Abstract
To acquire the ability to fertilize, spermatozoa undergo complex, but at present poorly understood, activation processes. The intracellular rise of cAMP produced by the bicarbonate-dependent soluble adenylyl cyclase (sAC) has been suggested to play a central role in initiating the cascade of the events that culminates in spermatozoa maturation. Here, we show that targeted disruption of the sAC gene does not affect spermatogenesis but dramatically impairs sperm motility, leading to male sterility. sAC mutant spermatozoa are characterized by a total loss of forward motility and are unable to fertilize oocytes in vitro. Interestingly, motility in sAC mutant spermatozoa can be restored on cAMP loading, indicating that the motility defect observed is not caused by a structural defect. We, therefore, conclude that sAC plays an essential and nonredundant role in the activation of the signaling cascade controlling motility and, therefore, in fertility. The crucial role of sAC in fertility and the absence of any other obvious pathological abnormalities in sAC-deficient mice may provide a rationale for developing inhibitors that can be applied as a human male contraceptive.
Spermatozoa undergo several essential activation processes, including the initiation of motility on the release from the epididymis, induction of hyperactivated motility, capacitation, and acrosome reaction. As a result, the sperm cells acquire the ability to fertilize (1). Despite the biological relevance, the molecular mechanisms underlying these processes are still poorly understood. It has been suggested that all three events depend on the intracellular rise of cAMP induced by bicarbonate ions (2-4). More recently, it has been shown that bicarbonate ions directly stimulate soluble adenylyl cyclase (sAC) in a pH-independent manner (5). Adenylyl cyclases (ACs) are the enzymes responsible for the intracellular production of cAMP, an important second messenger relevant in a number of biological processes in various cell types. Several isoforms of ACs have been described (6, 7) that are generally expressed in many cell types. In testes, at least two categories have been described: first, the membrane-associated ACs that are regulated by G protein-associated receptors (8-11) and second, the structurally distinct soluble Mn2+/--dependent form that is modulated by bicarbonate and is insensitive to G protein modifiers (12, 13). The amino acid sequence of the two catalytic domains (C1 and C2) indicates that sAC is related more closely to the ancestral cyanobacterial ACs than to the mammalian membrane-associated ACs (5, 14). Moreover, the ability of the cyanobacterial ACs to respond to bicarbonate stimuli shows that this metabolic pathway is highly conserved. Consequently, an essential and distinct function in the sAC-mediated cAMP signaling during late spermatogenesis has been hypothesized (5). In addition to testes, sAC protein has also been detected in choroid plexus and kidneys (5), which are tissues known to be able to modulate bicarbonate concentration and show AC activity (15). Intriguingly, in testes, sAC mRNA appears to be localized in cell types typical of late stages of spermatogenesis, i.e., from round spermatids to early elongated spermatids (16). The high expression levels observed may indicate an important role for sAC in generating cAMP in spermatozoa required for either sperm maturation, initiation of motility, hypermotility, and/or the acrosome reaction. Moreover, it has been suggested that sAC is produced as a high-molecular-weight precursor protein that is converted to the active form by proteolytic cleavage as the sperm cells proceed through the epididymal tract (5, 14). It has, therefore, been postulated that the activation of sAC protein results in an increase of the intracellular concentration of cAMP and the induction of the signaling cascade, leading to the completion of the sperm maturation. Alternative splicing of sAC mRNA may be an additional mechanism responsible for the production of the shorter active isoform of the enzyme (17).
Interestingly, targeted disruption of a number of genes (18-20) either suggested or shown to be involved in the cAMP-dependent pathway leading to sperm maturation resulted in fertility defects that were mainly due to impaired sperm motility. These findings suggest a role for sAC in male fertility.
In an attempt to elucidate the specific role of sAC during spermatogenesis, we generated mice deficient for sAC (sAC-/-). Whereas female mutant mice showed normal fertility, male sAC-/- mice were infertile because of a severe sperm-motility defect. These results indicate a critical role for sAC in spermatozoa maturation.
Materials and Methods
Generation of sAC-Deficient Mice. The murine sAC gene was disrupted in embryonic stem cells (ES cells) by homologous recombination. A replacement vector was designed to delete exons 2-4 of the sAC wild-type locus, leaving both the splice acceptor site of exon 2 and the splice donor site of exon 4 intact. The deleted sequence was replaced by a LacZ/MC1-Neor selection cassette (Fig. 1A) such that the LacZ reporter gene was in frame with the sAC coding sequence. The targeting vector was electroporated into 129 Sv/Evbrd cells, after which colonies were selected in the presence of G418 and 1-(2′-deoxy-2′-fluoro-β-d-arabinofuranosyl)-5-iodouracil. G418-1-(2′-deoxy-2′-fluoro-β-d-arabinofuranosyl)-5-iodouracil-resistant colonies were isolated and analyzed for homologous recombination by Southern blot analysis by using a 5′ external probe (Fig. 1B). ES cell clones that were characterized by correct homologous recombination at both sides of the vector and a single integration event were selected for blastocyst injections. The chimeric mice obtained were mated to C57BL/6 females for germline transmission. Heterozygous mice were then interbred to produce homozygous mice. Genomic DNA isolated by tail biopsy was used for genotype screening by PCR analysis. The reactions were performed by using the following primer pairs specific for the wild-type allele (5′-GTCTGGCCACACACTAAGG-3′ and 5′-CTCCAGCTCCGATGAAGG-3′) and for the targeted allele (5′-CTGGGCTGTCCTCAAGCTC-3′ and 5′-GCAGCGCATCGCCTTCTATC-3′). Briefly, PCR was carried out in 50 μl of reaction mixture containing 100 ng of each primer, 1 unit of Taq polymerase (Amersham Biosciences), 200 μM dNTP (Amersham Biosciences), and 1× PCR buffer (Amersham Biosciences). Amplification was carried out by using the following protocols: 5 min at 95°C, 30 denaturing cycles at 95°C for 30 sec, and annealing at 57°C (mutant allele); and 59°C for 30 sec and extension at 72°C for 1 min in a DNA Thermal Cycler 9700 (Perkin-Elmer) (wild-type allele). Samples were subsequently analyzed on 2% agarose gels. A 500-bp fragment was generated from the wild-type allele, whereas the mutant allele generated a 600-bp fragment.
Fig. 1.
Generation of sAC-deficient mice. (A) Schematic representation of the gene targeting in the sAC locus by homologous recombination. Only exons 1-5 of the wild-type locus are shown. Rectangles represent coding DNA. Only the relevant EcoRI sites are shown. E, exon; RI, EcoRI site; P, 5′ external probe. (B) Targeted ES cells. A Southern blot of EcoRI-digested DNA from targeted ES and wild-type cells, respectively, is shown. A 5′ external probe was used. The wild-type fragment migrates at 16 kb, and the fragment from the targeted locus migrates at 8 kb. (C) PCR genotyping-confirmed gene disruption in mice. Tail genomic DNA was used to amplify the sAC targeted allele and the sAC wild-type allele. (D) In situ hybridization of testis from wild-type (msAC+/+) and sAC-/- (msAC-/-) mice, respectively.
Mice. Animals were housed in macrolon cages (four to six animals per cage) at 19-21°C; the relative humidity was 50-60%, and a 12:12 h artificial-light/dark cycle was offered. Standard pelleted food (SDS; Special Diet Services, Essex, England) and water was provided ad libitum during the in-life period. The animals were observed daily for morbidity and mortality during the in-life period. Additionally, the animals were observed regularly for any behavioral and physical abnormalities. All animal experiments were approved by the Organon Animal Ethics Committee.
RNA Isolation and RT-PCR Analysis. Total RNA was isolated from testes from wild-type, heterozygous, and homozygous littermates by using TRIzol (GIBCO/BRL). First-strand synthesis was performed by using oligo(dT)15 (Promega) and pd(N), (Amersham Biosciences) primers and SuperScript II RNase H reverse transcriptase (Invitrogen) according to the manufacturer's instructions. Reverse-transcribed products were then PCR amplified for 17, 20, 23, and 26 cycles with the following primers: mSAC RNA-509F, 5′-GGCGGTGGATGATGTCCGCC-3′; and mSAC RNA-1053R, 5′-GGGAAGCCGAAGACACAGAGG-3′. GAPDH-specific primers (5′-CCCTTCATTGACCTCAACTACATGG3′ and 5′-GGTCCACCACCCTGTTGCTGTAGCC) were used as a control. PCR products were analyzed on a 1% agarose gel.
In Situ Hybridization. In situ hybridization was performed as described (21). Briefly, a 444-bp fragment of the sAC cDNA was subcloned in pcDNA3.1/V5-His-TOPO vector and was used as template for the synthesis of radioactive 35S-labeled RNA probes. Mouse testes were fixed in 4% paraformaldehyde for 6 h and incubated in 0.5 M sucrose overnight at 4°C. The testes were embedded in OCT (Tissue-Tek, Torrance, CA), cut into 10-μm sections, and mounted. Slides were postfixed in paraformaldehyde and treated as described (21). Radioactive hybridized sections were exposed in NTB2 Emulsion (Eastman Kodak) for 7 days, developed photographically, counterstained with 0.25% (wt/vol) Gill's hematoxylin and eosin in ethanol, cleared with xylene, and mounted with Permount (Fisher). Sections were visualized and photographed with an Axiocam (Zeiss).
AC Activity in Testes. For AC assay, the testes were homogenized in lysis buffer (50 mM Tris·HCl, pH 7.5/1 mM DTT/10 μg/ml leupeptin/10 μg/ml soybean trypsin inhibitor/0.7 mg/ml pepstatin A/50 mM benzamidine/1 mM PMSF) at 4°C with a Dounce homogenizer by using 20 strokes. For enzyme preparation from spermatozoa, the washed cauda epididymal sperm cells were resuspended in lysis buffer and sonicated three times for 30 sec each by using a Sonifier 450 (Branson) at a power setting of 6 with 30-sec ice-cooling periods. In vitro adenylate cyclase assay was performed in these testis and sperm homogenates, as described in ref. 17. Briefly, enzyme preparations were incubated in a reaction buffer containing 40 mM Tris·HCl (pH 7.5), 5 mM MnCl2 (or 5 mM MgCl2), 0.2 mM cAMP, 10 mM phosphoenol pyruvate, 3 units of pyruvate kinase, 10 μM GTP, 1 mM ATP, and 2 μCi (1 Ci = 37 GBq) of [32P]ATP for 20 min at 37°C. Reaction was terminated by the addition of 20 μl of 2.2 M HCl containing [3H]cAMP (0.01 μCi), followed by boiling for 4 min and cooling in an ice-water bath. Labeled cAMP was added to estimate and correct for recovery of the cyclic nucleotide during column chromatography. The cAMP generated was then separated from ATP by alumina columns and eluted with 5 ml of 0.1 M ammonium acetate (pH 6.5). We added 12 ml of aquasol-2 scintillation fluid (Packard) to each tube and then counted them on a β-counter.
Determination of β-Galactosidase Activity. sAC expression profiling using the LacZ reporter gene was performed on homozygous male mutant mice. Wild-type littermates were used as control. We dissected ≈40 organs and tissues from a single mouse and prepared them for the analysis as described (22).
Histopathological Analysis. Phenotypic analysis was performed on 10-week-old males and females from the F2 generation (wild-type, heterozygous, and homozygous; six mice per group). Before autopsy, blood was obtained from each mouse. Hematology and blood-clotting parameters were determined as described (22). All internal organs were examined for visual signs of abnormalities. The following organs were dissected free of adjacent fat and other surrounding tissues, and their weights were recorded: heart, spleen, thymus, liver/gallbladder, kidneys, adrenal glands, brain, testes, epididymides, seminal vesicles/coagulating glands/prostate gland, ovaries, and uterus. The organ weight relative to terminal body weight, determined before autopsy, was calculated. For histopathology, organs and tissues from the animals were prepared as described (22).
Fertility Studies. Timed matings were carried out by housing one male with two females per cage for a period of 2 weeks to 2 months. Females were evaluated for the presence of vaginal plugs during the first 3 days of mating.
Sperm Count and Total Motility Determination. By cervical dislocation, 12-week-old mice were killed. The cauda epididymis was dissected and placed in L15 media (Life Technologies, Breda, The Netherlands) containing 0.3% (wt/vol) BSA (Sigma). Spermatozoa were allowed to swim out or diffuse for 15 min, and they were then prepared for counting by incubation with 40 μg/ml Hoechst 33342 (Hoechst Pharmaceuticals) in L15 media for 10 min at 37°C. Samples were placed in Leja counting chambers (Orange Medical, Tilburg, The Netherlands), and sperm concentration was evaluated by using the IVOS sperm analyzer (Hamilton). Total sperm motility was measured in the same conditions.
Elongated Spermatid Count. Testes were collected from 12-week-old mice killed by cervical dislocation and stored at -80°C. After thawing, the testes were decapsulated and the parenchyma was homogenized in physiological solution containing 0.05% (vol/vol) Triton X-100 (23, 24). Homogenization-resistant elongated spermatids were counted in Leja chambers with a phase-contrast microscope. The elongated-spermatid concentration was determined by counting the spermatids present in 100 fields of the grid on 14 positions.
cAMP Loading. cAMP-acetoxymethyl ester (AM) (Sigma) was dissolved in DMSO to a 20-mM stock solution. This solution was mixed 1:1 with 20% Pluronic F-127 in DMSO (Molecular Probes) and then diluted to 120 μM in Biggers, Whitten, and Whittingham (BWW) HCO3-free medium (120 mM NaCl/4.8 mM KCl/1.3 mM CaCl2/1.2 mM MgSO4/1.2 mM KH2PO4/20 mM C3H5NaO3/5 mM glucose/0.25 mM C3H3NeO3, pH 7.4), supplemented with 10 mM Hepes (pH 7.4) and 4 mg/ml BSA, and then immediately mixed with an equal volume of a sperm suspension. After 30 min, an aliquot of the sperm suspension (10 μl) was transferred to the hemocytometer. Total number of sperm (immotile and forward-moving) was counted. Motility is expressed as percentage of sperm moving forward.
In Vitro Fertilization Assay. In vitro fertilization was performed as described (25) with slight modifications. Briefly, 10 units of urinary FSH (Humegon, NV Organon) was used instead of 20 units of Humegon. In addition, 2.5 units of mouse hCG (Pregnyl, NV Organon) was administrated 48 h after Humegon for ovulation induction, instead of 7.5-20 units of Pregnyl per mouse.
Results
Generation of sAC-Deficient Mice. To inactivate sAC function, the murine sAC locus was modified by gene targeting. Exons 2, 3 and 4 encoding the C1 catalytic domain were replaced with an internal ribosome entry site (IRES)-LacZ cassette containing a neomycin resistance gene. The wild-type sAC locus and the targeted allele after homologous recombination are depicted in Fig. 1A. Correct homologous recombination was confirmed by Southern blot analysis (Fig. 1B). The targeted allele was transmitted into the germline (Fig. 1C). Homozygous sAC mutant mice were viable, present at the expected Mendelian ratios and did not exhibit overt abnormalities. In situ hybridization analysis performed with histological sections of testes from wild type and sAC-/- confirmed the absence of sAC message in the mutant animals (Fig. 1D). More sensitive RT-PCR experiments performed with RNA isolated from testes from wild-type, sAC+/-, and sAC-/- littermate mice, by using primers spanning exons 5 and 9, revealed the presence of sAC message in the testes of mutant mice, albeit at a very low level when compared with controls (data not shown). The presence of sAC mRNA is most likely because of transcription through the IRES-LacZ/neomycin cassette. However, because of the presence of frameshift mutations in the resulting mRNA, a functional sAC polypeptide cannot be produced.
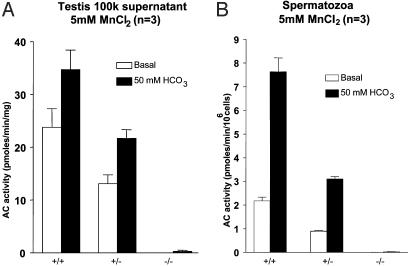
Testes and Spermatozoa from sAC-Deficient Mice Are Devoid of Bicarbonate-Induced AC Activity. A major property associated with sAC expression in the testis is the presence of a Mn2+-dependent, bicarbonate-stimulated AC (17). Whereas the Mn2+-dependent and bicarbonate-stimulated activity could be detected in the high speed supernatant of testes from wild-type mice, homozygous mutants were characterized by a complete absence of this activity, thereby confirming sAC gene inactivation (Fig. 2A). Similarly, no Mn2+-dependent bicarbonate stimulated activity could be detected in homogenates of spermatozoa from the sAC-/- mice (Fig. 2B).
Fig. 2.
AC activity in testes (A) and spermatozoa (B) from the sAC-/-, matched sAC+/-, and wild-type littermates, respectively. Testes and spermatozoa extracts were prepared as described in Methods. Aliquots of the extracts were used to measure AC activity in the presence of 5 mM MnCl2 without or with 50 mM HCO3-.
sAC-Deficient Animals Do Not Show Gross Abnormalities. No obvious behavioral or gross anatomical differences were noted when comparing 10-week-old sAC-/-, sAC+/-, and wild-type littermates. Also, no relevant changes in organ weights or hematological, blood clotting and biochemical parameters were observed among the different male and female groups (data not shown). Histopathological abnormalities were not observed in all organs and tissues studied. Whereas sAC has been shown to be present also in choroid plexus and kidneys, (5), we were unable to detect β-galactosidase activity in the latter organs from sAC mutant mice carrying a LacZ gene insertion (data not shown). Histopathological analysis of the brain and kidneys did not reveal any obvious abnormalities. Moreover, animals observed up to 8 months of age appeared to be healthy. However, more detailed functional studies may be required to assess whether sAC deficiency has an effect on brain and/or kidney function.
sAC-Deficient Mice Develop Normal Testes and Epididymides. In testes, sAC is specifically expressed during the late stages of spermatogenesis in round and elongated spermatids (16). This expression pattern was confirmed in sAC-/- mice carrying the LacZ reporter gene insertion (Fig. 3A). Recently, sAC protein was reported to be expressed in clear cells in the epididymis (26), however, because of the presence of endogenous β-galactosidase activity in the epididymis (22), we were unable to confirm this finding.
Fig. 3.
Characteristics of mutant testes. (A) β-Galactosidase activity in the testis from a 9-week-old male sAC-/- mouse. Strong positive staining (arrows) in the late stages of spermatogenesis, near the spermatids. (B) Bouin-fixed and PAS-stained section from the testis of a 10-week-old male sAC-/- mouse. Normal histological appearance of the testis with seminiferous tubules in all stages of spermatogenesis including stage 12 (*) with normal meiotic cells. (B Inset) Detailed view showing the acrosome staining in the elongated spermatids.
Surprisingly, the histopathological analysis of testes and epididymides of sAC mutants revealed that all stages of spermatogenesis were present. Also, testes weight (184 ± 24 mg in mutant and 188 ± 7 mg in wild type) and epididymides weight (60.7 ± 5.9 mg in mutant and 57.7 ± 2.7 mg in wild type) were not different. Elongated spermatids were present in testes (Fig. 3B) and spermatozoa were present in the epididymis. Moreover, the presence of an acrosome in spermatids (Fig. 3B Inset), indicates that the spermatids are able to reach late developmental stages.
sAC Deficiency Leads to Male Sterility. Whereas mating of female sAC-/- mice (n = 8) with either wild-type or heterozygous mutant mice gave rise to normal-sized litters (7-10 pups per litter), prolonged mating (from 2 weeks to 2 months) of sAC mutant male mice (n = 20) with wild-type females did not produce any pregnancies. Mating behavior of male sAC-deficient mice was normal because vaginal plugs were observed in the females. Fertility was not affected in heterozygous sAC mutant mice as control breeding involving both sAC+/- and wild-type littermate mice produced normal-sized litters (Fig. 4A).
Fig. 4.
sAC-/- male mice are infertile. (A) Shown is the percentage of litter production from sAC+/+ (n = 10) and sAC-/- females (n = 8) after a breeding period of 1 week. sAC-/- male mice (n = 20) were caged with three C57BL/6 females each for periods of 2 weeks to 2 months. (B) Sperm and elongated spermatid count. Only one epididymis per mouse was used for sperm isolation, and only one testis per mouse was used. The graphs represent data from 12 wild-type and 12 sAC-/- animals. (C) Linear motility measurement. A-C represent means ± SEM.
sAC Deficiency Impairs Sperm Motility. Elongated spermatids and sperm counts did not reveal substantial differences between wild-type and homozygous mutant animals (Fig. 4B). We also did not observe differences in the proportion of propidium iodide positive (dead) and SYBR14 positive (living) sperm cells from sAC-/- and sAC+/+ animals (data not shown). Sperm were collected from the cauda epididymis of sAC-/- and wild-type littermates at different ages (groups of mice of 12-18 weeks old) and motility was analyzed. The results obtained indicated that total sperm motility was drastically reduced in sAC-/- animals when compared with control littermates. Mutant sperm appeared to be able to produce only a trembling movement of the midpiece accounting for ≈30% of motility when compared with control sperm. Moreover, no forward movement could be detected in the sAC-deficient sperm cells (Fig. 4C). Although sAC mutant sperm cells appeared to be fully developed, it was observed that mutant sperm cells when placed in L15 or T6 media would assume a “curled up” position whereas wild-type sperm cells always showed normal posture and motility. This phenomenon was not observed when preparing the sperm cells in BWW media or in a saline physiological solution and may hint to a dramatic sensitivity of sAC-/- spermatozoa to specific media components. However, motility measurements were not influenced by the media composition.
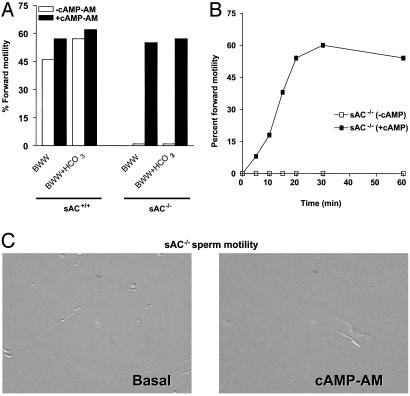
Motility in sAC-Deficient Sperm Is Restored by cAMP Loading. To test the hypothesis that motility would be restored by reintroducing cAMP, sAC-/- spermatozoa were loaded with cAMP-AM. After 30 min, cells were washed and their motility was assessed. Loading of sAC-/- spermatozoa with the cAMP analogue produced a time-dependent recovery of both overall motility as well as vigorous forward motility (Fig. 5), indicating that a deficit in cAMP in sAC-/- spermatozoa is the main cause for the absence of motility. Moreover, the possibility of restoring motility in the mutant spermatozoa by cAMP loading rules out an indirect effect by the epididymis environment resulting in the motility defect of sAC-/- sperm cells.
Fig. 5.
Forward motility is restored in the sAC-/- spermatozoa by cAMP loading. (A) Total number of sperm (immotile and forward-moving) was scored by two individuals independently. Motility is expressed as percentage of sperm moving forward. (B) Motility was monitored at various times after exposure to cAMP-AM. (C) Frame of sAC-/- control spermatozoa or spermatozoa exposed to cAMP-AM. For the complete movie, see Movies 1 and 2, which are published as supporting information on the PNAS web site.
sAC Is Required for in Vitro Fertilization. In vitro fertilization experiments were performed to test the ability of sAC-deficient spermatozoa to fertilize oocytes. Wild-type or mutant sperm exposed previously to capacitating media, were incubated with superovulated oocytes from wild-type mice (83 and 74 oocytes, respectively). The samples were scored for the presence of two-cell-stage embryos 24 h later. Although they were able to induce cumulus release, the mutant spermatozoa failed to produce two-cell-stage embryos, in contrast with the oocytes incubated with wild-type sperm (n = 58). Whereas the vast majority of the oocytes incubated with wild-type sperm developed into hatching blastocysts (48 plus 7 blastocysts and 6 morulae) 96 h after coincubation, oocytes incubated with mutant sperm underwent parthenogenesis to the same extent as oocytes (n = 30) that were not incubated with spermatozoa. We, therefore, conclude that, because of their severe motility defect, sAC-deficient sperm are unable to fertilize oocytes in vitro.
Discussion
Elevated cAMP concentrations serve as a switch to activate multiple pathways during sperm maturation rendering the spermatozoa competent for fertilization. Bicarbonate ions stimulate the sAC directly to convert ATP into cAMP. Most recent models of sperm capacitation and acquisition of hyperactivated motility postulate that cAMP synthesized by sAC will act on proximal effector targets, including ion channels such as CatSper (19) and protein kinase A (PKA). PKA stimulation will lead to the phosphorylation of a plethora of sperm proteins that, by means of structural and functional modifications, will lead to the completion of sperm maturation (2).
The finding that sAC is stimulated by bicarbonate concentrations similar to those observed in sperm cells in the epididymal tract (5), together with the recent observations that targeted disruption of genes linked to the cAMP cascade (18-20) led to male infertility due to severe motility defects prompted us to investigate the specific role of sAC during spermatogenesis by generating sAC-deficient mutant mice. Both male and female sAC-/- animals were viable and did not exhibit obvious abnormalities, although sAC expression has been detected also in kidneys and in the choroid plexus (5). The efficacy of sAC gene inactivation in the mutant mice was confirmed by the absence of bicarbonate-induced AC activity in testis homogenate.
sAC mutant mice developed normal testes and epididymides. Histopathological analysis of these organs did not reveal differences between tissues from mutant and control animals. These findings show that the sAC-mediated cAMP cascade is independent from the cAMP-responsive element-modulator (CREM) pathway because CREM-deficient mice show a complete block of spermiogenesis (27). Moreover, they confirm a direct role of ACT as an activator of CREM (28).
Fertility studies uncovered an essential role for sAC in sperm maturation: whereas sAC-/- females were fertile, sAC mutant male mice did not produce progeny even after prolonged mating with wild-type females. Infertility in sAC-deficient male mice could not be ascribed to a decreased in sperm production. For PKA, AKAP4, and CatSper mutant mice, the numbers and viability of sperm from mutant mice were also not different from those of wild-type control animals. Strikingly, determination of sperm motility revealed that the forward progressive movements were abolished in sAC-deficient spermatozoa. Moreover, the spermatozoa were able to produce only a trembling movement accounting for ≈30% residual motility when compared with controls by automated sperm analysis. Although sperm cells appeared to be fully developed (acrosomes also were visible in sections from testes), the striking features of their movements hinted to a possible structural defect, in addition to a functional blockade in the sperm-activation cascade, because of the absence of a functional sAC protein. However, the finding that cAMP loading restored motility excludes a role for sAC in the structural function of flagella and uncovers its key role in promoting sperm motility. It is tempting to suggest that the intracellular rise of cAMP produced by sAC is the initial switch necessary to activate the biochemical pathway leading to the acquisition of sperm motility. In this case, the trembling movement produced by the tail midpiece of the sAC-deficient spermatozoa could be ascribed to the action of traces of cAMP produced by membrane-associated ACs.
It has been shown that the cAMP-dependent PKA is directly activated by bicarbonate ions (2, 29). It is tempting to speculate that sAC-mediated production of cAMP is directly responsible for PKA activation in testis and spermatozoa. Interestingly, when comparing spermatozoa defects in sAC and PKA-Cα mutant mice, important differences can be noted. sAC mutation affects only motility, whereas PKA-Cα disruption leads to an increase in sperm-head aberrations, in addition to motility defects (20). To some extent, these findings may be explained by the effect of PKA targeted disruption in Leydig and Sertoli cells or by the role of PKA during earlier stages of spermiogenesis, when cAMP produced by transmembrane ACs can serve as an activator. In contrast, the absence of a functional sAC enzyme affects PKA function only at a specific stage of sperm maturation and uncovers the specific role of sAC in the functional cascade that leads to motility.
cAMP is an ubiquitous intracellular second messenger to several effector proteins that, in turn, regulate a variety of cellular functions, including gene expression. It may be speculated, therefore, that the absence of sAC effects the transcription of genes expressed during final stages of spermatogenesis. However, by using semiquantitative PCR analysis (data not shown), we did not observe any significant variation in the expression of genes like SPTRX-1 (30), SPTRX-2 (31), Spag6 (32), AKAP4 (18), CatSper (19), GAPDS (33), Calmegin (34), SP10 (35), and Act-1 (28) in sAC-/-, sAC+/-, and wild-type animals. Most of these genes have been shown to be essential for sperm motility and/or are expressed during the final stages of sperm development. Hence, our data indicate that sAC mutation may interfere with the correct activation or modification of proteins involved in sperm movement, confirming the hypothesis that sAC is a key player in the phenomenon of sperm motility and fertility.
In humans, motility defects are associated with 82% of the cases of male infertility and seem to be directly responsible for 20% of these cases (36). The finding that some infertile patients show decreased levels of bicarbonate ions in seminal fluids (37) or have altered AC functions (38), together with the reversibility of the phenotype of sAC mutant sperm on cAMP, could suggest a causal relationship between impaired sAC function and male infertility. No mutations in the sAC gene have been described so far, however, our data may provide a rationale to study sAC mutations in asthenozoospermic men. Furthermore, it could be hypothesized that sperm motility may be rescued or improved by cAMP loading. This hypothesis would support the idea that a sAC activator compound may have applications for the treatment of asthenozoospermia. Moreover, the normal development, normal (sexual) behavior, and lack of side effects in sAC-deficient mice, together with the inability of the mutant sperm to fertilize oocytes, indicate that sAC inhibitors could be used as a contraceptive drug.
Supplementary Material
Acknowledgments
We thank Jia Zhao for helpful discussion, Jasper E. Koenders, Ad J. M. van Disseldorp, and John Fundter for technical assistance. The sAC mutant mice were generated at and contracted from Lexicon Genetics (The Woodlands, TX). M.C., B.S.J., and F.X. were supported by National Institutes of Health Grant HD31544.
Abbreviations: AC, adenylyl cyclase; AM, acetoxymethyl ester; ES cells, embryonic stem cells; sAC, soluble AC.
References
- 1.Breihbart, H. (2002) Mol. Cell. Endocrinol. 187, 139-144. [DOI] [PubMed] [Google Scholar]
- 2.Visconti, P. E., Moore, G. D., Bailey, J. L., Leclerc, P., Connors, S. A., Pan, D., Olds-Clarke, P. & Kopf, G. S. (1995) Development (Cambridge, U.K.) 121, 1139-1150. [DOI] [PubMed] [Google Scholar]
- 3.Zippin, J. H., Levin, L. R. & Buck, J. (2001) Trends Endocrinol. Metab. 12, 366-370. [DOI] [PubMed] [Google Scholar]
- 4.Lee, M. A. & Storey, B. T. (1986) Biol. Reprod. 34, 349-356. [DOI] [PubMed] [Google Scholar]
- 5.Chen, Y., Cann, M. J., Litvin, T. N., Iourgenko, V., Sinclair, M. L., Levin, L. R. & Buck, J. (2000) Science 289, 625-628. [DOI] [PubMed] [Google Scholar]
- 6.Taussig, R. & Gilman, A. G. (1995) J. Biol. Chem. 270, 1-4. [DOI] [PubMed] [Google Scholar]
- 7.Sunhara, R. K., Dessauer, C. W. & Gilamn, A. G. (1996) Annu. Rev. Pharmacol. Toxicol. 36, 461-480. [DOI] [PubMed] [Google Scholar]
- 8.Gautier-Courteille, Salanova, M. & Conti M. (1998) Endocrinology 139, 2588-2599. [DOI] [PubMed] [Google Scholar]
- 9.Defer, N., Marinx, O., Povard, M., Lienard, M. O., Jegou, B. & Hanoune, J. (1998) FEBS Lett. 424, 216-220. [DOI] [PubMed] [Google Scholar]
- 10.Baxendale, R. W. & Fraser, L. R. (2003) Mol. Reprod. Dev. 66, 181-189. [DOI] [PubMed] [Google Scholar]
- 11.Adeoya-Osiguwa, S. A. & Fraser, L. R. (2002) Mol. Reprod. Dev. 63, 245-255. [DOI] [PubMed] [Google Scholar]
- 12.Braun, T. & Dods, R. F. (1975) Proc. Natl. Acad. Sci. USA 72, 1907-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gordeladze, J. O., Purvis, K. Clausen, O. P. Rommerts, F. F. & Hansson, V. (1981) Int. J. Androl. 4, 172-182. [DOI] [PubMed] [Google Scholar]
- 14.Buck, J., Sinclair, M. L., Schapal, L., Cann, M. J. & Levin, L. R. (1999) Proc. Natl. Acad. Sci. USA 96, 79-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mittag, T. W., Guo, W. B. & Kobayashi, K. (1993) Am. J. Physiol. 264, F1060-F1064. [DOI] [PubMed] [Google Scholar]
- 16.Sinclair, M. L., Wang, X. Y., Mattia, M. Conti, M. Buck, J. Wogelmuth, D. J. & Levin L. R. (2000) Mol. Reprod. Dev. 56, 6-11. [DOI] [PubMed] [Google Scholar]
- 17.Jaiswal, B. S. & Conti, M. (2001) J. Biol. Chem. 276, 31698-31708. [DOI] [PubMed] [Google Scholar]
- 18.Miki, K., Willis, W., D., Brown, P. R., Goulding, E. H., Fulcher, K. D. & Eddy, E. M. (2002) Dev. Biol. 248, 331-342. [DOI] [PubMed] [Google Scholar]
- 19.Ren, D., Navarro, B., Perez, G., Jackson, A. C., Hsu, S., Shi, Q., Tilly, J. L. & Clapham, D. E. (2001) Nature 413, 603-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skalhegg, B. S., Huang, Y., Su, T., Iddzerda, R. L. McKnight, G. S. & Burton, K. A. (2002) Mol. Endocrinol. 16, 630-639. [DOI] [PubMed] [Google Scholar]
- 21.Horner, K., Livera, G., Hinckley, M., Trinh, K. Storm, D. & Conti, M. (2003) Dev. Biol. 258, 385-396. [DOI] [PubMed] [Google Scholar]
- 22.Krajnc-Franken, M. A. M., van Disseldorp, A. J. M., Koenders, J. E., Mosselman, S., van Duin, M. & Gossen, J. (2004) Mol. Cell. Biol. 24, 687-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blazak, W. F., Treinen, K. A. & Juniewics, P. E. (1993) in Male Reproductive Toxicology, eds. Heindel, J. J. & Chapin, R. E., pp. 86-94.
- 24.Meistrich, M. L. & vn Beek, M. E. A. B. (1993) in Male Reproductive Toxicology, eds. Heindel, J. J. & Chapin, R. E., pp. 106-123.
- 25.Rose, U. M., Hanssen, R. G. & Kloosterboer, H. J. (1999) Biol. Reprod. 6, 503-511. [DOI] [PubMed] [Google Scholar]
- 26.Pastor-Soler, N., Beaulieu, V., Litvin, T. N., Da Silva, N., Chen, Y., Brown, D., Buck, J., Levin, L. R. & Breton, S. (2003) J. Biol. Chem. 278, 49523-49529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nantel, F., Monaco, L., Foulkes, N. S., Masquilier, D., LeMeur, M., Henriksen, K., Dierich, A., Pavinen, M. & Sassone-Corsi, P. (1996) Nature 380, 159-162. [DOI] [PubMed] [Google Scholar]
- 28.Fimia, G. M., Morlon, A., Macho, B., De Cesare, D., Sassone-Corsi, P. (2001) Mol. Cell. Endocrinol. 179, 17-23. [DOI] [PubMed] [Google Scholar]
- 29.Harrison, R. A. & Miller, N. G. (2000) Mol. Reprod. Dev. 55, 220-228. [DOI] [PubMed] [Google Scholar]
- 30.Jiménez, A., Oko, R., Gustafsson, J.-Å., Spyrou, G., Pelto-Huikko, M. & Miranda-Vizuete, A. (2002) Mol. Hum. Reprod. 8, 710-718. [DOI] [PubMed] [Google Scholar]
- 31.Sadek, C. M., Damdimopolus, A. E., Pelto-Huikko, M., Gustafsson, J.-Å, Spyrou, G. & Miranda-Vizuete, A. (2001) Genes Cells 12, 1077-1090. [DOI] [PubMed] [Google Scholar]
- 32.Sapiro, R., Kostetskii, I., Olds-Clarke, P., Gerton, G. L., Radice, G. L. & Strauss, J. F., III. (2002) Mol. Cell. Biol. 17, 6298-62305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Welch, J. E., Brown, P. L., O'Brien, D. A., Magyar, P. L., Bunch, D. O., Mori, C. & Eddy, E. M. (2000) J. Androl. 2, 328-338. [PubMed] [Google Scholar]
- 34.Yoshinaga, K. Tanij, I. & Toshimori, K. (1999) Arch. Histol. Cytol. 62, 283-293. [DOI] [PubMed] [Google Scholar]
- 35.Reddi, P. P., Naaby-Hansen, S., Aguolnik, I., Tsai, J-Y., Silver, L. M., Flickinger, C. J. & Herr, J. C. (1995) Biol. Reprod. 53, 873-881. [DOI] [PubMed] [Google Scholar]
- 36.Curi, S. M., Ariagno, J. I., Chenlo, P. H., Mendeluk, G. R., Pugliese, M. N., Sardi Segovia, L. M., Repetto, H. E. H. & Blanco, A. M. (2003) Arch. Androl. 49, 343-349. [DOI] [PubMed] [Google Scholar]
- 37.Okamura, N., Tajima, Y., Soejima, A., Masuda, H. & Sugita, Y. (1985) J. Biol. Chem. 260, 9699-96705. [PubMed] [Google Scholar]
- 38.Ishikawa, H., Tomomasa, H., Yoshii, S., Koiso, K., Tajima, Y., Okamura, N. & Sugita, Y. (1989) Andrologia 21, 437-440. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.