Abstract
Over the last decades many epidemiologic studies considered the morbidity patterns for respiratory diseases and lung function of children in the context of ambient air pollution usually measured in the postnatal period. The main purpose of this study is to assess the impact of prenatal exposure to fine particulate matter (PM2.5) on the recurrent broncho-pulmonary infections in early childhood.
The study included 214 children who had measurements of personal prenatal PM2.5 exposure and regularly collected data on the occurrence of acute bronchitis and pneumonia diagnosed by a physician from birth over the seven-year follow-up. The effect of prenatal exposure to PM2.5 was adjusted in the multivariable logistic models for potential confounders, such as prenatal and postnatal ETS (environmental tobacco smoke), city residence area as a proxy of postnatal urban exposure, children’s sensitization to domestic aeroallergens, and asthma. In the subgroup of children with available PM2.5 indoor levels, the effect of prenatal exposure was additionally adjusted for indoor exposure as well. The adjusted odds ratio (OR) for incidence of recurrent broncho-pulmonary infections (five or more spells of bronchitis and/or pneumonia) recorded in the follow-up significantly correlated in a dose-response manner with the prenatal PM2.5 level (OR = 2.44, 95%CI: 1.12 – 5.36).
In conclusion, the study suggests that prenatal exposure to PM2.5 increases susceptibility to respiratory infections and may program respiratory morbidity in early childhood. The study also provides evidence that the target value of 20 μg/m3 for the 24-hour mean level of PM2.5 protects unborn babies better than earlier established EPA guidelines.
Keywords: birth cohort study, fine particulate matter, prenatal and postnatal exposure, bronchitis, pneumonia
Introduction
The rising trends worldwide in respiratory diseases in children are of great public health concern since lower respiratory tract infections in early life can lead to decreased lung function, persistent lung damage and increased susceptibility to various lung diseases in later life (1 – 4) Many studies have analyzed the problem of respiratory health in terms of exposure to various outdoor air pollutants, such as particulate matter (PM) or polycyclic aromatic hydrocarbons (PAH), which are mostly generated by automobile traffic and power plants. Much smaller number of studies tried to explain the problem in the context of the effect of indoor air pollutants, which include emissions from the combustion of fuel for residential heating, unvented gas appliances, environmental tobacco smoke (ETS) and fumes from cooking (5 – 8).
Earlier epidemiologic studies considered the respiratory morbidity patterns in children in a wide range of context, but mainly for ambient air pollution measured in the postnatal period (9 – 14). Moreover, the studies on postnatal exposure have usually quantified the concentrations of outdoor air pollutants in the residence area, and assigned approximate exposure levels to the study subjects. In some studies, residential proximity to industrial plants was also the proxy for exposure to industrial pollution. Estimating individual average exposures during specific study period by relying on the ambient air monitoring stations even close to the children’s’ residence area may result in exposure misclassification. Networks of air pollution stations are usually located far away from the residences and may provide the inaccurate surrogate measures for personal exposure.
Reproductive epidemiology studies provide evidence that the fetus is likely to be significantly more sensitive to a variety of environmental toxicants than adults (15 – 17) This results from the fact that many environmental toxicants absorbed by the mother easily cross the placenta and accumulate in the fetus often at higher concentrations than in mothers (18). Although there is some epidemiologic evidence linking prenatal exposure to tobacco smoke and respiratory health in children (19 – 22), little research has been conducted on the effects of prenatal determinants of respiratory health in early childhood resulting from gestational exposure to fine particulate matter measured on an individual basis.
The main purpose of the study was to assess the impact of prenatal exposure to fine particulate matter, measured during the second trimester of pregnancy with personal monitors, on the incidence of recurrent broncho-pulmonary infections (physician diagnosed episodes of bronchitis and pneumonia) in the offspring over a seven-year postnatal period. The second trimester marks the halfway period of pregnancy, when the fetus starts to grow very quickly and the brain undergoes its most important period of growth. From now on, the fully developed placenta provides all the fetus’ needs until birth and this may also create favorable conditions for toxicants absorbed by mother to cross placenta and eventually put fetus at risk. The effect of prenatal exposure to PM2.5 on broncho-pulmonary infections was adjusted for a set of potential confounders, such as prenatal and postnatal ETS, city residence area as a proxy of postnatal outdoor exposure, children’s sensitization to domestic aeroallergens, and asthma diagnosed by a physician. In the subgroup of children with available indoor pollution levels at the age 3, the effect of prenatal exposure was additionally adjusted for indoor exposure to PM2.5. A secondary goal of the study was to assess the degree to which the present EPA 24-hour air quality standards (35 μg/m3) protect the developing fetus and young children (23).
Material and methods
The study initially enrolled 502 newborns, 214 of whom have completed the seven -year follow-up and had skin prick testing for common domestic allergens. This is the part of an ongoing longitudinal investigation on the health impact of prenatal exposure to outdoor/indoor air pollution in infants and children from the Krakow city area. The detailed description of the study design has been presented elsewhere (24). In short, pregnant women were recruited from ambulatory prenatal clinics in the first or second trimester of pregnancy. The study included women between 18 and 35 years of age, who claimed to be non-smokers, with singleton pregnancies, without illicit drug use and HIV infection, free from chronic diseases such as diabetes or hypertension, and resident in Krakow for at least one year prior to pregnancy. All women participating in the study had read and signed an informed consent. The Jagiellonian University Bioethical Committee approved the research.
Upon enrollment, a detailed questionnaire was administered to each subject to elicit information on demographic data, house characteristics, medical history of mothers, and smoking practices of others present in the home. Over the seven-year follow-up, regular data on the occurrence of broncho-pulmonary infections (acute bronchitis and pneumonia) diagnosed by a physician were regularly collected by interviewers visiting homes of the children at intervals of 3 months in the first two years of life and 6 months later. Prenatal ETS was defined by the number of cigarettes smoked daily at home; and postnatal ETS by the number of years the child has lived in the house where at least one of the household members was an active smoker. Atopic status of children was defined as sensitization to at least one common domestic aeroallergen.
Dosimetry of prenatal and postnatal exposure to fine particles
Prenatal exposure was monitored during the second trimester of pregnancy in recruited mothers with a personal environmental monitoring sampler (PEMS) designed in Dr Spengler’s Lab. The PEMS is designed to achieve the particle mass target size of < 2.5 μm at a flow rate of 4.0 liters per minute (LPM) with an array of 10 impactor nozzles. Flow rates are calibrated (with filters in place) using a bubble meter prior to the monitoring, and are checked again with a change of the battery pack on the second day and at the conclusion of the monitoring. Pumps operated continuously at 2 LPM over the 48-hour period. To modify the sampler to achieve the 2.5 μm size cut at 2 LPM, 5 of the nozzles were blocked. Particles were collected on a Teflon membrane filter (37 mm Teflo™, Gelman Sciences). The combination of low pressure drop (permitting use of a low power sampling pump), low hygroscopicity (minimizing bound water interference in mass measurements), and low trace element background (improving analytical sensitivity) of these filters make them highly appropriate for personal particle sampling. Before the field study the validation was a series of QA/QC checks on flows, timing, filter integrity, and weighting protocols.
During the monitoring session, the woman was instructed to wear the PEMS monitor during the daytime hours for 2 consecutive days and to place the monitor near the bed at night. On the second day of the air monitoring, the technician and interviewer visited the woman’s home to change the battery-pack and administer the full questionnaire. They also checked to see that the monitor had been running continuously and that there had been no technical or operating failures.
In the subset of 80 pregnant women, personal dosimetry was repeatedly taken once during each trimester to estimate how measurements of PM2.5 in the second trimester may be representative for the whole pregnancy period. Additionally, in the subgroup of children (n = 131) for whom a consent was obtained, the indoor levels of PM2.5 were measured using the same methodology.
Ascertainment of atopic status
All 5-year olds were invited to undergo a skin prick test (SPT) for 4 common domestic aeroallergens (Dermatophagoides pteronyssinus, Dermatophagoides farinae, dog and cat hair). The results were read after 15 minutes by measuring the largest diameter of the wheal. Atopic status was ascertained as a wheal reaching diameter of 3 mm which is greater than the histamine control. The participants were defined as atopic if they had at least one positive skin prick test.
Statistical analysis
Statistical analyses were performed in order to assess a possible association between prenatal PM2.5 levels with incidence of recurrent episodes of bronchitis and pneumonia occurring over the seven-year follow-up. As the distribution of PM2.5 concentrations was skewed to the right, values were ln-transformed to normalize the distribution. PM2.5 levels were provided as geometric mean values (average after ln-transformation, followed by back transformation) together with 95% confidence intervals. In the initial univariate analysis, the differences in continuous variables between groups were analyzed using a one-way analysis of variance; differences in the frequencies of categorical variables were evaluated with the Chi-square test. After the descriptive univariate analysis we used the multivariable nested logistic regression models to explore the relationship between recurrent broncho-pulmonary infections and prenatal PM2.5 exposure adjusted for a set of a priori selected covariates (prenatal and postnatal ETS, indoor PM2.5, at the age 3, residence city area, children’s atopy and asthma diagnosed by a medical doctor). The regression estimates are nested in the sense that the first regression is nested in the second since all the predictors in the first regression are included in the second. Similarly, the second regression is nested in the third regression and so on. Statistical analysis was performed by the statistical software STATA version 12.1 and two-sided p <0.05 was considered statistically significant.
Results
Table 1 presents the characteristics of the total study sample grouped by values of prenatal exposure to PM2.5 in the second trimester dichotomized by 35.0 μg/m3 level, which was very close to the median value (35.9 μg/m3, 95%CI: 23.3 – 50.4 μg/m3). Characteristics of children who did not participate in the follow-up did not differ significantly from the participants except for prenatal level of PM2.5(Table 2).
Table 1.
Characteristics of the children who completed the follow-up grouped by prenatal exposure to P PM2.5
| Variables | Low PM2.5 <=35 μg/m3 N=112 | High PM2.5 >35 μg/m3 N=102 | Total N=214 | P for difference |
|---|---|---|---|---|
|
| ||||
| Maternal age(yrs) | ||||
| mean | 27.73 | 27.85 | 27.79 | 0.7959 |
| SD | 3.12 | 3.70 | 3.40 | |
|
| ||||
| Maternal education (yrs) | ||||
| mean | 15.81 | 15.61 | 15.72 | 0.5837 |
| SD | 2.54 | 2.91 | 2.72 | |
|
| ||||
| Maternal allergy (+) | ||||
| n (%) | 30 (26.8) | 20(19.6) | 50(23.4) | 0.2812 |
|
| ||||
| Older siblings | ||||
| n (%) | 39 (34.8) | 39 (38.2) | 78 (36.4) | 0.7069 |
|
| ||||
| Gender, Boys | ||||
| n (%) | 58(51.8)) | 45(44.1) | 103 (48.1) | 0.3249 |
|
| ||||
| Gestational age (weeks) | ||||
| mean | 39.60 | 39.54 | 39.57 | 0.7040 |
| SD | 1.078 | 1.191 | 1.131 | |
|
| ||||
| Birth weight (g) | ||||
| mean | 3506.0 | 3383.6 | 3447.7 | 0.0424 |
| SD | 455.1 | 417.8 | 441.0 | |
|
| ||||
| Breastfeeding > 6 months | ||||
| n (%) | 31 (27.7) | 30 (29.4) | 61 (28.5) | 0.8974 |
|
| ||||
| Prenatal ETS (+) | ||||
| n (%) | 21 (18.8) | 31 (30.4) | 52 (24.3) | 0.0682 |
|
| ||||
| Postnatal ETS: | ||||
| 0 yrs n (%) | 90 (80.4) | 69 (67.6) | 159(74.3) | 0.0008 |
| 1–3 yrs n(%) | 17(15.2) | 11 (10.8) | 28(13.1) | |
| 4–7 yrs n (%) | 5 (4.5) | 22(21.6) | 27 (12.6) | |
|
| ||||
| Residence area: city center | ||||
| n (%) | 16(14.3) | 25 (24.5) | 41 (19.2) | 0.0847 |
|
| ||||
| Postnatal indoor PM2.5 μg/m3 measured at the age 3 | ||||
| mean | 42.89 | 45.85 | 44.36 | 0.6312 |
| SD | 32.39 | 37.74 | 35.04 | |
| Missing data | 46 | 37 | 83 | |
|
| ||||
| Bronchitis (l–7 yrs) number of episodes | ||||
| mean | 1.8 | 2.1 | 1.9 | 0.2209 |
| SD | 1.8 | 2.4 | 2.1 | |
|
| ||||
| Pneumonia (l–7 yrs) number of episodes | ||||
| mean | 0.5 | 0.6 | 0.5 | 0.3622 |
| SD | 0.8 | 1.2 | 1.0 | |
Table 2.
Characteristics of the children who completed and did not complete the seven-year follow-up
| Variables | Followed-up completed N=214 | Followed-up not completed N=266 | Total N= 480 | P for difference |
|---|---|---|---|---|
|
| ||||
| Maternal age(yrs): | ||||
| mean | 27.79 | 27.38 | 27.57 | 0.2180 |
| SD | 3.40 | 3.73 | 3.59 | |
|
| ||||
| Maternal education (yrs): | ||||
| mean | 15.72 | 15.46 | 15.57 | 0.3040 |
| SD | 2.72 | 2.78 | 2.75 | |
|
| ||||
| Maternal allergy (+): | ||||
| n (%) | 50 (23.4) | 68 (25.6) | 118 (24.6) | 0.6530 |
|
| ||||
| Older siblings | ||||
| n (%) | 78 (36.4) | 97 (36.5) | 175 (36.5) | 1.0000 |
|
| ||||
| Gender, Boys | ||||
| n (%) | 103 (48.1) | 142 (53.4) | 245 (51.0) | 0.2926 |
|
| ||||
| Gestational age (weeks) | ||||
| mean | 39.57 | 39.51 | 39.54 | 0.5750 |
| SD | 1.131 | 1.150 | 1.141 | |
|
| ||||
| Birth weight (g) | ||||
| mean | 3447.7 | 3442.2 | 3444.6 | 0.8914 |
| SD | 441.0 | 433.7 | 436.5 | |
|
| ||||
| Breastfeeding only > 6 months | ||||
| n (%) | 61(28.5) | 59 (23.1) | 120 (25.6) | 0.2222 |
| Missing data | 0 | 11 | 11 | |
|
| ||||
| Prenatal ETS (+) | ||||
| n (%) | 52 (24.3) | 77 (28.9) | 129 (26.9) | 0.2991 |
|
| ||||
| Residence area: city center | ||||
| n (%) | 41 (19.2) | 56 (21.1) | 97 (20.2) | 0.6897 |
|
| ||||
| Prenatal PM2.5 (μg/m3): | ||||
| mean | 42.37 | 45.27 | 43.98 | 0.3824 |
| SD | 27.55 | 41.79 | 36.14 | |
|
| ||||
| Postnatal indoor PM2.5 (μg/m3) at the age 3: | ||||
| mean | 44.36 | 36.29 | 40.19 | 0.0422 |
| SD | 35.04 | 29.98 | 32.71 | |
| Missing data | 83 | 126 | 209 | |
Prenatal personal PM2.5 values significantly correlated with the number of cigarettes smoked daily at home in pregnancy (nonparametric trend z = 3.57, p < 0.001) and the duration of ETS exposure in the postnatal period (z = 3.93, p < 0.001), and was significantly higher in subjects who lived in the city center compared with those from the outer city area (42.7 vs. 34.2 μg/m3, t = 2.26, p = 0.025).
Personal dosimetry taken once during each trimester in the subset of 80 pregnant women showed a consistent trend between PM2.5 levels over pregnancy trimesters. The nonparametric trend for PM2.5 concentrations (in tertiles) measured in the second and third pregnancy trimester was highly significant (z = 3.21, p = 0.001); the corresponding level of statistical association between the measurements taken in the first and the second trimester was significant as well (z = 2.42, p = 0.015).
The mean number of acute bronchitis episodes recorded in the follow-up was about four times higher than the number of pneumonia episodes (2.02; 95%CI: 1.86 – 2.19 vs. 0.57; 95%CI: 0.48 – 0.66). Both physician-diagnosed bronchitis and pneumonia cases were lower in the younger age group than in older children (Table 3) and the frequency of bronchitis/pneumonia spells greatly depended on the asthma status. Children with asthma showed a two-fold higher number of bronchitis episodes and more than a three-fold higher number of pneumonia episodes. Importantly, the incidence of recurrent bronchitis (four or more spells) and pneumonia (two or more spells) significantly correlated with the level of prenatal PM2.5 exposure level (Table 4).
Table 3.
Cumulative incidence of respiratory infections diagnosed by a physician in the follow-up grouped by the age of children and medical diagnosis of asthma (in brackets 95% confidence intervals)
| Asthma (−) | Asthma (+) | Total | |
|---|---|---|---|
| Bronchitis | |||
| Age 1 – 3 yrs | 0.75(0.65–0.87) | 1.60(1.19–2.10) | 0.85(0.74–0.96) |
| Age 4 – 7 yrs | 1.05(0.92–1.19) | 2.19(1.71–2.76) | 1.17(1.04–1.30) |
| Age 1 – 7 yrs | 1.80(1.63–1.98) | 3.79(3.14–4.51) | 2.02(1.86–2.19) |
| Pneumonia | |||
| Age 1 – 3 yrs | 0.21 (0.15–0.27) | 0.56(0.33–0.89) | 0.25(1.19–0.31) |
| Age 4 – 7 yrs | 0.25(0.20–0.33) | 0.90(0.61–1.30) | 0.32(0.26–0.40) |
| Age 1–7 yrs | 0.46(0.38–0.55) | 1.47(1.08–1.92) | 0.57(0.48–0.66) |
Table 4.
Frequency of recurrent cases of acute bronchitis and pneumonia reported in the follow-up grouped by the prenatal PM2.5 level (in tertiles)
| Variable | PM2.5 concentrations in tertiles | P-value | ||
|---|---|---|---|---|
| 11.1–26.6 | 26.7–45.9 μg/m3 | 46.3–43.9 μg/m3 | ||
| Recurrent acute bronchitis | 10 (14.3%) | 8(10.1%) | 20 (30.8%) | Chi-square11.263, p = 0.004 |
| Recurrent pneumonia | 3 (4.3%) | 7 (8.9%) | 12(18.5%) | Chi-square 7.617, p = 0.022 |
| Number of children | 70 | 79 | 65 | 214 |
In the next step of the statistical analysis, we used nested multivariable logistic regression models to assess the relationship between broncho-pulmonary infections (5 or more episodes of acute bronchitis and/or pneumonia) and prenatal PM2.5 exposure. Table 5 shows the results of the hierarchical regression analysis for variables predicting odd ratios (ORs) of broncho-pulmonary infections for PM2.5 ln-transformed values adjusted for covariates in participants of the seven-year follow-up. The variables added to each block are stepwise listed and the change in Wald Chi-square for a given block is reported with its significance level. Prenatal PM2.5 exposure entered in the first model was significant (p = 0.026), however, neither prenatal or postnatal ETS separately added in subsequent models, nor atopy or the residence area were significant. The inclusion of asthma in the fifth model produced a substantial increment in the Wald Chi-square (p = 0.004).
Table 5.
Summary of hierarchical logistic regression analysis for variables predicting ORs for recurrent broncho-pulmonary infections (defined as five or more episodes) in the seven-year follow-up (N = 214)
| Variables | OR | Pr>|z| | 95% Confidence intervals | Wald Chi-square | df | Pr> F | |
|---|---|---|---|---|---|---|---|
| Model I | |||||||
| Prenatal PM2.5 exposure a | 2.00 | 0.026 | 1.09 | 3.70 | 4.97 | 1 | 0.0257 |
| Model II | |||||||
| Prenatal PM2.5 exposure a | 2.17 | 0.018 | 1.14 | 4.12 | 0.69 | 1 | 0.4063 |
| Prenatal ETSb | 0.78 | 0.406 | 0.43 | 1.40 | |||
| Model III | |||||||
| Prenatal PM2.5 exposure a | 2.22 | 0.015 | 1.17 | 4.23 | 0.43 | 1 | 0.5109 |
| Prenatal ETSb | 0.97 | 0.963 | 0.40 | 2.37 | |||
| Postnatal ETSc | 0.76 | 0.511 | 0.33 | 1.73 | |||
| Model IV | |||||||
| Prenatal PM2.5 exposure a | 2.20 | 0.017 | 1.15 | 4.19 | 0.22 | 1 | 0.6404 |
| Prenatal ETSb | 0.95 | 0.913 | 0.39 | 2.32 | |||
| Postnatal ETSc | 0.78 | 0.559 | 0.34 | 1.79 | |||
| Atopy Y/N | 1.26 | 0.640 | 0.48 | 3.26 | |||
| Model V | |||||||
| Prenatal PM2.5 exposure a | 2.10 | 0.028 | 1.08 | 4.06 | 8.08 | 1 | 0.0045 |
| Prenatal ETSb | 0.92 | 0.885 | 0.36 | 2.39 | |||
| Postnatal ETSc | 0.79 | 0.597 | 0.33 | 1.91 | |||
| Atopy Y/N | 1.19 | 0.715 | 0.45 | 3.17 | |||
| Asthma Y/N | 3.81 | 0.004 | 1.52 | 9.59 | |||
| Model VI | |||||||
| Prenatal PM2.5 exposure a | 2.05 | 0.035 | 1.05 | 3.99 | |||
| Prenatal ETSb | 0.93 | 0.885 | 0.36 | 2.39 | |||
| Postnatal ETSc | 0.79 | 0.597 | 0.33 | 1.91 | |||
| Atopy Y/N | 1.19 | 0.735 | 0.45 | 3.14 | |||
| Asthma Y/N | 3.87 | 0.004 | 1.53 | 9.75 | |||
| Residenced | 1.31 | 0.553 | 0.54 | 3.17 | 0.35 | 1 | 0.5525 |
ln transformed concentrations
0: no prenatal ETS exposure, 1: up to 5 cigarettes smoked daily, 2 : more than 5 cigarettes smoked daily
0: no postnatal ETS exposure, 1: short exposure (<=3 years), 2: long exposure (>3 years)
0: outer city area residents, 1: city center residents
The same analysis performed for recurrent broncho-pulmonary infections in the subgroup of 131 children with available indoor PM2.5 measurement confirmed a significant effect of prenatal PM2.5 level (Table 6). The impact of indoor PM2.5 measured at the age 3 showed a border significance level (p = 0.089).
Table 6.
Summary of hierarchical logistic regression analysis for variables predicting ORs for recurrent broncho-pulmonary infections (defined as five or more episodes) in the seven-year follow-up (N = 131). The subgroup of children with indoor PM2.5 measurements repeated at the age 3.
| Variables | OR | Pr>|z| | 95% Confidence intervals | Wald Chi-square | df | Pr> F | |
|---|---|---|---|---|---|---|---|
| Model I | |||||||
| Prenatal PM2.5 exposure a | 2.44 | 0.025 | 1.12 | 5.36 | 5.00 | 1 | 0.0253 |
| Model II | |||||||
| Prenatal PM2.5 exposure a | 2.47 | 0.026 | 1.12 | 5.50 | 1.82 | 1 | 0.1772 |
| Postnatal PM2.5a | 1.53 | 0.177 | 0.83 | 2.83 | |||
| Model III | |||||||
| Prenatal PM2.5 exposure a | 2.84 | 0.016 | 1.22 | 6.63 | 1.18 | 1 | 0.2784 |
| Postnatal PM2.5 exposure a | 1.76 | 0.102 | 0.89 | 3.48 | |||
| Prenatal ETSb | 0.66 | 0.278 | 0.31 | 1.40 | |||
| Model IV | |||||||
| Prenatal PM2.5 exposure a | 3.08 | 0.013 | 1.27 | 7.46 | 0.46 | 1 | 0.4986 |
| Postnatal PM2.5 exposure a | 1.84 | 0.086 | 0.92 | 3.67 | |||
| Prenatal ETSb | 0.93 | 0.908 | 0.27 | 3.19 | |||
| Postnatal ETSc | 0.67 | 0.499 | 0.21 | 2.12 | |||
| Model V | |||||||
| Prenatal PM2.5 exposure a | 3.16 | 0.011 | 1.30 | 7.58 | 0.34 | 1 | 0.5575 |
| Postnatal PM2.5 exposure a | 1.83 | 0.089 | 0.91 | 3.66 | |||
| Prenatal ETSb | 0.92 | 0.889 | 0.27 | 3.15 | |||
| Postnatal ETSc | 0.69 | 0.402 | 0.22 | 2.16 | |||
| Atopy Y/N | 1.47 | 0.558 | 0.41 | 5.28 | |||
| Model VI | |||||||
| Prenatal PM2.5 exposure a | 2.79 | 0.026 | 1.13 | 6.88 | |||
| Postnatal PM2.5 exposure a | 1.87 | 0.080 | 0.93 | 3.78 | |||
| Prenatal ETSb | 0.81 | 0.745 | 0.23 | 2.90 | |||
| Postnatal ETSc | 0.77 | 0.657 | 0.24 | 2.48 | |||
| Atopy | 1.32 | 0.667 | 0.36 | 4.90 | |||
| Asthma | 2.51 | 0.110 | 0.81 | 7.75 | 2.56 | 1 | 0.1096 |
| Model VII | |||||||
| Prenatal PM2.5 exposure a | 2.82 | 0.025 | 1.14 | 7.00 | |||
| Postnatal PM2.5 exposure a | 1.85 | 0.089 | 0.91 | 3.75 | |||
| Prenatal ETSb | 0.78 | 0.518 | 0.22 | 2.05 | |||
| Postnatal ETSc | 0.78 | 0.674 | 0.24 | 2.52 | |||
| Atopy Y/N | 1.32 | 0.885 | 0.35 | 4.91 | |||
| Asthma Y/N | 2.53 | 0.107 | 0.82 | 7.83 | |||
| Residence aread | 0.83 | 0.777 | 0.23 | 2.98 | 0.08 | 1 | 0.7769 |
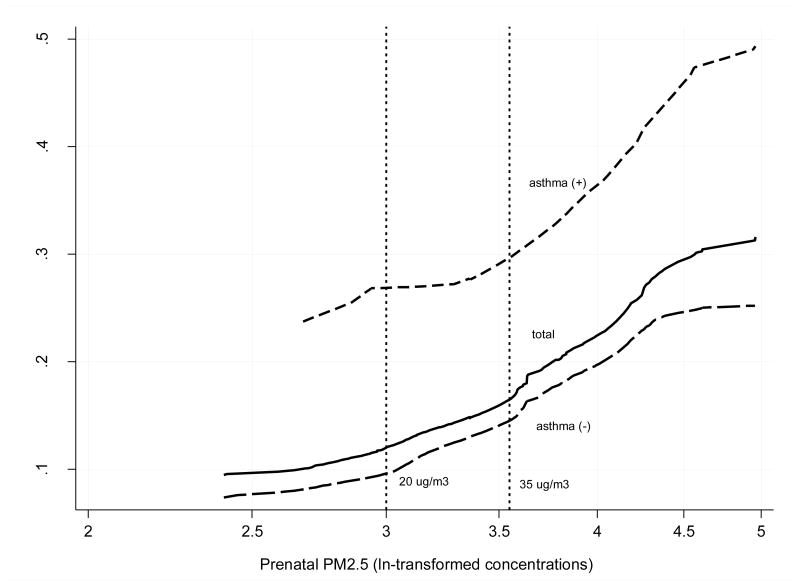
Figure 1 presents the dose-effect relationship between the prenatal PM2.5 exposure level and the probability of recurrent broncho-pulmonary infections in the total sample and in the subgroups of children with and without asthma. The probability of recurrent spells of respiratory infections increased linearly, but a little more steeply in asthmatic children than in those without asthma. Furthermore, the dose-effect relationship between recurrent infections and prenatal exposure showed that an increment of respiratory episodes even begins at PM2.5 level below 35 μg/m3.
Figure 1.
Predicted probability of recurrent broncho-pulmonary infections (five or more episodes treated by physician in the follow-up) related to prenatal exposure (ln-transformed PM2.5 μg/m3).
Discussion
While most of the previous studies were mainly concerned with the effect of the postnatal ambient exposure to particulate matter on respiratory morbidity in children, this is the first study of its kind that has evaluated the association between the individual prenatal PM2.5 and the recurrent broncho-pulmonary episodes as an indicator of children’s susceptibility to respiratory tract infections. It is important to mention that the estimates of the effect remained significant after adjustment for a set of potential confounders. The impact of prenatal exposure to PM2.5 on the risk of recurrent broncho-pulmonary infections during early childhood appeared to be independent of the effects of ETS, residence area and sensitization to common domestic aeroallergens, which were a proxy of quality of postnatal indoor/outdoor air quality. Prenatal exposure was also independent of indoor PM2.5 levels measured in children’s household at the age 3. The study supports a causal relationship between the prenatal PM2.5 level and respiratory health and suggests that the prenatal exposure may program postnatal respiratory morbidity in childhood. Though the clear threshold level of PM2.5 was not identified, the results of our study suggest that the PM2.5 level of 20 μg/m3 would better protect children against respiratory outcomes than higher values of 35 μg/m3 established by EPA (23). However, our proposed standard value would be very close to the 24-hour mean limit of 25 μg/m3 recommended by the present WHO guidelines (25).
The biological mechanism whereby prenatal exposure to prenatal PM2.5 may lead to the increased susceptibility is yet unclear. PM2.5 is a proxy for a wide spectrum of environmental hazards, such as constituents of tobacco and wood smoke, organic compounds, sulfates, polycyclic aromatic hydrocarbons (PAHs), metals and many other chemicals, which may be implicated in generating oxidative stress (8). Fine particles containing a very high proportion of organic carbon add to the biologic oxidative potency of these particles. While inhaled particles of 2.5 μm are linked to bronchial inflammatory effects, smaller particles (0.25 μm or less) are thought to move beyond the respiratory system and reach the bloodstream across placenta.
It is believed that the one of the key mechanism by which air pollutants is linked with the increased risk of respiratory infections is the inhibition of the production of immunocompetent cells contributing to immunosuppression. For example, exposure to absorbed airborne toxicants inhibits the differentiation of human monocytes, but mature differentiated immune cells also constitute their targets. (26 – 30). On the other hand, transplacental exposure of newborns to higher prenatal PM2.5 and its compounds may result in the production of an “allergic response” typified by the proliferation of Th2 type T lymphocytes which secrete proinflammatory cytokines in the body tissues. As the Th2 cytokines promote allergen-specific IgE antibody and induce eosinophile-dominated inflammatory tissue responses, allergic reactions are enhanced within the bronchial tract and lead to an increased susceptibility of newborns and young infants to pulmonary infections (31 – 36).
A strength of our study is the prospective birth cohort design that also enabled us to limit measurement error in estimating prenatal exposure to fine particles by assigning an individual prenatal personal exposure level to each child. The personal monitoring of ambient PM2.5 exposure is a relevant measure incorporating outdoor and indoor exposures. Good agreement between the personal PM2.5 measurements across all trimesters of pregnancy carried out in a subsample of 80 subjects provided evidence that the measurements of fine particles in the second trimester is also a good reflection of mean exposure level over pregnancy. Additional advantage of the study is the adjustment of prenatal PM2.5 effect for repeated indoor measurement of PM2.5 at the age 3 and constant monitoring of prenatal and postnatal ETS exposure over the follow-up. Another strong point of our study stems from the fact that we were able to monitor medical diagnoses of respiratory infections over regular time points in the course of face-to-face interviews with mothers of children. Since the mobility of the subjects under study was very moderate and mainly restricted to the same urban air pollution area, this gave us an additional confidence that the estimates of effects were unbiased. On the other hand, we are aware of the limitations of our study, which are mainly related to a relatively small sample size and the lack of data on time spent by children outdoors and their outdoor activity patterns.
In summary, the study suggests that prenatal exposure to PM2.5 increases susceptibility to respiratory infections and may program respiratory morbidity in early childhood. The observed effect of the increased susceptibility to respiratory infections may result from cytokine deregulation and an “allergic response” phenotype possibly established in the fetal period as a result of transplacental exposure to fine particulate matter. The study also provides evidence that the daily exposure below 20 μg/m3 may better protect unborn babies than that proposed by EPA.
Acknowledgments
This is part of an ongoing comparative longitudinal investigation on the health impact of prenatal exposure to outdoor/indoor air pollution in infants and children being conducted in New York City and Krakow. In part, the study received funding from an ROI grant entitled, “Vulnerability of the Fetus/Infant to PAH, PM2.5 and ETS” (5 ROI ES10165 NIEHS; 02/01/00 - 01/31/04) and from the NIEHS (ROI ES010165-0451) the Lundin Foundation and the Gladys T. and Roland Harriman Foundation.
Principal investigator: Prof. FP Perera; Co-investigator Prof. WA Jedrychowski
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shaheen SO, Barker DJP, Shiell AW. The relationship between pneumonia in early childhood and impaired lung function in late adult life. Am J Respir Crit Care Med. 1994;149:616–619. doi: 10.1164/ajrccm.149.3.8118627. [DOI] [PubMed] [Google Scholar]
- 2.Tennant PWG, Gibson CJ, Pearce MS. Life course predictors of adult respiratory function: results from the Newcastle thousand families study. BMJ. doi: 10.1136/thx.2008.096388. [DOI] [PubMed] [Google Scholar]
- 3.Johnston IDA, Strachan DP, Anderson HR. Effect of pneumonia and whooping cough in childhood on adult lung. N Engl J Med. 1998;338:581–587. doi: 10.1056/NEJM199802263380904. [DOI] [PubMed] [Google Scholar]
- 4.Barker DJ, Godfrey KM, Fall C, Osmond C, Winter PD, Shahleen SO. Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airway disease. BMJ. 1991;393:671–675. doi: 10.1136/bmj.303.6804.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zedeck MS. Polycyclic aromatic hydrocarbons: a review. J Environ Path Toxicol. 1980;3:537–567. [PubMed] [Google Scholar]
- 6.Environmental and Experimental Data (Monographs on the Evolution of Carcinogenic Risks to Humans) IARC Press; Lyon: 1983. IARC Polynuclear Aromatic Compounds, Part 1: Chemical. [PubMed] [Google Scholar]
- 7.Knize MG, Salmon CP, Pais P, Felton JS. Food heating and the formation of heterocyclic aromatic amine and polycyclic aromatic hydrocarbon mutagens/carcinogens. Adv Exper Med Biol. 1999;459:179–193. doi: 10.1007/978-1-4615-4853-9_12. [DOI] [PubMed] [Google Scholar]
- 8.Spengler JD, Samet JM, McCarthy JF. Air cleaning - particles. Chapter 9:9.1–9.28. [Google Scholar]; Risk analysis framework. 70:70.3–70.38. [Google Scholar]; Indoor Air Quality Handbook. McGraw-Hill; 2001. [Google Scholar]
- 9.Schwartz J, Neas EVI. Fine particles are more strongly associated than coarse particles with acute respiratory health effects in school children. Epidemiology. 2000;11:6–10. doi: 10.1097/00001648-200001000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Chauhan AJ, Johnston S. Air pollution and infection in respiratory illness. Brit Med Bull. 2003;68:95–112. doi: 10.1093/bmb/ldg022. [DOI] [PubMed] [Google Scholar]
- 11.Peters A, Dockery DW, Heinrich J, Weichmann HE. Short-term effects of particular air pollution on respiratory morbidity in asthmatic children. Eur Resp J. 1997;10:872–879. [PubMed] [Google Scholar]
- 12.Gouveia N, Fletcher T. Respiratory diseases in children and outdoor air pollution in Sao Paulo, Brazil: a time series analysis. Occup Environ Med. 2000;57:477–83. doi: 10.1136/oem.57.7.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnett AG, Williams GM, Schwartz J, Neller AH, Best TL, Petroeschevsky AL, Simpson RW. Air pollution and child respiratory health. A case-crossover study in Australia and New Zealand. Am J Respir Crit Care Med. 2005;171:1272–1278. doi: 10.1164/rccm.200411-1586OC. [DOI] [PubMed] [Google Scholar]
- 14.Lewis TC, Robins TG, Dvonch JT, Keeler GJ, Yip FY, Mentz GB, Lin X, Parker EA, Israel BA, Gonzalez L, Hill Y. Air pollution-associated changes in lung function among asthmatic children in Detroit. Environ Health Perspect. 2005;113:1068–1075. doi: 10.1289/ehp.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson LM, Diwan BA, Fear NT, Roman E. Critical windows of exposure for children’s health: cancer in human epidemiological studies and neoplasms in experimental animal models. Environ Health Perspect. 2001;109:573–594. doi: 10.1289/ehp.00108s3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perera FP, Tang D, Tu YH, Cruz LA, Borjas M, Bernert T, et al. Biomarkers in maternal and newborn blood indicate heightened fetal susceptibility to procarcinogenic DNA damage. Environ Health Perspect. 2004;112:1133–1136. doi: 10.1289/ehp.6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jedrychowski W, Galas A, Pac A, Flak E, Camman D, Rauh V, Perera F. Prenatal ambient air exposure to polycyclic aromatic hydrocarbons and the occurrence of respiratory symptoms over the first year of life. Eur J Epidemiol. 2005;20:775–782. doi: 10.1007/s10654-005-1048-1. [DOI] [PubMed] [Google Scholar]
- 18.Perera FP, Rauh V, Whyatt RM, Tsai WY, Bernert JT, Tu YH. Molecular evidence of an interaction between prenatal environmental exposures and birth outcomes in a multiethnic population. Environ Health Perspect. 2004;112:626–630. doi: 10.1289/ehp.6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stick SM, Burton PR, Gurrin L, Sly PD, LeSouef PN. Effects of maternal smoking during pregnancy and a family history of asthma on respiratory function in newborn infants. The Lancet. 1996;348:1060–1064. doi: 10.1016/s0140-6736(96)04446-7. [DOI] [PubMed] [Google Scholar]
- 20.Tager IB, Hanrahan JP, Tosteson TD, Castile RG, Brown RW, Weiss ST, Speizer FE. Lung function, pre- and post-natal smoke exposure, and wheezing in the first year of life. Am RevRespDis. 1993;147:811–817. doi: 10.1164/ajrccm/147.4.811. [DOI] [PubMed] [Google Scholar]
- 21.DiFranza JR, Aligne CA, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children’s health. Pediatrics. 2004;113:1007–1015. [PubMed] [Google Scholar]
- 22.Miller RI, Garfinkel R, Horton M, Camann D, Perera FP, Whyatt R, Kinney PL. Polycyclic aromatic hydrocarbons, environmental tobaccosmoke, and respiratory symptoms in an inner-city birth cohort. Ches. 2004;126:1071–1078. doi: 10.1378/chest.126.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.EPA. National Ambient Air Quality Standards for Particulate Matter. 2006. (71FR 61144) [Google Scholar]
- 24.Jedrychowski W, Whyatt R, Camann D, Bawle U, Peiki K, Spengler JD, Dumyahn T, Perera FP. Effect of prenatal PAH exposure on birth outcomes and neurocognitive development in a cohort of newborns in Poland. Study design and preliminary ambient data. Int J Occup Med Environ Health. 2003;16:21–29. [PubMed] [Google Scholar]
- 25.Air quality guidelines for Europe. 2. Copenhagen: World Health Organization Regional Office for Europe; 2000. (WHO Regional Publications, European Series, No. 91). [PubMed] [Google Scholar]
- 26.Holgate ST. The Effects of Air Pollution on Children’s Health and Development: A Review of Evidence. WHO, European Centre for Environment and Health; Bonn: 2005. Mechanisms by which air pollution injures the child’s respiratory system; pp. 29–43. [Google Scholar]
- 27.Lyte M, Bick PH. Modulation of interleukin-1 production by macrophages following benzo(a)pyrene exposure. Int J Immunopharmacology. 1986;8:377–381. doi: 10.1016/0192-0561(86)90120-7. [DOI] [PubMed] [Google Scholar]
- 28.Saxon A, Diaz-Sanchez D. Diesel exhaust as a model xenobiotic in allergic inflammation. Immunopharmacology. 2000;48:325–327. doi: 10.1016/s0162-3109(00)00234-4. [DOI] [PubMed] [Google Scholar]
- 29.Ward EC, Murray MJ, Lauer LD, House RV, Irons R, Dean JH. Immunosuppression following 7,12-dimethylbenz[a]anthracene exposure in B6C3F1 mice. Effects on humoral immunity and host resistance. Toxicol Appl Pharmacol. 1984;75:299–308. doi: 10.1016/0041-008x(84)90212-6. [DOI] [PubMed] [Google Scholar]
- 30.Wojdani A, Alfred IJ. Alterations in cell-mediated immune functions induced in mouse splenic lymphocytes by polycyclic aromatic hydrocarbons. Cancer Res. 1984;44:942–945. [PubMed] [Google Scholar]
- 31.Kaan PM, Hegele RG. Interaction between respiratory syncytial virus and particulate matter in guinea pig alveolar macrophages. Am J Respir Cell Mol Biol. 2003;28:697–704. doi: 10.1165/rcmb.2002-0115OC. [DOI] [PubMed] [Google Scholar]
- 32.Davila DR, Romero DL, Burchiel SW. Human T cells are highly sensitive to suppression of mitogenesis by polycyclic aromatic hydrocarbons and this effect is differentially reversed by alpha-naphthoflavone. Toxicology and Applied Pharmacology. 1996;139:333–341. doi: 10.1006/taap.1996.0173. [DOI] [PubMed] [Google Scholar]
- 33.Nel AE, Diaz-Sanchez D, Ng D, Hiura T, Saxon A. Enhancement of allergic inflammation by the interaction between diesel exhaust particles and the immune system’. J Allergy Clin Immunol. 1998;102:539–554. doi: 10.1016/s0091-6749(98)70269-6. [DOI] [PubMed] [Google Scholar]
- 34.Devouassoux G, Saxon A, Metcalfe DD, Prussin C, Colomb MG, Brambilla C. Chemical constituents of diesel exhaust particles induce IL-4 production and histamine release by human basophils. J Allergy Clin Immunol. 2002;109:847–853. doi: 10.1067/mai.2002.122843. [DOI] [PubMed] [Google Scholar]
- 35.Laupeze B, Amiot L, Sparfel L, Le Ferrec E, Fauchet R, Fardel O. Polycyclic aromatic hydrocarbons affect functional differentiation and maturation of human monocyte derived dendritic cells. J Immunol. 2002;168:2652–2658. doi: 10.4049/jimmunol.168.6.2652. [DOI] [PubMed] [Google Scholar]
- 36.Van Grevenynghe J, Rion S, Le Ferrec E, Le Vee M, Amiot L, Fauchet R, Fardel O. Polycyclic aromatic hydrocarbons inhibit differentiation of human monocytes into macrophages. J Immunol. 2003;170:2374–2381. doi: 10.4049/jimmunol.170.5.2374. [DOI] [PubMed] [Google Scholar]