Abstract
Genetic analysis in Caenorhabditis elegans has uncovered essential roles for DAF-16 in longevity, metabolism, and reproduction. The mammalian orthologs of DAF-16, the closely-related FOXO subclass of forkhead transcription factors (FKHR/FOXO1, FKHRL1/FOXO3a, and AFX/FOXO4), also have important roles in cell cycle arrest, apoptosis and stress responses in vitro, but their in vivo physiological roles are largely unknown. To elucidate their role in normal development and physiology, we disrupted each of the Foxo genes in mice. Foxo1-null embryos died on embryonic day 10.5 as a consequence of incomplete vascular development. Foxo1-null embryonic and yolk sac vessels were not well developed at embryonic day 9.5, and Foxo1 expression was found in a variety of embryonic vessels, suggesting a crucial role of this transcription factor in vascular formation. On the other hand, both Foxo3a- and Foxo4-null mice were viable and grossly indistinguishable from their littermate controls, indicating dispensability of these two members of the Foxo transcription factor family for normal vascular development. Foxo3a-null females showed age-dependent infertility and had abnormal ovarian follicular development. In contrast, histological analyses of Foxo4-null mice did not identify any consistent abnormalities. These results demonstrate that the physiological roles of Foxo genes are functionally diverse in mammals.
The FOXO subclass of the forkhead box transcription factors is evolutionarily conserved, and its origin may predate the divergence of the vertebrate and invertebrate lineage (1). Genetic analysis has shown that the insulin-signaling pathway negatively regulates DAF-16, the Caenorhabditis elegans ortholog of Foxo (2, 3), and modulates the lifespan, metabolism, and fertility of the worm (4). Activation of insulin signaling pathways in mammals also negatively regulates members of the FOXO transcription factors (FOXO1/FKHR, FOXO3a/FKHRL1, and FOXO4/AFX) through direct phosphorylation of three conserved residues by the Akt (PKB) serine/threonine kinase, resulting in their active nuclear export and thereby inhibition of their transcriptional activities (5-8). Several in vitro overexpression studies have suggested that FOXO genes play important roles in several biological processes such as control of the cell cycle, apoptosis, and stress response, and some shared downstream transcriptional targets have been identified (9, 10). However, each FOXO gene has a unique pattern of expression in tissues (11-14) and exhibits a distinct response under a variety of conditions (15-17), suggesting that their in vivo physiological roles might be different. To test this, we disrupted each of the three Foxo genes in mice. We have previously shown that Foxo1 haploinsufficiency restores insulin sensitivity and rescues the diabetic phenotype in insulin-resistant mice (18). Here, we describe the phenotype of mice completely lacking each Foxo gene. Our results demonstrate that the physiological roles of Foxo genes are quite diverse in mammals.
Materials and Methods
Mutant Mice. Generation of the targeted Foxo1 allele has been described (18). The mice are maintained as heterozygotes and homozygous embryos were obtained by intercrossing. Foxo3a mutant mice were generated by using embryonic stem (ES) cell clones from OmniBank(R) ES cell library of randomly targeted cell lines (Lexicon Genetics, The Woodlands, TX) (19). The gene trap vector was inserted into the Foxo3a intron 1 as shown schematically in Fig. 4. Targeted 129/SvEvBrd-derived ES cells were injected into C57BL/6 blastocysts. The chimeric male was crossed with C57BL/6 females to generate the heterozygotes, and heterozygotes were crossed to generate the homozygotes. The Foxo4 targeting vector contained 6.5 kb of genomic sequence immediately 5′ side of the first exon followed by a 1.3-kb phosphoglycerate kinase promoter-driven neomycin phosphotransferase (PGK-Neo) cassette and 1.5 kb of genomic sequence of intron 1. The Foxo4 targeting vector also had a polyoma enhancer/herpes simplex virus thymidine kinase (MC1-TK) cassette for negative selection. The targeting vector was linearized and electroporated into 129/SvJ-derived ES cells. G418-resistant ES clones were screened for homologous recombination by Southern blot analysis and injected into C57BL/6 blastocysts. Because the Foxo4 locus is located on the X chromosome, the chimeric male was crossed with C57BL/6 females to generate the heterozygous females or hemizygous males, which were subsequently crossed to generate homozygous females. All protocols used in this study were approved by the Animal Subjects Program of the University of California at San Diego and conformed to National Institute of Health guidelines and public law. All mice were maintained on a 12-h light/dark cycle with food and water provided ad libitum.
Fig. 4.
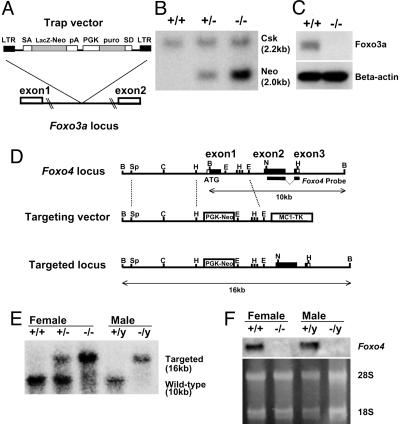
Targeted disruption of the Foxo3a gene and Foxo4 gene. (A) Targeting strategy for Foxo3a. A retroviral promoter trap vector was inserted in intron 1 of Foxo3a. SA, splice acceptor site; SD, splice donor site; LTR, long terminal repeat sequence; pA, polyadenylation signal; puro, puromycin. (B) Southern analysis of SacI-digested genomic DNA obtained from a mating of Foxo3a+/- mice by using the Neo- and Csk-specific probes. Different intensities demonstrated by hybridization of the Neo-specific probe discriminate zero, one, or two Foxo3a gene disruptions, representing +/+, +/-, and -/- mice, respectively. (C) Western analysis of lung tissue lysates from Foxo3a+/+ and Foxo3a-/- mice by using antibodies against Foxo3a and β-actin. (D) Restriction maps of the wild-type Foxo4 locus, the targeting vector, and the targeted locus. The targeting vector contained a PGK-Neo cassette instead of the Foxo4 exon1. A targeting probe (3′ region of Foxo4 cDNA) was also indicated. Restriction enzymes were indicated as B, BamHI; C, ClaI; E, EcoRI; H, HindIII; N, NcoI; and Sp, SpeI. (E) Southern analysis of BamHI-digested genomic DNA obtained from Foxo4 female (+/+, +/-, and -/-) and male (+/y and -/y) mice by using a probe from the 3′ region of Foxo4 cDNA. The Foxo4 wild-type allele (10 kb) and targeted allele (16 kb) were indicated. (F) Northern analysis of total RNA from skeletal muscle of Foxo4 female (+/+ and -/-) and male (+/y and -/y) mice by using the same probe used for Southern blot analysis. The ethidium bromide-staining 28S and 18S bands are also indicated.
Genotyping. DNA from tails was analyzed by Southern blotting. Foxo1 genotyping has been described (18). Foxo3a genotyping was performed by comparing autoradiographic intensities of 1.5-kb SacI fragments hybridized to a Neo-specific probe as described (20). The c-src tyrosine kinase (Csk)-specific probe was used as a control for copy number. This strategy discriminates zero-, one-, or two-targeted gene disruptions, representing +/+, +/-, and -/- mice, respectively, at any trapped genetic locus. For Foxo4 genotyping, BamHI-digested DNA was hybridized with a probe from the 3′ region of Foxo4 cDNA. To genotype the Foxo1 mutant embryo and yolk sac, genomic DNA from small portions of embryo or yolk sac were analyzed by PCR. Primers used for genotyping were 5′-TGG GAG CCC TGA AGT GAG TA-3′ (primer 1), 5′-ATG TCC CAC CTG TGA TTC TG-3′ (primer 2), and 5′-CCA TCA TGG CTG ATG CAA TG-3′ (primer 3). Primers 1 and 2 amplified 301-bp fragments from the wild-type Foxo1 allele. Primers 2 and 3 amplified 815-bp fragments from the mutant allele.
Northern Blot Analysis. Total RNA was prepared from skeletal muscle of wild-type and Foxo4-null mice by using TRIzol (Invitrogen). Ten micrograms of total RNA was electrophoresed through 1% agarose gels, transferred to Hybond-N+ membranes (Amersham Biosciences), and hybridized to a probe from the 3′ region of Foxo cDNA.
Western Blot Analysis. Twenty micrograms of lysate were prepared from tissues from both wild-type and Foxo1-null embryonic day (E) 9.5 embryos and wild-type and Foxo3a-null adult mice, separated by electrophoresis, and blotted to nitrocellulose membranes. Antibodies used for Western analysis were polyclonal anti-FKHR antibody (C-20, Santa Cruz Biotechnology), polyclonal anti-FKHRL1 antibody (Upstate Biotechnology, Waltham, MA), monoclonal anti-β-actin antibody (clone AC-15, Sigma), and horseradish peroxidase (HRP)-conjugated secondary antibodies (Dako).
Immunohistochemistry of Whole-Mount Embryos and Yolk Sacs. Embryos and yolk sacs were fixed overnight in 4% paraformaldehyde/PBS and bleached with 5% H2O2 in methanol for 5 h. Staining, washing, and developing procedures were essentially the same as described (21). Antibodies used for immunohistochemistry were the monoclonal anti-PECAM-1 antibody (clone MEC 13.3, BD Pharmingen) and HRP-conjugated secondary antibodies (Jackson ImmunoResearch).
LacZ Staining. Whole-mount embryo or yolk sac tissues were fixed in 0.25% glutaraldehyde/PBS for 10 min, rinsed three times with PBS, and stained overnight at 37°C with X-Gal buffer (5 mM potassium ferrocyanide/5 mM potassium ferricyanide/1 mM magnesium chloride/0.2% Triton X-100/1 mg/ml X-Gal in PBS, pH 7.3). For lacZ staining of sections, embryos were fixed in 4% paraformaldehyde/PBS at 4°C for 1 h and equilibrated with 30% sucrose/PBS at 4°C. Embryos were then embedded in OCT compound, cryosectioned, and stained with X-Gal buffer. After lacZ staining, samples were counterstained by nuclear fast red. Some tissues were cryosectioned after whole-mount lacZ staining.
Histological Analysis. Tissues embedded in paraffin were sectioned and stained with hematoxylin/eosin. The tissues that were examined for initial screening of Foxo3a- and Foxo4-null animals were white and brown adipose tissue, adrenal glands, femur, sternum, brain, reproductive tract, clitoral glands, eyes, Harderian glands, gastrointestinal tract, liver with gall bladder, heart, kidneys, lung, mesenteric lymph node, mammary gland, skin, pancreas, salivary gland, skeletal muscle, spinal cord, spleen, thymus, thyroid gland, trachea, urinary bladder from females, testes, epididymides, kidneys, preputial glands, prostate, salivary gland, seminal vesicles, and coagulating gland from males. A board-certified veterinary pathologist (American College of Veterinary Pathologists) at the Charles River Laboratory performed the initial examination of all histological sections. A detailed analysis of the ovarian phenotype in the Foxo3a-null mice was performed in conjunction with the University of California at San Diego Cancer Center Core Histology facility. Histology of mouse ovarian follicles was categorized by the standard classification (22).
Results
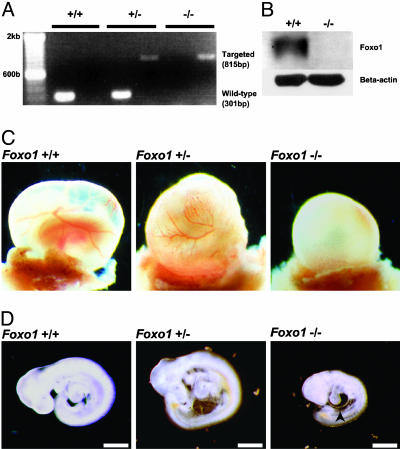
Foxo1 Is Required for Embryonic Vascular Development. While generating mice with Foxo1 haploinsufficiency to test its role in insulin sensitivity, we noticed the absence of homozygous Foxo1-/- mice in litters derived from heterozygous matings suggesting that Foxo1-/- animals were unable to complete embryonic development (18). To address this possibility, embryos were isolated from timed matings, and their genotypes and Foxo1 expression levels were assessed by genomic PCR and Western analyses, respectively (Fig. 1 A and B). At E9.5, there was no apparent difference between Foxo1+/+ and Foxo1+/- yolk sacs, but Foxo1-/- yolk sacs lacked well developed blood vessels (Fig. 1C). Foxo1+/+ and Foxo1+/- embryos were also phenotypically indistinguishable in appearance, whereas Foxo1-/- embryos were ≈50% the size of their Foxo1+/+ littermates (Fig. 1D). Furthermore, cardiac looping of Foxo1-/- embryos was retarded and the pericardium was distended compared to Foxo1+/+ and Foxo1+/- embryos at E9.5. Despite these developmental defects, the hearts of Foxo1-/-embryos were still beating at E9.5. However, none of them survived beyond E10.5.
Fig. 1.
Disruption of the Foxo1 gene results in embryonic lethality. (A) PCR analysis with genomic DNA from E9.5 yolk sacs obtained from a mating of Foxo1+/- mice. PCR amplification from the wild-type and Foxo1-targeted loci resulted in fragment sizes of 301 bp and 815 bp, respectively. Samples were independently amplified with each primer set. (B) Western blot analysis of lysates from E9.5 Foxo1+/+ and Foxo1-/- embryos by using antibodies against Foxo1 and β-actin. (C) At E9.5, both Foxo1+/+ and Foxo1+/- yolk sacs had well developed blood vessels, whereas Foxo1-/- yolk sacs lacked them. (D) At E9.5, Foxo1+/+ and Foxo1+/- embryos were phenotypically indistinguishable in appearance, whereas Foxo1-/- embryos were approximately half their size. In addition, cardiac looping was retarded and the pericardium was distended (arrowhead). (Scale bar, 500 μm.)
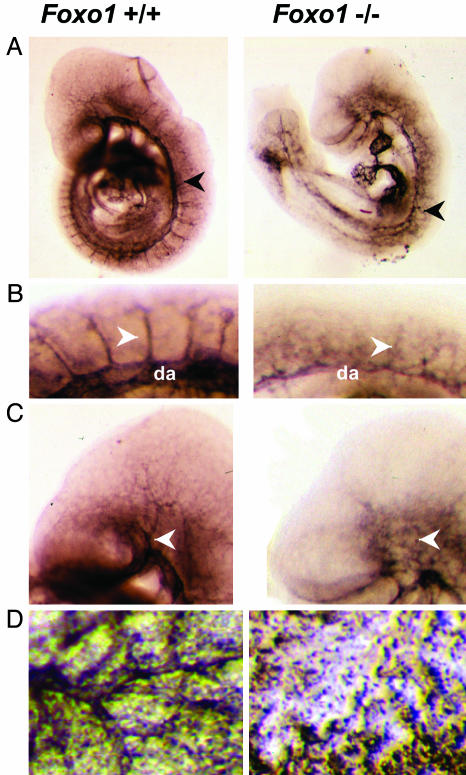
We next examined the vascular structures of Foxo1-/- embryos and yolk sacs and compared them with control littermates by whole-mount immunostaining with a monoclonal antibody to PECAM-1, a specific marker for vascular endothelial cells. Significant defects in the formation of the vascular system were observed in Foxo1-/- embryos compared to those of Foxo1+/+ (Fig. 2A). The dorsal aorta in Foxo1-/- embryos appeared thin and disorganized (Fig. 2 A and B), and the intersomitic vessels in Foxo1-/- embryos were also irregularly developed (Fig. 2B). The vasculature in the heads of Foxo1-/- embryos appeared to lack properly formed branches of the internal carotid artery (Fig. 2C). In addition, developed vasculature was not present in Foxo1-/- yolk sacs (Fig. 2D). Thus, the primary defect in Foxo1-/- embryos and yolk sacs appears to be a disruption of normal vascular formation.
Fig. 2.
Foxo1-/- embryos and yolk sacs show defective vascular development. (A) PECAM-1 immunostaining of whole-mount E9.5 Foxo1+/+ and Foxo1-/- embryos, respectively. Note the thin and disorganized dorsal aorta in Foxo1-/- embryo compared with Foxo1+/+ embryo (arrowheads). (B) Magnified view of the E9.5 Foxo1+/+ and Foxo1-/- intersomitic vessels. Intersomitic vessels in Foxo1-/- embryo were disorganized compared with Foxo1+/+ (arrowheads). da, dorsal aorta. (C) Magnified view of the E9.5 Foxo1+/+ and Foxo1-/- head vessels. The vessels of the head, including the branches of the internal carotid artery, were properly developed in Foxo1+/+ but not in Foxo1-/- embryos (arrowheads). (D) PECAM-1 staining of Foxo1+/+ and Foxo1-/- yolk sacs. Properly developed vasculature was present in Foxo1+/+ yolk sacs but not in Foxo1-/- yolk sacs.
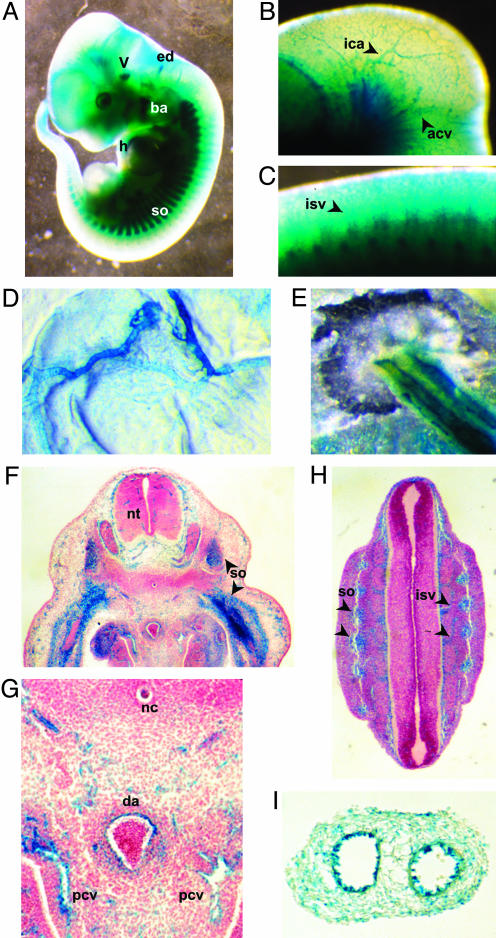
The targeting construct we used to disrupt the Foxo1 gene included a promoterless lacZ gene (18), allowing Foxo1 promoter activity (i.e., normal Foxo1 expression) to be monitored by surrogate lacZ staining (14). In whole-mount heterozygous Foxo1+/- embryos, lacZ staining was observed in several tissues and organs such as head vasculature, somite, branchial arch, heart, trigeminal ganglia, and the endolymphatic diverticulum of otocyst (Fig. 3A). A magnified view showed that branches of the internal carotid artery and anterior cardinal vein in the head and intersomitic vessels in the trunk were also stained (Fig. 3 B and C). In addition, the extraembryonic vasculature including the vitelline and umbilical vessels were stained (Fig. 3 D and E). To examine the precise location of expression, we performed lacZ staining on cryosectioned Foxo1+/- embryos at E11.5. Staining was apparent in the somites (Fig. 3 F and H) and endothelium of variously sized vessels, including the capillaries in the neural tube (Fig. 3F), the dorsal aorta, the posterior cardinal veins (Fig. 3G), and the intersomitic vessels (Fig. 3H). The endothelia of both the common umbilical artery and vein stained for lacZ (Fig. 3I). These results indicate that Foxo1 is highly expressed in developing embryonic vasculature.
Fig. 3.
LacZ expression from the insertional targeting vector in heterozygous Foxo+/- embryos and extraembryonic tissues at E11.5. (A) Whole-mount lacZ staining of embryo. LacZ was expressed in several tissues such as the somites (so), branchial arch (ba), heart (h), endolymphatic diverticulum of otocyst (ed), and trigeminal ganglia (V). (B and C) Magnified view of the lacZ stained head and trunk, respectively. Lac Z staining was found in branches of the internal carotid artery (ica), anterior cardinal vein (acv), and intersomitic vessels (isv). (D and E) Whole-mount lacZ staining of the yolk sac and umbilical cord, respectively. Both vitelline vessels and both umbilical vessels were stained. (F-H) LacZ staining for sectioned E11.5 embryo. Various vessels including the capillaries in the neural tube (nt), dorsal aorta (da), posterior cardinal vein (pcv) and intersomitic vessels (isv) were stained. (G) Magnified view of F. so, somite; nc, notocord. (I) Section of lacZ-stained umbilical cord. The endothelial layers of the two vessels (common umbilical artery and vein) were stained.
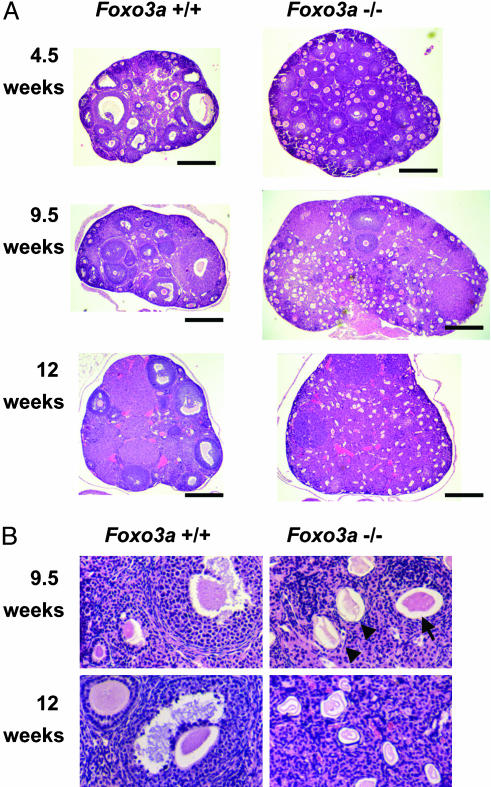
Foxo3a Is Required for Ovarian Follicular Development, Whereas Foxo4-/- Mice Are Grossly Normal in Appearance. To explore the in vivo roles of other Foxo members, we generated mice with disruptions of either Foxo3a or Foxo4. Disruption of Foxo3a was accomplished by retroviral-mediated gene trapping within intron 1 (Fig. 4A). Progeny were genotyped by quantitative Southern blot analysis (Fig. 4B; see Materials and Methods) and the resultant lack of Foxo3a expression was confirmed by Western blot analysis (Fig. 4C). Disruption of Foxo4 was accomplished by homologous gene targeting (Fig. 4D). Genotyping was performed by Southern blot analysis (Fig. 4E) and lack of Foxo4 expression was determined by Northern blot analysis (Fig. 4F). In contrast to Foxo1-null mice, both Foxo3a- and Foxo4-null mice were viable, born with the predicted Mendelian frequencies and grossly indistinguishable from their wild-type littermates. Histological analysis of >30 tissues (see Materials and Methods) from Foxo3a-null, Foxo4-null, and their normal counterparts at ≈9 weeks of age revealed no consistent histological difference among them with one exception. The ovaries from Foxo3a-/- female mice were clearly abnormal (see below). Foxo3a-/- females were completely sterile when mated at the age of 10 weeks or more (n = 9), while being fertile when mated at the age of 7 weeks or less (n = 4). On the other hand, Foxo4-/- males and females, as well as Foxo3a-/- males, showed no impairment of reproductive fitness. Further histological analysis of Foxo3a-/- ovaries showed abnormal follicular development (Fig. 5). At 4.5 weeks of age (i.e., the onset of puberty), developing follicles containing growing oocytes but no antrum (type 3b-5b) were more prominent, but mature follicles, containing antrum (type 6-7), were much less common in Foxo3a-/- ovaries than in Foxo3a+/+ ovaries (Fig. 5A). At 9.5 weeks of age, Foxo3a-/- ovaries contained plenty of developing follicles and only a few mature follicles compared to Foxo3a+/+ ovaries, similar in appearance to those observed at 4.5 weeks (Fig. 5A). The most prominent finding in Foxo3a-/- ovaries at 9.5 weeks was that the considerable number of oocytes in developing follicles had appeared to have undergone degeneration, indicating initiation of atretic change (Fig. 5B). At 12 weeks of age, Foxo3a-/- ovaries had no developing follicles and all oocytes present had undergone degeneration (Fig. 5). As Foxo3a+/- females were fertile and their ovaries were histologically indistinguishable from wild-type ovaries (data not shown), the presence of one Foxo3a allele appears to be sufficient for the normal regulation of ovarian follicular development.
Fig. 5.
Histological analysis of ovaries with hematoxylin/eosin staining. (A) At 4.5 weeks of age, developing follicles containing growing oocytes but no antrums (type 3b-5b) were prominent, but mature follicles containing antrums (type 6-7) were less common in Foxo3a-/- ovaries. At 9.5 weeks of age, Foxo3a-/- ovaries had many developing follicles and few mature follicles, similar to those at 4.5 weeks. At 12 weeks of age, Foxo3a-/- ovaries had no developing follicles, and all oocytes had undergone degeneration. Each specimen was sectioned at its largest diameter. (Scale bar, 500 μm.) (B) Various stages of follicles were found in wild-type ovaries at both 9.5 and 12 weeks of age. Normal oocytes (arrow) were present, but degenerating oocytes (arrowheads) were prominent in Foxo3a-/- ovaries at 9.5 weeks of age. All oocytes had undergone degeneration in Foxo3a-/- ovaries at 12 weeks of age.
Discussion
Here we showed that Foxo1 is expressed in developing embryonic vasculature and complete disruption of Foxo1 resulted in embryonic lethality due to vascular defects. Thus, Foxo1 is an essential regulator in embryonic vessel formation. Formation of the embryonic vasculature has been separated into two major processes, vasculogenesis and angiogenesis (23). During the initial stages of vascular development, endothelial cell precursors form a network of homogeneous and primitive blood vessels (the primary vascular plexus) by the process of vasculogenesis. The primary vascular plexus is then remodeled through the process of angiogenesis by sprouting and pruning blood vessels and recruiting mural cells to establish the vascular structure. In Foxo1-null mutant embryos, vasculature stained by PECAM-1 can be seen at E9.5, although it is immature compared to wild-type embryos. This finding suggests that Foxo1 may not have a major role in the process of embryonic vasculogenesis. However, the failure to establish normal vasculature in Foxo1-null embryos indicates that Foxo1 plays an important role in the process of embryonic angiogenesis.
Our results raise several interesting questions related to the molecular mechanisms of Foxo1 regulation in vascular development. First, which signaling pathways regulate Foxo1? Studies in C. elegans have revealed that the regulation of DAF-16 by DAF-2 signaling modulates several physiological reactions (2-4). Because C. elegans is devoid of a vascular circulatory system, the role of Foxo1 in this system cannot be inferred from its study. It is also unclear whether the focused regulation of Foxo1 by insulin signaling is conserved in mammalian vascular development. In fact, other receptor tyrosine kinase signaling, such as those involved with the VEGF, PDGF, angiopoietin, and EphrinB pathways might be expected to have more direct roles in physiological vessel formation than insulin/IGF signaling (23). Second, how does the phosphorylation status of Foxo1 in vessels change during vascular development? A recent report showed that increased phosphorylation of Foxo1 is associated with proliferation of human umbilical vascular endothelial cells (24). Although it is not clear whether the in vitro proliferation of cultured endothelial cells reflects physiological vessel formation, our results do show that Foxo1 is highly expressed in developing vessels.
The observation that Foxo3a-/- female mice have an age-dependent reduced fertility is interesting in light of the observations in C. elegans that daf-16 mutations suppress all of the phenotypes of daf-2 mutants, including reduced fertility (4). This finding may suggest that the role of Foxo3a in reproduction has been modified during evolution. Early and widespread initiation of follicular growth and subsequent early atretic change occurred by 9.5 weeks of age, followed by a noticeable absence of growing follicles. This is likely to be the primary cause of the observed age-dependent reduced fertility. These latter results are consistent with a recent report (25) that showed the dependence of follicular development on Foxo3a by using a different gene disruption approach. Ovarian follicular development is regulated by intragonadal factors and extragonadal factors (26). Intragonadal factors initiate follicular growing and coordinate development of the oocyte, granulosa cell, and thecal cell in the follicle at early stages. Extragonadal factors regulate granulosa cell and thecal cell function synchronously at later stage. Our results suggest that Foxo3a may have a suppressive effect on initiation of follicular growth by affecting the mechanisms intrinsic to the ovary, but further analyses including extragonadal factors such as FSH and LH are needed to support this hypothesis.
Initial screening of Foxo4-/- mice failed to detect an obvious phenotype. However, studies in C. elegans showed that the phenotype of daf-16 mutants becomes more obvious under nonphysiological conditions, such as in combination with daf-2 or age-1 mutations (4). In fact, as described (18), we could identify the roles of Foxo1 in insulin metabolism by analyzing Foxo1+/- mice under conditions of abnormal insulin resistance. Thus, further analysis under a variety of conditions may yet reveal the physiological function for Foxo4.
A new Foxo family member, Foxo6, was reported recently (27). Interestingly, Foxo6 has several different characteristics when compared to the other Foxo family members, such as lack of some phosphorylation sites conserved in other members and predominant nuclear localization under serum or insulin stimulation. Whether Foxo6 participates in the regulation of cell cycle, apoptosis, metabolism, and stress responses is not yet known. Further study of Foxo6 both in vitro and in vivo will contribute to our understanding of the functional roles of the Foxo transcription factors.
The work described here represents the first step in determining essential roles for the Foxo family of transcription factors. Foxo1 clearly has an essential role in embryonic vascular development and Foxo3a a role in ovarian follicular physiology, whereas Foxo4, in this preliminary study, is seemingly nonessential. The diverse functions of the Foxo members demonstrated here may be due, at least in part, to differences in their transcriptional targets. Future work toward the identification of physiological targets specific to each Foxo family member, especially targets of Foxo1 in vascular development and targets of Foxo3a in the ovarian follicular development, will be crucial to our understanding of the various roles played by this family of transcription factors. Furthermore, by crossing the three mouse lines described here, insight can be gained into possible redundancy among these three Foxo family members.
Acknowledgments
We thank Dr. P. Soriano for providing the β-geo construct, Dr. C. MacLeod for help with ES cell culture, M. Paulus and the University of California at San Diego transgenic core for help with targeting and blastocyst injection, the University of California at San Diego histology core for histology preparation, and Dr. Arthur Sands and Lexicon Genetics for the Foxo3a targeted ES cells. This work was supported in part by National Institutes of Health National Research Service Award CA68672 and the National Foundation for Cancer Research.
Abbreviations: En, embryonic day n; ES, embryonic stem.
References
- 1.Arden, K. C. & Biggs, W. H., III (2002) Arch. Biochem. Biophys. 403, 292-298. [DOI] [PubMed] [Google Scholar]
- 2.Lin, K., Dorman, J. B., Rodan, A. & Kenyon, C. (1997) Science 278, 1319-1322. [DOI] [PubMed] [Google Scholar]
- 3.Ogg, S., Paradis, S., Gottlieb, S., Patterson, G. I., Lee, L., Tissenbaum, H. A. & Ruvkun, G. (1997) Nature 389, 994-999. [DOI] [PubMed] [Google Scholar]
- 4.Tissenbaum, H. A. & Ruvkun, G. (1998) Genetics 148, 703-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biggs, W. H., III, Meisenhelder, J., Hunter, T., Cavenee, W. K. & Arden, K. C. (1999) Proc. Natl. Acad. Sci. USA 96, 7421-7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunet, A., Bonni, A., Zigmond, M. J., Lin, M. Z., Juo, P., Hu, L. S., Anderson, M. J., Arden, K. C., Blenis, J. & Greenberg, M. E. (1999) Cell 96, 857-868. [DOI] [PubMed] [Google Scholar]
- 7.Kops, G. J., de Ruiter, N. D., De Vries-Smits, A. M., Powell, D. R., Bos, J. L. & Burgering, B. M. (1999) Nature 398, 630-634. [DOI] [PubMed] [Google Scholar]
- 8.Nakae, J., Park, B. C. & Accili, D. (1999) J. Biol. Chem. 274, 15982-15985. [DOI] [PubMed] [Google Scholar]
- 9.Burgering, B. M. & Medema, R. H. (2003) J. Leukocyte Biol. 73, 689-701. [DOI] [PubMed] [Google Scholar]
- 10.Tran, H., Brunet, A., Griffith, E. C. & Greenberg, M. E. (2003) Sci. STKE 2003, RE5. [DOI] [PubMed] [Google Scholar]
- 11.Anderson, M. J., Viars, C. S., Czekay, S., Cavenee, W. K. & Arden, K. C. (1998) Genomics 47, 187-199. [DOI] [PubMed] [Google Scholar]
- 12.Biggs, W. H., III, Cavenee, W. K. & Arden, K. C. (2001) Mamm. Genome 12, 416-425. [DOI] [PubMed] [Google Scholar]
- 13.Furuyama, T., Nakazawa, T., Nakano, I. & Mori, N. (2000) Biochem. J. 349, 629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitamura, T., Nakae, J., Kitamura, Y., Kido, Y., Biggs, W. H., III, Wright, C. V., White, M. F., Arden, K. C. & Accili, D. (2002) J. Clin. Invest. 110, 1839-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakae, J., Kitamura, T., Kitamura, Y., Biggs, W. H., III, Arden, K. C. & Accili, D. (2003) Dev. Cell 4, 119-129. [DOI] [PubMed] [Google Scholar]
- 16.Richards, J. S., Sharma, S. C., Falender, A. E. & Lo, Y. H. (2002) Mol. Endocrinol. 16, 580-599. [DOI] [PubMed] [Google Scholar]
- 17.Bois, P. R. & Grosveld, G. C. (2003) EMBO J. 22, 1147-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakae, J., Biggs, W. H., III, Kitamura, T., Cavenee, W. K., Wright, C. V., Arden, K. C. & Accili, D. (2002) Nat. Genet. 32, 245-253. [DOI] [PubMed] [Google Scholar]
- 19.Zambrowicz, B. P., Friedrich, G. A., Buxton, E. C., Lilleberg, S. L., Person, C. & Sands, A. T. (1998) Nature 392, 608-611. [DOI] [PubMed] [Google Scholar]
- 20.Finch, R. A., Donoviel, D. B., Potter, D., Shi, M., Fan, A., Freed, D. D., Wang, C. Y., Zambrowicz, B. P., Ramirez-Solis, R., Sands, A. T. & Zhang, N. (2002) Cancer Res. 62, 3221-3225. [PubMed] [Google Scholar]
- 21.Hogan, B., Beddington, F., Constantini, F. & Lacy, E. (1994) Manipulating the Mouse Embryo: A Laboratory Manual (Cold Spring Harbor Lab. Press, Woodbury, NY).
- 22.Pedersen, T. & Peters, H. (1968) J. Reprod. Fertil. 17, 555-557. [DOI] [PubMed] [Google Scholar]
- 23.Jain, R. K. (2003) Nat. Med. 9, 685-693. [DOI] [PubMed] [Google Scholar]
- 24.Potente, M., Fisslthaler, B., Busse, R. & Fleming, I. (2003) J. Biol. Chem. 278, 29619-29625. [DOI] [PubMed] [Google Scholar]
- 25.Castrillon, D. H., Miao, L., Kollipara, R., Horner, J. W. & DePinho, R. A. (2003) Science 301, 215-218. [DOI] [PubMed] [Google Scholar]
- 26.Elvin, J. A. & Matzuk, M. M. (1998) Rev. Reprod. 3, 183-195. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs, F. M., Van Der Heide, L. P., Wijchers, P. J., Burbach, J. P., Hoekman, M. F. & Smidt, M. P. (2003) J. Biol. Chem. 278, 35959-35967. [DOI] [PubMed] [Google Scholar]