Abstract
Background
Leiomyosarcoma is a soft tissue sarcoma whose outcome has historically been confounded by the inclusion of gastrointestinal stromal tumors. Thus, we sought to determine the factors that predict survival and recurrence in patients with primary leiomyosarcoma alone.
Methods
During 1982–2006, 353 patients with primary resectable leiomyosarcoma were identified from a prospective database. Multivariate analysis was used to assess clinicopathologic factors for association with disease-specific survival (DSS). Competing risk survival analysis was used to determine factors predictive for local and distant recurrence.
Results
Of 353 patients, 170 (48%) presented with extremity, 144 (41%) with abdominal/retroperitoneal, and 39 (11%) with truncal tumors. Median age was 57 years (range, 18–88), and median follow-up was 50 months (1–270). Most tumors were high grade (75%), deep (73%), and completely resected (97%); median size was 6.0 cm (range, 0.3–45 cm). Abdominal/retroperitoneal location was associated with worse long-term DSS compared to extremity or trunk (P=0.005). However, by multivariate analysis, only high grade and size were significant independent predictors of DSS. Overall, 139 patients (39%) had recurrence: 51% of abdominal/retroperitoneal, 33% of extremity, and 26% of truncal patients. Significant independent predictors for local recurrence were size and margin, whereas predictors for distant recurrence were size and grade. Site was not an independent predictor of recurrence; however, late recurrence (> 5 years) occurred in 9% of abdominal/retroperitoneal and 4% of extremity lesions.
Conclusions
Grade and size are significant independent predictors of DSS and distant recurrence. Long-term follow-up in leiomyosarcoma is important, as late recurrence continues in 6–9% patients.
Introduction
Leiomyosarcoma is a common form of soft tissue sarcoma that is composed of cells showing distinct smooth muscle features [1]. The term leiomyosarcoma encompasses a spectrum of disease ranging from low grade cutaneous lesions with relatively benign behavior to aggressive deep lesions of the abdomen or extremity with significant metastatic potential [1–3]. The natural history of gastrointestinal leiomyosarcoma has been confounded with gastrointestinal stromal tumor (GIST), since these tumors have similar gross and microscopic appearance [4–6]. The vast majority of so-called smooth muscle tumors arising in the gastrointestinal tract, mesentery, and omentum are GISTs, defined by the presence of activating mutations in KIT or PDGFRA and expression of CD117 and/or CD34. Leiomyosarcoma, characteristically positive for smooth muscle actin and desmin [1], forms a significant percentage of retroperitoneal and pelvic sarcomas and is the predominant sarcoma arising from large blood vessels. Thus, with the advent of molecular pathology, a better definition of GISTs, and more frequent use of immunohistochemical stains, distinguishing leiomyosarcoma histologically has become more accurate [2, 7].
There is a paucity of literature that defines the outcomes for primary leiomyosarcoma patients alone. A recent paper on non-visceral leiomyosarcomas investigated clinical characteristics associated with outcome [8]. Although the authors found that grade, depth, and size correlated with metastasis-free survival, they did not report outcome in patients with primary disease alone and did not define sites of recurrence. Furthermore, since abdominal lesions were excluded, there were no outcome data for this site. Similarly, in a previous study from our institution, the majority (63%) of leiomyosarcoma patients presented with metastasis and/or local recurrence [9]; this study also included patients with uterine leiomyosarcoma, a biologically different disease.
The goal of our study is to report the natural history of primary leiomyosarcoma, specifically how site of the primary tumor relates to outcome as determined by disease-specific survival (DSS). Secondarily, we sought to determine the common sites of failure and the clinicopathologic features that are predictive of local and distant recurrence.
Methods
Between July 1, 1982 and June 30, 2006, 7066 adult patients admitted and treated at Memorial Sloan-Kettering Cancer Center (MSKCC) were identified from a prospective soft tissue sarcoma database, following IRB approval. The diagnosis of leiomyosarcoma was defined by characteristic pathologic features on H&E staining consisting of ovoid or cigar-shaped nuclei with a blunt end, variably eosinophilic cytoplasm, and uniform positive staining for α-sma, desmin and/or h-caldesmon, whereas all GISTs (CD117+ and CD34+) were excluded [1]. All patient pathology that included the diagnosis of leiomyosarcoma or GIST in the database from 1982 to 2000 had been previously re-reviewed by Dr Christina Antonescu as part of previous GIST studies, and thus all the patient tumors included in this study had the immunohistochemical support to characterize them as leiomyosarcoma.
Tumors in a uterine site were excluded, since they are a distinct biologic entity [2]. Patients were excluded with metastatic or local recurrence at presentation (n=247), or if deemed unresectable at the time of surgery (n=32). Thus, the study cohort consisted of 353 patients with primary leiomyosarcoma who presented for surgical resection.
Clinicopathologic data included age at presentation, gender, depth, grade, size, site, and margin status. Anatomic depth was evaluated relative to the investing superficial fascia. Tumor grade was classified as high or low based on the degree of cellularity, degree of differentiation, number of mitoses per 10 high-powered fields, and amount of tumor necrosis [10]. Tumor size was recorded as the largest dimension of the primary tumor and also stratified as ≤5 cm, >5 cm to 10 cm, or >10 cm. Margins of resected specimen were defined as R0 (negative), R1 (microscopically positive), or R2 (grossly positive). Sites of disease were defined as: (1) extremity: upper and lower extremity, (2) abdominal/retroperitoneal: any lesion in the abdomen or retroperitoneum, and (3) trunk: chest wall, groin, and thoracic.
The primary end-point of the analysis was disease-specific survival (DSS), defined as time from date of initial presentation to date of death as a result of disease or complication. The influence of clinicopathologic features on DSS was analyzed using the competing risk survival analysis method, and the effect of each prognostic factor was examined using the Gray's test. Finally, a competing risk analysis was performed to determine predictive factors for local and distant recurrence.
Survival analysis was performed using the R software and the cmprsk package. A P value less than 0.05 was considered significant.
Results
Patient and Tumor Characteristics
The study included 353 patients (Table 1), with a median age of 57 years (range, 18–88 years). Extremity was the most common site (n =170), followed by abdominal/retroperitoneal sites (n=144), whereas truncal lesions (n=39) represented 11% of the group. The majority of primary leiomyosarcomas were high grade (75%) and deep (73%). Nevertheless, we were able to obtain complete (R0 + R1) resection in 97% of patients (Table 1), although the percentage varied by site (not shown).
Table 1.
Clinicopathologic features of 353 patients with primary leiomyosarcoma
| Patient Characteristic | N | % of Total |
|---|---|---|
| Age, years, median (range) | 57 (18–88) | |
| Sex | ||
| Female | 157 | 44 |
| Male | 196 | 56 |
| Site | ||
| Extremity | 170 | 48 |
| Abdominal/RP | 144 | 41 |
| Trunk | 39 | 11 |
| Grade | ||
| High | 265 | 75 |
| Low | 88 | 25 |
| Size,a cm, median (range) | 6.0 (0.3–45) | |
| Depth | ||
| Deep | 257 | 73 |
| Superficial | 96 | 27 |
| Margin | ||
| Negative (R0) | 289 | 82 |
| Microscopically positive (R1) | 52 | 15 |
| Grossly positive (R2) | 12 | 3 |
size unknown in 4 patients
Disease-Specific Survival Analysis
At a median follow-up of 50 months (range, 1 to 270 months), 172 patients were alive. On univariate analysis the known prognostic markers of grade, size, depth, and primary site were significant variables (Table 2). The 5-year DSS by site was 75%, 81%, and 67% for extremity, truncal, and abdominal/retroperitoneal groups, respectively (Supp Figure 1; P=0.005, Table 2). Strikingly, we found no disease-related deaths in the extremity or truncal groups after approximately 8 years; in the abdominal/retroperitoneal group, patients continued to succumb to their disease over the long term (Supp Figure 1).
Table 2.
Cumulative incidence rates of disease-specific survival (DSS) and factors predictive of DSS in primary leiomyosarcoma patients
| Prognostic Factor | N | 5-yr DSS (%) | Univariate P value | Multivariate P value | Hazard Ratio | 95% CI |
|---|---|---|---|---|---|---|
| Age (yrs) | ||||||
| ≤60 | 203 | 77 | 0.104 | – | ||
| >60 | 150 | 66 | ||||
| Sex | ||||||
| Female | 157 | 72 | 0.605 | – | ||
| Male | 196 | 72 | ||||
| Grade | ||||||
| High | 265 | 65 | <0.001 | 0.001 | 3.7 | 1.7–8.2 |
| Low | 88 | 98.5 | ||||
| Size, (cm)a | ||||||
| ≤5 | 155 | 91 | <0.001 | |||
| >5to≤10 | 95 | 71 | 0.049 | 1.8 | 1.0–3.3 | |
| >10 | 99 | 47 | <0.001 | 3.4 | 1.9–6.3 | |
| Depth | ||||||
| Deep | 257 | 66 | <0.001 | 0.076 | 2.0 | 0.9–4.5 |
| Superficial | 96 | 90 | ||||
| Site | ||||||
| Extremity | 170 | 75 | 0.005 | |||
| Abdominal/RP | 144 | 67 | 0.696 | 0.9 | 0.6–1.4 | |
| Trunk | 39 | 81 | ||||
| Margin | ||||||
| Negative | 289 | 74 | 0.226 | – | ||
| Microscopically positive | 52 | 70 | ||||
| Grossly positive | 12 | 42 |
size not available for 4 patients
Multivariate analysis was performed using a competing risk analysis to identify prognostic factors that are important for DSS (Table 2). The independent predictors of DSS were high grade (HR 3.7; 95% CI, 1.7–8.2), size >10 cm (HR 3.4; 95% CI, 1.9–6.3), and size >5–10 cm (HR 1.8; 95% CI, 1.0–3.3).
Features of leiomyosarcoma recurrence by site
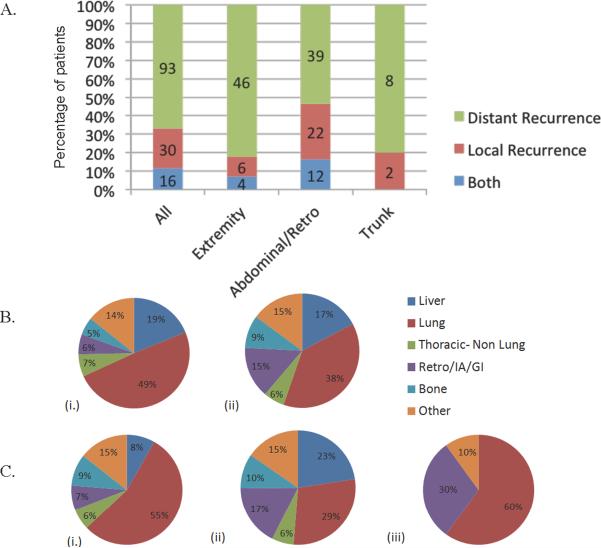
A recurrence occurred in 139 of the 353 patients (39%). The rate of first recurrence varied by site: 51% of abdominal/retroperitoneal, 33% of extremity, and 26% of truncal patients. Among the 139 patients with recurrence, the pattern of initial recurrence was predominately distant recurrence (DR), which occurred in 93 patients (67%), whereas 30 patients (22%) had a local recurrence (LR) and 16 patients (11%) had both local and distant recurrences (Figure 1A). First recurrences that were local recurrence only were substantially higher for abdominal/retroperitoneal than for truncal and extremity patients (30% vs. 20% and 11%). In contrast, distant only first recurrences were 82% for extremity patients, 80% for truncal patients, and 53% for abdominal/retroperitoneal patients (Figure 1A).
Figure 1.
Patterns of recurrence and sites of metastasis in primary leiomyosarcoma. A. Percentage of initial recurrence location in primary leiomyosarcoma patients. B. Sites of metastasis in all patients; site of first distant recurrence (i.) and all distant recurrences (ii). C. All distant recurrences by site; extremity (i.), abdominal/retroperitoneal (ii.), and trunk (iii). Thoracic – non lung = chest wall, soft tissue and/or mediastinum, Retro/IA/GI = retroperitoneal/intra-abdominal; Other = brain or lymph node.
The most common site of metastasis as either the site of first recurrence or subsequent disease dissemination was lung, followed by liver and soft tissue (Figure 1B). Less frequent sites of metastasis included bone, soft tissue metastasis in the chest wall exclusive of lung parenchyma, and abdomen or retroperitoneum. Although lung was the most common site of distant recurrence, the frequency of soft tissue, bone, and hepatic involvement varied substantially by primary site. Metastasis to bone occurred in 9% of extremity patients and 10% of abdominal/retroperitoneal patients, whereas no truncal patients developed boney metastasis. Similarly, hepatic metastasis occurred in 23% of abdominal/retroperitoneal patients and 8% of extremity patients, but was not observed in truncal patients (Figure 1C).
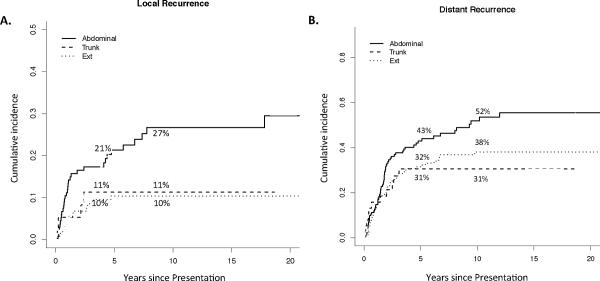
The rates of distant and local recurrence varied by primary leiomyosarcoma site (Figure 2). Local recurrence rates at both 5 and 10 years were 10% for extremity and 11% for truncal patients, with no local failures for either primary site after 5 years (Figure 2A). In contrast, abdominal/retroperitoneal patients had a much higher local recurrence rate at 5 years (21%), which continued to rise to 27% at 10 years. The 5-year distant recurrence rates were 43%, 32%, and 31% for the abdominal/retroperitoneal, extremity, and truncal patients, respectively. Truncal patients did not have any distant recurrences beyond 5 years, whereas late distant recurrences (5 to 10 years after diagnosis) occurred in 6% of extremity patients and in 9% of abdominal/retroperitoneal patients (Figure 2B).
Figure 2.
Local (A) and distant recurrence (B) rates by site in primary leiomyosarcoma. Five-year and 10-year recurrence rates are displayed. Ext = extremity.
Factors predictive for local and distant recurrence
Finally, we performed a competing risk analysis for both local and distant recurrence (Table 3). Prognostic factors for local recurrence were size >10 cm (HR 2.9; 95% CI, 1.2–6.7) and margin status R1 vs. R0 (HR 2.1; 95% CI, 1.1–3.9), as we excluded R2 margins in our analysis. For distant recurrence, the prognostic factors were grade (HR 3.9; 95% CI, 1.9–7.8) and size (HR 2.6; 95% CI, 1.5–4.6).
Table 3.
Competing risk analysis for local and distant recurrence in primary leiomyosarcoma
| Local Recurrence | Distant Recurrence | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Hazard | Hazard | |||||
| Clinicopathologic Variable | P value | Ratio | 95% CI | P value | Ratio | 95% CI |
| Margin R1 vs. R0a | 0.024 | 2.1 | 1.1–3.9 | – | – | – |
| Site Abd/RP vs. Extremity/Trunk | 0.744 | 1.1 | 0.6–2.2 | 0.258 | 0.8 | 0.5–1.2 |
| Size >10 cm vs. ≤ 10cm | 0.013 | 2.9 | 1.2–6.7 | 0.001 | 2.6 | 1.5–4.6 |
| High vs. low grade | 0.266 | 1.7 | 0.7–4.4 | <0.001 | 3.9 | 1.9–7.8 |
| Deep vs. superficial | 0.125 | 2.7 | 0.8–9.2 | 0.059 | 1.9 | 1.0–3.6 |
R2 margins were excluded
Discussion
In this study, we present a natural history study of primary leiomyosarcoma ranging over 25 years. This is the largest series of its kind that excludes GIST and uterine site, as these two tumor types are separate biologic diseases. Prior to advent of molecular pathology, published studies fail to discriminate between LMS of the GI tract and GIST [5, 6]. Estimates of the true incidence of leiomyosarcoma range from 2% to 10% of all smooth muscle sarcomas of the GI tract [11–14].
Predictive factors for DSS in leiomyosarcoma
The most important independent predictors for DSS in patients with primary leiomyosarcoma are grade (HR =3.7 for high vs. low) and size (HR = 3.4 for >10 cm vs. <5; HR =1.8 for 5–10 cm vs. <5 cm). Although site was a significant univariate predictor of DSS and we found a higher frequency of late leiomyosarcoma-specific deaths to be associated with the abdominal/retroperitoneal site, site was not an important predictor of DSS after adjusting for grade and size. The fact that 27% of abdominal/retroperitoneal leiomyosarcoma patient and 7% of extremity and truncal patients will succumb to disease more than 5 years after diagnosis supports the need for follow-up beyond 5 years, particularly for patients with abdominal/retroperitoneal tumors.
Our survival data cannot easily be compared with those from previous studies, since those studies included metastatic patients [15, 16], unresectable patients [16], local recurrence [8], uterine disease [9], or GISTs [5, 6] or excluded abdominal and/or retroperitoneal sites [8, 17]. Nevertheless, grade has also been found to be a predictor of leiomyosarcoma-specific survival in other series [8, 9, 16, 17]. However, in contrast to previous reports, we did not find margin status predictive [9, 17]. This is likely explained by different R0 resection rates: 55% [17] and 48% [9] compared to 82% in our series. Also, we did not find age to be a DSS predictive factor, unlike previous studies [15, 17]. In one series, elderly patients had a much higher incidence of metastasis during follow-up (67% vs. 25% for those above vs. below age 62); however, the reasons for this observation are unclear [17]. Furthermore, we did not find a higher incidence of female patients than male patients [9] (Table 1). Finally, mitotic index was not examined in our study; although it can be useful in discriminating leiomyomas from leiomyosarcoma [2] and in determining grade, its use as an independent prognostic factor has not been validated in larger LMS series [3, 16, 17].
Patterns of leiomyosarcoma recurrence: sites of failure and predictive factors for local and distant disease
To our knowledge, this study represents the first comprehensive analysis of the timing and sites of recurrence according to site of primary disease (Figure 1). Collectively, our recurrence data define which leiomyosarcoma sites are prone to distant and local recurrence and when and where these events are likely to occur.
The cumulative incidence of recurrence varied significantly by site (Figure 1). Extremity and truncal patients had approximately a 10% cumulative incidence of local recurrence, all of which occurred within 5 years of resection (Figure 2A). This is consistent with a previous study of similar primary sites in which an 8% local recurrence rate was observed, and all recurrences occurred within 2 years of resection [17]. Abdominal/retroperitoneal patients in our study had a higher incidence of local recurrence (21% at 5 years), and also a substantial incidence of late local recurrence (Figure 2A). With respect to distant recurrence, we surprisingly found that 6% of extremity and 9% of abdominal/retroperitoneal patients developed distant recurrence more than 5 years out from primary tumor diagnosis (Figure 2B), emphasizing the need for long-term follow-up in both the retroperitoneal and extremity subgroups.
Lung was the most common site of metastasis regardless of the site of primary (Figure 1Bi), which has been previously reported [1, 9, 17]. The second most common site of metastasis was other soft tissue sites in extremity patients (15% of all distant recurrences) and liver in abdominal/retroperitoneal patients (23% of all distant recurrences). In contrast, primary truncal leiomyosarcomas did not metastasize to liver, bone, or non-lung thoracic sites, yet the second most common site of metastasis in truncal patients was abdominal/retroperitoneal soft tissue (30% of all distant recurrences).
In our competing risk survival analysis, size and margin status are important prognostic factors for local recurrence, whereas size and grade are prognostic for distal recurrence. In the recent Scandinavian study, which did not include abdominal and/or retroperitoneal leiomyosarcoma, decreased metastasis-free survival was similarly associated with grade, size, and depth [8]. This study, however, did not define clinicopathologic features associated with local recurrence except for inadequate local treatment, which was defined as marginal tumor resection or an intralesional surgical margin with or without radiation therapy [8]. Thus, our study is the first to define the clinical characteristics associated with local recurrence for the most common sites of leiomyosarcoma.
Patients with low grade, superficial, or small (<5 cm) leiomyosarcomas (n=43) had more favorable outcomes as the 5-year DSS was 98.5, 90, and 91% respectively (Table 2). None of the 5 truncal patients in this subgroup recurred. However, of the 38 “favorable” extremity patients, 1 patient developed a local recurrence at 3 years, followed by distant disease at 5 years but currently has no evidence of disease, and another patient died of disease after the development of distant disease 6 years after diagnosis.
Although our study did not examine survival post-metastasectomy in leiomyosarcoma patients, we have found reasonable median survival in patients with complete resection of liver metastasis, especially when recurrence develops later than 2 years post-diagnosis [18].
We observed significant late disease-specific mortality in patients with abdominal and/or retroperitoneal disease, as approximately 6% die of disease after 8 years. Such late disease-specific mortality has not been previously reported, presumably because of smaller patient series [19] or shorter follow-up [9]. These abdominal/retroperitoneal patients also continue to experience late local and distant recurrence even 10 years or more from primary diagnosis (Figure 2). Therefore, we strongly recommend sarcoma centers to continue surveillance for recurrence for the lifetime of these patients.
Conclusions
Grade and size are the most important prognostic factors for disease-specific survival and distant recurrence in patients with primary leiomyosarcoma. Site is not an important independent prognostic factor for local recurrence in this series; however, size and margin are. Long-term follow-up of leiomyosarcoma patients is important as late local or distant recurrence occurs in 9% of abdominal/retroperitoneal patients and 6% of extremity patients.
Supplementary Material
Synopsis.
Analysis of 353 patients with primary leiomyosarcoma, excluding gastrointestinal stromal tumor, demonstrates that grade and size are independent predictors of metastasis and disease-specific survival. Although location did not independently predict outcome, abdominal/retroperitoneal location was associated with late recurrence.
Acknowledgments
This work was supported by Soft Tissue Sarcoma Program Project grant P01 CA 047179 (SS, LXQ, CA, MFB).
References
- 1.Weiss SW, Goldblum JR. Enzinger & Weiss's Soft Tissue Tumors. Mosby; St. Louis, MO: 2008. [Google Scholar]
- 2.Miettinen M, Fetsch JF. Evaluation of biological potential of smooth muscle tumours. Histopathology. 2006;48:97–105. doi: 10.1111/j.1365-2559.2005.02292.x. [DOI] [PubMed] [Google Scholar]
- 3.Pijpe J, Broers GH, Plaat BE, et al. The relation between histological, tumor-biological and clinical parameters in deep and superficial leiomyosarcoma and leiomyoma. Sarcoma. 2002;6:105–10. doi: 10.1080/1357714021000065404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katz SC, DeMatteo RP. Gastrointestinal stromal tumors and leiomyosarcomas. Journal of Surgical Oncology. 2008;97:350–9. doi: 10.1002/jso.20970. [DOI] [PubMed] [Google Scholar]
- 5.Ng EH, Pollock RE, Romsdahl MM. Prognostic implications of patterns of failure for gastrointestinal leiomyosarcomas. Cancer. 1992;69:1334–41. doi: 10.1002/1097-0142(19920315)69:6<1334::aid-cncr2820690606>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 6.Lee YT. Leiomyosarcoma of the gastro-intestinal tract: general pattern of metastasis and recurrence. Cancer Treatment Reviews. 1983;10:91–101. doi: 10.1016/0305-7372(83)90007-5. [DOI] [PubMed] [Google Scholar]
- 7.Price ND, Trent J, El-Naggar AK, et al. Highly accurate two-gene classifier for differentiating gastrointestinal stromal tumors and leiomyosarcomas. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3414–9. doi: 10.1073/pnas.0611373104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Svarvar C, Bohling T, Berlin O, et al. Clinical course of nonvisceral soft tissue leiomyosarcoma in 225 patients from the Scandinavian Sarcoma Group. Cancer. 2007;109:282–91. doi: 10.1002/cncr.22395. [DOI] [PubMed] [Google Scholar]
- 9.Clary BM, DeMatteo RP, Lewis JJ, Leung D, Brennan MF. Gastrointestinal stromal tumors and leiomyosarcoma of the abdomen and retroperitoneum: a clinical comparison. Ann Surg Oncol. 2001;8:290–9. doi: 10.1007/s10434-001-0290-3. [DOI] [PubMed] [Google Scholar]
- 10.Hajdu SI, Shiu MH, Brennan MF. The role of the pathologist in the management of soft tissue sarcomas. World J Surg. 1988;12:326–31. doi: 10.1007/BF01655665. [DOI] [PubMed] [Google Scholar]
- 11.Miettinen M, Furlong M, Sarlomo-Rikala M, Burke A, Sobin LH, Lasota J. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the rectum and anus: a clinicopathologic, immunohistochemical, and molecular genetic study of 144 cases. The American Journal of Surgical Pathology. 2001;25:1121–33. doi: 10.1097/00000478-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Miettinen M, Kopczynski J, Makhlouf HR, et al. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the duodenum: a clinicopathologic, immunohistochemical, and molecular genetic study of 167 cases. The American Journal of Surgical Pathology. 2003;27:625–41. doi: 10.1097/00000478-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Miettinen M, Sarlomo-Rikala M, Sobin LH, Lasota J. Gastrointestinal stromal tumors and leiomyosarcomas in the colon: a clinicopathologic, immunohistochemical, and molecular genetic study of 44 cases. The American Journal of Surgical Pathology. 2000;24:1339–52. doi: 10.1097/00000478-200010000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Agaimy A, Wunsch PH. True smooth muscle neoplasms of the gastrointestinal tract: morphological spectrum and classification in a series of 85 cases from a single institute. Langenbeck's Archives of Surgery / Deutsche Gesellschaft fur Chirurgie. 2007;392:75–81. doi: 10.1007/s00423-006-0092-y. [DOI] [PubMed] [Google Scholar]
- 15.Gustafson P, Willen H, Baldetorp B, Ferno M, Akerman M, Rydholm A. Soft tissue leiomyosarcoma. A population-based epidemiologic and prognostic study of 48 patients, including cellular DNA content. Cancer. 1992;70:114–9. doi: 10.1002/1097-0142(19920701)70:1<114::aid-cncr2820700119>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 16.Miyajima K, Oda Y, Oshiro Y, et al. Clinicopathological prognostic factors in soft tissue leiomyosarcoma: a multivariate analysis. Histopathology. 2002;40:353–9. doi: 10.1046/j.1365-2559.2002.01361.x. [DOI] [PubMed] [Google Scholar]
- 17.Farshid G, Pradhan M, Goldblum J, Weiss SW. Leiomyosarcoma of somatic soft tissues: a tumor of vascular origin with multivariate analysis of outcome in 42 cases. The American Journal of Surgical Pathology. 2002;26:14–24. doi: 10.1097/00000478-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 18.DeMatteo RP, Shah A, Fong Y, Jarnagin WR, Blumgart LH, Brennan MF. Results of hepatic resection for sarcoma metastatic to liver. Annals of Surgery. 2001;234:540–7. doi: 10.1097/00000658-200110000-00013. discussion 7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajani B, Smith TA, Reith JD, Goldblum JR. Retroperitoneal leiomyosarcomas unassociated with the gastrointestinal tract: a clinicopathologic analysis of 17 cases. Mod Pathol. 1999;12:21–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.