Summary
Streptococcus agalactiae (Group B Streptococcus, GBS) is a frequent commensal organism of the vaginal tract of healthy women. However, GBS can transition to a pathogen in susceptible hosts, but host and microbial factors that contribute to this conversion are not well understood. GBS CovR/S (CsrR/S) is a two component regulatory system that regulates key virulence elements including adherence and toxin production. We performed global transcription profiling of human vaginal epithelial cells exposed to WT, CovR deficient, and toxin deficient strains, and observed that insufficient regulation by CovR and subsequent increased toxin production results in a drastic increase in host inflammatory responses, particularly in cytokine signaling pathways promoted by IL-8 and CXCL2. Additionally, we observed that CovR regulation impacts epithelial cell attachment and intracellular invasion. In our mouse model of GBS vaginal colonization, we further demonstrated that CovR regulation promotes vaginal persistence, as infection with a CovR deficient strain resulted in a heightened host immune response as measured by cytokine production and neutrophil activation. Using CXCr2 KO mice, we determined that this immune alteration occurs, at least in part, via signaling through the CXCL2 receptor. Taken together, we conclude that CovR is an important regulator of GBS vaginal colonization and loss of this regulatory function may contribute to the inflammatory havoc seen during the course of infection.
Keywords: Streptococcus agalactiae, transcription, regulation, two component system, vaginal tract
Introduction
Streptococcus agalactiae (Group B Streptococcus, GBS) is an encapsulated Gram-positive bacterium that commensally colonizes the lower gastro-intestinal tract, and in females, the lower reproductive tract, of 20–30% of healthy adults (Doran et al., 2004). However, in immune compromised individuals, such as neonates, pregnant women, and the elderly, GBS may transition to an invasive pathogen, resulting in pneumonia, sepsis, urinary tract infections, and meningitis (Maisey et al., 2008). Despite currently recommended intrapartum antibiotic prophylaxis for GBS-positive mothers, GBS remains the leading cause of early-onset neonatal sepsis (Verani et al., 2010). Whether in utero or during labor, neonatal exposure to GBS requires survival, transversal through a number of host environments and barriers, immune evasion, and in the case of meningitis, crossing of the highly regulated blood-brain barrier (Maisey et al., 2008). Upstream of these virulent interactions within the neonate, is the initial commensal colonization of the maternal vaginal tract.
Vaginal colonization by GBS is believed to be somewhat transient, and likely dependent on vaginal pH, normal flora, pregnancy, and estrous cycle, among many other constituents. Factors that favor the persistence of GBS in this complex biological niche are not well understood. GBS biofilm formation has been demonstrated in simulated vaginal fluids, with bacterial survival and growth improving as pH approaches neutral (Borges et al., 2012, Ho et al., 2012). However, optimal pH for GBS biofilm formation is unclear, as one group reported biofilm production increased as pH rose from 4.2 to 6.5 (Borges et al., 2012), and another group, that GBS biofilm formation is enhanced at pH 4.5 compared to pH 7.0 (Ho et al., 2012). Although so much is still unknown, a few GBS determinants have been shown to contribute to vaginal cell adherence and colonization, including surface Serine Rich Repeat (Srr) proteins, Srr-1 and Srr-2, and pili protein, PilA of GBS Pilus Island (PI)-2a (Sheen et al., 2011). Interestingly, it has been recently observed that GBS PI-1 does not mediate attachment to vaginal cells in vitro (Jiang et al., 2012). Others have also begun exploring bacteriocin-like inhibitory substances produced by native vaginal species that negatively impact GBS growth (Ruiz et al., 2012). Nevertheless, the molecular mechanisms governing GBS vaginal persistence and competition with normal microbiota remain to be elucidated.
GBS has several regulatory systems in place that may control the transition of the organism from a commensal niche (e.g. vaginal tract) to invasive niches (e.g. blood, lung, brain, and other organs). Bacteria respond to changes in environmental stimuli using two-component systems (TCS) to alter gene expression. TCS typically consist of a membrane-associated histidine kinase and a cytoplasmic transcriptional regulator (Beier et al., 2006). In GBS, a TCS consisting of a sensor histidine kinase CovS (Cov, control of virulence), also known as CsrS (Csr, capsule synthesis regulator), and a response regulator CovR (CsrR) down regulates the expression of 27 genes and activates the expression of 3 genes in all GBS strains examined (Lamy et al., 2004, Jiang et al., 2008, Lembo et al., 2010). The conserved regulatory functions of CovR in GBS include repression of fibrinogen-binding proteins A and B (FbsA, FbsB), genes involved in iron uptake, and in particular, repression of cylE, the gene encoding GBS toxin β-hemolysin/cytolysin (β-H/C) (Pritzlaff et al., 2001), as CovR-deficient strains exhibit increased hemolytic activity (Lamy et al., 2004, Rajagopal et al., 2006, Jiang et al., 2008, Lembo et al., 2010). The β-H/C toxin is a well-established virulence factor known to promote GBS cellular interactions, and to provoke host innate immune responses in human epithelial and endothelial cell models by activating transcription of host genes encoding the chemokines IL-8, CXCL1, and CXCL2 for neutrophil recruitment (Doran et al., 2002, Doran et al., 2003). In vivo studies have further indicated that the β-H/C contributes to the development and severity of meningitis (Doran et al., 2003), pneumonia (Hensler et al., 2005), arthritis (Puliti et al., 2000), and sepsis (Ring et al., 2002). The cytotoxic and proinflammatory properties of the β-H/C toxin work to the detriment of the host. The CovR regulatory system itself has also been shown to contribute to disease progression (Lembo et al., 2010). GBS CovR/S mutation has been observed in clinical isolates (Sendi et al., 2009) and CovR/S mutations are frequently observed in Streptococcus pyogenes (Group A Streptococcus) (Engleberg et al., 2001, Sumby et al., 2006).
We hypothesized that the CovR/S TCS plays an important role in modulating GBS colonization and virulence, and that loss of CovR regulation in the vaginal environment will impact host response and GBS persistence. In this study, we examine for the first time the acute response of vaginal epithelium to GBS using microarray, real-time RT-PCR, and protein analysis. We show that human vaginal epithelial cells respond to GBS with the increased production of genes involved in the immune response and tissue remodeling compared to a native vaginal bacterium, Lactobacillus crispatus. Infection with GBS deficient in CovR induces a much more aggressive inflammatory response than does WT GBS, which is at least partially due to increased β-H/C production. Differentially induced genes were primarily proinflammatory chemokines such as those involved in neutrophil activation and recruitment. Experiments with isogenic GBS mutants lacking CovR, β-H/C, or both factors demonstrate that CovR regulated factors, independent of the β-H/C toxin, contribute to GBS adherence and invasion. Using an in vivo model of GBS vaginal colonization, we demonstrate that functional CovR regulation dampens cytokine production and promotes bacterial persistence in the vaginal tract. Our studies suggest that the host vaginal epithelium plays an active role in immune surveillance and that GBS precisely modulates gene expression to promote survival and colonization.
Experimental Procedures
Bacterial strains and growth conditions
Streptococcus agalactiae (GBS) strains were grown in Todd-Hewitt broth (THB) (Hardy Diagnostics) at 37°C. The wild-type (WT) clinical isolates used in this study are A909 (serotype Ia) (Madoff et al., 1991) and COH1 (serotype III) (Wessels et al., 1991). Both A909 and COH1 ΔcylE mutants were constructed previously (Pritzlaff et al., 2001), as were the A909ΔcovR and ΔcovR/ΔcylE mutants (Rajagopal et al., 2006, Lembo et al., 2010). The COH1ΔcovR was derived using methods described (Rajagopal et al., 2006). The A909ΔfbsB/Δ0956 strain was constructed using methods described (Shelver et al., 2003), where the gene encoding kanamycin (Ωkm-2) was used for allelic replacement. The complemented covR strain (pcovR) was generated using methods as described previously (Rajagopal et al., 2003). Briefly, the gene encoding CovR was amplified using primers 5’GCGCGGAGCTCTTGTTAAGTAAAGAATAAG 3’ and 5’GCGCGAGGATCCTTTATTTTTCACGAATCAC 3’ and the PCR products were digested and ligated into the complementation vector pDC123 (Chaffin et al., 1998) downstream to the tetracycline promoter, Ptet followed by electroporation into the GBS A909ΔcovR mutant. When necessary, mutants were maintained and grown in antibiotics at the following concentrations: spectinomycin (300 µg/mL), chloramphenicol (2 µg/mL), and kanamycin (500 µg/mL). Lactobacillus crispatus (LC) (Strain # 33820, ATCC) was grown in Lactobacilli MRS broth (BD Biosciences) at 37°C.
Cell lines
Immortalized human vaginal epithelial cell (HVEC) line, VK2/E6E7, and ectocervical epithelial cell line, Ect1/E6E7, were obtained from the American Type Culture Collection (ATCC CRL-2616 and ATCC CRL-2614 respectively) (Fichorova et al., 1997). Passages 5–20 were used for all cell assays. Cells were maintained at 37°C in a 5% CO2 atmosphere in keratinocyte serum-free medium (KSFM) (Invitrogen) with 67.419 pg/mL human recombinant epidermal growth factor and 65 µg/mL bovine pituitary extract as described previously (Sheen et al., 2011).
Vaginal cell infection and microarray analysis
HVEC were grown to confluency in 24 well tissue culture treated plates and washed prior to bacterial exposure. Bacteria were grown to mid-log phase and then added to cells at a multiplicity of infection (MOI) of 50. After an infection period of 4 hours, total RNA was extracted (Macherey-Nagel) and microarray analysis (HumanHT-12_v4, Illumina) was performed at BIOGEM at the University of California, San Diego. HVEC microarrays were performed with two independent biological replicates of each strain (A909, ΔcovR, and ΔcylE) and media only controls, and one replicate of L. crispatus. Heatmap analysis was performed using Cluster 3.0 and TreeView (Eisen Laboratories), and the Venn Diagram calculated with the assistance of Area-Proportional Venn Diagram (BioInfoRx).
In vitro cell assays
GBS adherence and invasion assays of HVEC were conducted as described previously (Sheen et al., 2011) with minor modifications. Concisely, cells were grown to confluency in 24 well tissue culture treated plates and washed prior to bacterial addition. Bacteria were grown to mid-log phase and added at an MOI of 1 for adherence assays. After 30 minutes of incubation, cells were washed 6 times with PBS and lysed by adding trypsin-EDTA and Triton X-100. Lysate was serially diluted and plated on THB agar plates to enumerate bacterial cfu. Total adherent cfu was calculated as (total cfu recovered/total cfu of original inoculum)×100%. To quantify intracellular bacteria, cells were incubated at MOI of 10 for 2 hours, monolayers washed, treated with antibiotics, and incubated for an additional 2 hours (Sheen et al., 2011). Cells were washed 3 times with PBS, lysed as described above, and intracellular GBS determined by serial dilution plating and total intracellular bacteria quantified as above.
RT-qPCR and ELISA
For analysis of gene expression induction, HVEC were grown to confluency in 24 well tissue culture-treated plates and washed prior to bacterial exposure. Bacteria were grown to mid-log phase and added at an MOI of 10 and incubated for 4 hours. Cells were then lysed, total RNA extracted (Macherey-Nagel), and qPCR performed (Quanta Biosciences). Primers and primer efficiencies for IL-8, CXCL1, CXCL2, CCL20 and GAPDH were utilized as previously described (van Sorge et al., 2008). For ELISA assays, HVEC were infected as described above with several modifications. Bacteria were added at an MOI of 10–50 as listed in figure legends, and cells were incubated with bacteria for 1.5–2 hours as indicated. After initial incubation, cells were washed with once with PBS, and fresh KSFM added containing 5 µg/mL penicillin and 50 µg/mL gentamicin. Cells were incubated an additional 3.5–4 hours in the presence of antibiotics and cell supernatants were analyzed for chemokine secretion using human IL-8 (R&D Systems), CXCL1 (R&D Systems), and CXCL2 (Antigenix America) ELISA kits according to manufacturer’s instructions.
In vitro HVEC viability assay
To determine viability of HVEC infected with GBS in vitro, adherence and invasion assays were performed as described above. Afterwards, supernatant was aspirated and placed into microfuge tubes. To dislodge adherent cells, 100 µL of 0.25% trypsin-EDTA (Gibco) was added to each well and incubated at 37°C for 5 minutes. Trypsin activity was halted by adding 200 µL of DMEM F12 (Cellgro) with 10% fetal bovine serum (Invitrogen). Cells were gently removed from wells by pipetting up and down several times and suspended cells were added to supernatant. To distinguish live cells from dead cells (with permeable membranes), 0.4% Trypan Blue (Life Technologies) was added at 1:10, and live cells (unstained) and dead cells (stained) were quantified using a hemocytometer. Percent of live cells per sample was calculated as (number of live cells/total cells counted)×100%. Two grids per sample were counted and results averaged.
In vivo mouse model of GBS vaginal colonization
All mouse work was approved by the Office of Lab Animal Care at San Diego State University and conducted under accepted veterinary standards. Female CD1 and BALB/c mice (8–16 weeks old) were obtained from Charles River Laboratories and used for colonization assays adapted from previous work (Sheen et al., 2011). Breeding pairs of CXCr2 (CXCL2 receptor) knock out (KO) mice (formerly IL8r KO mice), were originally purchased (C.129S2(B6)-Cxcr2tm1Mwm/J, Jackson Laboratories). The mutation was crossed onto a BALB/c background prior to being deposited at Jackson Laboratories. We established a homozygous×homozygous breeding colony at the UCSD VA Hospital using mice that were maintained on water containing co-trimoxazole (200 µg/mL sulfamethoxazole and 40 µg/mL trimethoprim). For the 17 week old females used in this study, antimicrobial treatment was terminated 48 hours prior to inoculation with GBS. To synchronize estrus and promote bacterial colonization (Furr et al., 1989, Cheng et al., 2005), mice were injected intraperitoneally with 0.5mg 17β-estradiol suspended in sesame oil (Sigma) 24 hours prior to inoculation. Mice were inoculated with ~1×107 cfu (in 10 µL PBS) GBS in the vaginal lumen. Immediately prior to inoculation, vaginal lavage was performed by pipetting the lumen with 20 µL of PBS several times to collect cells and cytokines as described elsewhere (Sonoda et al., 1998, Caligioni, 2009). On successive days, the vaginal lumen of each mouse was first lavaged for cytokine analysis and then swabbed with ultrafine calcium alginate-tipped swabs. Bacterial load was determined by serial dilution plating of swab samples. WT or mutant GBS strains were identified as mauve or light pink-pigmented colonies on CHROMagar Strep B agar (DRG International Inc.) (Poisson et al., 2011). For tissue collection, mice were sacrificed using CO2 asphyxiation and reproductive tracts excised from mid-uterine horn to just proximal of the vulva. Tissues were fixed in 4% paraformaldehyde and embedded in paraffin. ELISA assays were performed on vaginal lavages for KC (R&D Systems), MIP-2 (R&D Systems) and IL-1β (eBiosciences) as described by manufacturer.
Myeloperoxidase Assay
Neutrophil recruitment and activation was determined by quantifying the neutrophil enzyme, myeloperoxidase (MPO), as described previously (Banerjee et al., 2011), but modified for murine vaginal samples. Cells were collected from swabs as described above and suspended in 100 µL PBS. 50 µL of the swab sample was added to 100 µL of 0.05% hexadecyltrimethylammonium bromide (HTAB) buffer and MPO was released from neutrophils by bead-beating with 1.0 mm diameter zirconia beads (BioSpec) for 1 minute using a Mini BeadBeater (BioSpec). Samples were centrifuged at 13,000 rpm for 15 minutes at 4°C, and 10 µL of supernatant was added to 190 µL phosphate buffer containing 0.167 mg/mL ο-dianisidine dihydrochloride and 0.0005% hydrogen peroxide. Absorbance was measured at 450 nm in 96-well flat bottom plates and MPO activity calculated as milliunits per volume of homogenate supernatant (mU/mL of supernatant).
Microscopy
Vaginal cell monolayers were propagated on glass cover slips within 24 well plates. Following a standard adherence assay (MOI = 100), monolayers were washed 6 times with DPBS and the cover slips were then removed from the trays. Cover slips were air-dried and heat fixed then subjected to a standard Gram stain protocol as described previously (Sheen et al., 2011). Paraffin embedded reproductive tracts were sectioned on a Leica RM 2125 microtome at 5 µm and stained with a standard H&E staining protocol. All images were taken on a Zeiss upright microscope with attached Axiocam Icc3 camera at indicated magnification.
Statistical Analysis
GraphPad Prism version 5.04 was used for statistical analyses. Differences in in vitro assays including adherence, invasion, qPCR, ELISA and cell viability were calculated using unpaired Student’s t test analysis. Differences in bacterial loads recovered from mouse vaginal tracts were calculated using Kruskal-Wallis test (nonparametric) with Dunn’s multiple comparisons post-test, or Fisher’s exact test analysis as indicated in figure legends. Differences in cytokine and MPO levels from vaginal lavage were calculated using either Mann-Whitney test (nonparametric) or Fisher’s exact test analysis as indicated in figure legends. Statistical significance was determined at a p<0.05.
Results
Differential gene expression profile of vaginal epithelium induced by GBS
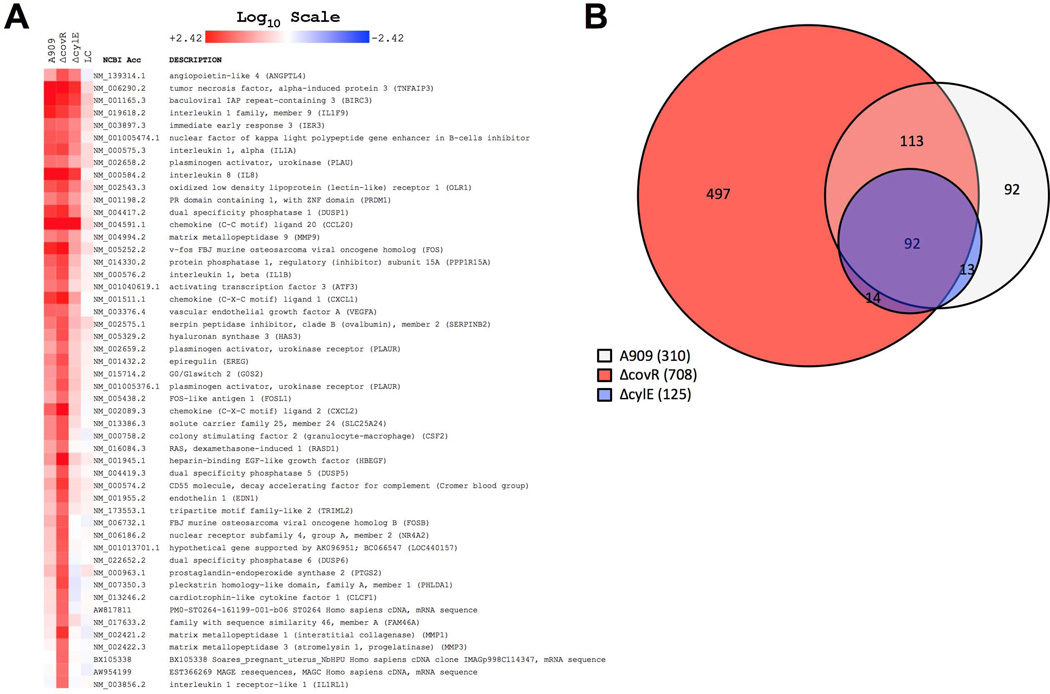
To determine the response of vaginal epithelium to GBS colonization, we performed global transcriptional profiling on human vaginal epithelial cells (HVEC) during infection with WT GBS (strain A909, serotype Ia) or isogeneic ΔcovR and ΔcylE mutant strains. As a control, we exposed HVEC to a nonpathogenic vaginal organism, L. crispatus (Wira et al., 2011). As can be expected, exposure to the native flora species, L. crispatus, did not induce an inflammatory response compared to media alone (Fig. 1A and Suppl. Table 1). However, when HVEC were infected with WT GBS, numerous immune pathways were activated including proinflammatory cytokines IL1-α and IL1-β, and chemokines involved in leukocyte recruitment and activation such as CCL20, IL8, CXCL1, and CXCL2 (Fig. 1A and Suppl. Table 1). A similar global pattern of gene induction was observed during infection with another WT (serotype V) GBS strain (data not shown). Of particular interest, infection with the ΔcovR mutant induced higher levels of expression of several of these genes, including IL-8 (2-fold) and CXCL2 (5-fold) when compared to WT. GBS β-H/C has previously been shown to activate neutrophil recruitment in other models of infection (Doran et al., 2003), and here we show that infection with the ΔcylE mutant resulted in lower induction of genes required for leukocyte recruitment and activation as a whole, including 4-fold lower transcription of IL-8 and CXCL2, indicating that β-H/C mediates similar responses in the vagina. Moreover, infection with the ΔcovR mutant resulted in the upregulation of 708 genes (>2-fold) in vaginal epithelial cells as compared to 310 or 125 genes induced by WT or ΔcylE infection respectively (Fig. 1B). These results illustrate the impact of β-H/C alone in the vaginal tract by comparing effects of WT and ΔcylE, and demonstrate a tremendous shift in vaginal epithelium response to GBS when CovR regulation is removed.
Figure 1. Microarray of gene expression levels in HVEC upon infection with A909, ΔcovR, ΔcylE or Lactobacillus crispatus (LC).
(A) Each column represents the mean of two biological replicates (one replicate for LC) of a microarray experiment. Shown are the top 50 genes upregulated by the ΔcovR mutant. Red and blue coloring indicates induced or down regulated genes respectively. Values expressed are on a Log10 scale and clustering was performed as described in Experimental Procedures. (B) Venn Diagram of all genes upregulated >2 fold. Circumferences and overlap were calculated as described in Experimental Procedures.
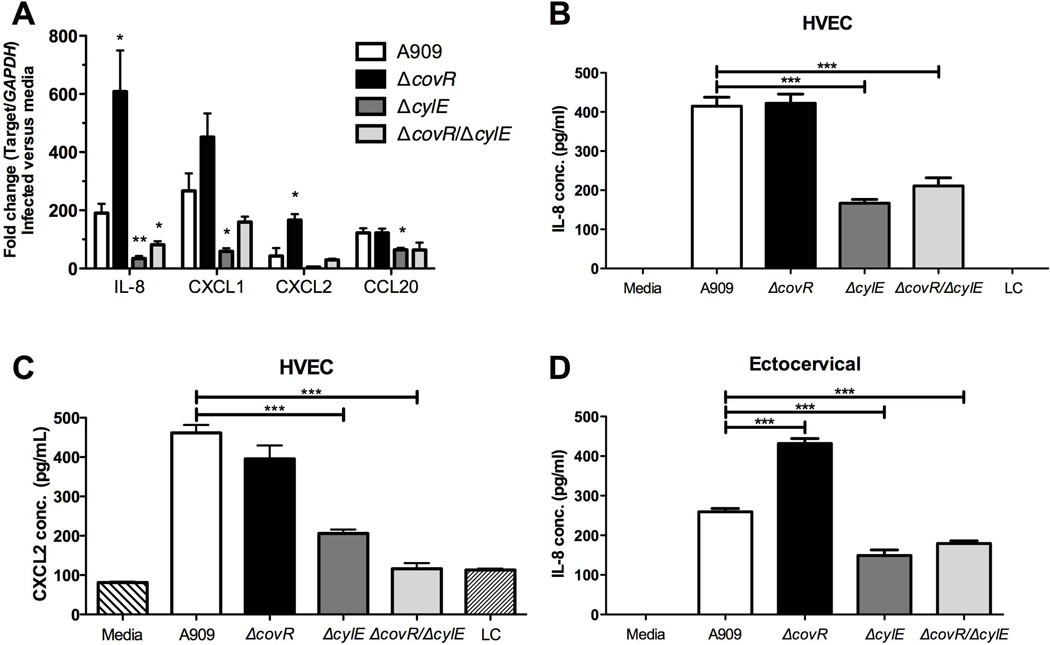
To verify the microarray data in vitro, we examined differences in transcript and protein levels of highly induced genes involved in neutrophil recruitment. Additionally, we examined the effect of infection with a ΔcovR/ΔcylE double mutant to determine the proportion of increased inflammatory response contributed by increased β-H/C production in ΔcovR. We observed that infection with the ΔcovR mutant resulted in increased transcription of IL-8 and CXCL2 compared to WT infection, while transcription of CXCL1 and CCL20 was not significantly different. When comparing the response to WT and ΔcylE infection, loss of β-H/C reduced chemokine transcription of IL-8, CXCL1, CXCL2, and CCL20. Interestingly, transcription of all chemokines was also down regulated in the ΔcovR/ΔcylE mutant compared to WT, highlighting the inflammatory role of β-H/C in human vaginal cells.
We further analyzed protein secretion of the most potent and differentially induced neutrophil chemokines during infection with WT, ΔcovR, ΔcylE, and ΔcovR/ΔcylE mutant stains. Production of IL-8 and CXCL2 protein was drastically increased in cells infected with A909 WT and ΔcovR compared to infection with commensal bacterium, L. crispatus or to the media control (Fig. 2B,C). As was observed at the transcriptional level, IL-8 and CXCL2 secretion was significantly lower in cells infected with ΔcylE and ΔcovR/ΔcylE strains as compared to WT (Fig. 2B,C). Similar results were observed for CXCL1 production (Suppl. Fig. 1A). We observed evidence of toxicity in HVEC during infection with the hyper-hemolytic A909 ΔcovR strain at the length of time required to detect protein production (data not shown), which may explain why protein levels of IL-8 and CXCL2 are not higher during A909 ΔcovR infection compared to that observed during WT A909 infection. However, differential IL-8 secretion was detected during infection of ectocervical cells (Fig. 2D). Furthermore, we observed that infection of HVEC with a less hemolytic GBS strain, COH1, a serotype III clinical isolate which is a sequence type (ST)-17 strain, and its isogenic ΔcovR mutant resulted in increased protein secretion of IL-8, CXCL1 and CXCL2 after exposure to the COH1 ΔcovR mutant strain compared to WT COH1 (Suppl. Fig. 1B).
Figure 2. mRNA expression of IL-8, CCL20, CXCL1 and CXCL2 and protein production of IL-8 in HVEC and ectocervical cells upon infection with WT and mutant strains.
(A) mRNA expression levels of IL-8, CCL20, CXCL1 and CXCL2 in HVEC infected with either A909, ΔcovR, ΔcylE or ΔcovR/ΔcylE using quantitative RT-PCR. Fold change was calculated using GAPDH. Data is one representative experiment of at least 3 independent experiments performed in 5 replicates. Protein expression of IL-8 (B) or CXCL2 (C) in HVEC supernatants 5 hours post-infection with A909, ΔcovR, ΔcylE, ΔcovR/ΔcylE or L. crispatus (LC) at MOI of 50. (D) Protein expression of IL-8 in ectocervical supernatants 5 hours post-infection with A909, ΔcovR, ΔcylE or ΔcovR/ΔcylE at MOI of 50. Experiments were performed at least two times with at least four replicates, and one representative experiment is shown. Data was analyzed by unpaired Student’s t test. * p<0.05, ** p<0.01, *** p<0.001.
CovR moderates adherence and invasion in vaginal epithelial cells
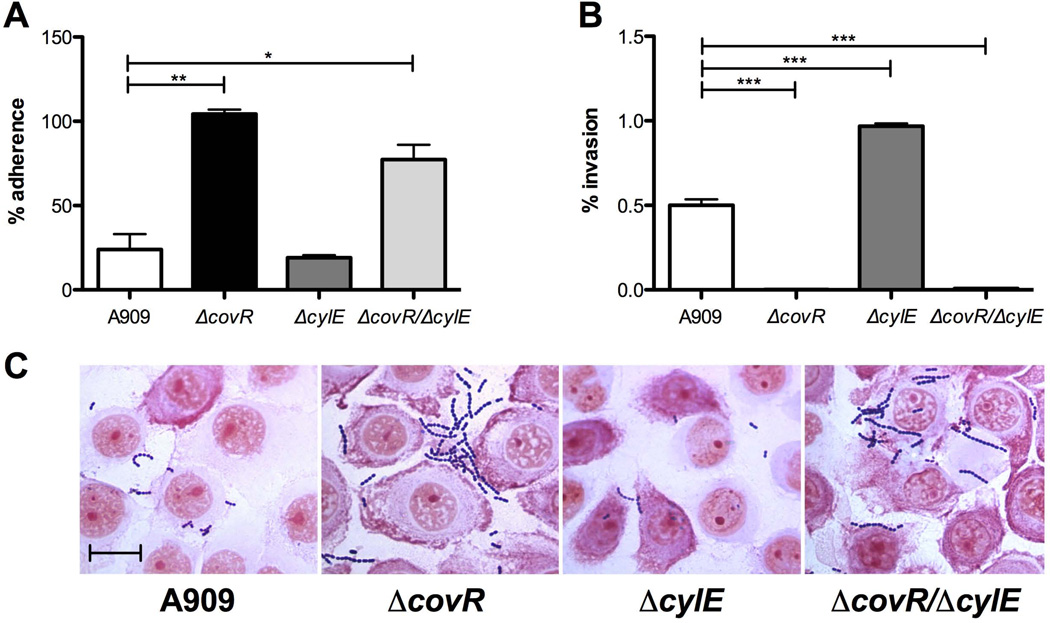
Several GBS WT strains, including A909, have already been shown to readily attach to HVEC in vitro (Sheen et al., 2011, Jiang et al., 2012). To determine the effect of CovR regulation on GBS interaction with vaginal epithelium, we characterized attachment and invasion of WT, ΔcovR, ΔcylE, and ΔcovR/ΔcylE strains in HVEC using standard methods as previously described (Doran et al., 2005, Sheen et al., 2011). After 30 minutes of incubation, and multiple washes to remove nonspecific interactions, adherent GBS was quantified from monolayer lysates. We observed that both ΔcovR (p = 0.0068) and ΔcovR/ΔcylE (p = 0.0136) mutants adhered significantly more than WT, with ΔcovR approaching 100% adherence of original inoculum (Fig. 3A). Adherence of the ΔcylE mutant did not significantly differ from that observed for WT GBS. Adherent GBS in association with vaginal epithelium were visualized by microscopy following multiple wash steps and Gram staining (Fig. 3C). Additionally, we quantified viable intracellular bacteria from cell lysates following a 2 hour incubation period and a 2 hour antibiotic treatment to kill any extracellular bacteria. Our data show that, when compared to WT, ΔcovR and ΔcovR/ΔcylE mutants exhibited reduced recovery of intracellular GBS (Fig. 3B). We recovered a relatively low level of intracellular bacteria (0.5% of the initial inoculum of WT GBS), but we were able to recover only 0.002% of the ΔcovR mutant. Thus approximately 0.021% and 0.000016% of the adherent WT and ΔcovR GBS, respectively, had invaded the intracellular compartment. Similar results were observed when CovR was deleted from serotype III GBS strain, COH1 (Suppl. Fig. 2A,B). Interestingly, the A909ΔcylE mutant had significantly more intracellular colony forming units than WT, indicating expression of β-H/C may decrease intracellular survival in vaginal epithelial cells, however, this effect was not seen in ΔcovR/ΔcylE, suggesting that additional genes regulated by CovR impact GBS intracellular survival.
Figure 3. GBS interaction with vaginal epithelium in vitro.
Adherence (A) and invasion (B) of GBS A909 and mutant strains to HVEC. Values are expressed as the total of cell-associated cfu (A) or total intracellular cfu (B) recovered compared to original inoculum. Assays were performed at an MOI of 1 (A) or MOI of 10 (B). (C) Gram-stain of HVEC infected with WT and mutant strains. Magnification = 1000×, scale bar = 20 µm. Experiments were repeated at least 3 times in triplicate and data from a representative experiment is shown. Data was analyzed by unpaired Student’s t test. * p<0.05, ** p<0.01, *** p<0.001.
To verify that the ΔcovR mutant phenotype could be complemented, we cloned the A909 covR sequence into a GBS expression vector and transformed this plasmid (pcovR) into the ΔcovR mutant as described in Experimental Procedures. GBS strains expressing more β-H/C typically produce more red pigment (Liu et al., 2004), such as our ΔcovR mutants, and we observed a loss of pigmentation when the ΔcovR mutant was complemented with pcovR (Suppl. Fig. 3C). Additionally the complemented strain exhibited WT levels of β-H/C activity (data not shown). In both adherence and invasion assays, the complemented pcovR strain differed significantly from the ΔcovR strain, exhibiting significantly lower adherence (Suppl. Fig. 3A) and significantly greater invasion (Suppl. Fig. 3B), approaching WT levels. Additionally, because we had observed toxicity during infection with the ΔcovR mutant at higher MOIs and longer incubation times, we sought to confirm that increased toxin production of ΔcovR was not compromising HVEC integrity during our invasion assays. As described in Experimental Procedures, we stained cells with Trypan Blue under the same conditions used in the invasion assay. We observed no difference in cell viability during incubation with WT or any of the mutant strains, and all treatment groups were >95% viable (Suppl. Fig. 3D).
Role of CovR in vaginal persistence in vivo
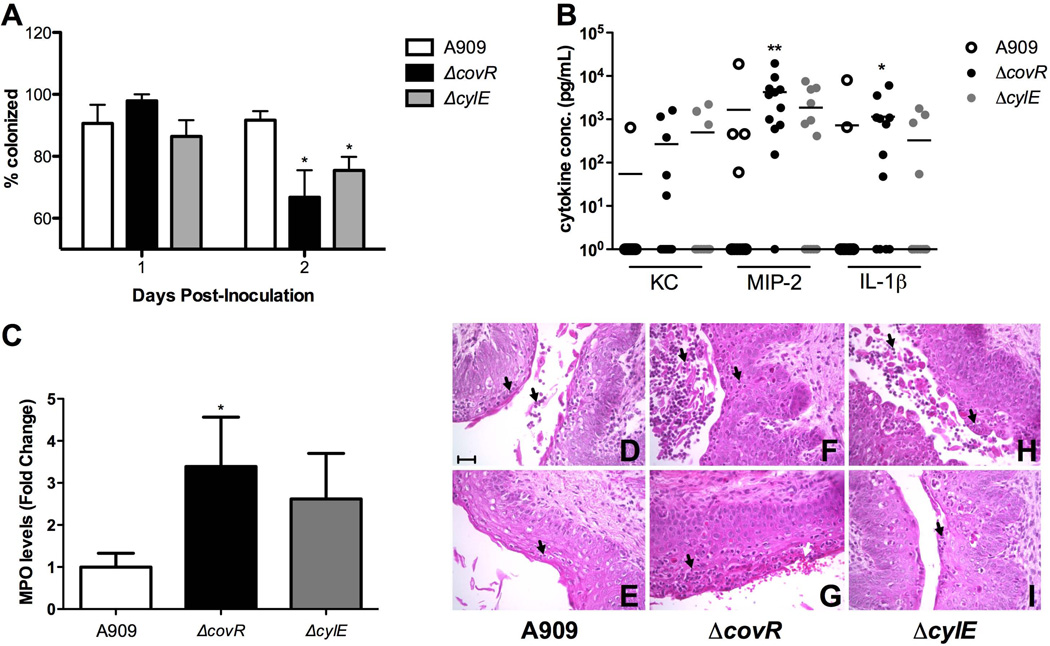
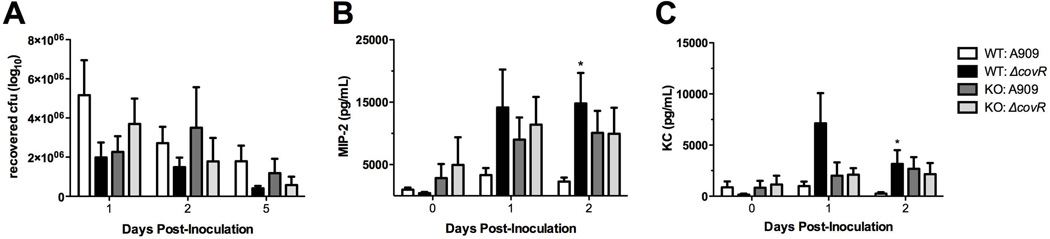
Our in vitro data indicates that CovR regulation alters both vaginal epithelial interactions (Fig. 3) as well as host immune response (Fig. 1, 2). To confirm these results in vivo, we used a mouse model of GBS vaginal colonization adapted from previous work by our lab (Sheen et al., 2011) and others (Cox et al., 1993, Cheng et al., 2005). In rodents, normal flora load and novel bacterial colonization ability appear to peak at estrus (Koiter et al., 1977, Larsen et al., 1977, Furr et al., 1989). We found this to be true in our model of GBS colonization (Suppl. Fig. 4A,B). Consequently, we treated 8-week old CD1 mice with 17β-estradiol one day prior to bacterial inoculation. We inserted ~1×107 cfu GBS into the vagina, and on successive days, the vaginal lumen was swabbed and recovered bacteria quantified on agar plates to determine changes in bacterial load over time. The ΔcovR mutant exhibited decreased persistence in the vaginal tract by day 2 post-inoculation, as significantly more mice treated with ΔcovR had cleared GBS when compared to WT (p = 0.0108) (Fig. 4A) in the combined results of 4 independent experiments. Similarly, a significant reduction was observed when analyzing the isogenic ΔcovR mutant in the COH1 background (Suppl. Fig. 2C). We also examined the colonization of other GBS mutants deficient in genes highly regulated by CovR in the A909 strain: ΔcylE, ΔcovR/ΔcylE, and a double mutant in fbsB (SAK_0955) and adjacent gene (SAK_0956) encoding a hypothetical protein. Although in all instances, the mean number of mutant bacterial cfu recovered from mice decreased during colonization compared to the WT strain over the course of 5 days, the differences were not significant largely due to the high level of variation observed in this model (Suppl. Fig 4C). However, at day 5 there was an 8.0-fold decrease in mean bacterial load recovered from mice inoculated with ΔcovR compared to only a 1.6-fold reduction in WT bacteria recovered.
Figure 4. CovR regulation affects host immune response in vivo soon after inoculation.
(A) Approximately 1×107 cfu GBS was inoculated into the vaginal lumen of 8 week old CD1 mice in 4 independent experiments and combined data at day 2 post inoculation is shown (n = 39 total per group). (B) KC, MIP-2 and IL-1β levels in vaginal lavage fluid from mice on day 2 post inoculation were quantified by ELISA (n = 12 per group). Lines represent mean cytokine concentration. (C) In three independent experiments, 8 week old CD1 mice were inoculated with 1×107 cfu GBS (n = 30 total per group), and neutrophils collected by swabbing the vagina at day 2 post-inoculation. MPO levels were quantified, fold changes of mutants normalized to WT, and combined data is shown. Mice that did not have detectable MPO levels were excluded. (D–I) Vaginal epithelium histology of CD1 mice inoculated as in (A) at day 2 post inoculation stained with H&E. Two representative images are shown per bacterial strain. (D,E) Mice inoculated with WT show some neutrophils present in the vaginal epithelium and lumen (black arrows). (F,G) Mice inoculated with ΔcovR show increased neutrophil infiltration (black arrows) and hemorrhaging (white arrow) compared to WT. (H,I) Mice inoculated with ΔcylE show variability in neutrophil infiltration (black arrows). Magnification = 400X, scale bar = 50 µm. Data was analyzed by Fisher’s exact test for colonization assays and ELISA’s, and Mann-Whitney test for MPO assays. * p<0.05, ** p<0.01.
Loss of CovR regulation heightens host immune response in vivo
Since we observed an increase in numerous innate immune factors in vaginal cells exposed to ΔcovR in vitro (Fig. 1, 2), as well as a decreased vaginal persistence (Fig. 4A), we sought to characterize host immune response in vivo. Vaginal lavage from CD1 mice was collected for cytokine analysis by gently pipetting PBS in the vaginal lumen multiple times as described (Sonoda et al., 1998) prior to GBS inoculation and then throughout GBS colonization. Cytokine levels of murine chemokines KC and MIP-2, as well as IL-1β, were measured by ELISA. We observed large variation in cytokine concentrations between individual mice, which has been seen previously (Sonoda et al., 1998). Even so, by day 2 post-inoculation, significantly more mice inoculated with ΔcovR secreted MIP-2 (11/12 vs. 4/12, p = 0.0094) and IL-1β (8/12 vs. 2/12, p = 0.0361) compared to WT (Fig. 4B). Additionally, more ΔcovR treated mice secreted chemokine KC than WT treated mice (5/12 vs. 1/12), but this difference was not significant.
Since MIP-2 and KC are both key chemoattractants involved in the recruitment of neutrophils to the vaginal epithelium (Sonoda et al., 1998, Hickey et al., 2012), we assessed neutrophil activation and influx into the vaginal lumen by measuring neutrophil enzyme myeloperoxidase (MPO) during GBS colonization. MPO serves as an effective indication of neutrophil infiltration (Bradley et al., 1982). We observed that mice inoculated with ΔcovR expressed significantly higher fold levels of MPO than mice treated with WT (Fig. 4C). To visualize neutrophil infiltration, we collected vaginal tissues 2 days post GBS inoculation for histopathologic analysis. Consistent with our previous results there were fewer mice colonized with ΔcovR at day 2 post inoculation compared to WT (data not shown). At this time point, neutrophils were present in most of the mice, however, mice treated with ΔcovR showed greater inflammation, epithelial rearrangement and hemorrhaging than WT and ΔcylE treated mice, with two representative images shown (Fig. 4D–I). Mice inoculated with ΔcylE exhibited more variation within the epithelium than either of the other groups (Fig. 4H–I), in combination with significantly fewer cfu recovered compared to WT (Fig. 4A), which may be explained by individual immune system differences between mice in response to a less virulent form of GBS.
The CXCL2 receptor contributes to host immune response in vivo
Thus far, we have demonstrated that infection with the ΔcovR mutant results in a heightened inflammatory response in vitro and in vivo. Moreover, we have observed a decreased persistence of the ΔcovR mutant in the vaginal environment compared to WT, and we hypothesized that enhanced immune response during infection with ΔcovR is responsible for this difference. Because neutrophil chemokines were differentially induced by the ΔcovR mutant, both in vitro and in vivo, we used mice that lack a receptor for these chemokines. We inoculated CXCr2 KO mice, formerly known as IL-8r KO mice, and control BALB/c mice with either WT GBS or the ΔcovR mutant as described in Experimental Procedures. Mice were swabbed and lavage fluid collected daily to enumerate bacterial load and cytokine production respectively. GBS persisted longer in BALB/c mice than in CD1 mice (data not shown), but by day 5, mean cfu values, although not significant, were distinctly different with fewer ΔcovR (4.1×105) recovered compared to WT (1.6×106) (Fig. 5A). However, we did not observe a difference in recovered bacteria between the WT and ΔcovR over time in CXCr2 KO mice (Fig. 5A). Our results revealed that, like CD1 mice, WT BALB/c exhibited significantly increased MIP-2 (p = 0.0372) and KC (p = 0.0328) levels in mice receiving ΔcovR as compared to WT by day 2 post-inoculation (Fig. 5B,C). At no time point examined did we observe significant differences in KC and MIP-2 levels in CXCr2 KO mice in either group (Fig. 5B,C). Furthermore, we did not observe neutrophils in vaginal lavage fluid of CXCr2 KO mice at any time point examined (Suppl. Fig. 5). In combination, this data suggests that increased host inflammatory response to ΔcovR occurs at least in part by signaling through the CXCL2 receptor.
Figure 5. Role of the CXCL2 receptor during GBS infection in vivo.
(A) Approximately 1×107 cfu GBS was inoculated into the vaginal lumen of 16–17 week old WT BALB/c and CXCr2 KO mice. GBS persistence was measured by swabbing the vagina and enumerating recovered bacteria as described in Experimental Procedures. For all groups, n = 10, or n = 9 for WT BALB/c mice inoculated with WT GBS. (B) MIP-2 and (C) KC levels in vaginal lavage from same mice as (A) measured by ELISA. Data was analyzed by Kruskal-Wallis test for persistence and Mann-Whitney test for cytokine production. * p<0.05.
Discussion
GBS poses a severe threat to newborn infants worldwide. GBS propagates through vertical transmission as 50–70% of infants born to colonized mothers become colonized (Baker et al., 2001). A better understanding of mechanisms important for GBS colonization of the vaginal tract, identification of host and bacterial factors contributing to colonization, and genetic and environmental stimuli that promote GBS colonization and subsequent transmission is essential. Through combining our in vitro and in vivo models, we have continued to identify host and microbe features that impact GBS persistence in the vaginal environment. Herein, we have demonstrated that the CovR/S regulatory system is necessary for limiting the expression of virulence factors during vaginal colonization, thereby reducing the host innate immune response to promote colonization.
GBS CovR/S has been shown to positively and negatively regulate a variety of genes, of which, cylE (β-H/C) is one of the most highly repressed (Lamy et al., 2004, Jiang et al., 2008, Lembo et al., 2010). Host global transcriptional profiling during infection with WT, ΔcovR, or ΔcylE GBS revealed the β-H/C toxin as a key mediator in provoking an acute inflammatory response in the vaginal epithelium, which was further confirmed by analysis of specific gene transcripts during infection with a ΔcovR/ΔcylE mutant. Most dramatic was the shift in gene induction profiles when CovR regulation was absent, including chemokines IL-8 and CXCL2, which are involved in neutrophil activation and recruitment (Baggiolini et al., 1994). Additionally, vaginal epithelial cells responded to GBS infection with increased production of proinflammatory factors such as IL-1β, which promotes neutrophil recruitment and bacterial clearance (Miller et al., 2007), GM-CSF (CSF2), which controls the production, differentiation, and function of granulocytes, macrophages and other leukocytes (Zhan et al., 2012), and oxidized low density lipoprotein (lectin-like) receptor 1 (OLR1), a leukocyte cell adhesion molecule (Honjo et al., 2003). Furthermore, GBS infection stimulated an increased production of extracellular matrix modifiers, including those associated with inflammation and epithelial disruption such as VEGF, MMP1, MMP3, MMP9, plasminogen activators and HAS3 (Mannelqvist et al., 2011, Yang et al., 2011, Noel et al., 2012, Rilla et al., 2012, Rodriguez-Flores et al., 2012). While these proteins are associated with inflammation and neutrophil migration, their expression may also allow GBS access to underlying tissues and bloodstream by disruption of the epithelial barrier.
CovR regulates many secreted or cell wall and envelope-associated GBS factors (Lamy et al., 2004, Lembo et al., 2010); thus logically, CovR regulation may impact host cell interactions. We have shown previously that the A909 CovR deficient strain exhibits increased adherence and decreased invasion in brain endothelium and lung epithelium (Lembo et al., 2010). Although the exact mechanism is not known, we reveal that loss of CovR in two different GBS serotypes, Ia and III, promotes adherence to and limits invasion of vaginal epithelial cells independent of cylE expression. Additionally, we observed that WT GBS adherence and invasion levels can be restored by complementing the CovR deficient strain. However, the invasion phenotype was not completely rescued, which may suggest a more complex regulation of this function. Consistent with our results, a recent study demonstrated that a CsrR/S (CovR/S) deficient strain exhibited increased adherence to epithelial and abiotic surfaces (Park et al., 2012). Environmental signals such as vaginal pH may alter CovR regulation as neutral, and not acidic, pH enhanced GBS binding to vaginal epithelial cells, and this was partially dependent on CsrR/S (CovR/S) (Park et al., 2012). Furthermore, CovR/S down regulates virulence factors such as cylE and scpB (a C5a peptidase) in acidic pH (Santi et al., 2009). The enhanced adherence observed in the CovR-deficient strain may prove detrimental to colonization, as increased host-microbe interaction may provoke the increased immune activation and cytokine production that we observed. Additionally, host intracellular responses may also explain the decreased invasion seen in ΔcovR and ΔcovR/ΔcylE mutants (Fig. 3B), because it has been demonstrated that, although a CovR deficient mutant was phagocytosed more efficiently by macrophages, it exhibited decreased ability to survive intracellularly (Cumley et al., 2012). Therefore, we cannot exclude the possibility that CovR regulation effects both bacterial invasion and intracellular survival, which consequently may impact colonization.
Other CovR-regulated factors, FbsA and FbsB, which are fibrinogen binding proteins, have been shown to contribute to bacterial attachment and invasion of other host cells (Gutekunst et al., 2004, Schubert et al., 2004, Tenenbaum et al., 2005). In the A909ΔcovR mutant, these genes are highly expressed; transcription of FbsA (SAK_1142) was increased 25-fold, and FbsB (SAK_0955) and an adjacent gene (SAK_0956) encoding a hypothetical protein was increased 151-fold and 157-fold respectively (Lembo et al., 2010). We constructed a mutant lacking FbsB and the adjacent gene (ΔfbsB/Δ0956), and subsequent analysis revealed that it exhibited similar levels of vaginal cell adherence and invasion to the WT strain (data not shown), and further, it was not cleared from the mouse vagina as readily as the ΔcovR mutant (Suppl. Fig. 4C). This suggests that these factors are not responsible for the altered adherence and invasion phenotype of the CovR deficient strain. However, fewer ΔfbsB/Δ0956 bacteria were recovered from the mouse vaginal tract compared to WT (Suppl. Fig. 4C) indicating fibrinogen binding may contribute to GBS colonization in vivo.
Murine chemokines KC (CXCL1) and MIP-2 (CXCL2) are both functional homologs of human CXCL8 (IL-8) and bind to CXCr2 (IL-8 receptor homolog) on neutrophils, resulting in their migration to the site of chemokine production (Olson et al., 2002). To determine the functional role of chemokine signaling during GBS colonization, we utilized CXCr2 KO mice in our murine vaginal model and observed no difference in cytokine levels between GBS WT or ΔcovR groups, starkly contrasting our results in CD1 and BALB/c mice. Cytokine levels in CXCr2 KO mice steadily increased in vaginal lavage over the time course examined when compared to BALB/c mice, indicating that chemokine production was not directly affected by the lack of the receptor. Steady increase of MIP-2 levels in CXCr2 KO mice has been observed in other models of pathogenic infection and is believed to occur because of lack of effective down-regulation and/or continued stimuli (Hang et al., 2000); KC was not examined in this particular study. Of note, CXCr2 KO mice did not have neutrophils visible in vaginal lavage fluid either prior to or during the experimental period when viewed under the microscope, whereas neutrophils were present in the control mice, both prior to inoculation as part of the estrous cycle, and following GBS inoculation (Suppl. Fig. 5). This supports previous work showing MIP-2 expression is necessary to recruit neutrophils to the vaginal lumen during the normal estrous cycle (Sonoda et al., 1998). This data also suggests that the increased cytokine levels observed in BALB/c mice treated with ΔcovR may be explained by paracrine signaling and secretion by neutrophils that have infiltrated the vaginal epithelium.
Within the vaginal tract, mucosal epithelial immunity is tightly controlled by the estrous cycle, with sex steroids affecting all aspects of innate and adaptive immunity, whether directly or indirectly (Hickey et al., 2011). In human vaginal fluid samples, IL-8 and IL-1β production positively correlate with pattern-recognition proteins, and these cytokines levels vary with stage of estrous cycle (Macneill et al., 2012). In the mouse, neutrophils are recruited after the estrogen peak in each estrous cycle with MIP-2 (CXCL2) controlling the majority of neutrophil migration (Sonoda et al., 1998). KC (CXCL1) and IL-1β are also implicated in this process (Hickey et al., 2012). In our mouse model of vaginal colonization, we have observed GBS persist for several weeks (Sheen et al., 2011), or up to several months (Patras, Doran, unpublished data), depending on the stage of estrus, estradiol treatment regime, or specific mouse strain used. We have observed nearly 100% colonization of mice within the first 24 hours post-inoculation, and subsequently, GBS bacterial strain, immune response, and normal flora determine the course of persistence (Patras, Doran, unpublished data). Furthermore, we have also observed that mice were either chronically or intermittently colonized, mimicking human GBS persistence (Yow et al., 1980). We recognize that there are many differences between humans and our murine model counterparts including vaginal pH, normal flora, length of estrous cycle, and immune repertoire. However, given the success of other vaginal disease mouse models (Furr et al., 1989, Jerse, 1999, Escario et al., 2010), and our findings thus far, we believe this model has useful applications in studying host-GBS interactions in the vaginal environment. Herein, we show CovR deficiency hinders GBS persistence in vivo, coinciding with increased innate immune markers known to recruit neutrophils, which have been described as first responders in other GBS infection models (Doran et al., 2003). To our knowledge, this is the first time vaginal immune response to GBS has been characterized in vivo.
In summary, we have shown that CovR deficiency provokes increased inflammatory response both in vitro and in vivo, as well as increased adherence to a vaginal epithelial cell line. This heightened response may contribute to the decreased persistence observed in our mouse model of colonization. Finally, a functional chemokine receptor, CXCr2, may contribute to the differential increase in host immune signaling pathways in response to loss of CovR regulation in vivo. Collectively, our work indicates that GBS virulence regulation by the CovR/S two-component system is critical for niche establishment and maintaining a commensal state in the female vaginal tract.
Supplementary Material
Acknowledgements
This work was supported by the SDSU University Grant Program, the MBRS/IMSD Program 2R25GM058906, a Howell-CSUPERB Research Scholar Award to E.M.F. and A.J., a Merit grant from the Department of Veterans Affairs to J.F., Grant RO1 AI070749 and RO1 AI100989 from the NIH to L.R, and grant RO1 NS051247 from the NIH to K.S.D. The host microarray analysis was performed at the Biogem Core Facility of the University of California San Diego, director Gary Hardiman. We thank Wan-Jung Lin and Kellie Burnside for construction of the CovR-complemented strain, and Sharon Okamoto for the breeding and maintenance of CXCr2 KO mice.
Footnotes
The authors declare no conflict of interest.
References
- Baker CJ, Edwards MS. Group B streptococcal infections. In: Remington JS, Klein JO, editors. Infectious Diseases of the Fetus and Newborn Infant. Philadelphia, PA: W.B. Saunders; 2001. pp. 1091–1156. [Google Scholar]
- Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines--CXC and CC chemokines. Advances in immunology. 1994;55:97–179. [PubMed] [Google Scholar]
- Banerjee A, Kim BJ, Carmona EM, Cutting AS, Gurney MA, Carlos C, et al. Bacterial Pili exploit integrin machinery to promote immune activation and efficient blood-brain barrier penetration. Nature communications. 2011;2:462. doi: 10.1038/ncomms1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier D, Gross R. Regulation of bacterial virulence by two-component systems. Current opinion in microbiology. 2006;9:143–152. doi: 10.1016/j.mib.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Borges S, Silva J, Teixeira P. Survival and biofilm formation by Group B streptococci in simulated vaginal fluid at different pHs. Antonie van Leeuwenhoek. 2012;101:677–682. doi: 10.1007/s10482-011-9666-y. [DOI] [PubMed] [Google Scholar]
- Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. The Journal of investigative dermatology. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- Caligioni CS. Assessing reproductive status/stages in mice. Current protocols in neuroscience / editorial board, Jacqueline N. Crawley … [et al.] 2009;Appendix 4 doi: 10.1002/0471142301.nsa04is48. Appendix 4I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffin DO, Rubens CE. Blue/white screening of recombinant plasmids in Gram-positive bacteria by interruption of alkaline phosphatase gene (phoZ) expression. Gene. 1998;219:91–99. doi: 10.1016/s0378-1119(98)00396-5. [DOI] [PubMed] [Google Scholar]
- Cheng Q, Nelson D, Zhu S, Fischetti VA. Removal of group B streptococci colonizing the vagina and oropharynx of mice with a bacteriophage lytic enzyme. Antimicrobial agents and chemotherapy. 2005;49:111–117. doi: 10.1128/AAC.49.1.111-117.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox F, Taylor L, Eskew EK, Mattingly SJ. Prevention of group B streptococcal colonization and bacteremia in neonatal mice with topical vaginal inhibitors. The Journal of infectious diseases. 1993;167:1118–1122. doi: 10.1093/infdis/167.5.1118. [DOI] [PubMed] [Google Scholar]
- Cumley NJ, Smith LM, Anthony M, May RC. The CovS/CovR acid response regulator is required for intracellular survival of group B Streptococcus in macrophages. Infection and immunity. 2012;80:1650–1661. doi: 10.1128/IAI.05443-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran KS, Chang JC, Benoit VM, Eckmann L, Nizet V. Group B streptococcal beta-hemolysin/cytolysin promotes invasion of human lung epithelial cells and the release of interleukin-8. The Journal of infectious diseases. 2002;185:196–203. doi: 10.1086/338475. [DOI] [PubMed] [Google Scholar]
- Doran KS, Engelson EJ, Khosravi A, Maisey HC, Fedtke I, Equils O, et al. Blood-brain barrier invasion by group B Streptococcus depends upon proper cell-surface anchoring of lipoteichoic acid. The Journal of clinical investigation. 2005;115:2499–2507. doi: 10.1172/JCI23829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran KS, Liu GY, Nizet V. Group B streptococcal beta-hemolysin/cytolysin activates neutrophil signaling pathways in brain endothelium and contributes to development of meningitis. The Journal of clinical investigation. 2003;112:736–744. doi: 10.1172/JCI17335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran KS, Nizet V. Molecular pathogenesis of neonatal group B streptococcal infection: no longer in its infancy. Molecular microbiology. 2004;54:23–31. doi: 10.1111/j.1365-2958.2004.04266.x. [DOI] [PubMed] [Google Scholar]
- Engleberg NC, Heath A, Miller A, Rivera C, DiRita VJ. Spontaneous mutations in the CsrRS two-component regulatory system of Streptococcus pyogenes result in enhanced virulence in a murine model of skin and soft tissue infection. The Journal of infectious diseases. 2001;183:1043–1054. doi: 10.1086/319291. [DOI] [PubMed] [Google Scholar]
- Escario A, Gomez Barrio A, Simons Diez B, Escario JA. Immunohistochemical study of the vaginal inflammatory response in experimental trichomoniasis. Acta tropica. 2010;114:22–30. doi: 10.1016/j.actatropica.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Fichorova RN, Rheinwald JG, Anderson DJ. Generation of papillomavirus-immortalized cell lines from normal human ectocervical, endocervical, and vaginal epithelium that maintain expression of tissue-specific differentiation proteins. Biology of reproduction. 1997;57:847–855. doi: 10.1095/biolreprod57.4.847. [DOI] [PubMed] [Google Scholar]
- Furr PM, Hetherington CM, Taylor-Robinson D. The susceptibility of germ-free, oestradiol-treated, mice to Mycoplasma hominis. Journal of medical microbiology. 1989;30:233–236. doi: 10.1099/00222615-30-3-233. [DOI] [PubMed] [Google Scholar]
- Gutekunst H, Eikmanns BJ, Reinscheid DJ. The novel fibrinogen-binding protein FbsB promotes Streptococcus agalactiae invasion into epithelial cells. Infection and immunity. 2004;72:3495–3504. doi: 10.1128/IAI.72.6.3495-3504.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang L, Frendeus B, Godaly G, Svanborg C. Interleukin-8 receptor knockout mice have subepithelial neutrophil entrapment and renal scarring following acute pyelonephritis. The Journal of infectious diseases. 2000;182:1738–1748. doi: 10.1086/317599. [DOI] [PubMed] [Google Scholar]
- Hensler ME, Liu GY, Sobczak S, Benirschke K, Nizet V, Heldt GP. Virulence role of group B Streptococcus beta-hemolysin/cytolysin in a neonatal rabbit model of early-onset pulmonary infection. The Journal of infectious diseases. 2005;191:1287–1291. doi: 10.1086/428946. [DOI] [PubMed] [Google Scholar]
- Hickey DK, Fahey JV, Wira CR. Mouse estrous cycle regulation of vaginal versus uterine cytokines, chemokines, alpha-/beta-defensins and TLRs. Innate immunity. 2012 doi: 10.1177/1753425912454026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey DK, Patel MV, Fahey JV, Wira CR. Innate and adaptive immunity at mucosal surfaces of the female reproductive tract: stratification and integration of immune protection against the transmission of sexually transmitted infections. Journal of reproductive immunology. 2011;88:185–194. doi: 10.1016/j.jri.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho YR, Li CM, Yu CH, Lin YJ, Wu CM, Harn IC, et al. The enhancement of biofilm formation in Group B streptococcal isolates at vaginal pH. Medical microbiology and immunology. 2012 doi: 10.1007/s00430-012-0255-0. [DOI] [PubMed] [Google Scholar]
- Honjo M, Nakamura K, Yamashiro K, Kiryu J, Tanihara H, McEvoy LM, et al. Lectin-like oxidized LDL receptor-1 is a cell-adhesion molecule involved in endotoxin-induced inflammation. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:1274–1279. doi: 10.1073/pnas.0337528100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerse AE. Experimental gonococcal genital tract infection and opacity protein expression in estradiol-treated mice. Infection and immunity. 1999;67:5699–5708. doi: 10.1128/iai.67.11.5699-5708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Park SE, Yadav P, Paoletti LC, Wessels MR. Regulation and function of pilus island 1 in group B streptococcus. Journal of bacteriology. 2012;194:2479–2490. doi: 10.1128/JB.00202-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang SM, Ishmael N, Dunning Hotopp J, Puliti M, Tissi L, Kumar N, et al. Variation in the group B Streptococcus CsrRS regulon and effects on pathogenicity. Journal of bacteriology. 2008;190:1956–1965. doi: 10.1128/JB.01677-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koiter TR, Hazenberg MP, van der Schoot P. Regulation of the bacterial microflora of the vagina in cyclic female rats. The Journal of experimental zoology. 1977;202:121–128. doi: 10.1002/jez.1402020114. [DOI] [PubMed] [Google Scholar]
- Lamy MC, Zouine M, Fert J, Vergassola M, Couve E, Pellegrini E, et al. CovS/CovR of group B streptococcus: a two-component global regulatory system involved in virulence. Molecular microbiology. 2004;54:1250–1268. doi: 10.1111/j.1365-2958.2004.04365.x. [DOI] [PubMed] [Google Scholar]
- Larsen B, Markovetz AJ, Galask RP. Relationship of vaginal cytology to alterations of the vaginal microflora of rats during the estrous cycle. Applied and environmental microbiology. 1977;33:556–562. doi: 10.1128/aem.33.3.556-562.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembo A, Gurney MA, Burnside K, Banerjee A, de los Reyes M, Connelly JE, et al. Regulation of CovR expression in Group B Streptococcus impacts blood-brain barrier penetration. Molecular microbiology. 2010;77:431–443. doi: 10.1111/j.1365-2958.2010.07215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu GY, Doran KS, Lawrence T, Turkson N, Puliti M, Tissi L, Nizet V. Sword and shield: linked group B streptococcal beta-hemolysin/cytolysin and carotenoid pigment function to subvert host phagocyte defense. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:14491–14496. doi: 10.1073/pnas.0406143101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macneill C, de Guzman G, Sousa GE, Umstead TM, Phelps DS, Floros J, et al. Cyclic changes in the level of the innate immune molecule, surfactant protein-a, and cytokines in vaginal fluid. Am J Reprod Immunol. 2012;68:244–250. doi: 10.1111/j.1600-0897.2012.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madoff LC, Michel JL, Kasper DL. A monoclonal antibody identifies a protective C-protein alpha-antigen epitope in group B streptococci. Infection and immunity. 1991;59:204–210. doi: 10.1128/iai.59.1.204-210.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisey HC, Doran KS, Nizet V. Recent advances in understanding the molecular basis of group B Streptococcus virulence. Expert reviews in molecular medicine. 2008;10:e27. doi: 10.1017/S1462399408000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannelqvist M, Stefansson IM, Bredholt G, Hellem Bo T, Oyan AM, Jonassen I, et al. Gene expression patterns related to vascular invasion and aggressive features in endometrial cancer. The American journal of pathology. 2011;178:861–871. doi: 10.1016/j.ajpath.2010.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LS, Pietras EM, Uricchio LH, Hirano K, Rao S, Lin H, et al. Inflammasome-mediated production of IL-1beta is required for neutrophil recruitment against Staphylococcus aureus in vivo. J Immunol. 2007;179:6933–6942. doi: 10.4049/jimmunol.179.10.6933. [DOI] [PubMed] [Google Scholar]
- Noel A, Gutierrez-Fernandez A, Sounni NE, Behrendt N, Maquoi E, Lund IK, et al. New and paradoxical roles of matrix metalloproteinases in the tumor microenvironment. Frontiers in pharmacology. 2012;3:140. doi: 10.3389/fphar.2012.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson TS, Ley K. Chemokines and chemokine receptors in leukocyte trafficking. American journal of physiology. Regulatory, integrative and comparative physiology. 2002;283:R7–R28. doi: 10.1152/ajpregu.00738.2001. [DOI] [PubMed] [Google Scholar]
- Park SE, Jiang S, Wessels MR. CsrRS and environmental pH regulate group B Streptococcus adherence to human epithelial cells and extracellular matrix. Infection and immunity. 2012 doi: 10.1128/IAI.00699-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poisson DM, Evrard ML, Freneaux C, Vives MI, Mesnard L. Evaluation of CHROMagar StrepB agar, an aerobic chromogenic medium for prepartum vaginal/rectal Group B Streptococcus screening. Journal of microbiological methods. 2011;84:490–491. doi: 10.1016/j.mimet.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Pritzlaff CA, Chang JC, Kuo SP, Tamura GS, Rubens CE, Nizet V. Genetic basis for the beta-haemolytic/cytolytic activity of group B Streptococcus. Molecular microbiology. 2001;39:236–247. doi: 10.1046/j.1365-2958.2001.02211.x. [DOI] [PubMed] [Google Scholar]
- Puliti M, Nizet V, von Hunolstein C, Bistoni F, Mosci P, Orefici G, Tissi L. Severity of group B streptococcal arthritis is correlated with beta-hemolysin expression. The Journal of infectious diseases. 2000;182:824–832. doi: 10.1086/315773. [DOI] [PubMed] [Google Scholar]
- Rajagopal L, Clancy A, Rubens CE. A eukaryotic type serine/threonine kinase and phosphatase in Streptococcus agalactiae reversibly phosphorylate an inorganic pyrophosphatase and affect growth, cell segregation, and virulence. The Journal of biological chemistry. 2003;278:14429–14441. doi: 10.1074/jbc.M212747200. [DOI] [PubMed] [Google Scholar]
- Rajagopal L, Vo A, Silvestroni A, Rubens CE. Regulation of cytotoxin expression by converging eukaryotic-type and two-component signalling mechanisms in Streptococcus agalactiae. Molecular microbiology. 2006;62:941–957. doi: 10.1111/j.1365-2958.2006.05431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilla K, Pasonen-Seppanen S, Karna R, Karjalainen HM, Torronen K, Koistinen V, et al. HAS3-induced accumulation of hyaluronan in 3D MDCK cultures results in mitotic spindle misorientation and disturbed organization of epithelium. Histochemistry and cell biology. 2012;137:153–164. doi: 10.1007/s00418-011-0896-x. [DOI] [PubMed] [Google Scholar]
- Ring A, Braun JS, Pohl J, Nizet V, Stremmel W, Shenep JL. Group B streptococcal beta-hemolysin induces mortality and liver injury in experimental sepsis. The Journal of infectious diseases. 2002;185:1745–1753. doi: 10.1086/340818. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Flores E, Campuzano J, Aguilar D, Hernandez-Pando R, Espitia C. The response of the fibrinolytic system to mycobacteria infection. Tuberculosis (Edinb) 2012 doi: 10.1016/j.tube.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Ruiz FO, Gerbaldo G, Garcia MJ, Giordano W, Pascual L, Barberis IL. Synergistic effect between two bacteriocin-like inhibitory substances produced by Lactobacilli Strains with inhibitory activity for Streptococcus agalactiae. Current microbiology. 2012;64:349–356. doi: 10.1007/s00284-011-0077-0. [DOI] [PubMed] [Google Scholar]
- Santi I, Grifantini R, Jiang SM, Brettoni C, Grandi G, Wessels MR, Soriani M. CsrRS regulates group B Streptococcus virulence gene expression in response to environmental pH: a new perspective on vaccine development. Journal of bacteriology. 2009;191:5387–5397. doi: 10.1128/JB.00370-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert A, Zakikhany K, Pietrocola G, Meinke A, Speziale P, Eikmanns BJ, Reinscheid DJ. The fibrinogen receptor FbsA promotes adherence of Streptococcus agalactiae to human epithelial cells. Infection and immunity. 2004;72:6197–6205. doi: 10.1128/IAI.72.11.6197-6205.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendi P, Johansson L, Dahesh S, Van-Sorge NM, Darenberg J, Norgren M, et al. Bacterial phenotype variants in group B streptococcal toxic shock syndrome. Emerging infectious diseases. 2009;15:223–232. doi: 10.3201/eid1502.080990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen TR, Jimenez A, Wang NY, Banerjee A, van Sorge NM, Doran KS. Serine-rich repeat proteins and pili promote Streptococcus agalactiae colonization of the vaginal tract. Journal of bacteriology. 2011;193:6834–6842. doi: 10.1128/JB.00094-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelver D, Rajagopal L, Harris TO, Rubens CE. MtaR, a regulator of methionine transport, is critical for survival of group B streptococcus in vivo. Journal of bacteriology. 2003;185:6592–6599. doi: 10.1128/JB.185.22.6592-6599.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda Y, Mukaida N, Wang JB, Shimada-Hiratsuka M, Naito M, Kasahara T, et al. Physiologic regulation of postovulatory neutrophil migration into vagina in mice by a C-X-C chemokine(s) J Immunol. 1998;160:6159–6165. [PubMed] [Google Scholar]
- Sumby P, Whitney AR, Graviss EA, DeLeo FR, Musser JM. Genome-wide analysis of group a streptococci reveals a mutation that modulates global phenotype and disease specificity. PLoS pathogens. 2006;2:e5. doi: 10.1371/journal.ppat.0020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenenbaum T, Bloier C, Adam R, Reinscheid DJ, Schroten H. Adherence to and invasion of human brain microvascular endothelial cells are promoted by fibrinogen-binding protein FbsA of Streptococcus agalactiae. Infection and immunity. 2005;73:4404–4409. doi: 10.1128/IAI.73.7.4404-4409.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Sorge NM, Ebrahimi CM, McGillivray SM, Quach D, Sabet M, Guiney DG, Doran KS. Anthrax toxins inhibit neutrophil signaling pathways in brain endothelium and contribute to the pathogenesis of meningitis. PloS one. 2008;3:e2964. doi: 10.1371/journal.pone.0002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verani JR, McGee L, Schrag SJ. Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010. MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports / Centers for Disease Control. 2010;59:1–36. [PubMed] [Google Scholar]
- Wira CR, Ghosh M, Smith JM, Shen L, Connor RI, Sundstrom P, et al. Epithelial cell secretions from the human female reproductive tract inhibit sexually transmitted pathogens and Candida albicans but not Lactobacillus. Mucosal immunology. 2011;4:335–342. doi: 10.1038/mi.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Zhao Z, Wu R, Lu H, Zhang X, Huan C, et al. Expression and biological relationship of vascular endothelial growth factor-A and matrix metalloproteinase-9 in gastric carcinoma. The Journal of international medical research. 2011;39:2076–2085. doi: 10.1177/147323001103900603. [DOI] [PubMed] [Google Scholar]
- Yow MD, Leeds LJ, Thompson PK, Mason EO, Jr, Clark DJ, Beachler CW. The natural history of group B streptococcal colonization in the pregnant woman and her offspringIColonization studies. American journal of obstetrics and gynecology. 1980;137:34–38. doi: 10.1016/0002-9378(80)90382-8. [DOI] [PubMed] [Google Scholar]
- Zhan Y, Xu Y, Lew AM. The regulation of the development and function of dendritic cell subsets by GM-CSF: more than a hematopoietic growth factor. Molecular immunology. 2012;52:30–37. doi: 10.1016/j.molimm.2012.04.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.