Abstract
Intracellular zinc homeostasis is regulated by an extensive network of transporters, ligands and transcription factors. The zinc detoxification functions of three transporters and a periplasmic protein regulated by the BaeSR two-component system were explored in this work by evaluating the effect of single gene knockouts in the BaeSR regulon on cell growth rate, free zinc, total zinc and total copper after zinc shock. Two exporters, MdtABC and MdtD, and the periplasmic protein, Spy, are involved in zinc detoxification based on the growth defects at high cell density and increases in free (>1000-fold) and total zinc/copper (> 2-fold) that were observed in the single knockout strains upon exposure to zinc. These proteins complement the ATP-driven zinc export mediated by ZntA in E. coli to limit zinc toxicity. These results highlight the functions of the BaeSR regulon in metal homeostasis.
Introduction
Zinc plays important catalytic, structural and regulatory roles in the cell. Hundreds of metalloproteins bind zinc as catalytic cofactors and/or structural elements1, 2. While zinc is an essential nutrient, excess zinc is toxic to the cell, possibly through inhibition of key enzymes and competition with other relevant metal ions1, 3. For these reasons, intracellular zinc is normally maintained at low “free” or readily exchangeable concentrations (pM levels) 4, 5 and regulated by an extensive network of transporters, ligands and transcription factors6. In E. coli, zinc detoxification is primarily achieved by exporters. ZntA, a P-type ATPase, and ZitB, a cation diffusion facilitator, are two well-studied zinc transporters7. Previous studies have shown that ZntA is up-regulated under high zinc stress to effectively lower the intracellular zinc concentration, while ZitB is constitutively expressed to function as a first-line defense against zinc influx7–9. Other transporters are also proposed to be involved in zinc detoxification, as the zntA knockout cells retain the ability to decrease the free and total zinc concentration, although more slowly5. Potential additional zinc transporters that could function in zinc detoxification include the ZitB homolog Yiip and several amphiphilic transporters10. Transcriptional analysis of cells after zinc shock demonstrated up-regulation of a number of transporter genes other than zntA, including acrD, mdtA, mdtC, mdtD, yfiK, and amtB, etc11. Among these transporters, acrD, mdtA, mdtC and mdtD are all controlled by the BaeSR (bacterial adaptive response) two-component system12, which suggests that the BaeSR regulon may play a role in zinc regulation.
The BaeSR two-component signal transduction system is one of three extra-cytoplasmic stress response (ESR) systems in E. coli that control the adaptive response to changes in the environment13. BaeS is a membrane-bound histidine kinase that senses environmental changes and transduces the information to the cell by catalyzing phosphorylation of the transcription factor BaeR 12. BaeR activates the expression of eight genes, which encode three transporters, one periplasmic protein and both BaeS and BaeR 12, 14 (Fig. 1). These genes are involved in the envelope stress response, drug resistance, and metal resistance of E. coli and Salmonella. The BaeSR regulon responds to a wide range of environmental stresses, including spheroplasting, and exposure to indole, tannin, flavonoid, sodium tungstate, and high levels of zinc or copper11, 12. Previous studies have analyzed the role of the BaeSR regulon in conferring drug resistance, while the function of these proteins in zinc detoxification and homeostasis is still emerging15. The transporters and the periplasmic protein on the BaeSR regulon are up-regulated upon exposure of cells to high concentrations of zinc11, and Salmonella strains lacking genes on this regulon exhibit significant growth defects under high zinc conditions15. Here we analyze the effects of single gene knockouts in this gene cluster on the cellular response to zinc toxicity.
Fig. 1. Schematic illustration of the BaeSR regulon.
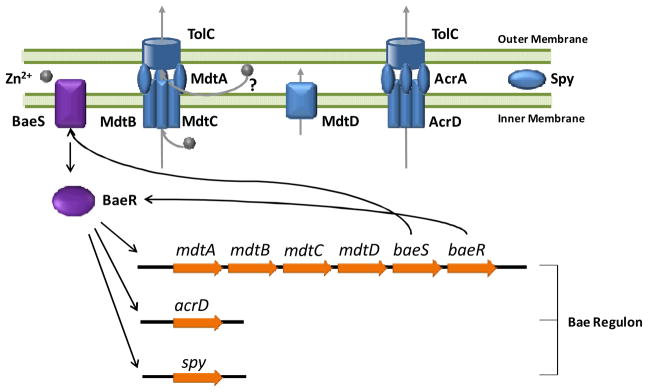
The BaeSR regulon encodes three transporters and one periplasmic protein. The membrane-bound BaeS senses environmental signals and transduces them to the transcription activator BaeR, which up-regulates the expression of eight genes on this regulon including baeR and baeS. MdtABC forms a RND-type trans-envelope efflux complex with TolC. MdtD belongs to the major facilitator family for metal transport. AcrD forms another RND-type efflux system in complex with AcrA and TolC. Spy is a periplasmic protein that exhibits ATP-independent chaperone activities in vitro. The regulator BaeS and BaeR are also up-regulated, forming a positive feedback loop to amplify the transcriptional response.
BaeS is an inner-membrane-bound histidine kinase containing a periplasmic sensing domain16. BaeR is a cytoplasmic transcription factor that can be phosphorylated by BaeS, which increases the affinity of BaeR for DNA, leading to initiation of transcription of the entire regulon. Genetic studies revealed a consensus BaeR binding sequence, 5′-TTTTTCTCCATDATTGGC-3′ (where D is G, A, or T), in the promoter regions of the mdtABCD-baeSR operon, acrD gene and spy gene 14. The mdtABC and acrD genes encode components of two RND (Resistance-Nodulation-Cell Division)-type transporters, mdtD encodes a putative transporter that belongs to the major facilitator superfamily (MFS), and spy encodes a small periplasmic protein. Activation of the BaeSR regulon results in a positive feedback loop as the expression of the two-component system, BaeS and BaeR, is also up-regulated.
MdtA, MdtB and MdtC form a RND-type trans-envelope efflux multiplex with the outer membrane pore protein TolC in E. coli17. MdtB and MdtC form a heterotrimeric proton-substrate antiporter across the inner membrane, which is connected to the outer membrane protein TolC through the membrane fusion protein MdtA17. Exposure of E. coli to 0.2 – 1 mM zinc resulted in a 2- to 3-fold increase in the transcription levels of mdtA and mdtC via regulation of BaeSR11, 15. Over-expression of MdtABC confers resistance to drugs such as novobiocin (16-fold) and deoxycholate (32-fold)18. MdtB is not necessary for drug resistance as trans-expression of mdtA and mdtC are sufficient to induce this phenotype in E. coli17. Under these conditions it is possible that the MdtB/MdtC heterotrimer may be replaced by an MdtC homotrimer in the transmembrane channel as MdtB shares ~ 50% amino acid sequence similarity with MdtC. Similar to all RND-type transporters in E. coli (except the copper transporter CusABC), MdtABC requires TolC as an outer membrane factor to function15. The fourth gene on the MdtABCD-BaeSR operon, mdtD, encodes a putative transporter that belongs to the major facilitator superfamily (MFS) based on sequence analysis19. MFS transporters are single-polypeptide carriers capable of transporting small solutes in response to chemiosmotic gradients20. They typically have 12 – 14 transmembrane domains. Similar to mdtA/B/C, exposure of cells to high zinc resulted in an ~ 2-fold increase in mdtD transcription11. The transport mechanism and biological function of MdtD is unknown, including no evidence that MdtD is involved in multidrug resistance18,19. acrD encodes an aminoglycoside efflux pump21, 22 that is a homologue to the well-known multidrug resistance transporter, AcrB. Like AcrB, AcrD forms a trans-inner-membrane homotrimer which complexes with the membrane fusion protein AcrA and outer membrane channel TolC as an RND-type efflux pump23. Exposure to zinc caused a 4- to 6-fold increase in acrD transcription, activated by BaeSR15. Spy, another gene product modulated by the BaeSR system, is a small periplasmic protein that was first demonstrated to be up-regulated during spheroplasting24. Additionally, denatured proteins and metal stress induce expression of Spy. Copper and zinc exposure lead to over-expression of Spy through regulation by CpxA/R and BaeSR, respectively25. The transcription of Spy is upregulated 20- to 50-fold upon exposure of cells to zinc. Although Spy has not been shown to bind zinc with high affinity11, 26, homologues of Spy have been identified with bound metals27, 28. Recent findings suggest that Spy functions as a periplasmic molecular chaperone to suppress protein aggregation and enhance protein folding under envelope stress conditions29. Therefore, Spy may protect against zinc-induced protein denaturation and aggregation in the periplasm.
Although the BaeSR regulon is up-regulated upon high zinc exposure, the biological functions of the individual proteins are not clear. The roles of the BaeSR-regulated transporters in protecting cells against high zinc stress were first studied in Salmonella enterica15. The mutant strains ΔacrDΔmdtABC and ΔbaeSR are much more sensitive to zinc and copper stress than the wild type strain, while no changes in drug resistance were observed. These results suggest that the BaeSR regulon may play an important physiological role in metal homeostasis, similar to the function of the RND-type copper transporter, CusABC, in copper resistance30. To test this, we investigated the function of each component of the BaeSR regulon in the regulation of zinc homeostasis in E. coli. We demonstrate that individual deletions of mdtC, mdtD and spy in E. coli both decrease the growth rate at high cell density in the presence of high zinc and lead to a significant increase in the total zinc concentration after zinc shock. Furthermore, the free zinc concentration increases dramatically after zinc shock, as measured by an expressed carbonic anhydrase-based excitation ratiometric fluorescence resonance transfer zinc sensor5. These data provide clear evidence that multiple components of the BaeSR regulon are involved in the regulation of intracellular zinc homeostasis, maintaining low cellular zinc concentrations upon exposure to high extracellular zinc.
Methods and Materials
E. coli Strains and Growth Conditions
E. coli strains from the Keio Knockout Collection were obtained from the E. coli Genetic Stock Center of Yale University31. The parent strain of this single knock-out collection, BW25113 {F, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), λ−, rph-1, Δ(rhaD-rhaB)568, hsdR514} was derived from E. coli K-12, and is considered “wild type” in this work. The mutant strains in this collection (ΔmdtA (JW5338-1), ΔmdtB (JW2060-1), ΔmdtC (JW2061-3), ΔmdtD (JW2062-1), ΔacrD (JW2454-1), ΔbaeS (JW2063-1), ΔbaeR (JW2064-3), and Δspy (JW1732-1)) were constructed from BW25113 by replacing the target gene with a kanamycin insert 31. E. coli cells were cultured in MOPS minimal medium supplemented with thiamine (1 μg/ml) and uracil (20 μg/ml) at 25°C 32. The doubling time (TG) for growth of these cells was determined by a fit of Equation 1 to the growth curves (OD600 versus time).
| Eq. 1 |
Measurement of Intracellular Total Zinc
E. coli cells transformed with the plasmid pTH_H94N_CA_TagRFP (see 2.3 for plasmid construction) that encodes a carbonic anhydrase-based zinc sensor were diluted 1:50 from an overnight culture grown in MOPS minimal media with 100 μg/ml ampicillin into MOPS medium without antibiotics8, and grown at 25°C to OD600 ~ 0.3 before a final concentration of 50 μM ZnSO4 was added to the flask. An aliquot (1 ml) of the cell culture was centrifuged (15,000 × g, 1 min) at various time points after addition of Zn. The pellets were washed twice with 500 μl ice-cold MOPS medium without additional zinc and once with 1 ml ice-cold ddH2O. The pellets were then dissolved in 200 μl of 35% ultra pure nitric acid, and incubated at 37°C overnight. The total metal content in these samples was measured using inductively coupled plasma mass spectrometry (ICP-MS), and the total zinc concentration per cell was calculated by dividing the total metal content by the total cell volume. The total cell volume was derived by multiplying the estimated average cell volume for E. coli (0.5 × 10−18 m3) by the total number of cells, as measured by OD600. The number of cells per OD600 (~ 1.3 × 109) was calibrated by plating the cells and counting the colonies. The volume per cell of 0.5 × 10−18 m3 was derived from the average dimensions of an E. coli cell of 2 μm length and 0.5 μm diameter33 (all of the strains have similar cell dimensions as observed by light microscopy).
Measurement of Intracellular Free Zinc
The intracellular free zinc concentration was measured using carbonic anhydrase-based zinc sensors as described previously with slight modifications5. The sensor expression vectors pTH_CA_TagRFP and pTH_H94N_CA_TagRFP were constructed by inserting the DNA sequence of wild type CA_TagRFP or H94N CA_TagRFP fusion genes between the restriction sites NcoI and KpnI after the trc-lac promoter on the vector pTrcHisa (Invitrogen). The CA_TagRFP genes were sequenced at the University of Michigan Sequencing Core facility. E. coli cells chemically transformed with one of these expression vectors were grown in MOPS minimal medium with 100 μg/ml ampicillin to OD600 ~ 0.3 at 30°C then induced by addition of 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG). The induced cultures were incubated at 30°C overnight allowing expression and maturation of the sensor. The culture was then diluted 1:20 into fresh MOPS medium without antibiotics and incubated at 30°C allowing the cells to recover and grow to log-phase. At OD600 ~ 0.3, samples (2 μl) of the culture were placed on a poly-L-lysine-coated 96-well glass bottom plate (Matrical) and incubated with 100 μl 2 μM dapoxyl sulfonamide (DPS)34 in MOPS medium for 20 min at room temperature. Then excess DPS was removed and 100 μl MOPS medium was added. Before imaging, 11 μl of various 10× ZnSO4 stock solutions were added to the imaging medium to make the final total zinc concentrations (0, 10, 50, and 100 μM) for the zinc shock experiments. Each culture sample was repeated in 5 separate wells, and a maximum of 2 images was taken from each well to limit photobleaching. Images were acquired at room temperature on a Nikon TE2000U inverted fluorescence microscope from two channels: FRET channel (Ex 350 nm/Em 620 nm, exposure time 500 ms) and FP channel (Ex 530 nm/Em 620 nm, exposure time 200 ms). The average IFRET/IFP ratio was compared to the in situ calibration curve of the sensor to calculate the intracellular free zinc concentration. In situ calibrations of the sensors were carried out by incubating the cells with NTA-chelated zinc buffers plus digitonin and DPS, as described previously5.
mRNA Preparation and Quantification
E. coli cells with the plasmid pTH_H94N_CA_TagRFP were grown to OD600 ~ 0.3 in MOPS medium without antibiotics at 37°C, cooled for ~ 20 min to room temperature (~ 25°C), and then various concentrations of ZnSO4 (0, 10, 50, and 100 μM) were added. A 0.5 ml sample of the cell culture was collected at various times, and diluted into 1 ml of RNeasy Bacteria Protect (Qiagen) solution. The mixture was vortexed for 5 s, incubated at room temperature for 5 min, and centrifuged for 10 min at 5,500 × g. The cell pellets were flash frozen in a dry ice-ethanol bath, and then stored at −80°C. Total mRNA was extracted from the cell pellets using the RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions. On-column DNase I treatment was conducted to eliminate the residual DNA during the extraction. The purified total RNA was then re-incubated with DNase I for 30 min using the DNA-free DNase treatment and removal reagents (Ambion). The total mRNA concentration was measured using the NanoDrop spectrophotometer (Thermo Scientific) and an extinction coefficient of 27 (ng/ml)−1cm−1.
mRNA transcripts of the mtdC, baeR, acrD and spy genes were quantified by RT-PCR. Template cDNA was synthesized using the SuperScript III Reverse Transcriptase Kit (Invitrogen) with total mRNA as the template and random hexamers as the primers. ~ 50 ng of the synthesized first strand cDNA was used as template in the 20 μl RT-PCR reaction. The PCR primers for the target genes and the housekeeping gene rrsD (16S ribosomal RNA of rrnD operon) were designed using the software Primer3. These primers clone a ~ 200 bp DNA sequence within each gene (listed in Table 1). The RT-PCR experiments were set up in triplicate and conducted using the SYBR Green qPCR SuperMix (Invitrogen) on a Mastercycler® ep realplex real time PCR machine (Eppendorf).
Table 1.
Primers for quantification of gene transcripts by RT-PCR.
| rrsD-5′: CTCAAAGGAGACTGCCAGTGATAA |
| rrsD-3′: ACGATTACTAGCGATTCCGACTTC |
| mdtA-5′: TTAATAATCAGGATGATGCGCTGT |
| mdtA-3′: CACCACTTTCTGACTGTCCTGAAT |
| mdtB-5′: CGGTTATGGTGTTTCTCGATTTTT |
| mdtB-3′: AGGTCAGCGAGATAATGGTAAAGC |
| mdtC-5′: TCCAGACCAATGATGAGCTAAAAA |
| mdtC-3′: TGTCAACCGTCTGGATAATATTGG |
| mdtD-5′: AGATTGACGGTGATGAAAATCGTA |
| mdtD-3′: GTAGTTCGGCATTAACAGCAATGT |
| baeR-5′: GAGTTACCAATCGACGAAAACACA |
| baeR-3′: CAGGGAGCATCAGATCTAACAGG |
| acrD-5′: ACTTCACCCATGAAAAAGACAACA |
| acrD-3′: CGATAACGCGAGCTTCTTTAATTT |
| spy-5′: AGGACATGATGTTCAAAGACCTGA |
| spy-3′: ATGTTAGCTTTGCGCTGTTCTTC |
Results
Growth Rates of Bae Regulon Knockout Strains
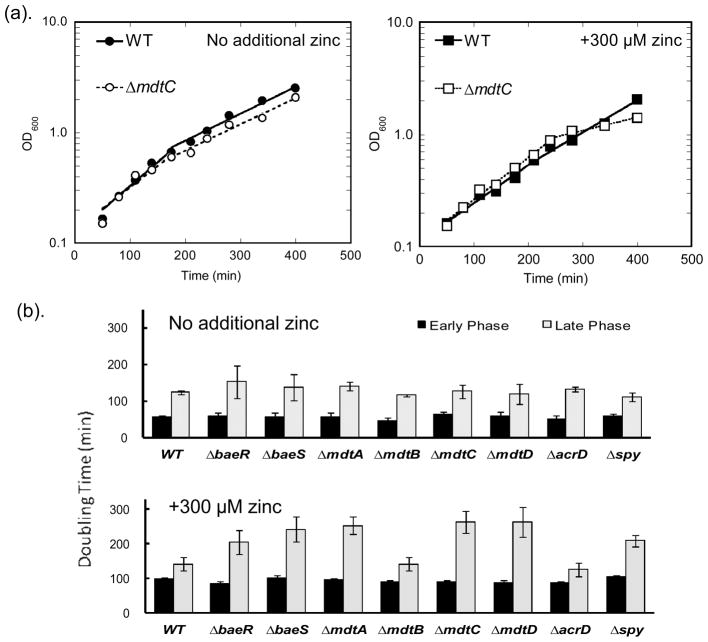
To evaluate the role of the Bae regulon in zinc homeostasis we first examined whether single gene knockouts affect the growth rate of E. coli in the presence or absence of high zinc. Overnight cultures of the wild type (WT) and single gene knockout E. coli strains (ΔbaeR, ΔbaeS, ΔmdtA, ΔmdtB, ΔmdtC, ΔmdtD, ΔacrD, and Δspy) were diluted to OD600 ~ 0.1 into MOPS minimal medium without additional zinc or with 300 μM ZnSO4, and the average doubling time was calculated from the time-dependent increase in OD600 using Eq. 1.
The wild type and knockout BW25113 E. coli strains exhibit biphasic growth in MOPS minimal media (as shown in Fig 2(a)); the doubling time at low cell density (OD600 < 0.8) is ~ 50 min which increases to ~ 130 min at higher cell density. Under these conditions, no significant difference in growth rate was observed between WT and any of the single knockout strains in either the early or late growth phase (Fig. 2(b)). This is not surprising given that the Bae regulon is primarily responsible for the cell’s adaptive response to environmental stress conditions and therefore may not be essential under normal growth conditions. Addition of 300 μM zinc to the medium decreases the growth of both the wild type and the deletion strains in the early growth phase by ~2-fold. Furthermore, in the late growth phase addition of zinc leads to slower growth rates compared to WT (TG = 142 ± 19 min) for the following single knockout strains (Fig. 2(b)): ΔbaeR (TG = 205 ± 34 min), ΔbaeS (TG = 243 ± 35 min), ΔmdtA (TG = 253 ± 26 min), ΔmdtC (TG = 264 ± 33 min), ΔmdtD (TG = 263 ± 43 min) and Δspy (TG = 209 ± 17 min). These results indicate that the proteins encoded by the Bae regulon enhance cell viability under zinc stress exacerbated by nutrition and energy constraints. One explanation for these results is that these proteins may alleviate zinc stress by complementing other energy-dependent zinc detoxification mechanisms that are less functional at this growth stage.
Fig. 2. Growth curves and doubling time of BaeSR regulon single gene knockout strains.
(a). Representative growth curves with the wild type and ΔmdtC strains. Cultures of E. coli strains were diluted into MOPS minimal medium without added zinc (left panel) or with 300 μM zinc (right panel). OD600 was monitored over time and Eq. 1 was fit to these data to calculate early and late stage average doubling time. (b). Doubling time of E. coli cells with 300 μM zinc (bottom chart) and without (top chart) zinc. Under exposure to 300 μM zinc, the ΔbaeR, ΔbaeS, ΔmdtA, ΔmdtC, ΔmdtD and Δspy strains (labeled with stars) exhibit slower growth rates (TG > 200 min) than WT (TG ~ 140 min) in the late stage.
The single knockouts ΔmdtB and ΔacrD do not exhibit significant growth defects relative to the wild type strain even upon addition of 300 μM zinc. Previous experiments have demonstrated that MdtB can be replaced by an MdtC in the transmembrane heterotrimer19. Therefore, ΔmdtB may not exhibit a growth phenotype due to this functional complementation by MdtC. The lack of a growth defect observed with the strain ΔacrD suggests that AcrD does not play a significant role in protection against metal stress.
Free Zinc Changes after Zinc Shock in Bae Regulon Deletion Strains
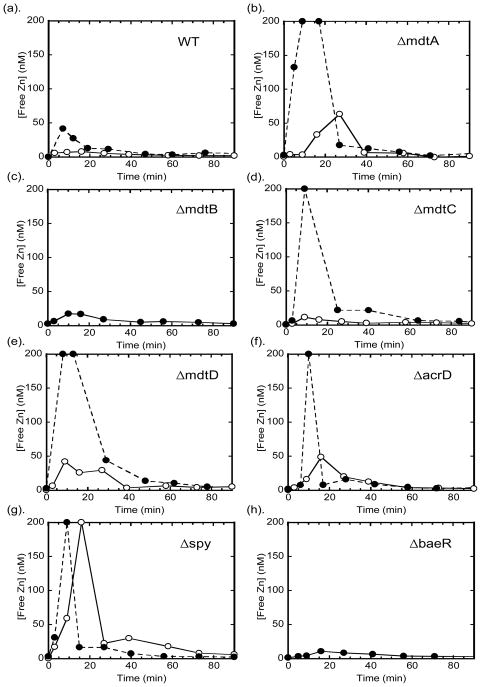
To examine whether the BaeSR regulon components play a role in regulating intracellular zinc concentration, we monitored the intracellular free zinc changes upon zinc shock in these single knockout strains using the expressible H94N CA_TagRFP sensor. This sensor quantifies intracellular free zinc concentrations in the range of 1 – 200 nM8. Consistent with previous observations8, the readily exchangeable zinc concentration in wild type E. coli in MOPS minimal medium is below the detection limit of this sensor (< 1 nM). However, after zinc shock a transient increase in the intracellular zinc concentration is observed where the maximal free zinc concentration varies with the extracellular zinc concentration, peaking at ~ 10 nM and ~ 50 nM intracellular zinc for addition of 10 μM and 50 μM zinc, respectively, to wild type E. coli (Fig. 3). The maximal concentration of intracellular zinc after the 50 μM zinc shock is dramatically increased (to > 200 nM which is beyond the dynamic range of the sensor) in the single knockout strains ΔmdtA, ΔmdtC, ΔmdtD, and Δspy, but not in the ΔmdtB, ΔbaeS, and ΔbaeR strains. The initial high zinc concentration dropped significantly within 20 – 40 min, and then gradually subsided to a level below the detection limit within 1 hr. The magnitude of the increase in free zinc depends on the concentration of the external zinc concentration, as the free zinc peak is lower at 10 μM ZnSO4 (15 – 70 nM) for all of the strains except Δspy where the duration of the elevated zinc concentration is decreased. These data clearly indicate that all three transporters (MdtABC, MdtD and AcrD) and the periplasmic protein (Spy) are involved in limiting the intracellular free zinc concentration to various extents under zinc stress, although the elevated free zinc does not always translate into growth defects as in the case of AcrD. The temporal patterns for intracellular free zinc changes for the ΔmdtA, ΔmdtC, and Δspy strains after zinc shock are comparable to the changes previously measured for deletion of ZitB8, a cation diffusion facilitator important for zinc detoxification.
Fig. 3. Intracellular free zinc changes after zinc shock.
E. coli cells transformed with the plasmid pTH_H94N_CA_TagRFP were grown in MOPS minimal medium to log phase (OD600 ~ 0.3 – 0.4), incubated with dapoxyl sulfonamide and imaged using a fluorescence microscope as described in Methods and Materials 2.3. 10 μM (open circles) or 50 μM ZnSO4 (solid circles) was added to the imaging medium and intracellular free zinc concentrations were measured over time from the IFRET/IFP ratio compared to the in situ calibration curve of the H94N CA_TagRFP sensor (in situ apparent KD for zinc ~ 9 nM).
The transporter MdtABC plays a role in alleviating the free zinc spike in the cell after zinc shock, consistent with the growth effects of the single MdtA and MdtC knockouts in the previous section. At the 10 μM zinc shock, the increase in the free zinc concentration is higher in the ΔmdtA strain compared to the ΔmdtC, although differential effects of zinc stress on the growth rates were not observed for these strains. The free zinc concentrations in the ΔmdtB strain after zinc shock are comparable to those observed for the wild type strain, consistent with the previous observations that the function of MdtB can be compensated by MdtC17 and no growth defects were observed in ΔmdtB. These results indicate that MdtABC participates in zinc detoxification by lowering the initial intracellular free zinc concentration when the cells encounter zinc stress, possibly by exporting zinc from the periplasm and/or the cytosol. Similarly, the increase in free zinc in the ΔmdtD strain indicates that MdtD is involved in zinc detoxification upon zinc shock. These data are consistent with the growth defects of the mdtD knockout, implicating MdtD as playing a role in zinc transport.
Although deletion of acrD does not affect cell growth in high zinc, the free zinc concentration in this strain increases significantly after zinc shock indicating that AcrD contributes to lowering free zinc content under these conditions. However, the transient intracellular zinc spike has the shortest duration for the AcrD knockout strain (<20 min) perhaps contributing to the lack of an effect of deletion of AcrD on the growth rate. These data suggest that AcrD may play a minor role in the cellular response to zinc toxicity.
Interestingly, Spy plays a remarkable role in regulating the intracellular free zinc concentration, even though there is no evidence that this protein is directly involved in zinc transport. The intracellular free zinc concentration is the highest (>200 nM) in the Δspy knockout strain, even at 10 μM ZnSO4 (Fig. 3(g)) and the zinc remains elevated for a longer time than any of the other knockout strains tested here. The effects on free zinc are consistent with the growth defects seen at high zinc. Two possible explanations for the free zinc changes in the Δspy strain are: (i) Spy may function as a molecular chaperone to enhance the activity of some of the zinc exporters so in the absence of Spy the constitutive metal transport system is less effective, resulting in higher initial zinc; and (ii) Spy may bind to and thereby decrease the free zinc concentration in the periplasm, reducing the initial zinc influx catalyzed by zinc transporters in the plasma membrane.
Although the expression of mdtABC, mdtD, acrD, and spy are upregulated under zinc stress11 (see Fig. 5), the baeS and baeR knockouts have a minimal effect on the intracellular free zinc concentration after zinc shock (<10 nM) (Fig. 3(h)) compared to the wild type strain, even though the growth rates of the ΔbaeS and ΔbaeR strains are slower in high zinc medium. This result suggests that the basal level of expression of the various components of the BaeSR regulon is sufficient to serve as the first line of defense against the transient, environmental zinc shock. Alternatively, deletion of baeS and baeR may result in overexpression of other protective mechanisms against metal stress, such as increased expression of zntA or zitB, that compensate for the impairment of the Bae regulon function.
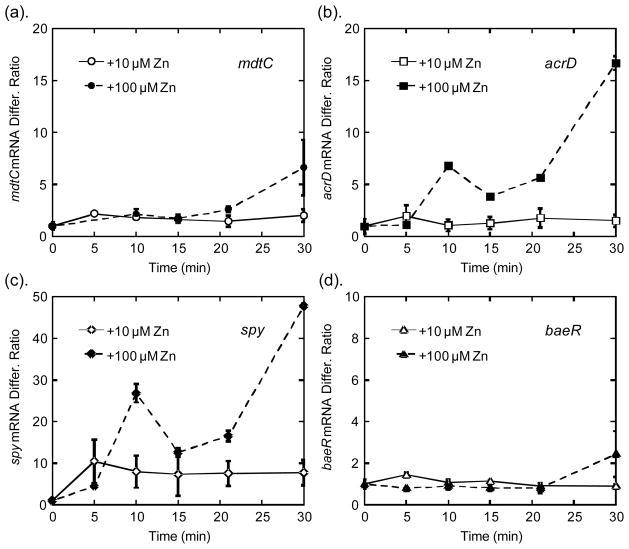
Fig. 5. Transcriptional response of BaeSR regulon upon exposure to zinc.
Wild type E. coli cells were exposed to 10 μM and 100 μM ZnSO4, and the transcript levels of each gene were measured by RT-PCR at various time points after zinc exposure. The transcription level of mdtC (a) and baeR (b) increased to 7-fold and 2-fold at 30 min, respectively. The mRNA of spy (c) and acrD (d) exhibited biphasic increases up to 48-fold and 17-fold at 30 min, respectively.
Total Metal Changes after Zinc Shock in Bae Regulon Deletion Strains
We then measured the total metal changes upon zinc shock at 50 μM ZnSO4 using ICP-MS in the wild type and knockout strains that exhibited both late stage growth defects and substantial intracellular free zinc increases upon zinc shock, including ΔmdtC and Δspy.
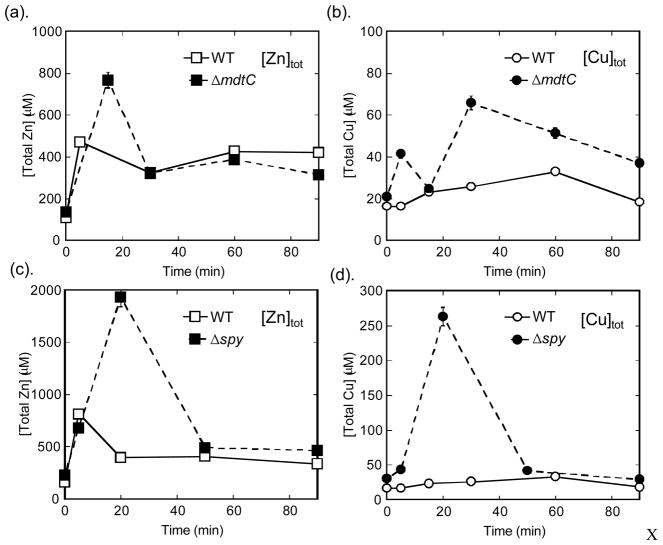
ΔmdtC and Δspy showed increases in both total zinc and copper compared to the wild type strain after zinc shock (Fig. 4). No significant alterations in total iron, calcium and magnesium contents were observed in these strains (data not shown). Previous studies have demonstrated that the Bae regulon can be up-regulated by addition of Cu(II) as well as zinc, and that the ΔmdtABCΔacrD and ΔbaeSΔbaeR mutants grow much more poorly than wild type in the presence of high copper in the medium15. However, it is interesting that exposure to external zinc can cause a significant increase in the intracellular copper homeostasis in these single knock-out mutants, perhaps due to competition between zinc and copper for binding to metal transporters and buffering proteins. The increased intracellular total copper levels may contribute to the growth defects observed under zinc stress conditions.
Fig. 4. Total zinc changes after zinc shock.
E. coli cells grown at log phase were exposed to 50 μM ZnSO4, and total cellular metal concentrations were measured by ICP-MS, as described in Section 2.2. Both total zinc (a) and copper (b) levels are much higher in the ΔmdtC strain than that of WT. Total zinc (c) and copper (d) also increased dramatically (>10-fold) in the Δspy strain compared to WT, highlighting its importance in metal homeostasis.
In the ΔmdtC strain, the total zinc concentration increased by 2-fold at ~ 20 min which then subsided to a level similar to that of wild type at 30 min (Fig. 4(a)). This increase in total zinc is much smaller than the increase in free zinc (< 1 nM to > 200 nM) reflecting the larger zinc buffering capacity of the cell8. In the WT cells, a modest increase in total copper (~50%) is observed after zinc shock, which then decreases to the initial level at 90 min. In contrast, in the ΔmdtC cells the intracellular total copper content increases by 2- to 3-fold and remains ~ 2-fold higher than the baseline level even after 90 min (Fig. 4(b)). It is worth noting that the copper efflux system, CusABC, a RND-type transporter, was previously reported to be significantly up-regulated upon exposure to zinc11, implying that the cells need to export copper under high zinc stress. The elevated copper levels in the MdtABC knockout suggest that this protein may export both zinc and copper.
In the Δspy knockout strain, dramatic increases in both total zinc (~ 10-fold) and copper (~ 12-fold) were observed at ~ 20 min (Fig. 4(d)(d)) with a similar temporal pattern. These data indicate that the loss of Spy affects both copper and zinc homeostasis, but not other metals such as Ca and Mg (data not shown). As suggested previously, Spy could functions either (or both) as: (i) a molecular chaperone to protect the membrane and periplasmic proteins against zinc and copper stress to limit uptake or enhance export and/or (ii) a metal binding protein to decrease the concentration of zinc and copper in the periplasm and limit metal uptake.
Transcriptional Response to Zinc Shock of Bae Regulon Deletion Strains
The Bae regulon had previously been shown to be up-regulated upon exposure to high zinc stress11, 15. Here we monitored the time course of the transcription of mdtC, acrD, spy, and baeR genes on the regulon to gain further insight into their functions. Two genes on the mdtABCD-baeSR operon, mdtC and baeR, are moderately up-regulated (2- and 7-fold, respectively) 30 min after exposure to high zinc. Interestingly, the time course of the changes in the mRNA levels does not correspond to that of free zinc changes. The maximal intracellular zinc peak occurs at ~20 min and then decreases while the mRNA levels increase after 30 min. The observed increases in transcription are consistent with previously published results11, 15 where the increase in transcription was shown to be primarily through regulation of BaeR, as the transcriptional response was abolished in a ΔbaeSR strain15.
The transcription levels of spy and acrD increase more significantly (up to 50-fold and 17-fold at 30 min), and interestingly exhibit two distinct peaks. The transcript levels peak at ~ 10 min after zinc shock, and then decline afterwards at 15 min., coinciding nicely with the increases in intracellular free zinc levels (Fig. 3 and Fig. 5). Therefore, it is possible that the BaeSR system directly senses and responds to changes in intracellular free zinc levels. However, unlike the transcription of zntA that plateaus or decreases as the free zinc level subsides8 a second phase of increasing transcript level is seen with spy and acrD, increasing to the highest level at 30 min. Two possible explanations to this second phase of transcriptional increase are: (i) the BaeSR two-component system may sense tertiary stress signals that escalate overtime under prolonged exposure to high zinc concentrations to trigger the stress response transcription; and (ii) the second phase of transcriptional increase could result from the intrinsic positive feedback loop of the BaeSR system, in which BaeS/baeR are up-regulated to amplify the stress signal and activate additional transcription14. However, since the transcript level of baeR did not significantly increase until after 20 min (Fig. 5(b)), it is unlikely that the positive feedback loop was responsible for the second phase of transcription increase.
Discussion
The BaeSR regulon is responsible for envelope-related stress responses13; however, the functions of each of its components remain unclear. Past explorations of the function of MdtABC has focused on its role in multidrug resistance12, 17, 19. However, over-expression of MdtABC only provides a minor enhancement in drug resistance compared to other drug resistance associated transporters, and the ΔmdtABC knockout strain did not alter the cellular drug resistance profile15. On the other hand, in Salmonella knockout of mdtABC/acrD or BaeSR dramatically reduced the cells viability under zinc and copper stress 15. This observation, together with the fact that BaeSR regulon is up-regulated under exposure to zinc, led to the proposal that the BaeSR regulon may play important roles in the cellular resistance to metal stress. Here we explored the functions of these transporters and periplasmic protein in zinc homeostasis by analyzing the effects of single knockouts of the genes of the BaeSR regulon in E. coli on the cellular response to zinc shock. These results demonstrate that the RND-type transporter MdtABC plays an important role in the cellular response to metal stress, especially under energy constraint and zinc shock conditions. The three knockout strains ΔmdtA, ΔmdtB, and ΔmdtC, exhibit different patterns in growth rates, and free and total zinc changes, coinciding with the proposed function of each component based on previous studies17, 19. MdtC is the trans-inner-membrane antiporter that drives efflux of a substrate into the periplasmic space through proton exchange19. The ΔmdtC knockout produced a growth defect in the late phase (Fig. 2) and a significant transient increases in cytosolic free zinc (Fig. 3(d)), total zinc and total copper levels (Fig. 4(a)(b)). This indicates that loss of mdtC leads to accumulation of zinc and copper in the cell which presumably accounts for the lower doubling time under higher energy constraints when it is more difficult for the cell to alleviate the metal stress by other mechanisms. MdtA is a membrane fusion protein that links the trans-inner-membrane trimer with the outer-membrane pore, TolC, to facilitate efficient extrusion of substrates to the outside of the cells17, 19. Growth defects and increases in cytosolic free zinc in ΔmdtA were similar to that of ΔmdtC (Fig. 2(b) and Fig. 3(b)). Loss of MdtA could potentially reduce the cells’ ability to lower the zinc concentration in the periplasm, as MdtC cannot form a trans-envelope complex with TolC to efficiently extrude zinc ions to the outside. This build-up of periplasmic zinc can be toxic by interfering with periplasmic metalloproteins or osmotic levels. Additionally, higher periplasmic zinc levels correlate with higher intracellular zinc levels due to enhancement of zinc influx via transporters and the cytosolic zinc could limit cell growth. No significant growth defects or changes in cytosolic free zinc were observed in ΔmdtB (Fig. 2(b) and Fig. 3(c)), which is consistent with the previous proposal that the MdtB-MdtC heterotrimer can be substituted with MdtC homotrimer without significant loss of function17.
Bioinformatic analysis indicates that the function of MdtABC may be different from both other E. coli drug efflux and metal efflux RND-type transporters. Although MdtABC is classified as a hydrophobic and amphiphilic efflux RND-type (HAE-RND) transporter, MdtC does not share high sequence identity with other known RND-type multidrug resistance transmembrane proteins (except for MdtB) in E. coli, including AcrB, AcrF, and MdtF (28%, 29%, and 30%, respectively)18. In contrast, the sequence similarities among AcrB, AcrF, AcrD and MdtF are fairly high (60 – 80%). Additionally, MdtC also has low sequence homology with the heavy metal efflux RND-type transporter CusA (23%), which transports Cu(I) into the cell employing a stepwise shuttle mechanism via a series of methionine pairs 35. Interestingly, MdtC shares 73% identity with a putative cation/protein antiporter, CvrA, that is proposed to be involved in coping with osmotic pressure changes and regulation of cell volume21. These analyses indicate that MdtABC may employ a different transport mechanism than other types of RND transporters.
Whether MdtABC exports zinc in the form of free ions or in a ligand complex is unclear. This exporter transports several structurally unrelated amphiphilic molecules (novobiocin, SDS, doxycycline and cholates, etc.), as extrapolated from the observed drug resistance in cells over-expressing MdtABC12, 17–19. This is similar to other drug resistance RND-type transporters that have nonspecific substrate binding sites36. Therefore, it is possible that MdtABC exports zinc in a ligand-complex form from the cytosol and/or the periplasm to lower the total zinc content. Nonetheless, the observation of a dramatic increase in intracellular free zinc content in the ΔmdtC and ΔmdtA knockout strains suggests that MdtABC reduces the intracellular free zinc concentration by a net extrusion of zinc ions from the cytosol. Overall, these results reveal that the MdtABC transporter lowers the cellular zinc content after zinc shock, and therefore plays an important role in cell survival under high zinc stress.
MdtD is an uncharacterized member of the major facilitator superfamily (MFS) of transporters16. MFS members are functionally diverse, transporting a wide range of molecules from inorganic phosphate to organic drug compounds20. Although proposed as a putative multidrug transporter, neither deletion of mdtD nor over-expression affect cellular drug resistance15, 17, 18. Here we showed that MdtD is involved in the zinc stress response in E. coli. The mdtD knockout moderately decreased the growth rate in the presence of zinc at higher cell density (Fig. 2(b)), and dramatically increased the intracellular free zinc concentration after zinc shock (Fig. 3(e)). These results indicate that MdtD is involved in zinc homeostasis under metal stress conditions. Since no known MFS family members transport divalent cations20, it is likely that zinc is co-transported in a complex with other ligands that are substrates of MdtD.
AcrD may also be implicated in alleviating zinc stress in addition to its function in drug resistance. AcrD shares 66% and 63% sequence identity with the well-known drug resistance transporters, AcrB and AcrF18. Furthermore, AcrD transports aminoglycosides in vitro in the presence of the membrane fusion protein AcrA22, 37. Our results showed that intracellular free zinc concentration is higher in the ΔacrD knockout compared to the wild-type strain upon zinc stress (Fig. 3(f)); however, this increased concentration is not sufficient to cause negative effects on the cell growth rates (Fig. 2(b)). The transcription of acrD increases in a biphasic manner up to 17-fold after zinc shock, a more dramatic increase than the transcription level of mdtC (7-fold) (Fig. 5(a)). The implication of this increase in transcription is unclear. One speculation is that up-regulation of AcrD may be a response to secondary effects caused by elevated zinc level. One clue is that AcrD is involved in L-cysteine efflux from the cell38, and exposure to high zinc could lead to over-production of cysteine, as shown in a previous study9. Therefore, up-regulation of AcrD could be responding to a high cellular cysteine level.
Spy, an ATP-independent molecular chaperone in the periplasmic, is also implicated in a cellular response to metal-associated stresses. The Δspy knockout strain exhibited a slower growth rate under high zinc stress (Fig. 2(b)), and large temporary increases in free zinc (Fig. 3(g)), and total zinc/copper upon zinc shock (Fig. 4(c)(d)). Spy is also aggressively up-regulated upon zinc exposure, mediated by the BaeSR system25. However, the specific function of Spy in zinc homeostasis is unclear. Spy shares structural homology with Zrap, a periplasmic zinc-dependent molecular chaperone that requires zinc for its activity and is up-regulated upon zinc exposure27, 39. Spy also shares superficial structural similarity of helix bundles to the copper-binding transcriptional regulator Mycobacterium tuberculosis CsoR (copper-sensitive operon repressor) and CnrX, the periplasmic metal sensing domain of an ECF-type sigma factor involved in cobalt and nickel resistance40. Both proteins have been reported to bind transition metals and involved in metal resistance. However, unlike these proteins, there is no evidence that Spy binds zinc with high affinity; previous data demonstrated that Spy does not bind tightly to iminodiacetic acid agarose equilibrated with zinc26. Also, the crystal structure of Spy revealed a long kinked hairpin-like structure of four α-helices that form an antiparallel dimer with no identifiable signature zinc binding motifs41. Previously published data indicate that Spy functions as a periplasmic molecular chaperone42. Therefore, the role of Spy in relieving zinc stress may be to facilitate the folding and to protect the integrity of transmembrane and periplasmic transporters that function in zinc export.
Overall, we have shown that the BaeSR regulon plays an important role in E. coli’s defense against high zinc stress. The MdtABC and MdtD transporters are involved in the initial efflux of zinc, possibly in the form of a small molecule-zinc complex. Spy possibly acts as a periplasmic chaperone to facilitate the folding of the newly synthesized zinc detoxification machinery. Deletion of key components of this regulon renders the cells more sensitive to zinc toxicity, especially under high cell density with energy constraints, highlighting their functions in defense against zinc that are complementary to the ATP-driven exporters, such as ZntA.
The MdtABC and MdtD transporters are not the primary transporters important for metal detoxification since deletion of these genes do not have as significant an effect on cell growth after zinc shock as deletion of zntA, an inducible ATP-dependent zinc exporter. However, the impact of deletion of these transporters on cell growth and zinc content is more substantial that the impact of deleting ZitB8, a known constitutive metal transporter. Therfore, these data demonstrate that MdtABC and MdtD are an important part of a a first-line, constitutive defense network that functions to lower the intracellular metal contents upon zinc shock, similar to the function of ZitB. Furthermore, these transporters are also up-regulated upon zinc exposure, making them responsive to metal stress, similar to ZntA. Overall, these attributes contribue to their functions in zinc detoxification.
Acknowledgments
We thank Dr. Ursula Jakob and Wei-Yun Chen at the University of Michigan Department of Molecular, Cellular and Developmental Biology for their help with the RT-PCR experiments. We thank Dr. Richard Thompson at the University of Maryland School of Medicine for his help with the fluorescence microscopy. We acknowledge the support of National Institute of Health and National Institute of Environmental Sciences for project grant R21 ES018993.
Notes and references
- 1.Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 2.Vallee BL, Auld DS. Active zinc binding sites of zinc metalloenzymes. Matrix Suppl. 1992;1:5–19. [PubMed] [Google Scholar]
- 3.Dineley KE, Votyakova TV, Reynolds IJ. Zinc inhibition of cellular energy production: implications for mitochondria and neurodegeneration. J Neurochem. 2003;85:563–70. doi: 10.1046/j.1471-4159.2003.01678.x. [DOI] [PubMed] [Google Scholar]
- 4.Bozym RA, Thompson RB, Stoddard AK, Fierke CA. Measuring picomolar intracellular exchangeable zinc in PC-12 cells using a ratiometric fluorescence biosensor. Acs Chemical Biology. 2006;1:103–111. doi: 10.1021/cb500043a. [DOI] [PubMed] [Google Scholar]
- 5.Wang D, Hurst TK, Thompson RB, Fierke CA. Genetically encoded ratiometric biosensors to measure intracellular exchangeable zinc in Escherichia coli. J Biomed Opt. 2011;16:087011. doi: 10.1117/1.3613926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eide DJ. Multiple regulatory mechanisms maintain zinc homeostasis in Saccharomyces cerevisiae. J Nutr. 2003;133:1532S–5S. doi: 10.1093/jn/133.5.1532S. [DOI] [PubMed] [Google Scholar]; Eide DJ. Zinc transporters and the cellular trafficking of zinc. Biochim Biophys Acta. 2006;1763:711–22. doi: 10.1016/j.bbamcr.2006.03.005. [DOI] [PubMed] [Google Scholar]; Blencowe DK, Morby AP. Zn(II) metabolism in prokaryotes. FEMS Microbiol Rev. 2003;27:291–311. doi: 10.1016/S0168-6445(03)00041-X. [DOI] [PubMed] [Google Scholar]; Hantke K. Bacterial zinc transporters and regulators. Biometals. 2001;14:239–49. doi: 10.1023/a:1012984713391. [DOI] [PubMed] [Google Scholar]
- 7.Rensing C, Mitra B, Rosen BP. The zntA gene of Escherichia coli encodes a Zn(II)-translocating P-type ATPase. Proc Natl Acad Sci U S A. 1997;94:14326–31. doi: 10.1073/pnas.94.26.14326. [DOI] [PMC free article] [PubMed] [Google Scholar]; Grass G, Fan B, Rosen BP, Franke S, Nies DH, Rensing C. ZitB (YbgR), a member of the cation diffusion facilitator family, is an additional zinc transporter in Escherichia coli. J Bacteriol. 2001;183:4664–7. doi: 10.1128/JB.183.15.4664-4667.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D, Hosteen O, Fierke CA. ZntR-mediated transcription of zntA responds to nanomolar intracellular free zinc. J Inorg Biochem. 2012;111:173–81. doi: 10.1016/j.jinorgbio.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto K, Ishihama A. Transcriptional response of Escherichia coli to external zinc. J Bacteriol. 2005;187:6333–40. doi: 10.1128/JB.187.18.6333-6340.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chao Y, Fu D. Thermodynamic studies of the mechanism of metal binding to the Escherichia coli zinc transporter YiiP. J Biol Chem. 2004;279:17173–80. doi: 10.1074/jbc.M400208200. [DOI] [PubMed] [Google Scholar]; Grass G, Otto M, Fricke B, Haney CJ, Rensing C, Nies DH, Munkelt D. FieF (YiiP) from Escherichia coli mediates decreased cellular accumulation of iron and relieves iron stress. Arch Microbiol. 2005;183:9–18. doi: 10.1007/s00203-004-0739-4. [DOI] [PubMed] [Google Scholar]
- 11.Lee LJ, Barrett JA, Poole RK. Genome-wide transcriptional response of chemostat-cultured Escherichia coli to zinc. J Bacteriol. 2005;187:1124–34. doi: 10.1128/JB.187.3.1124-1134.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leblanc SK, Oates CW, Raivio TL. Characterization of the induction and cellular role of the BaeSR two-component envelope stress response of Escherichia coli. J Bacteriol. 2011;193:3367–75. doi: 10.1128/JB.01534-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raffa RG, Raivio TL. A third envelope stress signal transduction pathway in Escherichia coli. Mol Microbiol. 2002;45:1599–611. doi: 10.1046/j.1365-2958.2002.03112.x. [DOI] [PubMed] [Google Scholar]
- 14.Nishino K, Honda T, Yamaguchi A. Genome-wide analyses of Escherichia coli gene expression responsive to the BaeSR two-component regulatory system. J Bacteriol. 2005;187:1763–72. doi: 10.1128/JB.187.5.1763-1772.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishino K, Nikaido E, Yamaguchi A. Regulation of multidrug efflux systems involved in multidrug and metal resistance of Salmonella enterica serovar Typhimurium. J Bacteriol. 2007;189:9066–75. doi: 10.1128/JB.01045-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagasawa S, Ishige K, Mizuno T. Novel members of the two-component signal transduction genes in Escherichia coli. J Biochem. 1993;114:350–7. doi: 10.1093/oxfordjournals.jbchem.a124180. [DOI] [PubMed] [Google Scholar]
- 17.Nagakubo S, Nishino K, Hirata T, Yamaguchi A. The putative response regulator BaeR stimulates multidrug resistance of Escherichia coli via a novel multidrug exporter system, MdtABC. J Bacteriol. 2002;184:4161–7. doi: 10.1128/JB.184.15.4161-4167.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishino K, Yamaguchi A. Analysis of a complete library of putative drug transporter genes in Escherichia coli. J Bacteriol. 2001;183:5803–12. doi: 10.1128/JB.183.20.5803-5812.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baranova N, Nikaido H. The baeSR two-component regulatory system activates transcription of the yegMNOB (mdtABCD) transporter gene cluster in Escherichia coli and increases its resistance to novobiocin and deoxycholate. J Bacteriol. 2002;184:4168–76. doi: 10.1128/JB.184.15.4168-4176.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pao SS, Paulsen IT, Saier MH., Jr Major facilitator superfamily. Microbiol Mol Biol Rev. 1998;62:1–34. doi: 10.1128/mmbr.62.1.1-34.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radchenko MV, Tanaka K, Waditee R, Oshimi S, Matsuzaki Y, Fukuhara M, Kobayashi H, Takabe T, Nakamura T. Potassium/proton antiport system of Escherichia coli. J Biol Chem. 2006;281:19822–9. doi: 10.1074/jbc.M600333200. [DOI] [PubMed] [Google Scholar]
- 22.Rosenberg EY, Ma D, Nikaido H. AcrD of Escherichia coli is an aminoglycoside efflux pump. J Bacteriol. 2000;182:1754–6. doi: 10.1128/jb.182.6.1754-1756.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elkins CA, Nikaido H. Substrate specificity of the RND-type multidrug efflux pumps AcrB and AcrD of Escherichia coli is determined predominantly by two large periplasmic loops. J Bacteriol. 2002;184:6490–8. doi: 10.1128/JB.184.23.6490-6498.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hagenmaier S, Stierhof YD, Henning U. A new periplasmic protein of Escherichia coli which is synthesized in spheroplasts but not in intact cells. J Bacteriol. 1997;179:2073–6. doi: 10.1128/jb.179.6.2073-2076.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamoto K, Ogasawara H, Ishihama A. Involvement of multiple transcription factors for metal-induced spy gene expression in Escherichia coli. J Biotechnol. 2008;133:196–200. doi: 10.1016/j.jbiotec.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Noll M, Petrukhin K, Lutsenko S. Identification of a novel transcription regulator from Proteus mirabilis, PMTR, revealed a possible role of YJAI protein in balancing zinc in Escherichia coli. J Biol Chem. 1998;273:21393–401. doi: 10.1074/jbc.273.33.21393. [DOI] [PubMed] [Google Scholar]
- 27.Appia-Ayme C, Hall A, Patrick E, Rajadurai S, Clarke TA, Rowley G. ZraP is a periplasmic molecular chaperone and a repressor of the zinc-responsive two-component regulator ZraSR. Biochem J. 2012;442:85–93. doi: 10.1042/BJ20111639. [DOI] [PubMed] [Google Scholar]
- 28.Sevcenco AM, Pinkse MW, Wolterbeek HT, Verhaert PD, Hagen WR, Hagedoorn PL. Exploring the microbial metalloproteome using MIRAGE. Metallomics. 2011;3:1324–30. doi: 10.1039/c1mt00154j. [DOI] [PubMed] [Google Scholar]
- 29.Ho E, Quan N, Tsai YH, Lai W, Bray TM. Dietary zinc supplementation inhibits NFkappaB activation and protects against chemically induced diabetes in CD1 mice. Exp Biol Med (Maywood) 2001;226:103–11. doi: 10.1177/153537020122600207. [DOI] [PubMed] [Google Scholar]
- 30.Long F, Su CC, Lei HT, Bolla JR, Do SV, Yu EW. Structure and mechanism of the tripartite CusCBA heavy-metal efflux complex. Philos Trans R Soc Lond B Biol Sci. 2012;367:1047–58. doi: 10.1098/rstb.2011.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol. 2006;2:2006 0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neidhardt FC, Bloch PL, Smith DF. Culture medium for enterobacteria. J Bacteriol. 1974;119:736–47. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akerlund T, Nordstrom K, Bernander R. Analysis of cell size and DNA content in exponentially growing and stationary-phase batch cultures of Escherichia coli. J Bacteriol. 1995;177:6791–7. doi: 10.1128/jb.177.23.6791-6797.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ali Azam T, Iwata A, Nishimura A, Ueda S, Ishihama A. Growth phase-dependent variation in protein composition of the Escherichia coli nucleoid. J Bacteriol. 1999;181:6361–70. doi: 10.1128/jb.181.20.6361-6370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson RB, Maliwal BP, Zeng HH. Zinc biosensing with multiphoton excitation using carbonic anhydrase and improved fluorophores. Journal of Biomedical Optics. 2000;5:17–22. doi: 10.1117/1.429963. [DOI] [PubMed] [Google Scholar]
- 35.Kim EH, Nies DH, McEvoy MM, Rensing C. Switch or funnel: how RND-type transport systems control periplasmic metal homeostasis. J Bacteriol. 2011;193:2381–7. doi: 10.1128/JB.01323-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitamura H, Morikawa H, Kamon H, Iguchi M, Hojyo S, Fukada T, Yamashita S, Kaisho T, Akira S, Murakami M, Hirano T. Toll-like receptor-mediated regulation of zinc homeostasis influences dendritic cell function. Nat Immunol. 2006;7:971–7. doi: 10.1038/ni1373. [DOI] [PubMed] [Google Scholar]; Nikaido H, Takatsuka Y. Mechanisms of RND multidrug efflux pumps. Biochim Biophys Acta. 2009;1794:769–81. doi: 10.1016/j.bbapap.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fukada T, Yamasaki S, Nishida K, Murakami M, Hirano T. Zinc homeostasis and signaling in health and diseases: Zinc signaling. J Biol Inorg Chem. 2011;16:1123–34. doi: 10.1007/s00775-011-0797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamada S, Awano N, Inubushi K, Maeda E, Nakamori S, Nishino K, Yamaguchi A, Takagi H. Effect of drug transporter genes on cysteine export and overproduction in Escherichia coli. Appl Environ Microbiol. 2006;72:4735–42. doi: 10.1128/AEM.02507-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thede GL, Arthur DC, Edwards RA, Buelow DR, Wong JL, Raivio TL, Glover JN. Structure of the periplasmic stress response protein CpxP. J Bacteriol. 193:2149–57. doi: 10.1128/JB.01296-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu T, Ramesh A, Ma Z, Ward SK, Zhang L, George GN, Talaat AM, Sacchettini JC, Giedroc DP. CsoR is a novel Mycobacterium tuberculosis copper-sensing transcriptional regulator. Nat Chem Biol. 2007;3:60–8. doi: 10.1038/nchembio844. [DOI] [PubMed] [Google Scholar]; Trepreau J, Girard E, Maillard AP, de Rosny E, Petit-Haertlein I, Kahn R, Coves J. Structural basis for metal sensing by CnrX. J Mol Biol. 2011;408:766–79. doi: 10.1016/j.jmb.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 41.Kwon E, Kim DY, Gross CA, Gross JD, Kim KK. The crystal structure Escherichia coli Spy. Protein Sci. 2010;19:2252–9. doi: 10.1002/pro.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quan S, Koldewey P, Tapley T, Kirsch N, Ruane KM, Pfizenmaier J, Shi R, Hofmann S, Foit L, Ren G, Jakob U, Xu Z, Cygler M, Bardwell JC. Genetic selection designed to stabilize proteins uncovers a chaperone called Spy. Nat Struct Mol Biol. 2011;18:262–9. doi: 10.1038/nsmb.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]