Summary
Classic galactosemia is a potentially lethal disorder that results from profound deficiency of galactose-1-phosphate uridylyltransferase (GALT), the second enzyme in the Leloir pathway of galactose metabolism. Although early diagnosis and rigorous dietary restriction of galactose prevent or resolve the potentially lethal acute symptoms, patients are at markedly increased risk of long term complications including significant cognitive, speech, and behavioral difficulties, among other problems. The mechanisms that underlie these long-term complications remain unclear, as do the factors that modify their severity. Here we explored the scholastic and behavioral outcomes experienced by a cohort of 54 school age children with classic galactosemia. Data collected included survey responses from parents and teachers, school records including standardized test scores, and GALT genotype data used to estimate predicted residual GALT activity based on a yeast expression system. As expected, many but not all of the children in our study demonstrated speech, scholastic, and behavioral difficulties. Perhaps most striking, we found that predicted cryptic residual GALT activity, often below the threshold of detection of clinical assays, appeared to modify scholastic outcome. These data raise the intriguing possibility that cryptic GALT activity might also influence the severity of other long-term complications in classic galactosemia.
Introduction
Classic galactosemia (OMIM 230400) is a potentially lethal genetic disorder that impacts more than 1/60,000 newborns and results from profound deficiency of galactose-1P uridylyltransferase (GALT, E.C. 2.7.7.12), the middle enzyme in the Leloir pathway of galactose metabolism (reviewed in Fridovich-Keil and Walter 2008). In many countries, infants with classic galactosemia are identified by population newborn screening, which enables early diagnosis and life-saving intervention in the form of dietary restriction of galactose. Unfortunately, despite even pre-symptomatic diagnosis and rigorous dietary modification, many affected infants grow to experience significant long-term complications (e.g. Coss et al 2012, reviewed in Fridovich-Keil and Walter 2008). Cognitive, speech, and behavioral disabilities are among the most prevalent long-term complications in classic galactosemia, impacting between 30% and 80% of all children and adults (Gitzelmann and Steinmann 1984; Waggoner et al 1990; Schweitzer et al 1993; Kaufman et al 1995; Shield et al 2000; Antshel et al 2004; Hughes et al 2009; Doyle et al 2010; Coss et al 2012; Jumbo-Lucioni et al 2012).
Cognitive outcomes
Measures of cognitive development and function, such as Intelligence Quotient (IQ) for children and adults with classic galactosemia range from high normal in some, to low normal in many, to significantly impaired in some. Waggoner and colleagues reported that of the 177 children in their study ≥6 years old, 45% were considered developmentally delayed (Waggoner et al 1990). Studies of teens and adults also report evidence of long-term cognitive disability: Hoffmann and colleagues (Hoffmann et al 2011) found a mean IQ of 76.2 ± 14.8 in their cohort of 32 patients in Germany, and an independent study of 20 volunteers in Britain found a mean IQ in the mid to high 80s (Doyle et al 2010); mean IQ in the general population is 100. Deficits in executive, adaptive, and visuoperceptual function of patients with classic galactosemia have also been reported (Doyle et al 2010; Waisbren et al 2012), as have deficits in working memory (Antshel et al 2004). Of note, although some early studies suggested that cognitive disability in galactosemia worsens with age (Waggoner et al 1990; Schweitzer et al 1993; Kaufman et al 1994; Kaufman et al 1995), more recent studies dispute that conclusion (Antshel et al 2004; Doyle et al 2010; Schadewaldt et al 2010).
A number of prior studies have also identified brain structural differences in patients with classic galactosemia. For example, white matter abnormalities were reported from brain magnetic resonance imaging (MRI) studies of pediatric and adult patients on galactose restricted diets (Nelson et al 1992; Kaufman et al 1995; Hughes et al 2009), and positron emission tomography (PET) scans of patients revealed metabolic differences in regions of the brain associated with both motor and cognitive function (Dubroff et al 2008). Whether these structural or metabolic differences underlie the functional deficits reported, or are a result of the same cause, but not themselves causal, remains unclear.
One question about cognitive outcome in classic galactosemia that has been addressed thoroughly is whether exposure to high levels of dietary galactose in infancy causes the developmental delay or long-term cognitive disability experienced later in life; the answer is no. This conclusion was reached both from studies of outcome among unrelated children diagnosed at different ages (Waggoner et al 1990) and also from comparisons of outcome severity among affected siblings (Waggoner et al 1990; Hughes et al 2009). The sibling comparisons reported by Waggoner (Waggoner et al 1990) and Hughes (Hughes et al 2009) from studies conducted almost 20 years apart, and on different continents, are particularly meaningful because, by definition, the sibling sets were matched for GALT genotype and, since they were raised by the same parents in the same household, were also matched for many other significant environmental influences. Both studies found that older siblings, who were exposed to milk and became clinically ill as infants before dietary galactose-restriction, fared no worse, long-term, than did their younger siblings who never consumed milk; some of these were even born to mothers who abstained from milk during pregnancy. This apparent independence of long-term outcome from milk exposure in infancy is also consistent with the apparent independence of long-term outcome severity from hemolysate gal-1P values, either at diagnosis, or later in childhood (Waggoner et al 1990).
Another proposed modifier of cognitive outcome in galactosemia that has been addressed by multiple studies, sometimes with contradictory results, is GALT genotype (Shield et al 2000; Tyfield 2000; Waisbren et al 2012). Most studies have examined whether homozygosity for the common Q188R allele correlates with outcome severity. Some studies have supported this viewpoint (Shield et al 2000) while others have not (Waisbren et al 2012). The relatively small sizes of the cohorts studied, combined with differences in the identity and prevalence of the non-Q188R alleles in the cohorts, may explain the apparent disparity. Of note, even sib-pairs who have the same GALT genotype may be discordant for cognitive or behavioral outcomes (Waggoner et al 1990; Hughes et al 2009), reinforcing the idea that unknown modifiers, some likely genetic and others perhaps environmental, contribute to the severity of these outcomes.
Speech and language outcomes
Speech/language disorders have been reported in close to 50% of all galactosemic patients (Waggoner et al 1990; Nelson et al 1991; Bosch 2006; Potter et al 2008; Hughes et al 2009; Potter 2011; Shriberg et al 2011) and are disproportionately common among galactosemic children with borderline to low cognitive development (Potter et al 2008). Even galactosemic children with typical cognitive function are at increased risk for speech/language difficulties (Potter et al 2008), although these children more often demonstrate an expressive language disorder, whereas galactosemic children with borderline or low cognitive development are more likely to demonstrate a mixed receptive expressive language disorder (Potter et al 2008). Of note, children with galactosemia who demonstrate a speech disorder are four to six times more likely than non-galactosemic children who demonstrate a speech disorder also to demonstrate language impairment (Potter et al 2008).
Behavioral outcomes
Behavioral or social disturbances that negatively impact quality of life are also frequent among both children and adults with classic galactosemia (Waggoner et al 1990; Antshel et al 2004; Jumbo-Lucioni et al 2012; Waisbren et al 2012). For example, in younger and school-age children behavior problems may include difficulty sitting still, difficulty paying attention, difficulty getting along with others, and/or a tendency to be victims of bullying (Lambert and Boneh 2004). Among adults, some of these same challenges hold, and in addition Waisbren and colleagues (Waisbren et al 2012) found that 39% of the adults in their study had experienced depression and more than half reported heightened anxiety. Whether these behaviors or psycho-social difficulties experienced by children and adults with classic galactosemia are secondary to cognitive or other complications, or occur as an independent outcome of GALT-deficiency, remains unclear.
The goal of the study reported here was to characterize the nature of scholastic and behavioral outcomes experienced by school age children with classic galactosemia and to explore the impact of cryptic GALT activity as a candidate modifier of that outcome. We therefore recruited 54 volunteers with classic galactosemia ranging in age from 4 to 18 years and gathered scholastic and behavioral outcome information about those volunteers using established survey instruments completed by parents and teachers, and school records including standardized test results. We also gathered GALT genotype data and from that predicted the GALT activity level in most volunteers. The results of this study demonstrated that children with classic galactosemia exhibit a variety of behavioral difficulties noted by both parents and teachers; many of the concerning behaviors were classified as indicative of internalizing rather than externalizing problems. Finally, we observed a statistically-significant association between predicted GALT activity and scholastic achievement in math, and also noted trends between predicted GALT activity and other outcomes that, while not statistically significant, were intriguing and merit follow up using larger samples with improved power to detect significant associations. These results both confirm prior reports and extend from prior conclusions to implicate cryptic residual GALT activity as a potential modifier of long-term outcome in classic galactosemia.
Methods
Study volunteers
Fifty-four study volunteers (25 boys and 29 girls) between the ages of 4 and 18 years old were recruited by self-referral through the Galactosemia Foundation (www.galactosemia.org) or by referral from a health care professional who treats patients with galactosemia. Our recruitment strategy did not allow us to calculate a response rate as we do not know how many eligible families saw our advertisements or heard about the study. All volunteers had been diagnosed previously with classic galactosemia, either by a clinical red blood cell GALT assay or by a recognized GALT genotype. This study was reviewed and approved as part of Protocol eIRB00024933 (formerly protocol 619–99, PI: JL Fridovich-Keil) by the Emory University Institutional Review Board. Informed consent, assent (where appropriate), and authorization to contact teachers and access school records were obtained for each volunteer.
Behavioral Assessments
We asked the parents of each study volunteer to provide the names of one or two teachers or other adults with whom their galactosemic child interacted on a regular basis in a scholastic setting (e.g. a classroom teacher, an instructional aide, or a special education tutor). Following written authorization to contact these individuals we sent hard copy surveys, together with an explanatory cover letter and a large self-addressed, stamped return envelope, to the parents and to the designated teachers. Parents received the age-appropriate child behavior checklist (CBCL for ages 1½ to 5 years, or for ages 6–18 years) from the Achenbach System of Empirically Based Assessment (ASEBA) and also the Social Skills improvement System (SSIS) parent form (Achenbach and Rescorla 2000; Achenbach and Rescorla 2001; Gresham and Elliott 2008). Each teacher received an age-appropriate (as above) Teacher Report Form (TRF from ASEBA) and also the SSIS teacher form. For the 54 children enrolled in this study we received completed parental surveys for 53, and we also received a total of 62 completed teacher surveys for 45 children; the data set includes two teacher observations for 17 of the children. Survey respondents each received a small gift card ($20) as compensation for their time spent taking the surveys.
Scholastic outcome measures
We attempted to collect school records for each study volunteer. Some of these records, such as standardized test scores, report cards, and IEP records, were provided by parents from their files; others came from teachers or other school officials following written authorization by the parents. We were not able to obtain all records for every volunteer. For example, essentially all of our youngest study volunteers were missing standardized test scores because most schools do not begin standardized testing until second or third grade. Nonetheless, the information we were able to gather was substantial, and where necessary we dealt with the problem of missing information in statistical analyses by using permutations to predict probabilities rather than a normal distribution to set significance (see below).
Survey respondents for each study volunteer were asked to send a copy of relevant state or national standardized test results from the current or prior year. We also asked whether the child was currently receiving speech therapy or any other form of special educational support in the classroom. Respondents for students who received special classroom assistance were asked to send any available files that might explain the reason why the child was receiving special assistance, the level of assistance provided, and any progress in the classroom (i.e. Individual Educational Plans, IEP). From this information we scored each study volunteer as either achieving at their grade level, or not achieving at their grade level in math and in language arts. Those students who did not take standardized tests were omitted from this analysis unless other clear documentation was received indicating that the child was excused from standardized testing because their achievement status was so low as to preclude testing. Of the 54 volunteers enrolled in our study, we received sufficient information for 33 to designate each as “achieves at grade level” or “achieves below grade level” for math and language arts, and also to understand what form of special classroom support, if any, they received.
GALT genotype analysis and predicted GALT activity level
We collected medical history information for each study volunteer, including the information upon which the diagnosis of classic galactosemia was originally based. Some volunteers had documentation of GALT genotype analysis performed as part of their diagnostic work-up. For volunteers whose GALT genotype information was either missing or incomplete (e.g. one or both alleles were designated as “unknown”) we determined GALT genotype by sequence analysis of DNA obtained from a blood or saliva sample, as described elsewhere (Gleason et al, in preparation).
All missense and nonsense mutations in the GALT open reading frame were assessed for residual GALT activity using our previously described yeast expression system for the human enzyme (Fridovich-Keil and Jinks-Robertson 1993; Chhay et al 2008). Volunteers with one or more GALT alleles that carried only non-coding sequence variants were excluded from analysis. Finally, a single “predicted GALT activity” value was calculated for each volunteer by averaging the predicted activity levels of the two GALT alleles identified in that individual.
Statistical analyses
To examine the potential relationship between the outcomes measured and GALT activity, we applied appropriate regression procedures that adjusted for the effects of age, gender, speech therapy, and special education status. Of these four covariates, only speech therapy was significantly associated with the various outcomes (p<0.05) so all other covariates were dropped to maintain parsimony. For dichotomous outcomes, such as achievement in math and/or language arts, we used logistic regression for inference. For the other continuous outcomes (internalizing problems, externalizing problems, adaptive functioning, total competence, problem behaviors, social skills), we used linear regression when analyzing data from parents and used generalized-estimating-equation (GEE) models when analyzing data from teachers (to account for the correlation in observations that arises when the same student has reports from multiple teachers). For each test performed, we assumed a one-sided alternative hypothesis based on prior belief of expected trends based on independent literature. To account for multiple testing, we used permutation-based inference to establish significance. For analyses of behavior and social outcomes based on teacher evaluations, we conducted permutations in a manner that preserved within-subject correlation of outcomes due to multiple teacher observations. All analyses were performed using the R programming language.
RESULTS
This section describes characteristics of our study population and presents data collected regarding the academic achievement and behavioral outcomes of these children. Due to the limited size of our study cohort we were only able to apply statistical analyses to a small subset of the questions addressed.
Characteristics of the study population
Most study volunteers were from European-American families, although a few were of mixed heritage, and most were of middle or upper-middle class socioeconomic status. The children were well distributed with regard to gender (25 males and 29 females) and age, though older teens were slightly under-represented (Table 1). Most volunteers (38/54) were enrolled in public school, though most of the youngest children (9/12) were enrolled in private school, and four children were homeschooled (Table 1). Of the 54 volunteers in this study, we were able to definitively classify the level of educational support for 33, and of those, 17 received some degree of formalized special education. The level of support received by different children in this group varied widely, however. Only one child spent the entire school day in a special education classroom. Five additional children received all-day support, but that extra support was provided either full-time by aides in a regular education classroom, or by aides part-time in a regular education classroom and part-time in a resource classroom. For the remaining 11 students who received special educational support, the services received were part-time and only in specific subjects. Some of these students remained in the regular classroom with an aide, while others relocated to a special classroom for less than 30% of the school day.
Table 1.
Demographics of the 54 children with classic galactosemia who participated in this study with regard to age, gender, school environment, scholastic achievement, and enrollment in special education classes or speech therapy:
| Overall | M:F ratio | 4–6 yr olds | 7–10 yr olds | 11–14 yr olds | 15–18 yr olds | |
|---|---|---|---|---|---|---|
| Study total: | 54 | 25:29 | 12 | 14 | 19 | 9 |
| School Environment | ||||||
| Public: | 70.4% (38/54) | 17:21 | 16.7% (2/12) | 71.4% (10/14) | 89.5% (17/19) | 100% (9/9) |
| Private: | 22.2% (12/54) | 7:5 | 75.0% (9/12) | 21.4% (3/14) | 0.0% (0/19) | 0.0% (0/9) |
| Other: | 7.4% (4/54) | 1:3 | 8.3% (1/12) | 7.1% (1/14) | 10.5% (2/19) | 0.0% (0/9) |
| Achievement1 | ||||||
| Math: | 47.2% (17/36) | 8:9 | NA | 36.4% (4/11) | 52.9% (9/17) | 50.0% (4/8) |
| Language Arts: | 52.8% (19/36) | 7:12 | NA | 63.6% (7/11) | 47.1% (8/17) | 50.0% (4/8) |
| Special Educational Services | ||||||
| Special Education Classes2: | 51.5% (17/33) | 5:12 | 20.0% (1/5) | 50.0% (4/8) | 71.4% (10/14) | 33.3% (2/6) |
| Speech Therapy3: | 47.1% (24/51) | 10:14 | 70.0% (7/10) | 42.9% (6/14) | 55.6% (10/18) | 11.1% (1/9) |
Achievement is presented as the percentile (and fraction) of volunteers in that group who met established achievement standards in either mathematics or language arts/English subjects.
Percentile (and fraction) of volunteers in that group who were enrolled in special education classes at the time surveyed.
Percentile (and fraction) of volunteers in that group who were receiving speech therapy at the time surveyed.
Finally, we classified each volunteer with regard to whether or not they received speech therapy at the time of the study. Other studies have indicated that a large percentage of school age children with classic galactosemia receive speech therapy (Potter et al 2008); our population was no exception, especially in the younger age group (Table 1).
Outcomes explored in this study
In this section we present results obtained from a review of school records and parent and teacher survey responses for each of the study volunteers. These data are presented in a descriptive format because the limited sample size restricted the number of hypotheses we could test in this dataset without incurring an overwhelming multiple-testing burden.
Scholastic achievement
Of the 54 volunteers in our study, we were able to gather records detailing scholastic achievement in math and language arts for 36; of the remaining 18 volunteers, 12 were under the age of 6 and therefore too young to have been formally assessed, and six were missing relevant records. Of the 36 students for whom appropriate records were available, in most cases achievement status, designated as “meets grade level” or “does not meet grade level” was based on scores from state or national standardized tests in math and/or language arts. Of note, some volunteers enrolled in special education classes took the same standardized tests as their “regular education” peers but were allowed extra time, verbal instruction, or other accommodations. In these instances we used the available standardized test scores to calculate achievement status. In some cases the scholastic files contained a phrase to the effect that the child was “exempted from standardized testing because achievement is well below grade level.” In these instances, where it was clearly stated that achievement was below grade level, we classified the student accordingly without standardized test scores. Volunteers for whom achievement status was unclear were excluded from this part of the study.
Close to half the volunteers in our study, for whom achievement status was documented, were not achieving at grade level in either math or language arts at the time of our survey (Table 1). Of note, not all volunteers receiving special educational support were classified as achieving below grade level; three of the 17 students receiving special services were achieving at or above their respective grade level in math, and four of the 17 were achieving at or above their respective grade level in language arts.
Behavioral outcomes
To measure behavioral outcomes in our study volunteers we used two different survey instruments: the Social Skills Improvement System (SSIS) and the Achenbach System of Empirically Based Assessment (ASEBA) (Achenbach and Rescorla 2000; Achenbach and Rescorla 2001; Gresham and Elliott 2008). These two surveys were selected because each has been used extensively in the general population, providing established reference values, and also in populations with known disabilities (Achenbach and Rescorla 2000; Achenbach and Rescorla 2001; Gresham and Elliott 2008). Both survey sets assessed similar behaviors, but they grouped these behaviors differently. Therefore, we used both surveys to capture the full spectrum of behavioral outcomes in our study volunteers. We also sought to distinguish problem behaviors that might exhibit only in one setting (e.g. at home) from those that exhibited consistently in different settings (e.g. at home and at school); we therefore requested survey responses for our study volunteers both from parents and teachers.
The SSIS surveys assessed both social skills (communication, cooperation, and engagement) and problematic behaviors (including bullying, hyperactivity/inattention, internalizing and externalizing behaviors) (Gresham et al 2010; Gresham et al 2010). SSIS survey scores were transformed to a standard scale for each age and gender group. Of note, the scores were also standardized to control for age and gender differences in the reference population, and were compared to established cut-off limits distinguishing a “clinical range”, or affected score, from an unaffected score.
The ASEBA surveys also yielded scores, standardized for age and gender, for problematic behaviors grouped into variables by category (Achenbach et al 1987; Verhulst et al 1988; Achenbach and Ruffle 2000; Achenbach and Dumenci 2001). Neither competence nor adaptive function variables were assessed on the preschool forms and therefore we do not have those scores for the youngest volunteers in this study. Problematic behaviors assessed via the ASEBA surveys were categorized as either Internalizing or Externalizing. The ASEBA splits problematic behaviors into these two groupings because the appearance of one, the other, or both is important for distinguishing the type of problem present (Achenbach 1966). Internalizing Problems, defined as problems “within the self,” such as anxiety, depression, somatic complaints, and withdrawal from social contact, may be present independently from Externalizing Problems, defined as specific problems stemming from “conflicts with other people and with their expectations,” such as bullying, disobedience, or oppositional or aggressive behavior (Achenbach and Rescorla 2000; Achenbach and Rescorla 2001).
Tables 2 to 4 provide details of the patterns of the observed behavioral data in our sample. Table 2 provides the mean scores in the entire sample, as well as scores stratified by the child’s gender. Table 3 provides the mean scores stratified by either special education or speech therapy status. Finally, Table 4 provides mean scores stratified by math and language-arts achievement. Within each Table, we also provide the mean scores for the reference population.
Table 2.
Behavioral outcomes for boys and girls:
| Respondent | Internalizing Problems (ASEBA) | Externalizing Problems (ASEBA) | Problem Behaviors (SSIS) | Total Competence or Adaptive Functioning (ASEBA) | Social Skills (SSIS) | |
|---|---|---|---|---|---|---|
| Reference Population (survey-specific) | ||||||
|
| ||||||
| mean value | 50.0–50.4 | 50.0–50.6 | 100 | 49.9–51.8** | 100 | |
| clinical range (CR) | >63 | >63 | >115 | <37 | <85 | |
| % ref group in CR | <10% | <10% | 16% | <10% | 16% | |
|
| ||||||
| Children with classic galactosemia (boys and girls combined) | ||||||
|
| ||||||
| Parent | mean ± SEM | 55.0 ± 1.55 | 48.2 ±1.34 | 102.7 ± 2.16 | 44.2 ± 1.74 | 93.7 ± 1.77 |
| % in CR (N) | 25% (52) | 8% (52) | 22% (51) | 28% (40) | 24% (51) | |
|
| ||||||
| Teacher* | mean ± SEM | 53.0 ± 1.25 | 50.5 ± 1.08 | 102.0 ± 1.57 | 48.4 ± 1.27 | 94.3 ± 1.74 |
| % in CR (N) | 16% (62) | 6% (62) | 16% (61) | 7% (44) | 20% (59) | |
|
| ||||||
| Children with classic galactosemia (boys only) | ||||||
|
| ||||||
| Parent | mean ± SEM | 54.6 ± 2.14 | 49.8 ± 1.70 | 104.1 ± 3.43 | 42.2 ± 2.84 | 96.0 ± 3.14 |
| % in CR (N) | 22% (23) | 4% (23) | 26% (23) | 41% (17) | 22% (23) | |
|
| ||||||
| Teacher* | mean ± SEM | 52.5 ± 1.68 | 49.8 ± 1.57 | 100.5 ± 2.39 | 51.3 ± 1.86 | 97.4 ± 2.53 |
| % in CR (N) | 11% (27) | 4% (27) | 15% (26) | 7% (15) | 13% (24) | |
|
| ||||||
| Children with classic galactosemia (girls only) | ||||||
|
| ||||||
| Parent | mean ± SEM | 55.4 ± 2.23 | 46.9 ± 1.97 | 101.6 ± 2.77 | 45.7 ± 2.18 | 91.8 ± 1.91 |
| % in CR (N) | 28% (29) | 10% (29) | 18% (28) | 17% (23) | 25% (28) | |
|
| ||||||
| Teacher* | mean ± SEM | 53.5 ± 1.81 | 51.1 ± 1.50 | 103.0 ± 2.09 | 46.9 ± 1.62 | 92.2 ± 2.33 |
| % in CR (N) | 20% (35) | 9% (35) | 17% (35) | 7% (29) | 26% (35) | |
The mean score ± standard error (SEM), percentage of responses in the clinical range, and number of responses from parents and teachers for each of the five score categories is presented. Note that the sample size was insufficient to make statistical comparisons across genders meaningful. Total Competence scores are from the ASEBA parent surveys while the Adaptive Functioning scores are from the ASEBA teacher surveys. The reference population mean values, percentage in the clinical range, and clinical range cut-offs were derived from the reference population for each survey (Achenbach and Rescorla 2000; Achenbach and Rescorla 2001; Gresham and Elliott 2008).
Means for teacher-rated variables are based on more than one observation for some volunteers (see Methods).
The range given is the combined range for both Total Competence and Adaptive Functioning; the range for Total Competence alone went only to 50.1.
Table 4.
Behavioral Outcomes and Scholastic Achievement:
| Respondent | Internalizing Problems (ASEBA) | Externalizing Problems (ASEBA) | Problem Behaviors (SSIS) | Total Competence or Adaptive Functioning (ASEBA) | Social Skills (SSIS) | |
|---|---|---|---|---|---|---|
| Reference Population (survey-specific) | ||||||
| mean value | 50.0–50.4 | 50.0–50.6 | 100 | 49.9–51.8** | 100 | |
| clinical range (CR) | >63 | >63 | >115 | <37 | <85 | |
| % ref group in CR | <10% | <10% | 16% | <10% | 16% | |
|
| ||||||
| Children with classic galactosemia achieving at grade level in math | ||||||
|
| ||||||
| Parent | mean ± SEM | 51.7 ± 2.31 | 42.6 ± 1.33 | 93.8 ± 2.48 | 50.0 ± 2.82 | 97.2 ± 2.48 |
| % in CR (N) | 18% (17) | 0% (17) | 0% (17) | 19% (16) | 12% (17) | |
|
| ||||||
| Teacher* | mean ± SEM | 50.0 ± 1.99 | 45.4 ± 1.44 | 93.5 ± 1.51 | 53.8 ± 1.91 | 103.9 ± 2.71 |
| % in CR(N) | 5% (20) | 0% (20) | 0% (19) | 0% (19) | 6% (17) | |
|
| ||||||
| Children with classic galactosemia achieving below grade level in math | ||||||
|
| ||||||
| Parent | mean ± SEM | 58.0 ± 2.47 | 50.6 ± 2.42 | 107.6 ± 3.60 | 41.2 ± 1.89 | 87.6 ± 2.99 |
| % in CR (N) | 32% (19) | 11% (19) | 28% (18) | 22% (18) | 44% (18) | |
|
| ||||||
| Teacher* | mean ± SEM | 53.5 ± 2.34 | 50.2 ± 1.54 | 102.8 ±2.33 | 45.1 ± 1.24 | 91.4 ± 1.91 |
| % in CR (N) | 18% (22) | 5% (22) | 14% (22) | 10% (21) | 23% (22) | |
|
| ||||||
| Children with classic galactosemia achieving at grade level in language arts | ||||||
|
| ||||||
| Parent | mean ± SEM | 51.9 ± 2.39 | 41.9 ±1.27 | 94.5 ± 2.74 | 48.4 ± 2.50 | 97.5 ± 2.61 |
| % in CR (N) | 16% (19) | 0% (19) | 5% (19) | 16% (19) | 16% (19) | |
|
| ||||||
| Teacher* | mean ± SEM | 48.7 ± 1.61 | 44.9 ±1.05 | 92.9 ± 0.10 | 52.0 ± 1.73 | 100.2 ±2.66 |
| % in CR (N) | 0% (23) | 0% (23) | 0% (22) | 0% (21) | 10% (20) | |
|
| ||||||
| Children with classic galactosemia achieving below grade level in language arts | ||||||
|
| ||||||
| Parent | mean ± SEM | 58.5 ± 2.40 | 52.3 ± 2.38 | 108.5 ± 3.56 | 41.5 ± 2.31 | 86.1 ± 2.73 |
| % in CR (N) | 35% (17) | 12% (17) | 25% (16) | 27% (15) | 44% (16) | |
|
| ||||||
| Teacher* | mean ± SEM | 55.6 ±2.63 | 51.5 ± 1.80 | 104.9 ± 2.56 | 46.2 ± 1.76 | 93.3 ± 2.45 |
| % in CR (N) | 26% (19) | 5% (19) | 16% (19) | 11% (19) | 21% (19) | |
The mean score ± standard error (SEM), percentage of responses in the clinical range, and number of responses from parents and teachers for each of the five scores is reported. Note that the sample size was insufficient to make statistical comparisons across achievement categories meaningful. Total Competence scores were derived from ASEBA parent surveys while the Adaptive Functioning scores were derived from ASEBA teacher surveys. The reference population mean values, percentage in the clinical range, and clinical range cut-offs come from the reference population for each survey (Achenbach and Rescorla 2000; Achenbach and Rescorla 2001; Gresham and Elliott 2008). Our study population was stratified by whether or not the child was meeting grade level achievement standards in math and language arts.
Means for teacher-rated variables are based on more than one observation for some volunteers (see Methods).
The range given is the combined range for both Total Competence and Adaptive Functioning; the range for Total Competence alone went only to 50.1.
Table 3.
Behavioral Outcome, Special Education and Speech therapy:
| Respondent | Internalizing Problems (ASEBA) | Externalizing Problems (ASEBA) | Problem Behaviors (SSIS) | Total Competence or Adaptive Functioning (ASEBA) | Social Skills (SSIS) | |
|---|---|---|---|---|---|---|
| Reference Population (survey-specific) | ||||||
|
| ||||||
| mean value | 50.0–50.4 | 50.0–50.6 | 100 | 49.9–51.8** | 100 | |
| clinical range (CR) | >63 | >63 | >115 | <37 | <85 | |
| % ref group in CR | <10% | <10% | 16% | <10% | 16% | |
|
| ||||||
| Children with classic galactosemia enrolled in special education | ||||||
|
| ||||||
| Parent | mean ± SEM | 60.2 ± 3.04 | 52.4 ± 2.69 | 108.6 ± 4.34 | 39.6 ± 2.21 | 86.3 ± 3.14 |
| % in CR (N) | 47% (17) | 18% (17) | 38% (16) | 36% (14) | 50% (16) | |
|
| ||||||
| Teacher* | mean ± SEM | 56.9 ±2.46 | 52.6 ±1.54 | 105.3 ± 2.42 | 44.7 ± 1.40 | 88.5 ± 1.94 |
| % in CR (N) | 30% (20) | 5% (20) | 20% (20) | 12% (17) | 30% (20) | |
|
| ||||||
| Children with classic galactosemia not enrolled in special education | ||||||
|
| ||||||
| Parent | mean ± SEM | 51.3 ± 2.86 | 44.3 ± 2.19 | 97.3 ± 2.82 | 56.3 ± 2.38 | 99.6 ± 2.45 |
| % in CR (N) | 13% (16) | 6% (16) | 6% (16) | 0% (12) | 6% (16) | |
|
| ||||||
| Teacher* | mean ± SEM | 48.1 ± 1.67 | 47.6 ± 1.62 | 95.9 ±1.75 | 55.3 ± 2.08 | 101.0 ± 2.74 |
| % in CR (N) | 0% (23) | 0% (23) | 0% (23) | 0% (15) | 14% (21) | |
|
| ||||||
| Children with classic galactosemia currently receiving speech therapy | ||||||
|
| ||||||
| Parent | mean ± SEM | 57.0 ± 2.51 | 50.1 ± 2.07 | 109.2 ± 3.28 | 38.3 ± 2.34 | 89.0 ± 2.63 |
| % in CR (N) | 30% (23) | 9% (23) | 35% (23) | 44% (16) | 39% (23) | |
|
| ||||||
| Teacher* | mean ± SEM | 56.8 ± 1.65 | 54.5 ± 1.55 | 106.5 ± 2.01 | 43.2 ±1.44 | 87.2 ± 2.22 |
| % in CR (N) | 21% (28) | 11% (28) | 21% (28) | 6% (17) | 36% (28) | |
|
| ||||||
| Children with classic galactosemia not currently receiving speech therapy | ||||||
|
| ||||||
| Parent | mean ± SEM | 53.5 ± 2.07 | 46.9 ± 1.84 | 98.3 ± 2.57 | 48.7 ± 2.13 | 96.7 ± 2.02 |
| % in CR (N) | 22% (27) | 7% (27) | 13% (26) | 13% (23) | 12% (26) | |
|
| ||||||
| Teacher* | mean ± SEM | 50.1 ± 1.72 | 47.2 ±1.32 | 98.3 ± 2.20 | 51.6 ±1.57 | 100.0 ±1.98 |
| % in CR (N) | 12% (33) | 3% (33) | 13% (32) | 7% (27) | 7% (30) | |
The mean score ± standard error (SEM), percentage of responses in the clinical range, and number of responses from parents and teachers for each of the five scores is reported. Note that the sample size was insufficient to make statistical comparisons across the special education or speech therapy categories meaningful. Total Competence scores were derived from ASEBA parent surveys while the Adaptive Functioning scores were derived from ASEBA teacher surveys. The reference population mean values, percentage in the clinical range, and clinical range cut-offs come from the reference population for each survey (Achenbach and Rescorla 2000; Achenbach and Rescorla 2001; Gresham and Elliott 2008). Our study population was stratified by whether the child was currently enrolled in or receiving special education or speech therapy.
Means for teacher-rated variables are based on more than one observation for some volunteers (see Methods).
The range given is the combined range for both Total Competence and Adaptive Functioning; the range for Total Competence alone went only to 50.1.
Social skills and adaptive behavior
We measured behavior in social and adaptive areas for our study volunteers using three different variables: Adaptive Functioning (ASEBA surveys), Total Competence (ASEBA surveys), and Social Skills (SSIS surveys). Adaptive Functioning was a composite of teachers’ observations of the student’s ability to work, behave, learn and interact with peers in the classroom. Total Competence was assessed from responses on the ASEBA parent survey concerning the number of activities, clubs, and sports the study volunteer was involved in, whether they were as capable at activities as their peers, and how they interacted with adults, peers and family. Social Skills were assessed from both the SSIS parent and teacher surveys from questions addressing the study volunteer’s ability to communicate, follow directions, and interact with their peers and adults. A clinical score in Social Skills could reflect either an inability to perform a given behavior or an inability to understand when a given behavior was appropriate for a specific situation. In Tables 2 to 4, we present the average values of these measures for our study volunteers, and for the reference population. Like the behavioral outcome data described above, we provide these data stratified by gender (Table 2), special-education/speech-therapy status (Table 3), and math/language arts achievement status (Table 4).
Cryptic GALT activity and scholastic and behavioral outcomes
Previous studies have looked at GALT genotype as a candidate modifier of long-term outcomes in classic galactosemia with mixed results (Shield et al 2000; Antshel et al 2004; Potter et al 2008); we addressed the question here from a slightly different angle, asking not whether GALT genotype itself correlates with scholastic outcome, but whether the residual GALT activity predicted by GALT genotype correlates with outcome.
Our study volunteers demonstrated a variety of GALT genotypes: 33 were homozygotes for the common Q188R allele, associated with no residual activity, 17 were compound heterozygotes for Q188R and another allele, and 4 carried only non-Q188R alleles. Of note, some non-Q188R alleles were predicted to encode residual GALT activity; others were not (see Supplemental Table 1). Residual GALT activity for each volunteer was estimated from the arithmetic mean of the predicted activities for each allele present in the genotype. To quantify the level of cryptic GALT activity associated with each allele we applied our previously described null background yeast expression system for human GALT (Riehman et al 2001), averaging the activities predicted for each allele in any given patient. This system allows over-expression of any hGALT open reading frame in a null-background strain of yeast, so that even very low levels of residual GALT activity, well below the threshold of detection by clinical assays of hemolysate, are detectable (see Methods and Gleason et al, in preparation). However, alleles that involve non-coding changes cannot be accurately modeled. We therefore excluded from this portion of the study any patients whose GALT genotype included an allele with only non-coding sequence changes; of the 54 volunteers in this study, only one had to be excluded for this reason.
For the purpose of our analyses we stratified volunteers into two groups: those with <0.4% predicted residual GALT activity, and those with ≥0.4% predicted residual GALT activity (Table 5). We set this cut-off level because it maximized the number of volunteers who could be categorized in the “greater than or equal to” group without straying too close to the threshold of detection of the enzymatic assay. Of the 25 volunteers with <0.4% predicted GALT activity for whom academic achievement status was known, only 8 were achieving at grade level in math, and only 11 were achieving at grade level in language arts; most of the children were achieving below grade level in one or both subjects (Table 5). In contrast, of the 10 volunteers with ≥0.4% predicted residual GALT activity for whom academic achievement status was known, all but two were achieving at grade level in math and all but three were achieving at grade level in language arts (Table 5). A similar finding, though not as striking, was seen when the volunteers were stratified with regard to special education status rather than scholastic achievement; of those with <0.4% predicted GALT activity more than half (14/23) were enrolled in special education, while of the volunteers with ≥0.4% predicted GALT activity only a third (3/9) were enrolled in special education. While these differences were certainly consistent, only achievement in math reached statistical significance in our model (p=0.03), likely due to the limited number of total volunteers in the study for whom adequate records were available, and the even more limited number of these volunteers with ≥0.4% predicted residual GALT activity (see Methods).
Table 5.
Scholastic achievement and predicted GALT activity:
| Predicted GALT activity (as a percentage of wild-type hGALT) | <0.4% | ≥0.4% |
|---|---|---|
| all study volunteers (n=53) | 42 | 11 |
| Special Educational Support | ||
| Enrolled in special education (n=17) | 14 | 3 |
| Not enrolled in special education (n=15) | 9 | 6 |
| Math Achievement1 | ||
| At grade level in math (n=16) | 8 | 8 |
| Below grade level in math (n=19) | 17 | 2 |
| Language Arts Achievement | ||
| At grade level in language arts (n=18) | 11 | 7 |
| Below grade level in language arts (n=17) | 14 | 3 |
Each study volunteer was classified by the level of residual GALT activity predicted for their GALT genotype based on yeast expression data (see Methods). One volunteer was omitted from this analysis because one of their mutations was non-coding and so could not be assessed using the yeast system. Scholastic achievement in language arts and as revealed by enrollment in special educational support shows a clear tendency with predicted residual GALT activity but due to the small number of volunteers with ≥0.4% predicted GALT activity this tendency is not statistically significant.
Math achievement and GALT activity do show a significant relationship (p-value= 0.03) after appropriate adjustment for multiple testing.
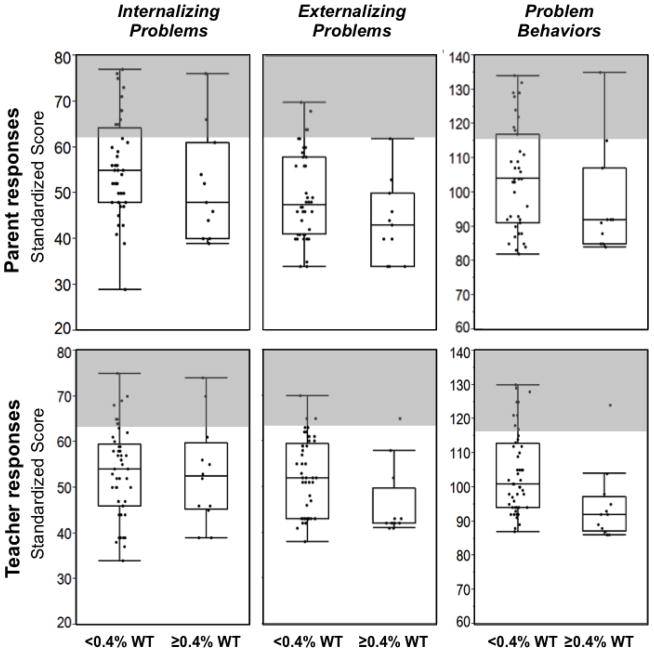
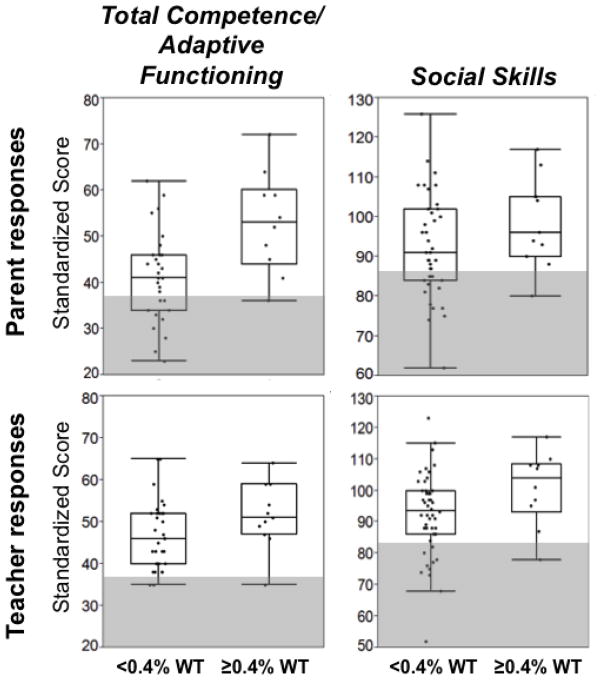
We also explored whether residual GALT activity might correlate with behavioral outcomes in our study volunteers (Figures 1 and 2). We found that the fraction of volunteers with scores for problematic behaviors in the clinical range was higher for volunteers with <0.4% predicted GALT activity than for volunteers with ≥0.4% predicted GALT activity (shaded areas, Figure 1). These findings were evident in the social and adaptive behavior scores as well (Figure 2). For example, the Social Skills scores from both the parent and teacher surveys regarding study volunteers with <0.4% predicted GALT activity showed a large percentage of scores in the clinical range (27% and 22%, respectively), while for study volunteers with ≥0.4% predicted GALT activity there was only one score in the clinical range (Figure 2). Presumably due to small sample size these findings were not statistically significant. However, this apparent relationship is especially intriguing in light of the statistically significant relationship observed between predicted residual GALT activity and achievement in math (Table 5).
Figure 1. Problematic Behavior scores are displayed as a function of predicted residual GALT activity.
For each data set illustrated, box and whisker plots indicate the median (center line in each box), limits of the 75th and 25th percentiles (top and bottom of each box), and 95th percentile confidence limits (top and bottom whiskers). Note that for some distributions the 95% percentile limits coincided, or nearly coincided, with the 75th and/or 25th percentile limits so no whiskers, or only tiny whiskers, are visible. Predicted residual GALT activity for each volunteer was estimated for that volunteer’s GALT genotype based on the level of residual activity associated with the relevant GALT alleles using a null-background yeast expression system for the human enzyme. Volunteers with non-coding mutations that could not be assessed using this system were excluded from analysis. Both parent and teacher surveys indicated that, on average, volunteers predicted to have <0.4% wild-type residual GALT activity demonstrated more problem behaviors, and were more likely to have scores in the clinical range, than volunteers predicted to have ≥0.4% wild-type GALT activity. However, likely reflecting the small sample size, none of these apparent differences reached statistical significance.
Figure 2. Social Interaction scores are displayed as a function of predicted residual GALT activity.
For each data set illustrated, box and whisker plots indicate the median (center line in each box), limits of the 75th and 25th percentiles (top and bottom of each box), and 95th percentile confidence limits (top and bottom whiskers). Predicted residual GALT activity for each volunteer was estimated for that volunteer’s GALT genotype based on the level of residual GALT activity associated with the relevant GALT alleles using a null-background yeast expression system for the human enzyme. Volunteers with non-coding mutations that could not be assessed using this system were excluded from analysis. Both parent and teacher surveys indicated that, on average, volunteers predicted to have ≥0.4% wild-type GALT activity demonstrated better Total Competence and Adaptive Functioning, and stronger Social Skills than volunteers predicted to have <0.4% wild-type GALT activity, although none of these differences reached statistical significance.
Discussion
The goal of this study was to characterize the nature of the scholastic and behavioral outcomes experienced by a group of 54 school age children with classic galactosemia and to ask whether cryptic GALT activity predicted for these children might influence parameters of their long-term outcomes. Our results both confirm earlier findings and extend them.
Scholastic achievement and behavioral outcomes
We used many different approaches to assess scholastic achievement and behavioral outcomes in our study volunteers, gathering information from parent and teacher surveys, standardized test scores, and school records. Our goal was to provide as comprehensive as possible a phenotypic description of scholastic and behavioral outcomes in school-aged children with classic galactosemia, recognizing that some of these outcomes might be highly correlated. Previous research, outside the field of galactosemia, has shown strong correlations between working memory, cognitive ability, social interactions and behavior (Vuontela et al 2012). Our results suggest that at least some of these relationships might also hold for children with classic galactosemia, though further studies with a substantially larger cohort will be required to confirm or refute this hypothesis in a statistically meaningful way.
Like other groups of patients with classic galactosemia who have been studied (e.g. (Waggoner et al 1990; Nelson et al 1991; Schweitzer et al 1993; Kaufman et al 1995; Antshel et al 2004; Lambert and Boneh 2004; Hughes et al 2009; Doyle et al 2010)), a large percentage of the children in our cohort struggled academically, many required speech therapy, and many exhibited problem behaviors. A small number of children demonstrated academic achievement at grade level for math but not language arts, or for language arts but not math, but most either achieved at grade level, or below grade level, for both subjects. This apparent concordance implies that the underlying deficit may be broad and that the achievement gap may not be simply a complication secondary to difficulties with speech.
Results from Table 2 appear to suggest that many of the volunteers in our cohort demonstrated internalizing rather than externalizing problem behaviors. This was observed both in the parents’ and teachers’ evaluation forms, which would imply these behaviors likely were not simply responsive to a specific environment (e.g. home vs. school). As above, studies in a larger cohort will be required to test the significance of this possible relationship. However, Antshel and colleagues (Antshel et al 2004) also noted internalizing problems in their study cohort of 25 children and adolescents with classic galactosemia, but all of their behavioral outcome data derived from parent surveys; they did not also survey teachers. Given the cognitive and speech challenges faced by many children with classic galactosemia, one might have anticipated that internalizing behaviors would be even more pronounced in the school context than at home, but that is not what we observed. For almost every behavioral parameter measured the parent and teacher survey responses were similar.
Cryptic GALT activity as a candidate prognostic indicator
Patients with classic galactosemia display a striking allelic heterogeneity at the GALT locus, leading to the logical supposition that these allelic differences might underlie at least some of the differences in outcome severity observed between patients. Indeed, a number of prior studies have addressed the hypothesis of genotype-phenotype correlation with sometimes contradictory results (Waggoner et al 1990; Kaufman et al 1994; Shield et al 2000; Tyfield 2000; Waisbren et al 2012). Here we have addressed this question from a slightly different angle, asking not whether GALT genotype itself predicts outcome severity, but rather whether the cryptic residual GALT activity associated with some alleles might impact outcome severity. This is a logical extension of early reports demonstrating that patients who carry an S135L GALT mutation, which is common in patients of African ancestry and associated with residual GALT activity in both clinical studies and model systems (Reichardt et al 1992; Landt et al 1997; Wells and Fridovich-Keil 1997; Lai and Elsas 2001; Henderson et al 2002; Crushell et al 2009), tend to experience milder long-term outcomes. Work in a Drosophila melanogaster (fruit fly) model of GALT deficiency also suggests that even very low levels of residual GALT activity can modify long-term outcome (Kushner et al 2010; Ryan et al 2012).
To address the role of cryptic GALT activity as a candidate modifier of scholastic or behavioral outcomes in our study population we first assessed the GALT genotype of each volunteer, and then applied our previously-described yeast model system (Fridovich-Keil and Jinks-Robertson 1993; Riehman et al 2001) to predict the level of residual GALT function associated with each missense or nonsense mutation. Finally, we averaged the activity levels predicted for each of the alleles in a given patient to derive a total predicted GALT activity value for that patient. This approach results in an approximation, at best, as for most sets of alleles we do not know whether there might be some allelic interaction (Elsevier and Fridovich-Keil 1996; Elsevier et al 1996; Christacos and Fridovich-Keil 2002). Nonetheless, the strength of this approach derives from the sensitivity of the null-background yeast model to detect even trace amounts of GALT function. Indeed, most of the patients predicted to have some residual GALT function by the yeast model demonstrated no detectable GALT activity by standard hemolysate clinical assays (Gleason et al, in preparation).
Of the 53 volunteers in our study whose GALT function we could assess using the yeast system (one was excluded from study due to a non-coding mutation), only 11 had ≥0.4% predicted residual GALT function. This low prevalence of patients with clearly detectable predicted residual GALT activity in our cohort may reflect the predominantly northern European heritage of most of our volunteers, and the high prevalence of the null-activity Q188R allele that goes with that demography. Even with this limitation, however, we observed a relationship between predicted residual GALT activity and scholastic achievement in math that was statistically significant even after an appropriate adjustment for multiple testing. That said, even among volunteers with <0.4% predicted residual GALT activity we observed a range of scholastic and behavioral outcomes, implicating the existence of other genetic and/or environmental modifiers outside the GALT locus.
With regard to the level of GALT activity detected in yeast expressing a specific allele of hGALT, it is important to recognize that the yeast system asks whether a given amino acid substitution impacts expression or catalytic function of hGALT in a yeast cell; how these numbers compare with the actual expression or function of the encoding allele in different human cells or tissues, or at different times in development, remains unknown. If a given hGALT allele exhibits substantial residual GALT activity in yeast we therefore conclude that in some patient cells this allele might also encode GALT function, although the level is unclear. In keeping with this uncertainty, we note that one of the alleles tested here (E308K) demonstrated very high residual GALT function when expressed in yeast; clearly this level cannot match what is seen in red blood cells or presumably this child would never have been diagnosed with classic galactosemia in the first place. However, we also note that the patient who carries this hGALT allele has one of the mildest outcomes we have seen, raising suspicion that there might be strong residual GALT function in some tissue.
Limitations and implications
The major limitation of this work was the small sample size; a problem common among studies of patients with rare conditions. This problem was compounded here with regard to testing the impact of predicted residual GALT activity on the outcomes measured because such a small fraction of patients with classic galactosemia in the US have evidence of residual GALT activity. This study is further limited by the absence of a matched control group, although we attempted to minimize that problem by using very well established survey instruments that have reference ranges already defined from prior studies. Finally, the results of this study reflect the population studied; these volunteers may or may not accurately represent the classic galactosemia population as a whole. This point is especially relevant given that the volunteers enrolled in this study were ascertained largely by self-referral from a family support group, or by referral from health care professionals. It is therefore possible that ascertainment bias may have skewed our volunteer base relative to the larger galactosemia population.
The implications of this work are two-fold. First, our results provide a more comprehensive overview of the types of scholastic and behavioral outcomes experienced by some school age children with classic galactosemia than has been reported previously. Recognizing the nature of these challenges empowers families and schools to provide the needed support structure to enable each child to reach his or her fullest potential. Second, our results demonstrate compelling, if limited, evidence that predicted cryptic GALT activity may be a modifier of long- term outcome severity in patients with classic galactosemia. The outcomes we have measured here are scholastic and behavioral, but our results raise suspicion that other outcomes might also be modified by the presence of even trace levels of residual GALT activity, at least in some tissues. Studies to test this hypothesis are currently underway. Until the proposed prognostic value of predicted residual GALT activity has been confirmed or refuted by studies of much larger patient cohorts they should be interpreted with caution, especially in the clinical setting.
Supplementary Material
1-sentence synopsis.
Studies of scholastic and behavioral outcomes among school children with classic galactosemia reveal predicted cryptic residual GALT activity as a potential modifier of scholastic outcome severity.
Acknowledgments
First and foremost, we are grateful to the many study volunteers and their families and teachers who participated in this project; without these amazing people this project could never have been completed. We are also grateful to our colleagues in the Departments of Human Genetics and Psychiatry and Behavioral Sciences at Emory for their many helpful discussions. This work was supported in part by funds from the National Institutes of Health grant R01 DK059904 (to JLFK); ELR was also supported in part by NIH Training Grants T32 MH087977, TL1 RR025010 and T32 GM008367.
Footnotes
Author contributions
This project was a true collaboration on many levels. JLFK and ELR initiated the project, recruited the study volunteers, gathered the survey and school records data, assembled the Tables and Figures, and wrote the majority of the manuscript; MEL and ET reviewed the school records and provided valuable insight and interpretation; TJG conducted all of the experiments related to patient GALT genotyping and GALT functional analysis using the yeast system; MPE performed the statistical analyses; all co-authors contributed to writing and editing the final manuscript.
References
- Achenbach T, Rescorla L. Book Manual for ASEBA Preschool Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2000. Manual for ASEBA Preschool Forms & Profiles. [Google Scholar]
- Achenbach T, Rescorla L. Book Manual for ASEBA School-Age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2001. Manual for ASEBA School-Age Forms & Profiles. [Google Scholar]
- Achenbach TM. The classification of children’s psychiatric symptoms: a factor-analytic study. Psychol Monogr. 1966;80(7):1–37. doi: 10.1037/h0093906. [DOI] [PubMed] [Google Scholar]
- Achenbach TM, Dumenci L. Advances in empirically based assessment: revised cross-informant syndromes and new DSM-oriented scales for the CBCL, YSR, and TRF: comment on Lengua, Sadowksi, Friedrich, and Fischer (2001) J Consult Clin Psychol. 2001;69(4):699–702. [PubMed] [Google Scholar]
- Achenbach TM, Rescorla L. Manual for the ASEBA preschool forms & profiles: an integrated system of multi-informant assessment. ASEBA; Burlington, VT: 2000. [Google Scholar]
- Achenbach TM, Rescorla L. Manual for the ASEBA school-age forms & profiles: an integrated system of multi-informant assessment. ASEBA; Burlington, VT: 2001. [Google Scholar]
- Achenbach TM, Ruffle TM. The Child Behavior Checklist and related forms for assessing behavioral/emotional problems and competencies. Pediatr Rev. 2000;21(8):265–271. doi: 10.1542/pir.21-8-265. [DOI] [PubMed] [Google Scholar]
- Achenbach TM, Verhulst FC, Baron GD, Althaus M. A comparison of syndromes derived from the Child Behavior Checklist for American and Dutch boys aged 6–11 and 12–16. J Child Psychol Psychiatry. 1987;28(3):437–453. doi: 10.1111/j.1469-7610.1987.tb01765.x. [DOI] [PubMed] [Google Scholar]
- Antshel K, Epstein I, Waisbren S. Cognitive strengths and weaknesses in children and adolescents homozygous for the galactosemia Q188R mutation: a descriptive study. Neuropsychology. 2004;18(4):658–664. doi: 10.1037/0894-4105.18.4.658. [DOI] [PubMed] [Google Scholar]
- Bosch AM. Classical galactosaemia revisited. J Inherit Metab Dis. 2006;29(4):516–525. doi: 10.1007/s10545-006-0382-0. [DOI] [PubMed] [Google Scholar]
- Chhay J, Openo K, Eaton J, Gentile M, Fridovich-Keil J. A yeast model reveals biochemical severity associated with each of three variant alleles of galactose-1P uridylyltransferase segregating in a single family. J Inherit Metab Dis. 2008;31:97–107. doi: 10.1007/s10545-007-0786-5. [DOI] [PubMed] [Google Scholar]
- Christacos N, Fridovich-Keil J. Impact of patient mutations on heterodimer formation and function in human galactose-1-P uridylyltransferase. Mol Genet Metab. 2002;76(4):319–326. doi: 10.1016/s1096-7192(02)00109-9. [DOI] [PubMed] [Google Scholar]
- Coss K, Doran P, Owoeye C, et al. Classical Galactosaemia in Ireland: incidence, complications and outcomes of treatment. J Inherit Metab Dis. 2012 Jul 3; doi: 10.1007/s10545-012-9507-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Crushell E, Chukwu J, Mayne P, Blatny J, Treacy E. Negative screening tests in classical galactosaemia caused by S135L homozygosity. J Inherit Metab Dis. 2009;32(3):412–415. doi: 10.1007/s10545-009-1081-4. [DOI] [PubMed] [Google Scholar]
- Doyle C, Channon S, Orlowska D, Lee P. The neuropsychological profile of galactosaemia. J Inherit Metab Dis. 2010;33(5):603–609. doi: 10.1007/s10545-010-9154-y. [DOI] [PubMed] [Google Scholar]
- Dubroff JG, Ficicioglu C, Segal S, Wintering NA, Alavi A, Newberg AB. FDG-PET findings in patients with galactosaemia. J Inherit Metab Dis. 2008;31(4):533–539. doi: 10.1007/s10545-008-0806-0. [DOI] [PubMed] [Google Scholar]
- Elsevier JP, Fridovich-Keil JL. The Q188R mutation in human galactose-1-phosphate uridylyltransferase acts as a partial dominant negative. J Biol Chem. 1996;271:32002–32007. doi: 10.1074/jbc.271.50.32002. [DOI] [PubMed] [Google Scholar]
- Elsevier JP, Wells L, Quimby BB, Fridovich-Keil JL. Heterodimer formation and activity in the human enzyme galactose-1-phosphate uridylyltransferase. Proc Natl Acad Sci USA. 1996;93:7166–7171. doi: 10.1073/pnas.93.14.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich-Keil JL, Jinks-Robertson S. A yeast expression system for human galactose-1- phosphate uridylyltransferase. Proc Natl Acad Sci USA. 1993;90:398–402. doi: 10.1073/pnas.90.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich-Keil JL, Walter JH. Galactosemia. In: Valle D, Beaudet A, Vogelstein B, Kinzler K, Antonarakis S, Ballabio A, editors. The Online Metabolic & Molecular Bases of Inherited Disease. McGraw Hill; 2008. http://www.ommbid.com/ [Google Scholar]
- Gitzelmann R, Steinmann B. Galactosemia: how does long-term treatment change the outcome? Enzyme. 1984;32(1):37–46. doi: 10.1159/000469448. [DOI] [PubMed] [Google Scholar]
- Gresham FM, Elliott SN. SSIS (Social Skills Improvement System) Rating Scales. NCS Pearson, Inc; Minneapolis, MN: 2008. [Google Scholar]
- Gresham FM, Elliott SN, Cook CR, Vance MJ, Kettler R. Cross-informant agreement for ratings for social skill and problem behavior ratings: an investigation of the Social Skills Improvement System-Rating Scales. Psychol Assess. 2010;22(1):157–166. doi: 10.1037/a0018124. [DOI] [PubMed] [Google Scholar]
- Gresham FM, Elliott SN, Kettler RJ. Base rates of social skills acquisition/performance deficits, strengths, and problem behaviors: an analysis of the Social Skills Improvement System--Rating Scales. Psychol Assess. 2010;22(4):809–815. doi: 10.1037/a0020255. [DOI] [PubMed] [Google Scholar]
- Henderson H, Leisegang F, Brown R, Eley B. The clinical and molecular spectrum of galactosemia in patients from the Cape Town region of South Africa. BMC Pediatr. 2002;2:7. doi: 10.1186/1471-2431-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann B, Wendel U, Schweitzer-Krantz S. Cross-sectional analysis of speech and cognitive performance in 32 patients with classic galactosemia. J Inherit Metab Dis. 2011;34(2):421–427. doi: 10.1007/s10545-011-9297-5. [DOI] [PubMed] [Google Scholar]
- Hughes J, Ryan S, Lambert D, et al. Outcomes of siblings with classical galactosemia. J Pediatr. 2009;154(5):721–726. doi: 10.1016/j.jpeds.2008.11.052. [DOI] [PubMed] [Google Scholar]
- Hughes J, Ryan S, Lambert D, et al. Outcomes of Siblings with Classical Galactosemia. The Journal of Pediatrics. 2009;154(5):721–726. doi: 10.1016/j.jpeds.2008.11.052. [DOI] [PubMed] [Google Scholar]
- Jumbo-Lucioni P, Garber K, Kiel J, et al. Diversity of approaches to classic galactosemia around the world: a comparison of diagnosis, intervention, and outcomes. J Inherit Metab Dis. 2012 doi: 10.1007/s10545-012-9477-y. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman FR, McBride-Chang C, Manis FR, Wolff JA, Nelson MD. Cognitive functioning, neurologic status and brain imaging in classical galactosemia. Eur J Pediatr. 1995;154(7 Suppl 2):S2–5. doi: 10.1007/BF02143794. [DOI] [PubMed] [Google Scholar]
- Kaufman FR, Reichardt JK, Ng WG, et al. Correlation of cognitive, neurologic, and ovarian outcome with the Q188R mutation of the galactose-1-phosphate uridyltransferase gene. J Pediatr. 1994;125(2):225–227. doi: 10.1016/s0022-3476(94)70197-0. [DOI] [PubMed] [Google Scholar]
- Kushner RF, Ryan EL, Sefton JM, et al. A Drosophila melanogaster model of classic galactosemia. Disease models & mechanisms. 2010;3(9–10):618–627. doi: 10.1242/dmm.005041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai K, Elsas LJ. Structure-Function Analyses of a Common Mutation in Blacks with Transferase-Deficiency Galactosemia. Mol Genet Metab. 2001;74:264–272. doi: 10.1006/mgme.2001.3230. [DOI] [PubMed] [Google Scholar]
- Lambert C, Boneh A. The impact of galactosaemia on quality of life--a pilot study. J Inherit Metab Dis. 2004;27(5):601–608. doi: 10.1023/b:boli.0000042957.98782.e4. [DOI] [PubMed] [Google Scholar]
- Landt M, Ritter D, Lai K, Benke PJ, Elsas LJ, Steiner RD. Black children deficient in galactose 1-phosphate uridyltransferase: Correlation of activity and immunoreactive protein in erythrocytes and leukocytes. The Journal of Pediatrics. 1997;130(6):972–980. doi: 10.1016/s0022-3476(97)70286-5. [DOI] [PubMed] [Google Scholar]
- Nelson CD, Waggoner DD, Donnell GN, Tuerck JM, Buist NR. Verbal dyspraxia in treated galactosemia. Pediatrics. 1991;88(2):346–350. [PubMed] [Google Scholar]
- Nelson MD, Jr, Wolff JA, Cross CA, Donnell GN, Kaufman FR. Galactosemia: evaluation with MR imaging. Radiology. 1992;184(1):255–261. doi: 10.1148/radiology.184.1.1319076. [DOI] [PubMed] [Google Scholar]
- Potter N. Voice disorders in children with classic galactosemia. J Inherit Metab Dis. 2011;34(2):377–385. doi: 10.1007/s10545-010-9213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter N, Lazarus J, Johnson J, Steiner R, Shriberg L. Correlates of language impairment in children with galactosaemia. J Inherit Metab Dis. 2008;31(4):524–532. doi: 10.1007/s10545-008-0877-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt JKV, Levy HL, Woo SL. Molecular characterization of two galactosemia mutations and one polymorphism: implications for structure-function analysis of human galactose-1-phosphate uridyltransferase. Biochemistry. 1992;31:5430–5433. doi: 10.1021/bi00139a002. [DOI] [PubMed] [Google Scholar]
- Riehman K, Crews C, Fridovich-Keil JL. Relationship between genotype, activity, and galactose sensitivity in yeast expressing patient alleles of human galactose-1-phosphate uridylyltransferase. J Biol Chem. 2001;276(14):10634–10640. doi: 10.1074/jbc.M009583200. [DOI] [PubMed] [Google Scholar]
- Ryan E, Duboff B, Feany M, Fridovich-Keil J. Mediators of a long-term movement abnormality in a Drosophila melanogaster model of classic galactosemia. Dis Model Mech. 2012 Jun 26; doi: 10.1242/dmm.009050. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schadewaldt P, Hoffmann B, Hammen H, Kamp G, Schweitzer-Krantz S, Wendel U. Longitudinal assessment of intellectual achievement in patients with classical galactosemia. Pediatrics. 2010;125(2):e374–381. doi: 10.1542/peds.2008-3325. [DOI] [PubMed] [Google Scholar]
- Schweitzer S, Shin Y, Jakobs C, Brodehl J. Long-term outcome in 134 patients with galactosaemia. European journal of pediatrics. 1993;152(1):36–43. doi: 10.1007/BF02072514. [DOI] [PubMed] [Google Scholar]
- Schweitzer S, Shin Y, Jakobs C, Brodehl J. Long-term outcome in 134 patients with galactosemia. Eur J Pediatr. 1993;152:36–43. doi: 10.1007/BF02072514. [DOI] [PubMed] [Google Scholar]
- Shield JP, Wadsworth EJ, MacDonald A, et al. The relationship of genotype to cognitive outcome in galactosaemia. Arch Dis Child. 2000;83:248–250. doi: 10.1136/adc.83.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shriberg L, Potter N, Strand E. Prevalence and phenotype of childhood apraxia of speech in youth with galactosemia. J Speech Lang Hear Res. 2011;54(2):487–519. doi: 10.1044/1092-4388(2010/10-0068). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyfield LA. Galactosaemia and allelic variation at the galactose-1-phosphate uridyltransferase gene: a complex relationship between genotype and phenotype. Eur J Pediatr. 2000;159(Suppl 3):S204–207. doi: 10.1007/pl00014404. [DOI] [PubMed] [Google Scholar]
- Verhulst FC, Achenbach TM, Althaus M, Akkerhuis GW. A comparison of syndromes derived from the child behavior checklist for American and Dutch girls aged 6–11 and 12–16. J Child Psychol Psychiatry. 1988;29(6):879–895. doi: 10.1111/j.1469-7610.1988.tb00760.x. [DOI] [PubMed] [Google Scholar]
- Vuontela V, Carlson S, Troberg AM, et al. Working Memory, Attention, Inhibition, and Their Relation to Adaptive Functioning and Behavioral/Emotional Symptoms in School-Aged Children. Child Psychiatry Hum Dev. 2012 doi: 10.1007/s10578-012-0313-2. [DOI] [PubMed] [Google Scholar]
- Waggoner DD, Buist NR, Donnell GN. Long-term prognosis in galactosaemia: results of a survey of 350 cases. Journal of inherited metabolic disease. 1990;13(6):802–818. doi: 10.1007/BF01800204. [DOI] [PubMed] [Google Scholar]
- Waisbren S, Potter N, Gordon C, et al. The adult galactosemic phenotype. J Inherit Metab Dis. 2012;35(2):279–286. doi: 10.1007/s10545-011-9372-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells L, Fridovich-Keil JL. Biochemical characterization of the S135L allele of galactose-1-phosphate uridylyltransferase associated with galactosemia. J Inher Metab Dis. 1997;20(5):633–642. doi: 10.1023/a:1005314207513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.