Abstract
Bone mineral density (BMD) declines significantly in HIV patients on antiretroviral therapy (ART). We compared the effects of intermittent versus continuous ART on markers of bone turnover in the Body Composition substudy of the Strategies for Management of AntiRetroviral Therapy (SMART) trial and determined whether early changes in markers predicted subsequent change in BMD. For 202 participants (median age 44 years, 17% female, 74% on ART) randomised to continuous or intermittent ART, plasma markers of inflammation and bone turnover were evaluated at baseline, months 4 and 12; BMD at the spine (dual X-ray absorptiometry [DXA] and computed tomography) and hip (DXA) was evaluated annually. Compared to the continuous ART group, mean bone-specific alkaline phosphatase (bALP), osteocalcin, procollagen type 1 N-terminal propeptide (P1NP), N-terminal cross-linking telopeptide of type 1 collagen (NTX), and C-terminal cross-linking telopeptide of type 1 collagen (βCTX) decreased significantly in the intermittent ART group, whereas RANKL and the RANKL:osteoprotegerin (OPG) ratio increased (all p≤0.002 at month 4 and month 12). Increases in bALP, osteocalcin, P1NP, NTX, and βCTX at month 4 predicted decrease in hip BMD at month 12, while increases in RANKL and the RANKL:OPG ratio at month 4 predicted increase in hip and spine BMD at month 12. This study has shown that compared with continuous ART, interruption of ART results in a reduction in markers of bone turnover and increase in BMD at hip and spine, and that early changes in markers of bone turnover predict BMD changes at 12 months.
Keywords: HIV, bone mineral density, antiretroviral therapy, bone turnover marker
Introduction
Although adults infected with the human immunodeficiency virus (HIV) have increased fracture risk (1) and lower bone mineral density (BMD) compared with the general population (2–4), the relative contributions of HIV infection versus the use of antiretroviral therapy (ART) are unknown. The immune system also plays a key role in bone homeostasis, as both B and T lymphocytes regulate osteoclast activity (5), and higher circulating levels of interleukin 6 (IL-6) are independently associated with greater bone loss (6). In untreated HIV infection, osteocalcin is lower, and beta C-terminal cross-linking telopeptide of type 1 collagen (βCTX) is higher, respectively, with more advanced HIV immunodeficiency (7–9). HIV may induce bone resorption by several mechanisms including increased receptor activator for nuclear factor-κβ ligand (RANKL) expression through chronic immune activation and increased synthesis of pro-inflammatory cytokines (10). Initiation of combination ART is associated with increases in markers of bone turnover, identified by increases in levels of both bone formation and resorption markers (7, 11–12). For ART-naïve patients initiating abacavir-lamivudine or tenofovir-emtricitabine with efavirenz, bone turnover markers increased to 24 weeks after ART initiation and remained relatively stable thereafter, and the increase in markers of bone turnover correlated with reduction in BMD, with r values ranging from −0.19 to −0.40 (11).
No prospective study has investigated the effects of cessation of ART on markers of turnover and inflammation, nor correlated changes in markers of bone turnover and inflammatory markers with subsequent changes in BMD on cessation of ART.
The INSIGHT Strategies for Management of AntiRetroviral Therapy (SMART) study was an international, randomized strategy treatment trial comparing intermittent, CD4+ T lymphocyte count-guided ART with continuous ART. BMD was measured at baseline and annually in a substudy (n=275) (13–14). In the intermittent ART group, participants stopped or deferred ART at baseline, and BMD increased or remained stable during the first year after study entry. In contrast, BMD decreased in the continuous ART group, by 0.8% per year at the hip and 0.4% per year at the spine by DXA, over 2.4 years mean follow-up (14).
In order to investigate why stopping/deferring ART resulted in higher BMD compared with continuous ART, we evaluated markers of bone turnover and inflammation at baseline, month 4 and month 12. The aims of the analyses were:
-
(1)
To evaluate the effect of intermittent compared with continuous ART on bone turnover markers, regulators of bone turnover and inflammatory markers by comparing the randomized treatment groups in a subset of the SMART study; and
-
(2)
To determine whether early changes in biomarkers (baseline to month 4) predict change in BMD at the lumbar spine and hip at 12 months.
Methods
Overall Study Design
Participants in SMART (n=5472) were randomized to two groups: (i) intermittent, CD4+ T cell count-guided ART, where ART was stopped or deferred at study entry, and re-started when the CD4+ T cell count declined below 250 cells/μL, and stopped again at CD4+ T cell counts above 350 cells/ μL; or (ii) continuous ART. (13) ART drugs were not protocol-specified. The SMART study was approved by the institutional review board (IRB) or ethics committee at each clinical site. All participants provided written, informed consent. The study was registered at ClinicalTrials.gov: NCT00027352.
Study Population
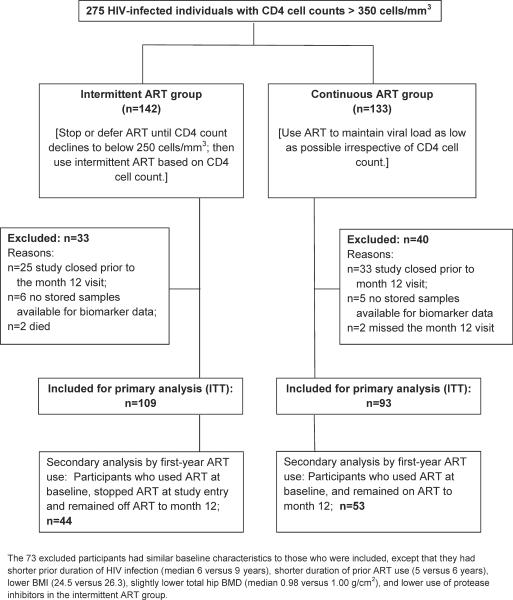
The DSMB closed the parent SMART study and its substudies early due to safety concerns in the intermittent ART arm of the parent study. This analysis includes the 202 of 275 participants co-enrolled in the SMART Body Composition substudy who had BMD measurements as well as stored samples for biomarker analysis available at baseline and month 12 (25 and 33 participants in the intermittent and continuous ART study arms had no month 12 follow-up visits), (see Figure 1, CONSORT diagram). Design and primary results of the Body Composition substudy were described elsewhere (14–15). Participants were enrolled at 32 sites in the United States, Australia and Spain.
Figure 1.
Study design and CONSORT flow diagram.
Measurement of BMD
BMD was assessed at baseline and month 12 by dual-energy X-ray absorptiometry (DXA) of the hip and lumbar spine (L1–L4) and quantitative computed tomography (qCT) of the spine (L2–L4). Each patient had all scans performed by the same imaging. Scans were obtained at 8 radiology centers and were centrally analyzed (Bio-Imaging Technologies, Inc., Newtown, PA, USA). All sites used a standardized scanning protocol, and the Central Reading Center corrected BMD measurements for cross-sectional and longitudinal consistency based on phantom scans (14). BMD assessments were blinded to treatment group.
Measurement of Markers of Bone Turnover and Inflammation
At baseline, months 4 and 12, plasma was collected into EDTA tubes, after participant fasting for at least 8 hours, processed within 4 hours of collection, and stored at −70°C. All assays were performed blinded to treatment group and BMD measurements.
Markers of bone formation assayed were bone-specific alkaline phosphatase (bALP) (Ostase BAP, ImmunoDiagnostic Systems, Fountain Hills, AZ) (reference range; men 3.7–20.9 μg/L, pre-menopausal women 2.9–14.5 μg/L, and postmenopausal women 3.8–22.6 μg/L, Inter-assay CV <5.3%), osteocalcin (N-MID Osteocalcin, Roche, Indianapolis, IN) (reference range; men 10.2–36.7 ng/ml, pre-menopausal women 9.7–35.1 ng/ml, post-menopausal women 7.3–37.8 ng/ml, Inter-assay CV <4.3%) and procollagen type 1 N terminal propeptide (P1NP) (Intact N-terminal propeptide of type 1 procollagen, ImmunoDiagnostic Systems, Fountain Hills, AZ) (Laboratory for Clinical Biochemistry Research [LCBR] reference range 25.91–132.5 μg/L, Inter-assay CV <12.4%). Markers of bone resorptions assayed were N-telopeptide (NTX) (Osteomark N-Tx, Wampole Laboratories, Princeton, NJ) (reference range; men 5.4–24.2 nM BCE, pre-menopausal women 6.2–19.0 nM BCE, Inter-assay CV <12.8%), C-terminal cross-linking telopeptide of type 1 collagen (βCTX) (B-Crosslaps, Roche, Indianapolis, IN) (reference range; men 0.158–0.584 ng/mL, pre-menopausal women 0.162–0.573 ng/ml, post-menopausal women 0.330–1.008 ng/ml, Inter-assay CV <8.9%). The bone remodelling markers assayed were osteoprotegerin (OPG) (Alpco Diagnostics, Salem, NH) (LCBR reference range 2.58–7.16 pmol/L, Inter-assay CV <12.5%), receptor of nuclear factor-κB ligand (RANKL) (Millipore, Billerica, MA) (LCBR reference range 0.160 pg/ml, Inter-assay CV <10.2%). Additionally, the inflammatory markers interleukin (IL)-1 (IL-1b, Panel B, Millipore, Billerica, MA) (LCBR reference range 0–1.35 pg/ml, Inter-assay CV <13%), IL-6 (R&D Systems, Minneapolis, MN) (LCBR reference range 0.68–6.36 pg.ml, Inter-assay CV <12.2%), and TNF-α (Panel B, Millipore, Billerica, MA) (LCBR reference range 0.79–9.41 pg/ml, Inter-assay CV <8.1%) were assayed.
Statistical Analyses
Treatment groups were compared for baseline characteristics using Kruskal-Wallis tests to compare medians and Chi-squared tests for proportions. For all analyses involving change in biomarkers, the biomarkers were analysed on the log2 scale, to satisfy distribution assumptions for the statistical tests. An increase in 1 log2 biomarker unit corresponds to a 2-fold increase (doubling) on the original biomarker scale (16). As the primary analysis for the first aim (see Introduction), the intermittent ART and continuous ART groups were compared for mean changes in bone biomarkers and in BMD by intent-to-treat, using unadjusted t-tests. Significant differences between the randomized treatment groups, where present, provide evidence for a causal relationship between ART use and biomarker changes. As sensitivity analyses, we repeated the comparisons with adjustment for sex, ART status at study entry, and baseline CD4 cell counts. Within treatment groups, statistical significance of changes in BMD and markers of bone turnover was assessed using two-sided t-tests. As secondary analyses, we compared participants who stopped ART, and stayed off ART through 12 months in the intermittent ART group with participants in the continuous ART group who were on ART at baseline and continued ART for 12 months (Figure 1).
For the second aim, associations of changes in biomarkers from baseline to Month 4 with changes in BMD from baseline to Month 12 were estimated using linear regression; all models were adjusted for age, sex, race, BMI, HIV duration, ART use at study entry, and baseline CD4 cell counts. We also included sex-by-race interaction terms, because by chance more women were randomized to the continuous ART arm and the race distribution varied by sex. To describe associations of each biomarker with BMD change (11 biomarkers, each with 4 BMD outcomes), estimated coefficients for the biomarker change were presented with 95% confidence intervals and p-values. These regressions were not adjusted for treatment group, since the treatment intervention was thought to cause changes in biomarkers, and such could mask the investigated relationship. For multiple regression, for each BMD outcome, we started with a linear regression model that included all 11 biomarkers and the adjustment factors, and performed stepwise backward selection with the Akaike Information Criterion (AIC) (17). For each of the BMD outcomes, we reported which markers remained in the model, and whether the association with BMD change was significant. Analyses used SAS version 9.2 (SAS Institute, Cary, NC, USA) and R version 2.15 (18). P-values ≤0.05 were considered statistically significant. All p-values are two-sided.
Results
Baseline characteristics
Baseline characteristics of the study population are shown in Table 1. Median age was 44 years, 17% were female, 27% were black, and 56% were white. The treatment groups were well balanced, except that 62% of the intermittent ART group had HIV RNA ≤400 copies/mL at baseline, compared with 47% in the continuous ART group (p=0.03 for difference), more participants in the intermittent ART group had used ART at baseline (80% versus 68%; p=0.05), and more participants in the intermittent ART group had used protease inhibitors (p=0.04). Among those using ART at study entry, however, protease inhibitor use was balanced across treatment groups (p=0.15). The intermittent ART group also had a lower proportion of women (13% versus 23%); the difference was not statistically significant. Few participants had osteoporosis and none was receiving osteoporosis therapy. Baseline values for the bone turnover and regulatory markers and inflammatory cytokines are described in Table 2; differences between treatment groups were not significant.
Table 1.
Baseline characteristics.
| Characteristic | Intermittent ART group (n=109) % or median | Continuous ART group (n=93) % or median | Total (n=202) % or median (IQR) |
|---|---|---|---|
| Demographics | |||
| Age (years) | 45.0 | 43.0 | 44.0 (40.0, 50.0) |
| Female participants (%) | 12.8 | 22.6 | 17.3 |
| Race/ethnicity (%) | |||
| Black | 25.7 | 28.0 | 26.7 |
| Latino or Hispanic | 16.5 | 14.0 | 15.3 |
| White | 56.0 | 57.0 | 56.4 |
| Other or unknown | 1.8 | 1.1 | 1.5 |
| HIV transmission modes (%) | |||
| MSM | 70.6 | 58.1 | 64.9 |
| Heterosexual | 30.3 | 34.4 | 32.2 |
| Intravenous drug use | 13.8 | 14.0 | 13.9 |
| Other or unknown | 4.6 | 10.8 | 7.4 |
| Clinical characteristics | |||
| BMI | 26.3 | 26.3 | 26.3 (23.6, 29.3) |
| Current smoker (%) | 41.3 | 49.5 | 45.0 |
| Corticosteroid use (%) | 0.9 | 3.2 | 2.0 |
| Duration of HIV infection (years) | 9.0 | 9.0 | 9.0 (5.0, 14.0) |
| Prior AIDS diagnosis (%) | 24.8 | 22.6 | 23.8 |
| Hepatitis B (%) | 3.7 | 5.5 | 4.5 |
| Hepatitis C (%) | 15.6 | 17.2 | 16.3 |
| CD4 cell count (cells/μ1) | 580 | 525 | 558 (441, 766) |
| Nadir CD4 cell count (cells/μL) | 225 | 268 | 250.0 (136, 371) |
| HIV-RNA ≤400 copies/mL plasma (%) | 62.4 | 47.3 | 55.4* |
| Antiretroviral therapy (ART) | |||
| Naive (%) | 5.5 | 6.5 | 5.9 |
| Duration of prior ART (years) | 6 | 7 | 6 (3, 8) |
| Current ART (%) | 79.8 | 67.7 | 74.3* |
| Current PI use (%) | 43.1 | 29.0 | 36.6* |
| Current tenofovir use (%) | 11.9 | 15.1 | 13.4 |
| Current TA-NRTI use (%) | 58.7 | 49.5 | 54.5 |
| Current NRTIsa other than tenofovir or TA-NRTIs (%) | 11.9 | 9.7 | 10.9 |
| Current NNRTI use (%) | 36.7 | 35.5 | 36.1 |
| Bone mineral density | |||
| Spine, qCT (mg/cm3) | 153 | 150 | 152 (128, 173) |
| Z-score | −0.10 | −0.11 | −0.11 (−0.88, 0.56) |
| T-score | −0.89 | −0.97 | −0.91 (−1.79, −0.02) |
| T-score < −2.5 (%) | 7.3 | 12.9 | 9.9 |
| Spine, DXA (g/cm2) | 1.11 | 1.09 | 1.10 (1.01, 1.21) |
| Z-score | −0.40 | −0.70 | −0.60 (−1.33, 0.30) |
| T-score | −0.64 | −0.70 | −0.70 (−1.40, 0.30) |
| T-score < −2.5 (%) | 4.6 | 4.3 | 4.5 |
| Total hip, DXA (g/cm2) | 1.00 | 1.00 | 1.00 (0.94, 1.12) |
| Z-score | −0.23 | −0.10 | −0.20 (−0.80, 0.49) |
| T-score | −0.50 | −0.41 | −0.46 (−1.00, 0.30) |
| T-score < −2.5 (%) | 2.8 | 1.1 | 2.0 |
| Femoral neck, DXA (g/cm2) | 0.92 | 0.91 | 0.91 (0.83, 1.05) |
| Z-score | −0.26 | −0.30 | −0.30 (−0.88, 0.42) |
| T-score | −0.80 | −0.70 | −0.79 (−1.40, 0.08) |
| T-score < −2.5 (%) | 0.9 | 3.3 | 2.0 |
| Low BMD, by DXA T-scores b (%) | 42.2 | 38.7 | 40.6 |
| Osteoporosis, by DXA T-scores b (%) | 5.5 | 4.3 | 5.0 |
Abbreviations: ART, antiretroviral therapy; IQR, interquartile range; MSM, Men who have Sex with Men; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; qCT, quantitative computed tomography; TA-NRTI, thymidine analogue-reverse transcriptase inhibitor.
P-value ≤ 0.05 for the comparison between the treatment groups; Chi-square tests were used to compare percentages, Kruskal-Wallis tests to compare medians.
includes abacavir (73%), didanosine (36%), emtricitabine (5%) and lamivudine (73%)
Low BMD, at least one of the T-scores for DXA spine and hip was below −1; osteoporosis, at least of the T- scores for DXA spine and hip was below −2.5.
Table 2.
Markers of bone turnover at baseline
|
Intermittent ART group
|
Continuous ART group
|
Total
|
||||
|---|---|---|---|---|---|---|
| Marker |
N
|
Median
|
N
|
Median
|
N
|
Median (IQR)
|
| Bone Formation | ||||||
| bALP (μg/L) | 109 | 20.3 | 93 | 18.7 | 202 | 19.6 (16.1, 26.1) |
| Osteocalcin (ng/mL) | 107 | 18.9 | 92 | 17.0 | 199 | 17.6 (13.9, 22.6) |
| P1NP (μg/L) | 109 | 49.4 | 93 | 45.9 | 202 | 47.6 (36.2, 62.1) |
| Bone Resorption | ||||||
| NTX (nM BCE) | 109 | 11.9 | 93 | 10.8 | 202 | 11.3 (9.7, 14.1) |
| βCTX (ng/mL) | 106 | 0.3 | 92 | 0.3 | 198 | 0.3 (0.2, 0.4) |
| Bone Regulators | ||||||
| OPG (pmol/L) | 107 | 4.0 | 90 | 3.8 | 197 | 3.9 (3.3, 4.6) |
| RANKL (pg/mL) | 109 | 7.9 | 91 | 8.6 | 200 | 8.3 (2.0, 23.2) |
| RANKL:OPG ratio | 107 | 2.0 | 89 | 3.2 | 196 | 2.2 (0.6, 6.1) |
| Inflammation | ||||||
| IL-6 (pg/mL) | 109 | 2.0 | 93 | 1.7 | 202 | 1.9 (1.2, 3.0) |
| IL-1 (pg/mL) | 104 | 0.1 | 86 | 0.1 | 190 | 0.1 (0.1, 0.1) |
| TNF-α (pg/mL) | 104 | 9.7 | 86 | 9.2 | 190 | 9.2 (6.7,12.2) |
Abbreviations: bALP, bone-specific alkaline phosphatase; βCTX, C-terminal cross-linking telopeptide of type 1 collagen; IL-1, interleukin lbeta; IL-6, interleukin 6; NTX, N-telopeptide; OPG, Osteoprotegerin; P1NP, procollagen type 1 N terminal propeptide; RANKL, receptor of nuclear factor-KB ligand; TNF-α, tumor necrosis factor α.
Antiretroviral therapy
Of the participants assigned to continuous ART, 68% were using ART at baseline, and 32% started ART; 97% were on ART at month 4 and 91% at month 12. Of those assigned to intermittent ART, 80% were using ART at baseline and ceased ART, the others continued to defer ART according to the CD4-guided intermittent ART strategy. Through follow-up, 18% had recommenced ART at month 4 and 46% at month 12 due to CD4 cell decline or clinical events.
Changes in BMD and Markers of Bone Turnover and Inflammation by treatment group
Changes in BMD and estimated between-group differences are shown in Figure 2. By year 1, each BMD measure was significantly higher in the intermittent ART group (increase of 0.33% at the hip and 1.64% at the spine by DXA) compared with the continuous ART group (decrease of −1.04% at the hip, and −0.13% at the spine by DXA) at M12. Within the continuous ART group, mean BMD declined by all measures at hip and spine, although the decline was not statistically significant for spine BMD by DXA. In the intermittent ART group, BMD increased, although only the increase in spine BMD by DXA was significant.
Figure 2.
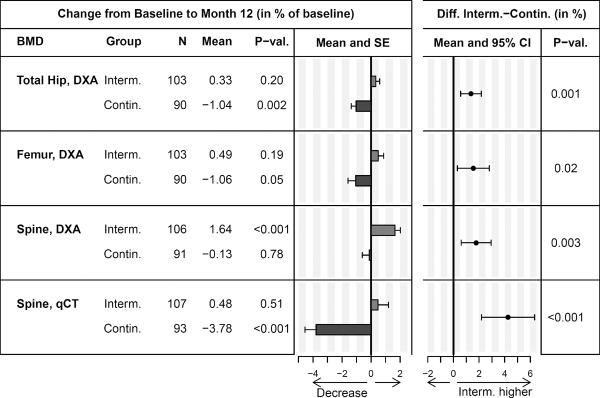
Change in BMD from baseline to year 1, and estimated difference between the intermittent and continuous ART groups.
Abbreviations: CI, confidence interval; SE, standard error.
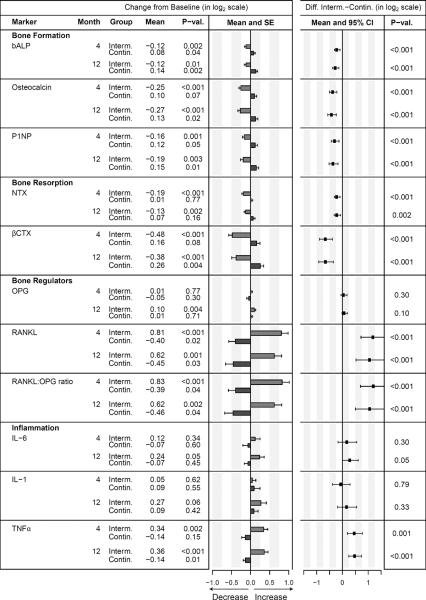
Mean changes from baseline for (log transformed) markers of bone turnover, bone regulation and chronic inflammation at 4 and 12 months, and the between-group differences (change in the intermittent ART minus the continuous ART group) are presented in Figure 3. In the intermittent ART group, bALP, osteocalcin, P1NP, NTX, and βCTX decreased, compared to an increase in the same markers in the continuous ART group. RANKL, the RANKL:OPG ratio and TNF-α increased in the intermittent ART group, and decreased in the continuous ART group. The changes were apparent by Month 4, and remained at about the same level at Month 12. Between-group differences in mean change were significant at both Months 4 and 12 for each of these biomarkers (all p-values ≤0.002). IL-6 followed a similar pattern to TNF-α, but the treatment difference was significant only at month 12 (p=0.05). There was no significant between-group difference in mean change in OPG or IL-1. Adjustment for sex, ART status at study entry, and baseline CD4 cell counts had minimal impact on the between-group comparison (data not shown).
Figure 3. Change in biomarkers from baseline to months 4 and 12 within the intermittent ART and continuous ART groups, and estimated treatment difference.
Bars denote the mean change from baseline. All biomarkers were analyzed on the log2 scale, an increase of 1 log2 biomarker unit corresponds to a 2-fold increase (doubling) in the original measurement scale.
Abbreviations: bALP, bone-specific alkaline phosphatase; βCTX, C-terminal cross-linking telopeptide of type 1 collagen; IL-1, interleukin 1beta; IL-6, interleukin 6; NTX, N-telopeptide; OPG, Osteoprotegerin; P1NP, procollagen type 1 N terminal propeptide; RANKL, receptor of nuclear factor-KB ligand; TNF-α, tumor necrosis factor α; CI, confidence interval; SE, standard error.
Changes in Markers of Bone Turnover and Inflammation by first-year ART use
When restricting the analyses to participants who were on ART at baseline, and either remained on ART for 12 months (n=53), or stopped and remained off ART for 12 months (n=44) (Figure 1), differences between the ART groups at month 4 were similar to the intent-to-treat comparisons in all 202 participants. Differences tended to increase further by month 12, although most of the treatment difference was attained by month 4 (data not shown). There was no evidence for a difference between the treatment groups in OPG or IL-1.
Effect of Early Change in Markers (Baseline-Month 4) on Change in BMD (Baseline-Month 12)
Associations between early changes in bone biomarkers (Baseline to Month 4) and changes in BMD between baseline and Month 12 are summarized in Figure 4. For example, an increase of 1 log2 unit in bALP (2-fold increase from baseline to Month 4) was associated with a 2.3% per year steeper decrease in hip BMD. Pooled across treatment groups, increases in bone formation markers (bALP, osteocalcin) as well as increases in bone resorption markers (NTX and βCTX) at Month 4 were significantly correlated with loss of total hip BMD, while increased RANKL and RANKL:OPG ratios were associated with increases of hip BMD. The association of increased P1NP with a decrease in hip BMD was borderline significant (p=0.06). The direction of the associations of these seven biomarkers with change in BMD were similar across all four BMD outcomes (total hip, femoral neck, spine by DXA and spine by qCT), although not all associations were significant. An increase in TNF-α by Month 4 was associated with increased spine BMD by qCT, but there was no evidence for an association of TNF-α, IL-1 or IL-6 with any of the other BMD outcomes. The analyses were adjusted for age, sex, race, BMI, HIV duration, ART use at study entry and baseline CD4 cell counts.
Figure 4. Associations of early changes in biomarkers with change in BMD.
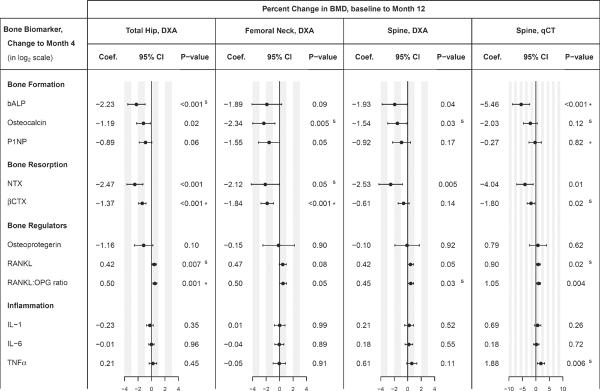
Coefficients, 95% confidence intervals and p-values for association were calculated in linear regression models, using the change in each biomarker (from baseline to month 4, log2 scale) to estimate the change in BMD at year 1; models were adjusted for age, sex, race, BMI, HIV duration, ART use at study entry, and baseline CD4 cell counts. The size of the coefficients is comparable across biomarkers, because an increase of 1 log2 biomarker unit corresponds to a 2-fold increase (doubling) in the original measurement scale. For each BMD outcome, superscripts for p-values denote the final multiple regression model obtained through backwards variable selection with AIC, starting from a model containing all 11 bone biomarkers (log2 scale) and adjustment factors. S = biomarker was selected, but association was not significant ; * = biomarker was independently associated with change in BMD in the final model (p ≤ 0.05).
Abbreviations: bALP, bone-specific alkaline phosphatase; βCTX, C-terminal cross-linking telopeptide of type 1 collagen; IL-1, interleukin 1beta; IL-6, interleukin 6; NTX, N-telopeptide; OPG, Osteoprotegerin; P1NP, procollagen type 1 N terminal propeptide; RANKL, receptor of nuclear factor-KB ligand; TNF-α, tumor necrosis factor α.
Changes in markers of bone turnover and inflammation were correlated (data not shown). When including all 11 bone biomarkers as predictors for change in BMD in multiple regression models, a backwards selection procedure retained early declines in βCTX (p<0.001) and the RANKL:OPG ratio (p=0.03) as independent predictors for increase in total hip BMD. Results for the other BMD outcomes are summarized in Figure 4; superscripts to the p-values denote which bone markers were selected for the final model (s, *), and whether they were independently associated with the BMD outcome at year 1 (* for p < 0.05).
Changes in CD4+ T cell counts
In the intermittent ART group, mean CD4+ T cell counts declined by 198 and 232 cells/μL from baseline to months 4 and 12, respectively. In the continuous ART group, mean CD4+ T cell counts increased by 25 cells/μL to month 4, and 33 cells/μL from baseline to month 12. A Month 4 increase in CD4+ T cells by 100 cells/μL was associated with a decrease in spine DXA BMD by −0.40% (95% CI: −0.71 to −0.08%, p=0.01), and a decrease in spine qCT BMD by −0.89% (95% CI: −1.44 to −0.35%, p=0.002) in univariate regression pooled across treatment groups. After adjustment for treatment group, these associations were not significant. There was no evidence for an association between changes in CD4+ T cell count at Month 4 and changes in hip BMD at Month 12.
Discussion
This is the first randomized study in HIV patients to compare changes in markers of bone turnover and inflammation between continuous ART (continue or start ART) and intermittent ART (stop or defer ART, and resume ART at CD4 counts <250 cells/μL). Relative to continuous ART, we found significant reductions in markers of both bone resorption and bone formation in the intermittent ART group. Markers of bone formation and resorption both decreased from baseline to month 4, and remained stable thereafter to month 12 in the intermittent ART group. Conversely, these markers tended to increase in the continuous ART group.
The RANKL: OPG ratio increased in the intermittent ART group by Month 4, and remained elevated through Month 12. In some studies of postmenopausal osteoporosis, increased RANKL:OPG was associated with net bone loss, while no association with BMD was demonstrated in others (19). In our study the association was inverse; higher RANKL:OPG ratios were associated with increased BMD and reduction in other markers of bone resorption. The inverse association was also seen in a prospective study in 87 patients with HIV, which reported significant reductions in levels of RANKL and OPG as well as inflammatory markers,on initiation of ART, while markers of bone formation and resorption significantly increased. The OPG/sRANKL ratio remained unchanged over time and there was no association with markers of bone turnover (20).
RANKL, IL-6 and TNF-α significantly increased in the intermittent ART group and decreased in the continuous ART group. In contrast, osteoprotegerin levels and IL-1 remained relatively stable. Conditions associated with chronic immune activation and inflammation are well known to be associated with increased bone loss (e.g. rheumatoid arthritis and inflammatory bowel disease)(21–23). Inflammation results in increased RANKL expression and production by activated T cells and B cells, increased numbers of osteoclast precursors and reduction in production in osteoprotegerin, with resultant greater bone resorption and net bone loss (24–26). It is counterintuitive that the increases in RANKL and the RANKL:OPG ratio in the intermittent ART group occurred in association with reduction in markers of bone resorption and increased BMD. It is possible that ART increases bone turnover through a pathway that is not mediated by RANKL or osteoprotegerin, and that the increase in RANKL at cessation of ART occurred as a compensatory response to the reduction in markers of bone turnover. Alternatively, the increase in RANKL may be a reflection of increased immune activation and subsequent expression and production of RANKL by activated T cells associated with untreated HIV infection.
Early increases (from baseline to Month 4) in the markers of bone formation and bone resorption predicted decreases in BMD at the hip and spine at Month 12. The directions of associations between biomarkers and BMD were consistent across BMD at the hip (femoral neck and total hip) and spine (lumbar spine measured by DXA and qCT). However, only a few markers (βCTX and the RANKL:OPG ratio for hip BMD, bALP and P1NP for spine BMD by qCT) remained independently associated with changes in BMD in multiple regression and the strength of the associations varied by BMD site and measurement modality. This reflects the complex interrelationships between the bone biomarkers, with none clearly dominating with respect to predicting change in BMD.
The randomized comparison provides evidence that the observed decline in markers of bone turnover in the continuous ART group versus the increase in the intermittent ART group are likely due to the differences in ART use. However, the difference between the two treatment groups may underestimate the effect of stopping ART, because in the intermittent ART arm, 36% of participants had recommenced ART by month 12, and 20% had not used ART at study entry. When the analysis was restricted to those participants in the intermittent ART arm who stopped ART and stayed off ART through month 12, the levels of markers of bone formation and resorption continued to decline through month 12, although most of the reduction was evident by month 4. Conversely, in the continuous ART group, when restricting the analysis to those who were using ART at study entry and continued to use ART through month 12, markers of bone turnover within this subset of participants tended to increase, but the magnitude of the increase was less than among those randomized to the continuous ART group who were not taking ART at baseline and started ART.
In the setting of ART, increased markers of bone turnover, as observed in the continuous ART group, have been consistently associated with loss of BMD, particularly over the first year of treatment (11–12). Elucidating the mechanism of ART-induced bone loss is confounded by changes in HIV-induced immune activation and inflammation. Is the significant early bone loss observed over 24–48 weeks after initiation of ART driven by immune reconstitution, as proposed by some (27–28), or by the direct effects of ART? In our study, increased CD4 T cell counts were associated with steeper decreases in spine BMD, pooled across treatment groups. It is not possible, however, to separate the effect of ART in our study from the effect of increased CD4 T cells. After adjustment for treatment group, there was no evidence for an association of CD4 T cell increases with bone loss.
The main strength of our study is the randomized comparison of continuous versus intermittent ART. There were several limitations to our study. About half of the participants randomized to intermittent ART had restarted ART by month 12. The ART regimens used in SMART were heterogeneous and may not be entirely representative of modern ART regimens; moreover, this study was not designed to evaluate the effects of specific drugs. The first follow-up time point was at month 4, so we could not determine which biomarker or group of biomarkers changed first. The analysis excluded 73 of the 275 randomized participants in the SMART Body Composition substudy, mostly because month 12 data were not available due to study closure. While the excluded participants were largely similar to those analyzed, they differed in some baseline characteristics. Finally, the proportion of women was higher and ART use at study entry was lower in one of the randomized treatment groups in the Body Composition substudy; however, after adjustment for sex and ART use at baseline in sensitivity analyses, results were similar to the unadjusted comparisons between treatment groups summarized in Figure 3.
In conclusion, this study has shown that interruption of ART results in a reduction in markers of bone turnover and increase in BMD at hip and spine relative to continuous ART, and that early changes in markers of bone turnover predict BMD changes at 12 months. The stabilization of bone loss after ART interruption, despite increased inflammation, suggests a deleterious effect of ART on bone, either directly or indirectly. The clinical implication of these findings is unknown given the imperative need for ART to prevent major HIV-related outcomes; once started, ART needs to be used continuously. The trend to initiate ART earlier in the course of HIV infection means that low BMD may become more prevalent as more patients use ART for longer, highlighting the need for prospective studies with longer follow-up.
Table 3.
Percent change in BMD from baseline to month 12.
| BMD |
Mean Change (% of baseline)
|
Estimated Difference Intermittent ART-Continuous ART | |||||
|---|---|---|---|---|---|---|---|
| Intermittent ART group | Continuous ART group | ||||||
|
| |||||||
| N | Mean | N | Mean | Est. Diff. | 95% CI | P-value | |
| Total Hip DXA | 103 | 0.33 | 90 | −1.04*** | 1.36 | 0.56–2.17 | 0.001 |
| Femoral Neck DXA | 103 | 0.49 | 90 | −1.06* | 1.55 | 0.30–2.79 | 0.02 |
| Spine DXA | 106 | 1.64*** | 91 | −0.13 | 1.77 | 0.60–2.94 | 0.003 |
| Spine qCT | 107 | 0.48 | 93 | −3.78*** | 4.26 | 2.19–6.34 | <0.001 |
Mean change is significantly different from 0, p ≤ 0.05 for two-sided t-test;
p ≤ 0.01;
p ≤ 0.001
Acknowledgements
The authors gratefully acknowledge the study participants, investigators (listed in (14)), study co-ordinators, and the staff at imaging centers and BioImaging, Inc., without whom this study could not have been completed. The authors also thank Dr Russell Tracy and his laboratory staff at the University of Vermont for performance of the bone turnover markers and immunology markers. Author's roles: Study design: JH, BG and AC. Study conduct: JH, BG, MR and AC. Data analysis: BG and MR. Data interpretation: JH, BG, KE, AC. Drafting manuscript JH, BG and AC. Revising manuscript JH, BG, MR, KE, IB, RC, NdC, MJ, AS, AC. Approval final version of the manuscript: JH, BG, MR, KE, IB, RC, NdC, MJ, AS, AC. BG and MR take responsibility for the integrity of the data analysis.
The study was funded by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, U01AI042170 and U01AI46362, and U01-AI068641. Clinical Trials.gov identifier: NCT00027352.
Disclosures Jennifer Hoy's institution has received funding for investigator-initiated research, service on Advisory Boards, lectures and conference sponsorship from Janssen-Cilag, Gilead Sciences, Merck, Sharp & Dohme and ViiV Healthcare (previously GlaxoSmithKline and Pfizer).
Birgit Grund, Mollie Roediger, and Anjali Sharma report no conflicts of interest.
Kris Ensrud has received travel support from Merck Sharp & Dohme for attendance at DMC meetings.
Indira Brar has received research funding from Gilead Sciences, Merck Sharp & Dohme, and ViiV Healthcare, and speaker's fees from Gilead Sciences and Tibotec.
Robert Colebunders received grants from Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, MSD, and Tibotec.
Nathalie De Castro received travel sponsorship from Janssen-Cilag, Abbott, ViiV Healthcare, Gilead Sciences and Merck and Co.
Margaret Johnson received funding for research grants and speaker's fees from Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, ViiV Healthcare, Merck Sharp & Dohme.
Andrew Carr has received research funding, consultancy fees, lecture sponsorships from, or has served on advisory boards for the following companies: Abbott, Baxter, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck, Pfizer, Roche, and Tibotec.
References
- 1.Hansen A-BE, Gerstoft J, Kronborg G, Larsen CS, Pedersen C, Pedersen G, Obel N. Incidence of low and high-energy fractures in persons with and without HIV infection: a Danish population-based cohort study. AIDS. 2012 Jan 28;26(3):285–93. doi: 10.1097/QAD.0b013e32834ed8a7. [DOI] [PubMed] [Google Scholar]
- 2.Dolan SE, Kanter JR, Grinspoon S. Longitudinal analysis of bone density in human immunodeficiency virus-infected women. Journal of Clinical Endocrinology & Metabolism. 2006 Aug;91(8):2938–45. doi: 10.1210/jc.2006-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yin MT, McMahon DJ, Ferris DC, Zhang CA, Shu A, Staron R, Colon I, Laurence J, Dobkin JF, Hammer SM, Shane E. Low bone mass and high bone turnover in postmenopausal human immunodeficiency virus-infected women. Journal of Clinical Endocrinology & Metabolism. 2010 Feb;95(2):620–9. doi: 10.1210/jc.2009-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnsten JH, Freeman R, Howard AA, Floris-Moore M, Lo Y, Klein RS. Decreased bone mineral density and increased fracture risk in aging men with or at risk for HIV infection. AIDS. 2007 Mar 12;21(5):617–23. doi: 10.1097/QAD.0b013e3280148c05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sims NA, Gooi JH. Bone remodeling: Multiple cellular interactions required for coupling of bone formation and resorption. Semin Cell Dev Biol. 2008 Oct;19(5):444–51. doi: 10.1016/j.semcdb.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 6.Ding C, Parameswaran V, Udayan R, Burgess J, Jones G. Circulating levels of inflammatory markers predict change in bone mineral density and resorption in older adults: a longitudinal study. J Clin Endocrinol Metab. 2008 May;93(5):1952–8. doi: 10.1210/jc.2007-2325. [DOI] [PubMed] [Google Scholar]
- 7.Aukrust P, Haug CJ, Ueland T, Lien E, Muller F, Espevik T, Bollerslev J, Froland SS. Decreased bone formative and enhanced resorptive markers in human immunodeficiency virus infection: indication of normalization of the bone-remodeling process during highly active antiretroviral therapy. J Clin Endocrinol Metab. 1999 Jan;84(1):145–50. doi: 10.1210/jcem.84.1.5417. [DOI] [PubMed] [Google Scholar]
- 8.Hernandez Quero J, Ortego Centeno N, Munoz-Torres M, Martinez Perez MA, Higuera Torres-Puchol JM. Alterations in bone turnover in HIV-positive patients. Infection. 1993 Jul-Aug;21(4):220–2. doi: 10.1007/BF01728893. [DOI] [PubMed] [Google Scholar]
- 9.Serrano S, Marinoso ML, Soriano JC, Rubies-Prat J, Aubia J, Coll J, Bosch J, Del Rio L, Vila J, Goday A, et al. Bone remodelling in human immunodeficiency virus-1-infected patients. A histomorphometric study. Bone. 1995 Feb;16(2):185–91. doi: 10.1016/8756-3282(94)00028-x. [DOI] [PubMed] [Google Scholar]
- 10.Yin MT, Shane E. Low bone-mineral density in patients with HIV: pathogenesis and clinical significance. Curr Opin Endocrinol Diabetes. 2006 Dec 1;13(6):497–502. doi: 10.1097/MED.0b013e3280109b6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stellbrink H-J, Orkin C, Arribas JR, Compston J, Gerstoft J, Van Wijngaerden E, Lazzarin A, Rizzardini G, Sprenger HG, Lambert J, Sture G, Leather D, Hughes S, Zucchi P, Pearce H, Group AS. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clinical Infectious Diseases. 2010 Oct 15;51(8):963–72. doi: 10.1086/656417. [DOI] [PubMed] [Google Scholar]
- 12.van Vonderen MG, Mallon P, Murray B, Doran P, Agtmael Mv, Danner S, Lips P, Reiss P, Group fMS. Changes in Bone Biomarkers in ARV-naïve HIV+ Men Randomized to NVP/LPV/r or AZT/3TC/LPV/r Help Explain Limited Loss of Bone Mineral Density over the First 12 Months after ART Initiation 18th Conference on Retroviruses and Opportunistic Infections (CROI); Boston. February 27-March 2, 2011. [Google Scholar]
- 13.The SMART Study Group CD4+ guided antiretroviral treatment interruption: Primary results of the SMART study. New England Journal of Medicine. 2006;355:2283–96. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 14.Grund B, Peng G, Gibert C, Hoy J, Isaksson R, Shlay J, Martinez E, Reiss P, Visnegarwala F, Carr A. Sub ISBC. Continuous antiretroviral therapy decreases bone mineral density. AIDS. 2009 Jul 31;23(12):1519–29. doi: 10.1097/QAD.0b013e32832c1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez E, Visnegarwala F, Grund B, Thomas A, Gibert C, Shlay J, Drummond F, Pearce D, Edwards S, Reiss P, El-Sadr W, Carr A, Grp ISS. The effects of intermittent, CD4-guided antiretroviral therapy on body composition and metabolic parameters. AIDS. 2010 Jan 28;24(3):353–63. doi: 10.1097/QAD.0b013e3283333666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grund B, Sabin C. Analysis of biomarker data: logs, odds ratios, and receiver operating characteristic curves. Curr Opin HIV AIDS. 2010 Nov;5(6):473–9. doi: 10.1097/COH.0b013e32833ed742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrell FE. Section 9.8.1. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. Springer; New York: 2001. [Google Scholar]
- 18.Team RDC. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2012. [Google Scholar]
- 19.Jabbar S, Drury J, Fordham JN, Datta HK, Francis RM, Tuck SP. Osteoprotegerin, RANKL and bone turnover in postmenopausal osteoporosis. Journal of Clinical Pathology. 2011 Apr;64(4):354–7. doi: 10.1136/jcp.2010.086595. [DOI] [PubMed] [Google Scholar]
- 20.Brown TT, Ross AC, Storer N, Labbato D, McComsey GA. Bone turnover, osteoprotegerin/RANKL and inflammation with antiretroviral initiation: tenofovir versus non-tenofovir regimens. Antiviral Therapy. 2011;16(7):1063–72. doi: 10.3851/IMP1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abitbol V, Roux C, Chaussade S, Guillemant S, Kolta S, Dougados M, Couturier D, Amor B. Metabolic bone assessment in patients with inflammatory bowel disease. Gastroenterology. 1995 Feb;108(2):417–22. doi: 10.1016/0016-5085(95)90068-3. [DOI] [PubMed] [Google Scholar]
- 22.Schett G, David JP. The multiple faces of autoimmune-mediated bone loss. Nat Rev Endocrinol. 2010 Dec;6(12):698–706. doi: 10.1038/nrendo.2010.190. [DOI] [PubMed] [Google Scholar]
- 23.Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, Capparelli C, Li J, Elliott R, McCabe S, Wong T, Campagnuolo G, Moran E, Bogoch ER, Van G, Nguyen LT, Ohashi PS, Lacey DL, Fish E, Boyle WJ, Penninger JM. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999 Nov 18;402(6759):304–9. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- 24.Clowes JA, Riggs BL, Khosla S. The role of the immune system in the pathophysiology of osteoporosis. Immunol Rev. 2005 Dec;208:207–27. doi: 10.1111/j.0105-2896.2005.00334.x. [DOI] [PubMed] [Google Scholar]
- 25.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997 Apr 18;89(2):309–19. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 26.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998 Apr 17;93(2):165–76. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 27.Ofotokun I, Weitzmann N, Vunnava A, Sheth A, Villinger F, Zhou J, al E. HAART-induced Immune Reconstitution: A driving force behind bone resorption in HIV/AIDS. 18th Conference on Retroviruses and Opportunistic Infections (CROI); Boston, USA. Feb, 2011. [Google Scholar]
- 28.Ofotokun I, McIntosh E, Weitzmann MN. HIV: inflammation and bone. Current HIV/AIDS Reports. 2012 Mar;9(1):16–25. doi: 10.1007/s11904-011-0099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]