum Mol Genet. 2001;10:1925–1933. doi: 10.1093/hmg/10.18.1925. [DOI] [PubMed] [Google Scholar]
- 37.Greenberg DA. Jin K. VEGF and ALS: the luckiest growth factor? Trends Mol Med. 2004;10:1–3. doi: 10.1016/j.molmed.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 38.Lambrechts D. Storkebaum E. Carmeliet P. VEGF: necessary to prevent motoneuron degeneration, sufficient to treat ALS? Trends Mol Med. 2004;10:275–282. doi: 10.1016/j.molmed.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki M. Svendsen CN. Combining growth factor and stem cell therapy for amyotrophic lateral sclerosis. Trends Neurosci. 2008;31:192–198. doi: 10.1016/j.tins.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 40.Keller-Peck CR. Feng G. Sanes JR. Yan Q. Lichtman JW. Snider WD. Glial cell line-derived neurotrophic factor administration in postnatal life results in motor unit enlargement and continuous synaptic remodeling at the neuromuscular junction. J Neurosci. 2001;21:6136–6146. doi: 10.1523/JNEUROSCI.21-16-06136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lie DC. Weis J. GDNF expression is increased in denervated human skeletal muscle. Neurosci Lett. 1998;250:87–90. doi: 10.1016/s0304-3940(98)00434-0. [DOI] [PubMed] [Google Scholar]
- 42.Kust BM. Copray JC. Brouwer N. Troost D. Boddeke HW. Elevated levels of neurotrophins in human biceps brachii tissue of amyotrophic lateral sclerosis. Exp Neurol. 2002;177:419–427. doi: 10.1006/exnr.2002.8011. [DOI] [PubMed] [Google Scholar]
- 43.Grundstrom E. Askmark H. Lindeberg J. Nygren I. Ebendal T. Aquilonius SM. Increased expression of glial cell line-derived neurotrophic factor mRNA in muscle biopsies from patients with amyotrophic lateral sclerosis. J Neurol Sci. 1999;162:169–173. doi: 10.1016/s0022-510x(98)00333-5. [DOI] [PubMed] [Google Scholar]
- 44.Lu P. Jones LL. Snyder EY. Tuszynski MH. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003;181:115–129. doi: 10.1016/s0014-4886(03)00037-2. [DOI] [PubMed] [Google Scholar]
- 45.Guillot S. Azzouz M. Deglon N. Zurn A. Aebischer P. Local GDNF expression mediated by lentiviral vector protects facial nerve motoneurons but not spinal motoneurons in SOD1(G93A) transgenic mice. Neurobiol Dis. 2004;16:139–149. doi: 10.1016/j.nbd.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 46.Li W. Brakefield D. Pan Y. Hunter D. Myckatyn TM. Parsadanian A. Muscle-derived but not centrally derived transgene GDNF is neuroprotective in G93A-SOD1 mouse model of ALS. Exp Neurol. 2007;203:457–471. doi: 10.1016/j.expneurol.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 47.Manabe Y. Nagano I. Gazi MS. Murakami T. Shiote M. Shoji M. Kitagawa H. Setoguchi Y. Abe K. Adenovirus-mediated gene transfer of glial cell line-derived neurotrophic factor prevents motor neuron loss of transgenic model mice for amyotrophic lateral sclerosis. Apoptosis. 2002;7:329–334. doi: 10.1023/a:1016123413038. [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto M. Ito Y. Mitsuma N. Li M. Hattori N. Sobue G. Parallel expression of neurotrophic factors and their receptors in chronic inflammatory demyelinating polyneuropathy. Muscle Nerve. 2002;25:601–604. doi: 10.1002/mus.10074. [DOI] [PubMed] [Google Scholar]
- 49.Manabe Y. Nagano I. Gazi MS. Murakami T. Shiote M. Shoji M. Kitagawa H. Abe K. Glial cell line-derived neurotrophic factor protein prevents motor neuron loss of transgenic model mice for amyotrophic lateral sclerosis. Neurol Res. 2003;25:195–200. doi: 10.1179/016164103101201193. [DOI] [PubMed] [Google Scholar]
- 50.Wang LJ. Lu YY. Muramatsu S. Ikeguchi K. Fujimoto K. Okada T. Mizukami H. Matsushita T. Hanazono Y, et al. Neuroprotective effects of glial cell line-derived neurotrophic factor mediated by an adeno-associated virus vector in a transgenic animal model of amyotrophic lateral sclerosis. J Neurosci. 2002;22:6920–6928. doi: 10.1523/JNEUROSCI.22-16-06920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bretzner F. Drew T. Changes in corticospinal efficacy contribute to the locomotor plasticity observed after unilateral cutaneous denervation of the hindpaw in the cat. J Neurophysiol. 2005;94:2911–2927. doi: 10.1152/jn.00254.2005. [DOI] [PubMed] [Google Scholar]
- 52.Nishimura Y. Isa T. Cortical and subcortical compensatory mechanisms after spinal cord injury in monkeys. Exp Neurol. 2012;235:152–161. doi: 10.1016/j.expneurol.2011.08.013. [DOI] [PubMed] [Google Scholar]